Abstract
Objectives
To assess the appropriateness of prescribing systemic antibiotics for different clinical conditions in primary care, and to quantify ‘ideal’ antibiotic prescribing proportions in conditions for which antibiotic treatment is sometimes but not always indicated.
Methods
Prescribing guidelines were consulted to define the appropriateness of antibiotic therapy for the conditions that resulted in antibiotic prescriptions between 2013 and 2015 in The Health Improvement Network (THIN) primary care database. The opinions of subject experts were then formally elicited to quantify ideal antibiotic prescribing proportions for 10 common conditions.
Results
Of the antibiotic prescriptions in THIN, 52.5% were for conditions that could be assessed using prescribing guidelines. Among these, the vast majority of prescriptions (91.4%) were for conditions where antibiotic appropriateness is conditional on patient-specific indicators. Experts estimated low ideal prescribing proportions in acute, non-comorbid presentations of many of these conditions, such as cough (10% of patients), rhinosinusitis (11%), bronchitis (13%) and sore throat (13%). Conversely, antibiotics were believed to be appropriate in 75% of non-pregnant women with non-recurrent urinary tract infection. In impetigo and acute exacerbation of chronic obstructive pulmonary disease, experts clustered into distinct groups that believed in either high or low prescribing.
Conclusions
In English primary care, most antibiotics are prescribed for conditions that only sometimes require antibiotic treatment, depending on patient-specific indicators. Experts estimated low ideal prescribing proportions in many of these conditions. Incomplete prescribing guidelines and disagreement about prescribing in some conditions highlight further research needs.
Introduction
Antibiotics are essential medicines for the treatment and prevention of bacterial infections, but their efficacy is increasingly threatened by the emergence of antimicrobial resistance (AMR).1 The consumption of antibiotics is directly linked to AMR2 and unnecessarily prescribed antibiotics contribute to AMR, patient side effects and economic costs while providing little to no patient benefit. Reducing inappropriate antibiotic prescribing [defined here as unnecessary prescribing, i.e. prescribing that (i) is likely to have marginal, if any, patient benefit and (ii) is outweighed by the potential risks of prescribing] has been identified as a key means of mitigating AMR,3 and at the 2016 G20 summit the UK government committed to halving inappropriate antibiotic prescribing by 2020.4 In England, primary care accounts for approximately three-quarters of human antimicrobial use, representing a clear target for government ambitions to reduce prescribing.1 However, before any such ambitions can be achieved, the appropriateness of prescribing antibiotics for different conditions in English primary care must first be defined.
Inappropriate antibiotic prescribing predominantly occurs in conditions that are often self-limiting and only sometimes caused by bacteria, such as acute respiratory tract infections (RTIs).5 These conditions are among the most common reasons for consultation in UK primary care, but prescribers generally lack the point-of-care testing necessary to reliably determine the aetiology of infection in such patients.4 This is problematic because although a large proportion of these patients will have non-bacterial and/or self-limiting infection, a small minority will have (potentially life-threatening) bacterial infections that require antibiotic treatment. To alleviate diagnostic uncertainty, prescribing guidelines tally symptoms, comorbidities and other patient characteristics to predict which patients are most likely to benefit from antibiotic therapy.6–8
In theory, the appropriateness of antibiotic prescribing could be assessed at the population level by comparing patient data with prescribing guidelines. However, many patient indicators that inform prescribing, such as symptom severity and duration, are not reliably available in primary care databases.9 With this information missing, and given that prescribers sometimes defy guidelines or prescribe for non-medical reasons,10 it is difficult to retrospectively assess the appropriateness of antibiotic prescribing using routinely collected electronic primary care records. Alternatively, population-level inappropriate antibiotic prescribing could be quantified by comparing observed prescribing with benchmark estimates of ‘ideal’ prescribing proportions (i.e. the proportions of patients that should be treated with antibiotics, balancing the competing risks of over- and under-treating patients).
Here, the appropriateness of prescribing antibiotics for different conditions was defined in the context of English primary care. First, prescribing guidelines were consulted to assess the appropriateness of antibiotic therapy for the conditions that resulted in antibiotic prescriptions in a large English primary care database. Then, to address the large proportion of prescriptions given for conditions in which antibiotic therapy is only sometimes appropriate (e.g. depending on symptom severity), an expert elicitation exercise was conducted to quantify ideal population-level prescribing proportions in 10 common conditions. These proportions serve as benchmarks of appropriate prescribing and can be compared with national prescribing data to help quantify the magnitude of inappropriate prescribing in English primary care.
