Abstract
Objectives
To analyse antibiotic prescribing behaviour in English primary care with particular regard to which antibiotics are prescribed and for which conditions.
Methods
Primary care data from 2013–15 recorded in The Health Improvement Network (THIN) database were analysed. Records with a prescription for systemic antibiotics were extracted and linked to co-occurring diagnostic codes, which were used to attribute prescriptions to clinical conditions. We further assessed which antibiotic classes were prescribed and which conditions resulted in the greatest share of prescribing.
Results
The prescribing rate varied considerably among participating practices, with a median of 626 prescriptions/1000 patients (IQR 543–699). In total, 69% of antibiotic prescriptions (n = 3 156 507) could be linked to a body system and/or clinical condition. Of these prescriptions, 46% were linked to conditions of the respiratory tract, including ear, nose and throat (RT/ENT); leading conditions within this group were cough symptoms (22.7%), lower respiratory tract infection (RTI) (17.9%), sore throat (16.7%) and upper RTI (14.5%). After RT/ENT infections, infections of the urogenital tract (22.7% of prescriptions linked to a condition) and skin/wounds (16.4%) accounted for the greatest share of prescribing. Penicillins accounted for 50% of all prescriptions, followed by macrolides (13%), tetracyclines (12%) and trimethoprim (11%).
Conclusions
The majority of antibiotic prescriptions in English primary care were for infections of the respiratory and urinary tracts. However, in almost one-third of all prescriptions no clinical justification was documented. Antibiotic prescribing rates varied substantially between practices, suggesting that there is potential to reduce prescribing in at least some practices.
Introduction
Antimicrobial resistance (AMR) compromises the effective treatment of bacterial infections and represents a global threat to public health.1,2 Antibiotic consumption is a key driver of the development and spread of AMR, and prudent antibiotic prescribing has been identified as an important strategy to curb this problem.3 Prudent prescribing includes avoiding unnecessary prescriptions, delaying prescriptions when possible, favouring narrow-spectrum over broad-spectrum antibiotics and optimizing treatment duration.4 Antimicrobial stewardship interventions can facilitate more-prudent antibiotic prescribing, but identifying specific challenges and goals for prescribing improvement in any given setting requires a thorough understanding of prescribing behaviour.
In 2016 the UK government set a target to halve inappropriate antibiotic prescribing by 2020.5 Primary care is a natural target for antimicrobial stewardship interventions, in part because outpatients are frequently prescribed antibiotics for self-limiting and/or non-bacterial infections,6 and because primary care accounts for approximately three-quarters of human antibiotic prescriptions in the UK.2 However, an up-to-date inventory of antibiotic prescribing in English primary care is lacking, yet is a prerequisite to identify and quantify potentials for improving prescribing in line with government ambitions. Although antibiotic prescribing in English primary care has been studied extensively (including the main indications for antibiotic prescribing and the specific drugs used to treat them6–10), most of the studies were conducted some years ago, and it is unclear how prescribing has shifted as a consequence of changing prescribing guidelines and various initiatives to reduce the unnecessary use of antibiotics.
The aim of this article is to provide thorough insight into the current use of antibiotics in English primary care. Here, we detail antibiotic prescribing by: (i) age; (ii) antibiotic class; (iii) body system and condition; and (iv) acute versus overall use.
Methods
Ethics
The Health Improvement Network (THIN) data were used for this work. The data collection scheme for THIN is approved by the UK Multicentre Research Ethics Committee (reference number 07H1102103). In accordance with this approval, the study protocol was reviewed and approved by an independent Scientific Review Committee (reference numbers 16THIN071 and 16THIN071-A1).
Database
Antibiotic prescribing events were extracted from THIN, a primary care electronic database that contains anonymized patient, prescribing practice, and consultation data. The database is representative (∼7% coverage) of the general UK population and rates of consultation and prescribing are similar to national estimates.11,12 We included data from English general practices that participated in THIN and provided data for at least one full calendar year between 1 January 2013 and 31 December 2015. Prescribing data were collected for a 3 year period to more accurately assess average annual prescribing rates. Patients of all ages were included in this study, provided that each individual patient’s record contained valid data on birthdate and practice registration. Patients were split into three age groups: children (aged <19 years), adults (19–64 years) and the elderly (≥65 years).
Selection and grouping of antibacterial drugs
We restricted our analyses to systemic antibiotics listed in the British National Formulary (BNF),13 chapter 5.1, excluding topical antibiotics as well as antituberculosis and antileprosy drugs. We used the Anatomical Therapeutic Chemical (ATC) classification system14 to group antibiotics, which facilitates international comparisons.12
Selection and grouping of diagnostic codes
To facilitate analysis of the vast number of different Read codes (the standard clinical diagnostic codes used in UK general practice) in the data, Read codes were organized using two different hierarchical groups. First, as in the BNF, Read codes were mapped to body systems (e.g. gastrointestinal, skin, cardiovascular, etc.). Second, within each body system, Read codes were mapped to ‘conditions’: concrete diagnoses or, if not possible, other broad diagnostic categories such as ‘symptoms’ and ‘examinations’ (for details see Supplementary data Figures S1–S4, available at JAC Online). Using this system, in the absence of a diagnosis, vague yet clinically informative Read codes (e.g. ‘miscellaneous urinary symptoms’) could still be mapped to conditions. However, some Read codes were clinically uninformative (e.g. ‘had a chat with patient’) and were not mapped to body systems or conditions.