Methods
Ethics
Data from the Health Improvement Network (THIN), a primary care electronic database that contains anonymized patient, prescribing practice, and consultation data covering ∼7% of the general UK population, were used for this work. The data collection scheme for THIN is approved by the UK Multicentre Research Ethics Committee (reference number 07H1102103). In accordance with this approval, the study protocol was reviewed and approved by an independent Scientific Review Committee (reference numbers 16THIN071 and 16THIN071-A1).
Classifying the appropriateness of antibiotic prescribing
Primary care data from English practices registered with THIN between 2013 and 2015 were analysed. In THIN, antibiotic prescriptions are not automatically linked to their corresponding diagnostic information, so algorithms were used to link as many antibiotic prescriptions as possible to body systems and, where possible, specific clinical conditions. These methods are described elsewhere,11 although slightly different assumptions were made for the algorithm used here (see the Supplementary data, available at JAC Online). For each condition that resulted in an antibiotic prescription, the appropriateness of antibiotic therapy was assessed by consulting English primary care prescribing guidelines6–8 (see the list of conditions in Table S1). Accordingly, conditions were grouped into three categories: conditions where antibiotic treatment is (i) appropriate (always justified); (ii) sometimes appropriate (conditionally justified, e.g. depending on symptoms); or (iii) inappropriate (never justified). In any conditions where guidelines were missing or ambiguous, antibiotic appropriateness was determined via consultation with a consultant in infectious disease epidemiology and members of the Primary Care Unit at Public Health England. If a single prescription was linked to multiple conditions, the condition giving the highest chance of appropriate prescribing was assumed to underlie the prescription. Finally, the total number of prescriptions for these three categories was counted.
Estimating ideal antibiotic prescribing proportions
Expert elicitation is a scientific consensus methodology that formally synthesizes the opinions of subject-matter experts to quantify parameter values and their ranges of uncertainty. Here, expert elicitation was used to generate benchmark estimates of ideal prescribing in conditions where guidelines indicate that antibiotic prescribing is only sometimes appropriate. Standardized expert elicitation protocols were followed to maximize the validity and reliability of this exercise.12–15
Elicitations were conducted as one-on-one scripted interviews in which experts estimated the proportions of the English primary care patient population that should be prescribed antibiotics when consulting with uncomplicated presentations of 10 common conditions (see the full expert elicitation methodology in the Supplementary data). The conditions included were those deemed most likely to contribute to inappropriate antibiotic prescribing in UK primary care, as identified by consultation of prescribing guidelines,6–8 a review of the literature5,16–19 and discussions with prescribers and public health professionals; however, with the exception of acute cough, syndromes and indistinct diagnoses (e.g. upper RTI) were excluded to minimize ambiguity. Experts estimated nine additional parameters about optimal treatment and the proportions of patients that have certain symptoms, conditions and comorbidities. (See the list of elicitation questions in Table S2).
To quantify parameter estimates, the ‘chips and bins’ method of parameter estimation was used, in which participants were asked to place probabilistic chips into a range of bins in order to build histograms that reflect their beliefs and uncertainties.13,20 Elicitation interviews were administered remotely by telephone and were facilitated by the MATCH Uncertainty Elicitation Tool, an online app that allows expert and elicitor to simultaneously access and manipulate a grid of chips and bins, and to fit these chips to probability distributions.21 Histograms were built using bins with a 10% range, and hence the most extreme average estimates experts could make—by putting all their chips into the lowest or highest bins—were 5% and 95%, respectively. If an expert remarked that his/her best estimate exceeded these extremes, sensitivity analyses were performed (e.g. for a desired estimate <5%, median estimates of 3% or 1% were assigned).
Potential experts were identified by recommendations from this project’s oversight group and a review of relevant publications in the literature. Experts were evaluated systematically using three inclusion criteria: (i) current academic appointment in the UK, (ii) ≥10 years of research experience; and (iii) ≥10 peer-reviewed publications in the fields of primary care and/or antibiotic prescribing between 1 January 2012 and 31 October 2016, with at least one publication in each field. All identified experts were invited via telephone call and/or e-mail. Prior to their interviews, participants were briefed on the research context, study methodology and common biases to avoid, but they were not provided with any figures or findings from the literature in order to avoid biasing them towards any particular conclusion.14 All participants agreed to be acknowledged as contributors, with their individual responses kept anonymous.
Concurrent with the formal elicitation, a separate online survey of antibiotic prescribers in the UK was also conducted. Prescribers were invited through non-academic channels (e.g. Royal College of General Practitioners newsletter, prescribing bulletins, personal correspondence), with the only inclusion criteria being that they currently prescribe antibiotics in UK general practice. This survey was composed of the same questions and was introduced with the same context and wording as the formal elicitation, but was designed to prioritize brevity and accessibility. Participants made point estimates for each parameter and qualified their uncertainties using a Likert scale. (See full online survey methodology in Supplementary data.)
All data were analysed using R version 3.3.1.22 Final estimates were calculated for each question by arithmetically pooling experts' chips.23 Pooled chips were fitted to continuous probability distributions using the R package fitdistrplus,24 except when expert consensus was not reached (e.g. pooled chips were bimodal), in which case chips were fitted to mixture probability distributions using the R package mixtools.25 From these distributions, final parameter values were estimated as the median and expert uncertainty as the IQR. Parameters were then compared with acceptable antibiotic prescribing ranges recently estimated by the European Surveillance of Antimicrobial Consumption Network (ESAC-Net).26
Results
Appropriateness of prescribing for different conditions
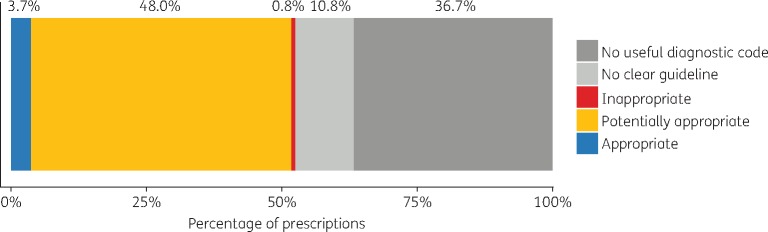
Of the 4 574 373 oral antibiotic prescriptions observed in THIN, 63.3% were linked to a clinical condition. In the remaining 36.7% of prescriptions, the reason for prescribing could not be determined because the prescription was linked to a clinically uninformative diagnostic code (e.g. ‘telephone encounter’, ‘had a chat with patient’) or no code whatsoever. The appropriateness of antibiotic therapy was assessed in 137 of the 177 conditions that resulted in antibiotic prescriptions, accounting for 52.5% of all prescriptions (Figure 1). Respectively, antibiotics were deemed appropriate, sometimes appropriate and inappropriate in 40, 72 and 25 of these conditions. In the remaining 40 conditions (10.8% of all prescriptions), antibiotic appropriateness was ambiguous and could not be assessed, e.g. for ‘miscellaneous symptoms’ or ‘other pain’.
Figure 1.
Appropriateness of antibiotic prescriptions according to guidelines. Approximately half (52.5%) of all antibiotic prescriptions in THIN were for conditions that could be assessed using prescribing guidelines. The vast majority of these (48.0% of all prescriptions) were for conditions where antibiotics are sometimes but not always appropriate, whereas a small proportion were for conditions where antibiotics are always (3.7%) or never (0.8%) appropriate. Appropriateness could not be assessed in 47.5% of prescriptions due to poor/ambiguous diagnostic coding (36.7%) and unclear/missing guidelines (10.8%).
Among the 137 conditions assessed using guidelines, the vast majority of prescriptions (91.4%) were for the 72 conditions for which antibiotics are sometimes appropriate. Of these, the conditions resulting in the most prescriptions were acute cough (15.0% of prescriptions assessed for appropriateness), lower respiratory tract infection (LRTI) (9.5%) and acute sore throat (9.3%). A further 7.0% of assessed prescriptions were for conditions for which antibiotic therapy is always appropriate, with cellulitis (2.6% of assessed prescriptions), chronic urinary tract infection (UTI) (0.7%) and bilateral/otorrhoeal acute otitis media (AOM) (0.6%) being the most common. Finally, 1.5% of assessed prescriptions were for conditions in which antibiotic therapy is never appropriate, the most common being miscellaneous nasal symptoms (0.3% of assessed prescriptions), fungal/viral skin infections (0.2%) and influenza-like illness (0.1%).