Linking prescriptions to diagnostic codes
Antibiotic prescriptions are not automatically linked to Read codes in THIN, and so two linking algorithms were developed. The first algorithm (used for all analyses presented here) linked all prescriptions of systemic antibiotics: (i) to Read codes entered on the same day as the prescription for the respective patients; and/or (ii) with the same consultation identifier, given that the Read codes were entered on or before the date of the prescription. A second, more comprehensive algorithm was developed to capture additional prescriptions that could not be linked to a condition using the baseline algorithm. This second algorithm used consultation and prescribing data in three ways in the following order: (i) if a prescription was part of a sequence of repeat prescriptions, it was linked to Read codes with the same date as the first antibiotic prescribed in the sequence; (ii) if not, Read codes coinciding with the same antibiotic prescribed within the previous 30 days were used; (iii) and lastly, if no Read code could be found in the previous steps, any codes dated <8 days before the prescription were used to capture delayed prescribing. For all analyses, except where noted in the detailed subdivision of antibiotics and conditions, we assumed that nitrofurantoin prescriptions with or without a diagnostic code were attributed to urinary tract infection (UTI), since nitrofurantoin is exclusively prescribed for management of UTI.
Multiple Read codes are often entered for the same patient and the same consultation. If a code describing a diagnosis co-occurred with a code describing symptoms or examinations within the same body system (e.g. ‘acute otitis media’ co-occurring with ‘ear pain’), precedence was given to the diagnosis. However, Read codes can coincide without being causally linked, such as when a patient consults a prescriber for multiple reasons during the same consultation (e.g. if a patient consulted with depression and UTI, both the depression and UTI codes would be documented for that consultation). To minimize false associations between antibiotic prescriptions and unrelated codes, we extracted for analysis only Read codes that were potentially related to antibiotic prescribing. However, some prescriptions were still linked to multiple Read codes from different body systems that could potentially underlie antibiotic prescribing, and so a ‘multiple body system’ group was created to identify when a specific body system could not be determined.
Analyses
The annual prescribing rate for each practice was determined by dividing the total number of antibiotic prescriptions by the number of registered patients at 1 July. Proportions of the total number of prescriptions were established for (i) antibiotic class, (ii) condition and (iii) age group. These analyses were performed separately for (i) all prescriptions and (ii) only acute prescriptions. Acute prescriptions were isolated by excluding prescriptions: (i) explicitly coded as a repeat prescription; (ii) that were part of a prescribing sequence where the same antibiotic was prescribed every month for at least 6 months; or (iii) that covered >162 exposure days over a period of 180 days; and (iv) that were preceded by an antibiotic prescription in the 30 days before, unless that prescription was made for another body system.
Results
Between 2013 and 2015, 4.57 million antibiotic prescriptions were issued in 349 practices in 2013, 285 practices in 2014 and 191 practices in 2015. Overall prescribing rates were 659, 654 and 607 per 1000 registered patients for 2013, 2014 and 2015, respectively. On average 30.3% of registered patients received at least one antibiotic prescription per year.
The median prescribing rate among participating practices was 626 prescriptions/1000 patients (IQR 543–699). The mean age of patients receiving antibiotics was 47 years and 62.6% of the antibiotics were prescribed to female patients. Antibiotic prescribing rates were highest in the elderly (aged ≥65 years), but approximately half of all antibiotics were prescribed to adults aged 19–64 years (Table 1).
Table 1.
Yearly antibiotic prescribing rates (number of prescriptions per 1000 mid-year registered patient population) by age group
| No. prescriptions/1000 registered patients in indicated age groups |
|||
|---|---|---|---|
| Year | <19 years | 19–64 years | ≥65 years |
| 2013 | 580 | 542 | 1138 |
| 2014 | 568 | 533 | 1145 |
| 2015 | 497 | 491 | 1113 |
| Total (%) 2013–15 | 849 539 (18.6) | 2 241 664 (49.0) | 1 483 170 (32.4) |
Practices that discontinued contribution to the database were comparable to practices with continuing contribution. Practices that participated in THIN for the whole period (n = 191) had, in 2013, a median of 7956 registered patients and a median prescribing rate of 661/1000 patients; practices that discontinued participation during this period (n = 158) had a median of 7831 patients and a median prescribing rate of 648 prescriptions/1000 patients.
Antibiotic prescriptions by antibiotic class
The distribution of antibiotic prescriptions by antibiotic class is shown in Tables 2 and 3. About half of all antibiotics prescribed were penicillins (50.3% in 2013 and 48.8% in 2015; Table 2), of which ∼55% was amoxicillin (Table 3). The proportion of penicillins was even higher in acute prescriptions (59.2% of all prescriptions in 2013 and 58.4% in 2015). The proportion of prescriptions for nitrofurantoin increased year on year.
Table 2.
Prescriptions by year and antibiotic class, comparing acute prescriptions (no repeat prescriptions, no other antibiotic prescriptions 30 days earlier and no long-term/prophylactic prescriptions) and all prescriptions
| Antibiotic prescriptions in indicated year (%) |
|||||||
|---|---|---|---|---|---|---|---|
| acute |
all |
||||||
| Antibiotic class | ATC code | 2013 | 2014 | 2015 | 2013 | 2014 | 2015 |
| Penicillinsa | J01C | 59.2 | 59.4 | 58.4 | 50.3 | 49.9 | 48.8 |
| Macrolides, lincosamides and streptogramins | J01F (99% macrolide) | 12.0 | 11.9 | 11.8 | 13.4 | 13.5 | 13.4 |
| Sulphonamides and trimethoprim | J01E (97% trimethoprim) | 10.6 | 10.9 | 10.8 | 11.0 | 11.3 | 11.2 |
| Tetracyclines | J01A | 7.6 | 7.4 | 8.0 | 11.7 | 11.7 | 12.4 |
| Nitrofurantoin | J01XE01 | 4.5 | 4.8 | 5.4 | 5.9 | 6.2 | 6.8 |
| Other β-lactam antibacterials | J01D (91% cefalexin) | 2.0 | 1.7 | 1.6 | 3.1 | 2.9 | 2.8 |
| Quinolones | J01M (93% ciprofloxacin) | 1.7 | 1.6 | 1.7 | 2.2 | 2.2 | 2.2 |
| Others | 2.4 | 2.3 | 2.3 | 2.4 | 2.3 | 2.4 | |
The breakdown by class of penicillin is shown in Table 3.