Eliciting ideal prescribing proportions
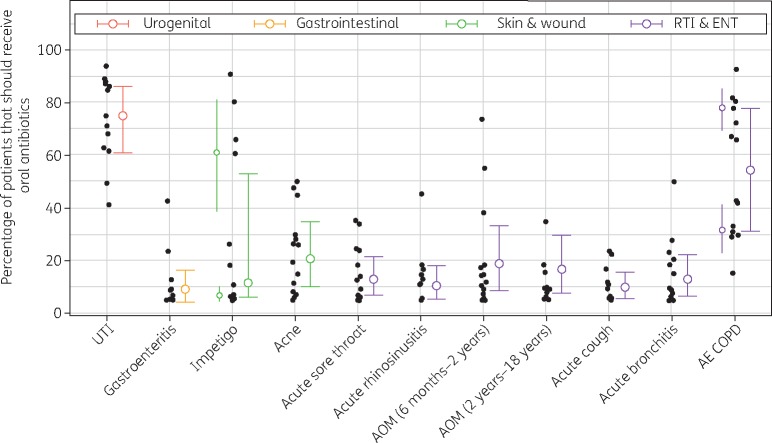
Thirty-five potential academic experts were identified, of whom 23 met our inclusion criteria and were invited to take part. Fourteen participated (response rate: 61%), including academics in primary care, pharmacy, medical microbiology and other fields (Table S3). Pooled expert estimates were fitted to continuous β distributions, except in prescribing proportions of impetigo and acute exacerbation of chronic obstructive pulmonary disease (AE COPD), which were bimodal and fitted to mixture β distributions (Figure 2), and in the duration of acne treatment, which was right-skewed and fitted to a log normal distribution. Ideal antibiotic prescribing proportions were highest in UTI (75%), AE COPD (54%) and acne (21%) (Table 1). For all other conditions included in the elicitation, which combined accounted for 967 313 prescriptions (21% of all prescriptions), experts estimated that <20% of patients presenting with acute illness and without comorbidity should receive an antibiotic prescription. The lowest estimate was in gastroenteritis (9%), although in this particular condition several experts believed that <5% of patients (the lowest allowable estimate) should receive antibiotics. In two sensitivity analyses these experts were assigned median estimates of 3% and 1%, both resulting in final pooled ideal prescribing estimates of 8%. Among other parameters, experts estimated the ideal duration of acne treatment as 14 weeks (Table S4).
Figure 2.
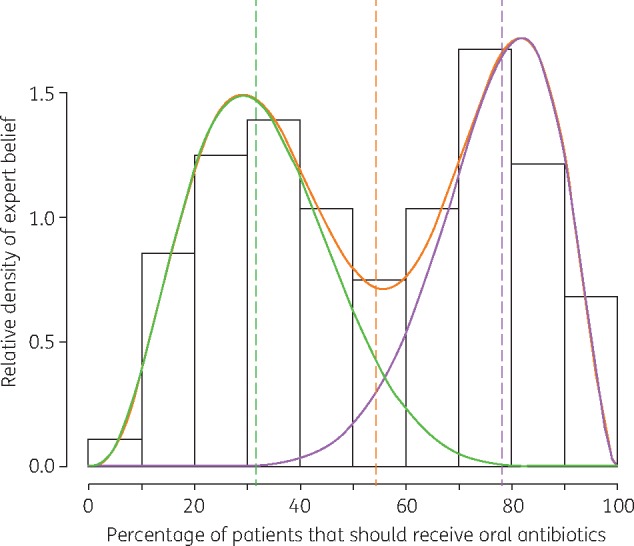
A pooled histogram of experts’ estimates of the percentage of patients that should be prescribed oral antibiotics when presenting to primary care with AE COPD. The component β distributions (green and purple lines) of the mixture distribution (orange line) indicate that experts are divided into two groups: those who believe in high versus low prescribing. Dashed coloured lines represent the medians of each distribution.
Table 1.