Table 3.
Penicillin prescriptions by year and class, comparing acute prescriptions (no repeat prescriptions, no other antibiotic prescriptions 30 days earlier and no long-term/prophylactic prescriptions) and all prescriptions
| Penicillin prescriptions in indicated year (%) |
|||||||
|---|---|---|---|---|---|---|---|
| acute |
all |
||||||
| Penicillin class | ATC code | 2013 | 2014 | 2015 | 2013 | 2014 | 2015 |
| Extended-spectrum penicillins | J01CA (99% amoxicillin) | 58.4 | 58.5 | 58.0 | 55.1 | 55.3 | 55.0 |
| β-Lactamase-sensitive penicillins | J01CE (99% phenoxymethylpenicillin) | 11.0 | 11.0 | 11.1 | 12.0 | 12.0 | 12.2 |
| β-Lactamase-resistant penicillins | J01CF (100% flucloxacillin) | 22.4 | 22.7 | 23.2 | 22.2 | 22.3 | 22.8 |
| Penicillin combinationsa | J01CR (99% co-amoxiclav) | 8.2 | 7.8 | 7.7 | 10.7 | 10.4 | 10.0 |
Including β-lactamase inhibitors.
The distribution of prescribed antibiotics varied substantially by age group (Tables 4 and 5). Penicillins were the most prescribed antibiotics in all age groups, but accounted for 66.7% of prescriptions in children, compared with 48.7% in adults and 41.9% in the elderly. After penicillins, the most prescribed antibiotics were macrolides in children (15.3%), tetracyclines in adults (14.0%) and trimethoprim in the elderly (15.8%).
Table 4.
Antibiotic prescriptions in patients of different age groups by antibiotic class, comparing acute (no repeat prescriptions, no other antibiotic prescriptions 30 days earlier and no long-term/prophylactic prescriptions) and all prescriptions
| Antibiotic prescriptions in indicated year age groups (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| acute |
all |
||||||||
| Antibiotic class | ATC code | <19 | 19–65 | ≥65 | all | <19 | 19–65 | ≥65 | all |
| Penicillinsa | J01C | 75.2 | 56.4 | 51.7 | 59.1 | 66.7 | 48.7 | 41.9 | 49.8 |
| Macrolides, lincosamides and streptogramins | J01F (99% macrolide) | 13.1 | 12.1 | 10.7 | 11.9 | 15.3 | 13.6 | 12.2 | 13.4 |
| Sulphonamides and trimethoprim | J01E (97% trimethoprim) | 5.9 | 10.3 | 15.5 | 10.7 | 6.4 | 9.9 | 15.8 | 11.1 |
| Tetracyclines | J01A | 3.4 | 9.0 | 8.3 | 7.6 | 7.9 | 14.0 | 10.9 | 11.9 |
| Nitrofurantoin | J01XE01 | 0.8 | 5.1 | 7.4 | 4.8 | 1.0 | 5.8 | 9.8 | 6.2 |
| Other β-lactam antibacterials | J01D (91% cefalexin) | 0.8 | 1.8 | 2.5 | 1.8 | 1.5 | 2.5 | 4.6 | 3.0 |
| Quinolones | J01M (93% ciprofloxacin) | 0.2 | 1.9 | 2.2 | 1.6 | 0.4 | 2.3 | 3.0 | 2.2 |
| Others | – | 0.6 | 3.4 | 1.7 | 2.5 | 0.8 | 3.2 | 1.8 | 2.4 |
The breakdown by class of penicillin is shown in Table 5.
Table 5.
Prescriptions for penicillins in patients of different age groups by antibiotic class, comparing acute (no repeat prescriptions, no other antibiotic prescriptions 30 days earlier and no long-term/prophylactic prescriptions) and all prescriptions
| Antibiotic prescriptions in indicated year age groups (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| acute |
all |
||||||||
| Penicillin class | ATC code | <19 | 19–65 | ≥65 | all | <19 | 19–65 | ≥65 | all |
| Extended-spectrum penicillins | J01CA (99% amoxicillin) | 63.4 | 54.4 | 60.8 | 58.3 | 60.8 | 56.4 | 51.6 | 55.2 |
| β-Lactamase-sensitive penicillins | J01CE (99% phenoxymethylpenicillin) | 17.8 | 11.4 | 2.3 | 11.0 | 18.5 | 5.4 | 12.5 | 12.0 |
| β-Lactamase-resistant penicillins | J01CF (100% flucloxacillin) | 14.2 | 25.3 | 26.8 | 22.7 | 14.6 | 24.7 | 25.0 | 22.3 |
| Penicillin combinationsa | J01CR (99% co-amoxiclav) | 4.6 | 8.9 | 10.1 | 8.0 | 6.1 | 13.5 | 10.9 | 10.5 |
Including β-lactamase inhibitors.