Ideal prescribing proportions: the proportions of patients that should receive oral antibiotics when presenting to primary care with different conditions
| Condition | Ideal prescribing proportions (%) |
|
|---|---|---|
| this study (IQR) | ESAC-Net study26 (acceptable range) | |
| Urinary tract infection | ||
| female, aged >14 years, no relevant comorbidities, non-recurrent bacterial infection | 75 (61–86) | |
| female, aged >19 years | 80–100 | |
| Gastroenteritis | ||
| aged >2 years | 9 (4–16) | |
| Impetigo | ||
| all patients | 12 (6–53) | |
| Acne | ||
| patients seeking treatment | 21 (10–35) | |
| Acute sore throat | ||
| no relevant comorbidities | 13 (7–22) | |
| aged >2 years (acute tonsillitis) | 0–20 | |
| Acute rhinosinusitis | ||
| no relevant comorbidities | 11 (5–18) | |
| aged >19 years with acute/ chronic sinusitis | 0–20 | |
| Acute otitis media | ||
| aged 6 months to 2 years, unilateral, non-otorrhoeal | 19 (9–33) | |
| aged 2–18 years, unilateral, non-otorrhoeal | 17 (8–30) | |
| aged >3 years with AOM/myringitis | 0–20 | |
| Acute cough | ||
| no relevant comorbidities | 10 (6–16) | |
| Acute bronchitis | ||
| no relevant comorbidities | 13 (6–22) | |
| aged 18–75 years with acute bronchitis/bronchiolitis | 0–30 | |
| Acute exacerbation of COPD | ||
| all patients | 54 (31–78) | |
There was relative consensus among experts for most prescribing proportions (Figure 3). The lowest IQRs were reported in acute cough (10%), gastroenteritis (12%) and acute rhinosinusitis (13%). By far the least consensus was observed in AE COPD and impetigo, with an IQR of 47% in both conditions. Among conditions with unimodal expert belief, the least consensus was observed in UTI and acne (25% IQR in both), followed by AOM (24% among children aged 6 months to 2 years; 22% among children aged 2–18 years). In general, there was greater expert uncertainty in questions about symptom proportions than prescribing proportions (Table S4).
Figure 3.
Individual and pooled estimates of ideal prescribing proportions. Black dots show each expert’s median estimate for each condition, and coloured dots and bars show median and IQRs for pooled estimates. In impetigo and AE COPD, additional coloured dots and bars to the left of the individual estimates show the median and IQRs of the component distributions that comprise their respective mixture distributions.
When asked to provide qualitative feedback on the elicitation, no experts identified any methodological difficulty, but three reported low confidence in their responses and relevant expertise. A sensitivity analysis was conducted in which these three experts were removed, resulting in median prescribing proportion estimates that differed by <3% from initial estimates, except in AE COPD, which was 5% lower when only confident experts were included, and in impetigo, which was 6% higher (although both distributions remained bimodal).
Prescriber survey
Forty-three prescribers participated in the online survey, the majority of whom were invited via personal correspondence from prescribing advisors, Clinical Commissioning Group representatives and other health professionals (Table S5). Ideal prescribing proportions were highest in UTI and AE COPD (median 80% for each). The majority of prescribers believed that antibiotics should be prescribed in <20% of patients without comorbidity presenting with the respiratory and ear, nose and throat (ENT) conditions included here (excluding AE COPD). In acne and impetigo, most prescribers believed that 20%–50% of patients should receive oral antibiotics. These survey results are consistent with estimates from the formal elicitation (Table S4), with the exception of gastroenteritis, where the median estimate from the online survey (1%) was substantially lower than that of the expert elicitation. In the online survey, gastroenteritis was also the condition with the least variation in prescriber belief, with a range of 10% between the lowest and highest estimates. In all other conditions this range was ≥40%, and in UTI, impetigo and AOM it was ≥90%. Despite this variation, IQRs of prescriber and academic estimates were similar.