Antibiotic prescriptions by body system and condition
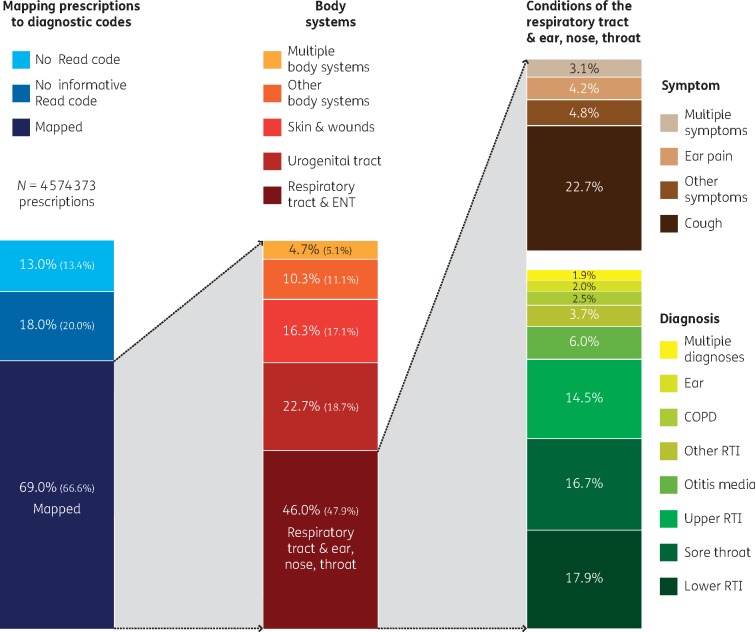
Of all antibiotic prescriptions, 69.0% were linked to one or more clinical conditions (i.e. were linked to a Read code that was mapped to a body system and a diagnosis, symptom, etc. using the hierarchical grouping of Read codes), 18.0% were linked to clinically uninformative codes (e.g. ‘telephone consultation’) and 13.0% could not be linked to any Read code whatsoever (Table 6). Using the second linking algorithm, an additional 6% of prescriptions (total 75%) were linked to a clinically informative Read code, but this did not have a substantial impact on the distribution of antibiotics across body systems (Table S1). Among prescriptions linked to an informative Read code, most were linked to conditions of the respiratory tract including ear, nose and throat (RT/ENT; 46.0%), the urogenital tract (22.7%) and the skin (including wounds) (16.3%). Among specific conditions, antibiotics were prescribed most often for UTI (20.6%), cough (10.4%) and lower respiratory tract infection (RTI) (8.2%) (Table 7).
Table 6.
Prescriptions by body system for acute (no repeat prescriptions, no other antibiotic prescriptions 30 days earlier and no long-term/prophylactic prescriptions) and all prescriptions
| Antibiotic prescriptions (%) |
||||
|---|---|---|---|---|
| acute |
all |
|||
| System the prescription was linked to | total | with informative diagnostic code | total | with informative diagnostic code |
| Informative diagnostic code | 80.6 | – | 69.0 | – |
| respiratory tract (including ENT) | 39.6 | 49.1 | 31.7 | 46.0 |
| urogenital tract | 15.8 | 19.6 | 15.7 | 22.7 |
| skin and wounds | 13.1 | 16.3 | 11.3 | 16.3 |
| other body systems | 8.1 | 10.0 | 7.1 | 10.3 |
| multiple body systems | 4.0 | 5.0 | 3.2 | 4.7 |
| Uninformative diagnostic code | 15.5 | – | 18.0 | – |
| No diagnostic code | 3.9 | – | 13.0 | – |
| Total no. of prescriptions | 3 144 367 | 4 574 373 | ||
Table 7.
Percentage of prescriptions for major conditions of the RT/ENT, urogenital tract and skin and wounds
| Body system/condition | Prescriptions within body system (%) | All linked prescriptions (%) | Acute linked prescriptions (%) |
|---|---|---|---|
| RT/ENT | |||
| cough | 22.7 | 10.4 | 11.1 |
| lower RTI | 17.9 | 8.2 | 8.8 |
| sore throat | 16.7 | 7.7 | 8.6 |
| upper RTI | 14.5 | 6.7 | 7.5 |
| ear-related diagnoses/ symptoms | 12.2 | 5.6 | 6.0 |
| other diagnoses/symptoms | 16.0 | 7.4 | 7.1 |
| Total RT/ENT conditions | 100.0 | 46.0 | 49.1 |
| Urogenital tract | |||
| urinary tract | 90.6 | 20.6 | 17.3 |
| genital tract | 6.7 | 1.5 | 1.7 |
| unspecific urogenital | 2.7 | 0.6 | 0.6 |
| Total urogenital tract conditions | 100.0 | 22.7 | 19.6 |
| Skin and wounds | |||
| boil, cyst, abscess | 13.9 | 2.3 | 2.4 |
| unspecific | 13.2 | 2.2 | 2.3 |
| cellulitis | 12.0 | 2.0 | 1.9 |
| acne | 9.2 | 1.5 | 1.3 |
| ingrown/infected nail | 7.4 | 1.2 | 1.2 |
| bites | 6.3 | 1.0 | 1.1 |
| other diagnoses/symptoms | 25.1 | 4.0 | 4.2 |
| wounds | 12.9 | 2.1 | 1.9 |
| Total skin conditions and wounds | 100.0 | 16.3 | 16.3 |
Table 8 shows how antibiotics grouped by antibiotic class were prescribed for different body systems, including selected conditions of the respiratory tract, urogenital tract and skin. The majority (81.6%) of all linked amoxicillin (in J01CA) prescriptions were prescribed for respiratory tract conditions, and 75.4% of all linked phenoxymethylpenicillin (in J01CE) prescriptions were for sore throat. Large differences between antibiotic classes were observed in the percentage of prescriptions not linked to a useful Read code, and less common or ‘other’ antibiotics were documented particularly poorly. As expected, nitrofurantoin was mainly prescribed for UTI (77.0% of linked prescriptions), with just 3% of prescriptions linked only to a clear non-urogenital condition.
Table 8.