Discussion
It is widely believed that antibiotics are overprescribed in general practice,19,27 but the extent of inappropriate prescribing in England is unknown. Here, English prescribing guidelines were used to define the appropriateness of prescribing antibiotics for every condition that resulted in an antibiotic prescription between 2013 and 2015 in a large English primary care database. Among all antibiotic prescriptions for the 137 conditions that could be assessed using guidelines, the vast majority (91.3%) were for conditions where antibiotic need is conditional on clinical characteristics (e.g. symptom severity), meaning that the appropriateness of these prescriptions cannot be known without detailed patient data. To generate benchmark estimates of ideal prescribing proportions in uncomplicated presentations of 10 of these conditions, the professional opinions of established academic experts in UK primary care and antibiotic prescribing were quantified using formal expert elicitation methodology. The resulting parameters represent informed estimates of ideal antibiotic prescribing that have been used to help quantify inappropriate antibiotic prescribing in England.28,29
Although many conditions only sometimes require antibiotic treatment, clinical studies and reviews rarely report ideal prescribing proportions. In acute sore throat, antibiotics may confer some symptom relief,30 but the proportion of patients that are clinically indicated for antibiotics due to the duration, number and severity of their symptoms is not known. In this study, experts estimated that 13% of acute sore throat patients without comorbidity should receive antibiotics. Similarly, in AOM, children with bilateral or otorrhoeal or prolonged infection are most likely to benefit from antibiotics,31–33 but the proportion of patients that have any such indicator for prescribing is not known. Here, in AOM patients aged 2–18 years, 17% were estimated to have otorrhoeal or bilateral disease, indicating them for antibiotics;6 this aligns with THIN, where 17.7% of AOM patients were indicated as having otorrhoeal or bilateral disease. In AOM patients without these symptoms, experts estimated that an additional 17% require antibiotics, suggesting that ∼31% of all AOM patients in this age group should be prescribed antibiotics.
In non-pregnant women presenting with symptoms of non-recurrent UTI, treatment should generally depend on the number and severity of symptoms and the use of point-of-care tests (dipsticks).6,7 The pre-test probability of a positive dipstick in symptomatic women ranges from 21% to 50%,34–38 but antibiotics are not indicated when bacteriuria is confirmed in asymptomatic women.39 Hence, the total proportion of women that should receive antibiotics when presenting with UTI symptoms or positive dipsticks is not clear. Here, experts estimated that approximately half (52%) of non-pregnant women that present with non-recurrent urinary symptoms have a bacterial infection, and that 75% should be prescribed antibiotics when bacterial UTI is confirmed (i.e. when the dipstick result is positive). However, dipstick sensitivity and bacterial count thresholds are variable, and these estimates apply best to the standards held most widely in UK primary care.40–43
In gastroenteritis, antibiotics are only indicated when infection is caused by specific bacteria, and in many cases only when infection is severe (e.g. Campylobacter enteritis, non-typhoid Salmonella, shigellosis),6–8,44 as reflected by experts' low prescribing estimate (8%–9%). Similarly, patients rarely benefit from antibiotics in acute cough, acute rhinosinusitis and acute bronchitis,45–49 which is consistent with experts’ estimates that, respectively, 10%, 11% and 13% of these patients should receive antibiotics. Conversely, in AE COPD there are conflicting reports of the benefit of antibiotic therapy in primary care,50 which is reflected by the divergent beliefs of experts in this study, and highlights the need for more research into the appropriateness of prescribing for this condition.
In impetigo, oral or topical antibiotics are recommended depending on subjective indicators such as severity and extent of infection.6,7 This lack of clear guidance may in part explain experts’ disagreement about using oral antibiotics to treat impetigo, again highlighting the need for further study and improved prescribing guidelines. By contrast, although acne has a suite of treatment options, including topical and oral antibiotics and other non-antimicrobial medications,7,51 experts were in relative agreement that ∼20% of patients should receive oral antibiotics when seeking treatment for their acne, and that this initial prescription should be for an average duration of 14 weeks.
Except in AE COPD and impetigo, experts were broadly in agreement in their estimates of antibiotic prescribing proportions for different conditions, and the relatively few outlier estimates came from a range of experts and had little to no impact on pooled estimates. However, there was substantially lower consensus (higher IQRs) in several questions about different symptoms and comorbidities, such as the proportion of patients presenting with acute cough that have an LRTI (IQR 33%) and the proportion of patients presenting with an LRTI that have acute bronchitis (IQR 44%). This variation in expert belief may reflect a need to better understand the causes and clinical presentations of lower respiratory symptoms at the population level. However, compared with prescribing proportions, which are well defined, it is also possible that relatively indistinct categories, such as LRTI, were not interpreted consistently between experts.
The parameter estimates in this study are consistent with the acceptable ranges of prescribing proportions estimated by ESAC-Net,26 and have several added benefits: they include more conditions, provide exact estimates along with associated uncertainty, are regionally specific, and allow consideration of comorbidity distributions, which inform antibiotic appropriateness and are often available in primary care data sets. Expert estimates in this study are also consistent with the beliefs of practising prescribers elicited in our online survey, suggesting relative consensus between these two groups. One exception was in gastroenteritis, where online prescribers estimated a lower ideal prescribing proportion than the academic experts. This may be due to a methodological artefact: the chips and bins parameter estimation tool is not conducive to extreme or highly specific estimates owing to the predetermined bin range, and this may have prevented experts from estimating a lower prescribing proportion.