Within antibiotic classes, the percentage of all antibiotic prescriptions by linked condition
| Percentage of total prescriptions per antibiotic class |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT/ENT |
Skin and wounds |
Urogenital tract |
Other and unknown body system |
|||||||||||||||
| Antibiotic or class | ATC code | GI tract | LRTI | URTI excl. sore throat | sore throat | cough | ear | other RTI | skin excl. acne | acne | urinary tract | genital tract | unspecific urogenitala | other body systemsb | misc. codes | multiple body systems | no (informative) diagnostic code | Total no. prescriptions |
| Doxycycline | J01AA02 | 0.3 | 12.6 | 10.5 | 0.7 | 10.1 | 0.8 | 9.1 | 3.7 | 1.7 | 0.6 | 1.3 | 0.8 | 2.0 | 4.3 | 3.4 | 38.1 | 302 879 |
| Oxytetracycline | J01AA06 | 0.3 | 2.0 | 1.1 | 0.3 | 1.9 | 0.3 | 1.4 | 7.0 | 9.3 | 0.3 | 0.2 | 0.1 | 0.6 | 2.4 | 2.3 | 70.5 | 75 754 |
| Other tetracyclines | other J01AA | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.6 | 6.4 | 16.7 | 0.2 | 0.3 | 0.1 | 0.4 | 2.2 | 2.7 | 69.3 | 163 972 |
| Extended-spectrum penicillins | J01CA | 0.7 | 14.5 | 10.4 | 3.3 | 17.3 | 9.8 | 8.2 | 1.3 | 0.0 | 2.3 | 0.2 | 0.2 | 1.5 | 4.3 | 3.7 | 22.3 | 1 257 935 |
| β-Lactamase-sensitive penicillins | J01CE | 0.2 | 0.1 | 2.4 | 54.7 | 0.8 | 0.4 | 1.6 | 4.3 | 0.0 | 0.2 | 0.2 | 0.1 | 0.5 | 4.0 | 3.0 | 27.5 | 274 197 |
| β-Lactamase-resistant penicillins | J01CF | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 1.9 | 0.6 | 56.6 | 0.2 | 0.3 | 0.8 | 0.2 | 2.7 | 7.7 | 3.7 | 24.8 | 509 259 |
| Combinations of penicillins | J01CR | 1.3 | 5.6 | 2.8 | 1.5 | 4.1 | 4.5 | 4.0 | 14.0 | 0.0 | 9.3 | 2.0 | 1.2 | 2.8 | 8.9 | 3.9 | 34.1 | 238 295 |
| Cephalosporins (1st generation) | J01DB | 1.2 | 1.9 | 1.1 | 1.0 | 2.0 | 1.0 | 1.4 | 2.7 | 0.0 | 22.3 | 0.9 | 2.1 | 0.7 | 6.1 | 3.0 | 52.6 | 130 279 |
| Trimethoprim and derivatives | J01EA | 0.3 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 0.6 | 0.2 | 46.1 | 0.5 | 5.3 | 0.3 | 4.2 | 4.0 | 37.4 | 496 114 |
| Erythromycin | J01FA01 | 0.7 | 5. 2 | 5.3 | 9.9 | 8.1 | 5.6 | 3.7 | 15.0 | 2.4 | 0.4 | 0.6 | 0.2 | 1.5 | 5.0 | 3.1 | 33.3 | 225 027 |
| Clarithromycin | J01FA09 | 2.0 | 12.6 | 5.5 | 6.1 | 12.9 | 3.9 | 8.0 | 11.4 | 0.1 | 0.3 | 0.3 | 0.1 | 1.1 | 4.9 | 3.6 | 27.2 | 320 462 |
| Other macrolides | other J01FA | 0.4 | 2.6 | 0.9 | 0.7 | 2.3 | 0.8 | 3.8 | 1.3 | 0.0 | 0.5 | 3.5 | 0.5 | 0.7 | 3.7 | 1.2 | 77.1 | 62 317 |
| Fluoroquinolones | J01MA | 4.1 | 2.7 | 0.6 | 0.2 | 1.9 | 2.4 | 2.2 | 3.2 | 0.0 | 18.9 | 3.5 | 5.9 | 0.9 | 7.3 | 3.2 | 43.0 | 100 323 |
| Imidazole derivatives | J01XD | 9.3 | 0.1 | 0.2 | 0.5 | 0.2 | 0.4 | 0.5 | 8.0 | 0.1 | 1.5 | 19.8 | 2.0 | 4.8 | 10.7 | 3.6 | 38.3 | 93 786 |
| Nitrofurantoinc | J01XE01 | 0.3 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.3 | 0.5 | 0.0 | 42.4 | 0.3 | 4.1 | 0.3 | 3.7 | 3.1 | 44.7 | 284 264 |
| Others | others | 1.0 | 1.3 | 0.5 | 0.4 | 1.3 | 0.7 | 1.8 | 7.0 | 0.0 | 2.4 | 0.5 | 0.3 | 0.7 | 5.4 | 1.1 | 75.6 | 39 510 |
| Total | 0.9 | 6.5 | 4.6 | 5.3 | 7.2 | 3.9 | 4.2 | 10.3 | 1.0 | 10.0 | 1.1 | 1.3 | 1.4 | 5.1 | 3.5 | 33.7 | 4 574 373 | |
GI, gastrointestinal; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection.
Unspecific urogenital: conditions where urinary tract and genital tract could not be distinguished.
Other body systems: includes but is not limited to cardiovascular, dental and prophylactic use.
Unlike in other analyses, nitrofurantoin prescriptions were here not automatically assumed to be for UTI.