Strengths and weaknesses
This study has several key strengths. The analysis of prescribing data allowed us to determine the appropriateness of prescribing for all conditions that resulted in antibiotic prescriptions in a representative sample of English primary care, and hence to globally describe the appropriateness of antibiotic prescribing in England as defined by English prescribing and treatment guidelines. The use of expert elicitation then allowed us to quantify appropriate antibiotic prescribing proportions in a suite of conditions that account for a large share of prescribing but rely on subjective patient assessment to determine antibiotic need. The formal and rigorous elicitation methodology ensured that the biases inherent in subjective parameter estimation were kept to a minimum (e.g. probabilistic betting ensured that parameter estimates were not affected by experts' relative numerical proficiency,13 and systematic inclusion criteria ensured that a diversity of experts were included, and not just those with similar academic networks and backgrounds14). The participation of 14 experts was consistent with elicitation guidelines, which recommend a minimum of 6 participants and state that, once ∼12 are included, the added benefits of additional participants are limited.14 Further, pooled estimates were robust to individual outliers, so the presence of a misinformed or aberrant expert is not likely to have had a marked impact on results. Finally, parameter validation with an anonymous survey of antibiotic prescribers showed that estimates from the invited academic experts were consistent with a second sample of health professionals.
There were also several limitations to this work. Even using the most rigorous expert elicitation methodology, subjective parameter estimation can be affected by biases held by participating experts. For example, experts may have been biased by their own patient or study populations—which may not be representative of the average patient population in England—and they may have had ideological or motivational biases that influenced their judgement. The chips and bins methodology may also have introduced some bias, as parameter estimates are inevitably constrained by the predetermined bin range, precluding extremely high or low estimates. This study was also limited to a selection of common conditions to avoid fatiguing experts. Further, although the online prescriber survey was advertised to all members of the Royal College of General Practitioners, there is likely to have been self-selection bias in those who chose to participate, and this convenience sample may not be representative of the UK prescriber population. Online survey participants were also unable to seek clarification or qualify their responses.
Conclusions
The majority of antibiotic prescribing in English primary care is for conditions where antibiotic appropriateness is conditional on patient-specific indicators that are not usually captured in primary care data. To establish levels of appropriate prescribing in 10 of the most common of these conditions, a formal expert elicitation study was conducted. Among other parameters, experts estimated that antibiotics should be prescribed in ∼10%–20% of patients without comorbidities presenting with acute RTIs, 75% of non-pregnant women without comorbidities presenting with a non-recurrent bacterial UTI, and 20% of patients seeking treatment for acne. This elicitation also revealed disagreement about prescribing in impetigo and AE COPD, with experts clustering into distinct groups that advocated either high or low prescribing. Poor consensus in these and other parameters highlights that prescribing guidelines are incomplete and further research is needed. Ultimately, the previously unavailable parameters that resulted from this study can be compared with prescribing data to facilitate the quantification of inappropriate antibiotic prescribing at the national level.
Supplementary Material
Acknowledgements
We thank the 14 subject-matter experts who participated in this elicitation: Drs Mark Ashworth, Christopher Butler, Jonathan Cooke, Nicholas Francis, Martin Gulliford, Kieran Hand, Anthony Harnden, Alastair Hay, Kerenza Hood, Paul Little, David Livermore, Cliodna McNulty, Michael Moore and Mike Sharland. We further thank Susan Hopkins, Cliodna McNulty and Christine Roberts for feedback on the interpretation of guidelines; Jaap Stiggelbout, Pien Pouwels and Eleanor Anderson for participating in trial elicitations; Diane Ashiru-Oredope, Heather Edmonds, Elizabeth Beech, Tim Chadborn, Rebecca Owens, Rebecca Howell-Jones, Imke Jahner and Andrew Simmons for facilitation of our online survey; three anonymous general practitioners for participating in a trial survey; and Harald Mieg and Carmen Keller for expertise on the subject of expertise. The authors are grateful for the support of a group of subject-matter experts who formed a Modelling Oversight Group (MOG) that convened bimonthly to discuss and review this work. Group members are listed in another paper in this Supplement.29 J. V. R. is affiliated with the National Institute for Health Research Health Protection Research Units (NIHR HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at both Imperial College London and University of Oxford in partnership with PHE.