Table 9 shows the distribution of different antibiotics that were prescribed to treat a selection of different conditions. RT/ENT conditions were treated with amoxicillin more often than any other antibiotic, except for sore throat, where 61.7% of all prescriptions were for phenoxymethylpenicillin. The distribution of antibiotic prescriptions linked to multiple body systems was markedly similar to the overall distribution of prescriptions. Conversely, the distribution of prescriptions that could not be mapped to a condition [i.e. no (informative) Read code] was substantially different from the overall distribution of prescriptions.
Table 9.
Within diagnoses, the percentage of all antibiotic prescriptions by antibiotic class
| Percentage of total prescriptions by body system/condition |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT/ENT |
Skin and wounds |
Urogenital tract |
Other and unknown body system |
||||||||||||||
| ATC codec | GI tract | LRTI | URTI excl. sore throat | sore throat | cough | ear | other RTI | skin excl. acne | acne | urinary tract | genital tract | unspecific urogenitala | other body systemsb | misc. codes | multiple body systems | no (informative) read code | Overall |
| J01AA02 | 2.3 | 12.8 | 15.1 | 0.9 | 9.3 | 1.3 | 14.2 | 2.4 | 10.7 | 0.4 | 8.4 | 4.1 | 9.5 | 5.6 | 6.5 | 7.5 | 6.6 |
| J01AA06 | 0.5 | 0.5 | 0.4 | 0.1 | 0.4 | 0.1 | 0.6 | 1.1 | 14.9 | 0.0 | 0.3 | 0.1 | 0.8 | 0.8 | 1.1 | 3.5 | 1.7 |
| Other J01AA | 1.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.5 | 2.2 | 57.8 | 0.1 | 1.0 | 0.2 | 1.1 | 1.5 | 2.8 | 7.4 | 3.6 |
| J01CA | 22.7 | 61.4 | 62.1 | 17.1 | 66.0 | 69.0 | 53.3 | 3.6 | 0.2 | 6.4 | 6.2 | 5.3 | 29.4 | 23.3 | 29.4 | 18.0 | 27.5 |
| J01CE | 1.3 | 0.1 | 3.1 | 61.7 | 0.7 | 0.5 | 2.2 | 2.5 | 0.1 | 0.1 | 1.4 | 0.3 | 2.1 | 4.8 | 5.1 | 4.9 | 6.0 |
| J01CF | 1.9 | 0.1 | 0.3 | 0.1 | 0.1 | 5.5 | 1.6 | 61.4 | 1.9 | 0.3 | 8.8 | 1.7 | 21.9 | 17.0 | 12.0 | 8.2 | 11.1 |
| J01CR | 7.9 | 4.5 | 3.1 | 1.5 | 3.0 | 6.0 | 4.9 | 7.1 | 0.2 | 4.9 | 10.0 | 4.8 | 10.4 | 9.1 | 5.8 | 5.2 | 5.2 |
| J01DB | 4.0 | 0.8 | 0.7 | 0.6 | 0.8 | 0.7 | 1.0 | 0.8 | 0.0 | 6.3 | 2.3 | 4.7 | 1.3 | 3.4 | 2.4 | 4.4 | 2.8 |
| J01EA | 4.1 | 0.3 | 0.2 | 0.1 | 0.3 | 0.5 | 0.9 | 0.6 | 1.9 | 50.0 | 4.8 | 44.2 | 2.0 | 9.0 | 12.5 | 12.0 | 10.8 |
| J01FA01 | 3.8 | 3.9 | 5.7 | 9.2 | 5.6 | 7.0 | 4.3 | 7.2 | 11.4 | 0.2 | 2.7 | 0.6 | 5.1 | 4.8 | 4.4 | 4.9 | 4.9 |
| J01FA09 | 15.5 | 13.6 | 8.4 | 8.1 | 12.5 | 7.0 | 13.2 | 7.8 | 0.6 | 0.2 | 2.0 | 0.6 | 5.6 | 6.8 | 7.3 | 5.6 | 7.0 |
| Other J01FA | 0.6 | 0.6 | 0.3 | 0.2 | 0.4 | 0.3 | 1.2 | 0.2 | 0.0 | 0.1 | 4.6 | 0.5 | 0.7 | 1.0 | 0.5 | 3.1 | 1.4 |
| J01MA | 10.1 | 0.9 | 0.3 | 0.1 | 0.6 | 1.3 | 1.1 | 0.7 | 0.0 | 4.1 | 7.2 | 10.0 | 1.3 | 3.2 | 2.0 | 2.8 | 2.2 |
| J01XD | 21.6 | 0.0 | 0.1 | 0.2 | 0.0 | 0.2 | 0.2 | 1.6 | 0.1 | 0.3 | 38.5 | 3.2 | 7.1 | 4.3 | 2.1 | 2.3 | 2.1 |
| J01XE01d | 2.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.4 | 0.3 | 0.0 | 26.3 | 1.5 | 19.5 | 1.4 | 4.5 | 5.6 | 8.3 | 6.2 |
| Others | 0.7 | 0.3 | 0.0 | 0.0 | 0.1 | 0.3 | 0.4 | 0.5 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.9 | 0.5 | 1.9 | 0.9 |
| Total no. prescriptions | 40 510 | 297 246 | 211 025 | 24 2932 | 329 443 | 177 875 | 193 775 | 469 437 | 47 250 | 457 852 | 48 339 | 59 082 | 63 896 | 231 805 | 158 497 | 1 545 409 | 4 574 373 |
GI tract, gastrointestinal tract; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection.
Unspecific urogenital: conditions where urinary and genital tract could not be distinguished.
Other body systems: includes but is not limited to cardiovascular, dental and prophylactic use.
J01AA02, doxycycline; J01AA06: oxytetracycline; other J01AA, other tetracyclines; J01CA, extended-spectrum penicillins; J01CE, β-lactamase-sensitive penicillins; J01CF, β-lactamase-resistant penicillins; J01CR, combinations of penicillins; J01DB, cephalosporins (1st generation); J01EA, trimethoprim and derivatives; J01FA01, erythromycin; J01FA09, clarithromycin; other J01FA, other macrolides; J01MA, fluoroquinolones; J01XD, imidazole derivatives; J01XE01, nitrofurantoin.
Unlike in other analyses, nitrofurantoin prescriptions were here not automatically assumed to be for UTI.
It was found that frequently used first-line antibiotics, in particular penicillins, were comparatively well documented. For instance, among extended-spectrum penicillins, which are dominated by amoxicillin, only 22.3% of prescriptions could not be linked to a diagnostic code (Table 8). Further, amoxicillin was dominant among linked prescriptions, accounting for 32.2% of all prescriptions, but only 17.8% among unlinked prescriptions. First-line antibiotics to treat UTI, i.e. nitrofurantoin and trimethoprim, were less well documented: 44.7% and 37.4% of prescriptions were unlinked to a diagnostic code, respectively. Infrequently used antibiotics were documented particularly poorly; for example, 70.5% of oxytetracycline prescriptions lacked an informative diagnostic code. Of the prescriptions that were linked to one or more diagnostic codes, 80.3% were for acute conditions. By contrast, just 43.0% of prescriptions without a diagnostic code were for acute conditions.
Discussion
Between 2013 and 2015, ∼4.6 million antibiotic prescriptions were registered in the THIN database. Most antibiotics were prescribed for infections of the respiratory and urinary tracts, although almost one-third (31%) of prescriptions could not be mapped to any clinical condition. Amoxicillin was the most commonly prescribed antibiotic in all age groups, but the distribution of prescribed antibiotics varied substantially between children, adults and the elderly. The overall prescribing rate decreased slightly and the overall distribution of prescribed antibiotics remained relatively stable over the 3 years included here.
This study represents an up-to-date assessment of antibiotic prescribing in England and is consistent with findings from previous work. A recent study by Shallcross et al.15 analysed data from THIN from 2011–13 and found a similar antibiotic prescribing rate (0.67 prescriptions per person year) as was found in this study (on average 0.66 prescriptions per patient per year for 2013, the only year with overlap). They also found that, on average, 30.1% of patients were prescribed at least one antibiotic per year, consistent with the 30.3% reported here.
In the current study, a slight increase in the use of nitrofurantoin was observed between 2013 and 2015. This is in line with recent changes in treatment guidelines, which now recommend the use of nitrofurantoin as first-line therapy to treat UTI.16 However, despite this increase in nitrofurantoin use, no decline was observed for trimethoprim (which is another standard treatment option for UTI). Furthermore, among all antibiotic prescriptions linked to a code for UTI (i.e. when not automatically assuming that all nitrofurantoin prescriptions are for UTI, as was done in other analyses), the proportions of prescriptions of trimethoprim (50.0%) and nitrofurantoin (26.3%) were similar to proportions reported by Hawker et al. from 2011 (53.5% and 24.0%, respectively, in England in women aged 16–74 years).9
Using data from 1998-2001, Petersen et al.10 found that the top five conditions for antibiotic prescriptions were RTI including productive cough (15%), upper RTI (14%), sore throat (11%), UTI including UTI symptoms (10%) and acute otitis media (8%). By comparison, here we found the leading condition to be UTI, accounting for 21% of prescriptions linked to a condition. This large difference may in part be explained by our assumption that all nitrofurantoin prescriptions were for UTI (Figure 1). Further, in contrast to Petersen et al.,10 we defined a separate category for all cough-related codes (not only productive cough), which accounted for 10% of all linked prescriptions and may partially, together with the increase in UTI, explain the lower relative share of prescriptions for lower RTI (8%) found in our study. Overall, it is difficult to infer the degree to which these studies differ owing to different methodologies as opposed to true changes in prescribing over time. In both studies, amoxicillin was the most frequently prescribed antibiotic (26.4% of all prescriptions then, 26.8% of all prescriptions in 2015), but our findings suggest that erythromycin (the second most prescribed drug in 1998–2001 with 9.5% of all antibiotic prescriptions, now 4.9%) has been partially replaced by clarithromycin (then 1.9%, now 7.0%). Between 1998–2001 and 2013–15, the most substantial shifts in antibiotic choice seem to have happened for UTI: nitrofurantoin use increased from 5.1% to 26.3%, first-generation cephalosporin use decreased from 19.9% to 6.3%, and slight decreases were also observed in use of trimethoprim (56.1% to 50.0%) and fluoroquinolones (5.9% to 4.1%).
Figure 1.
Mapping prescriptions to Read codes using a hierarchical system that mapped each Read code to (i) a body system and (ii) a condition (with varying specificity, e.g. a specific diagnosis or a general symptom). All columns add up to 100%. Percentage values with large font size assume that nitrofurantoin prescriptions without a linked Read code were used to treat UTI; values in parentheses with smaller font do not make this assumption.
Strengths and limitations
A major strength of this study is the use of recent individual patient data recorded in THIN, a large primary care database that is representative of the UK patient population.12 The extensive mapping of Read codes to body systems and clinical conditions allowed us to provide a more complete overview of prescribing behaviour in English primary care than has been done in previous studies. However, despite this extensive approach, 31% of antibiotic prescriptions could still not be linked to clinically informative information owing to missing or unspecific diagnostic codes (or 25% using our most sensitive algorithm).
Further, it appears that poor documentation of antibiotic prescribing is non-random, because the distribution of antibiotics unlinked to a clinical condition differed substantially from the overall distribution of prescribed antibiotics. Penicillin prescriptions were more likely than any other antibiotic class to be linked to a diagnostic code. Comparatively, prescriptions for first-line UTI treatment (nitrofurantoin, trimethoprim) were less well documented. Prescriptions for rarely used antibiotics and prescriptions issued to complex patients on long-term or repeated treatment were documented particularly poorly. One could speculate that, for some prescribers, an antibiotic with only one (primary) indication can supplant the need for a diagnostic code (e.g. nitrofurantoin or trimethoprim prescription signifying UTI). The lack of diagnostic codes in complex patients could potentially be explained by prescribers storing all relevant information in free text (unavailable to us) instead of using diagnostic codes. Another explanation could be that they prescribed on the advice of hospital clinicians and did not additionally document the indications.
We tried to minimize the risk of including clinical conditions that do not have a causal link to the prescription by prioritizing diagnostic Read codes for specific conditions over more general Read codes or Read codes corresponding to symptoms, and by excluding Read codes for conditions unrelated to antibiotic prescribing (e.g. depression). Despite these efforts, it cannot be ruled out that some prescriptions have been falsely mapped to certain conditions, since our algorithms could not account for every caveat and exception. An illustration of this can be found in Table 8, where 3.0% of nitrofurantoin prescriptions (with diagnostic code) were only linked to non-urogenital diagnoses (more than expected, given that nitrofurantoin is only indicated for use in UTI). Another limitation is the decline in the number of practices participating in THIN from 2013 to 2015. Although the annual variation in prescribing seems low, 2013 may be overrepresented in some results. However, we also showed that practices that withdrew did not differ markedly in their overall prescribing behaviour.
Conclusions
In English primary care, we found that antibiotics were most commonly prescribed to treat RTI (including cough) and UTI, although no clinical justification for prescribing could be determined in 31% of all antibiotic prescriptions. The most commonly prescribed antibiotics were amoxicillin, flucloxacillin and trimethoprim, and the majority of all prescriptions (69%) were for acute conditions. Results from this study can be used to support national and international policy on the reduction of inappropriate antibiotic prescribing, possibly by defining target indications for prescribing reductions. More efforts are needed to explain practice variation in the prescribing of antibiotics.
Supplementary Material
Acknowledgements
The authors are grateful for the support of a group of subject matter experts who formed a Modelling Oversight Group (MOG) that convened bimonthly to discuss and review this work. Group members are listed in another paper of this Supplement.17 J. V. R. is affiliated with the National Institute for Health Research Health Protection Research Units (NIHR HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at both Imperial College London and University of Oxford in partnership with PHE. Further, we thank Sandro Bösch for improving Figure 1 in this paper.
Funding
This paper was published as part of a Supplement supported and resourced by Public Health England (PHE). Only internal resources were used for this paper.
Transparency declarations
J. V. R. is a co-opted member of the UK Government Advisory Committee on Antimicrobial Prescribing, Resistance and Healthcare Associated Infection (APRHAI). All other authors: none to declare.
Supplementary data
Figures S1 to S4 and Table S1 are available as Supplementary data at JAC Online.
References
- 1. O’Neill J; on behalf of the Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: Review on Antimicrobial Resistance, 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf. [Google Scholar]
- 2. Public Health England PHE. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). Report2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/575626/ESPAUR_Report_2016.pdf.
- 3. Goossens H. Antibiotic consumption and link to resistance. Clin Microbiol Infect 2009; 15: 12–5. [DOI] [PubMed] [Google Scholar]
- 4. Spivak ES, Cosgrove SE, Srinivasan A.. Measuring appropriate antimicrobial use: attempts at opening the black box. Clin Infect Dis 2016; 63: 1639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AMR Policy Team. Government Response to the Review on Antimicrobial Resistance. Department of Health; https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/553471/Gov_response_AMR_Review.pdf. [Google Scholar]
- 6. Gulliford M, Latinovic R, Charlton J. et al. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. J Public Health 2009; 31: 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashworth M, Charlton J, Ballard K. et al. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995-2000. Br J Gen Pract 2005; 55: 603–8. [PMC free article] [PubMed] [Google Scholar]
- 8. Ashworth M, Latinovic R, Charlton J. et al. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the general practice research database. J Public Health 2004; 26: 268–74. [DOI] [PubMed] [Google Scholar]
- 9. Hawker JI, Smith S, Smith GE. et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother 2014; 69: 3423–30. [DOI] [PubMed] [Google Scholar]
- 10. Petersen I, Hayward AC; SACAR Surveillance Subgroup. Antibacterial prescribing in primary care. J Antimicrob Chemother 2007; 60 Suppl 1: i43–7. [DOI] [PubMed] [Google Scholar]
- 11. Bourke A, Dattani H, Robinson M.. Feasibility study and methodology to create a quality-evaluated database of primary care data. Inform Prim Care 2004; 12: 171–7. [DOI] [PubMed] [Google Scholar]
- 12. Blak BT, Thompson M, Dattani H. et al. Generalisability of the health improvement network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011; 19: 251–5. [DOI] [PubMed] [Google Scholar]
- 13. Joint Formulary Committee. British National Formulary. London: BMJ Group and Pharmaceutical Press, 2015. [Google Scholar]
- 14. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index https://www.whocc.no/atc_ddd_index/.
- 15. Shallcross L, Beckley N, Rait G. et al. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother 2017; 72: 1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Public Health England. Management of Infection: Guidance for Consultation and Adaptation 2017. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/643046/Management_and_treatment_of_common_infections.pdf.
- 17. Smieszek T, Pouwels KB, Dolk FCK. et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother 2018; 73Suppl 2: ii36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.