Funding
This paper was published as part of a Supplement supported and resourced by Public Health England (PHE). Only internal resources were used for this paper.
Transparency declarations
J. V. R. is a co-opted member of the UK Government Advisory Committee on Antimicrobial Prescribing, Resistance and Healthcare Associated Infection (APRHAI). All other authors: none to declare.
Supplementary data
Methods, Tables S1 to S5 and Figures S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/575626/ESPAUR_Report_2016.pdf.
- 2. Costelloe C, Metcalfe C, Lovering A. et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096. [DOI] [PubMed] [Google Scholar]
- 3. Department of Health. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf.
- 4. AMR Policy Team. Government Response to the Review on Antimicrobial Resistance. Department of Health; https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/553471/Gov_response_AMR_Review.pdf. [Google Scholar]
- 5. National Institute for Health and Care Excellence. Respiratory Tract Infections (Self-Limiting): Prescribing Antibiotics https://www.nice.org.uk/guidance/cg69/. [PubMed]
- 6. Public Health England. Management of Infection Guidance for Primary Care: For Consultation and Local Adaptation https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/591916/managing_common_infections.pdf.
- 7. National Institute for Health and Care Excellence. Clinical Knowledge Summaries https://cks.nice.org.uk/.
- 8. OpenPrescribing. BNF Section 5.1: Antibacterial Drugs https://openprescribing.net/bnf/0501/.
- 9. Herett E, Thomas SL, Schoonen WM. et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butler CC, Rollnick S, Pill R. et al. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ 1998; 317: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dolk FCK, Pouwels KB, Smith DRM. et al. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother 2018; 73Suppl 2: ii2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson SR, Tomlinson GA, Hawker GA. et al. Methods to elicit beliefs for Bayesian priors: a systematic review. J Clin Epidemiol 2010; 63: 355–69. [DOI] [PubMed] [Google Scholar]
- 13. Johnson SR, Tomlinson GA, Hawker GA. et al. A valid and reliable belief elicitation method for Bayesian priors. J Clin Epidemiol 2010; 63: 370–83. [DOI] [PubMed] [Google Scholar]
- 14. Knol AB, Slottje P, van der Sluijs JP. et al. The use of expert elicitation in environmental health impact assessment: a seven step procedure. Environ Health 2010; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan MG. Use (and abuse) of expert elicitation in support of decision making for public policy. Proc Natl Acad Sci USA 2014; 111: 7176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleming-Dutra KE, Hersh AL, Shapiro DJ. et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016; 315: 1864–73. [DOI] [PubMed] [Google Scholar]
- 17. van den Broek d'Obrenan J, Verheij TJM, Numans ME. et al. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother 2014; 69: 1701–7. [DOI] [PubMed] [Google Scholar]
- 18. Gulliford MC, Latinovic R, Charlton J. et al. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care. J Public Health 2009; 31: 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gulliford MC, Dregan A, Moore MV. et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014; 4: 3e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gore SM. Biostatistics and the Medical Research Council. Medical Research News 1987; 35: 19–20. [Google Scholar]
- 21. Morris DE, Oakley JE, Crowe JA.. A web-based tool for eliciting probability distributions from experts. Environ Model Softw 2014; 52: 1–4. [Google Scholar]
- 22. R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing https://www.R-project.org.
- 23. Dietrich F, List C.. Probabilistic opinion pooling In: Hitchcock C, Hájek A, eds. Oxford Handbook of Probability and Philosophy. Oxford: Oxford University Press, 2016. [Google Scholar]
- 24. Delignette-Muller ML, Dutang C.. fitdistrplus: an R package for fitting distributions. J Stat Softw 2015; 64: 1–34. [Google Scholar]
- 25. Benaglia T, Chauveau D, Hunter DR. et al. mixtools: an R package for analyzing finite mixture models. J Stat Softw 2009; 32: 1–29. [Google Scholar]
- 26. Adriaenssens N, Coenen S, Tonkin-Crine S. et al. European Surveillance of Antimicrobial Consumption (ESAC): disease-specific quality indicators for outpatient antibiotic prescribing. BMJ Qual Saf 2011; 20: 764–72. [DOI] [PubMed] [Google Scholar]
- 27. Hay AD. Can 88% of patients with acute lower respiratory infection all be special? Br J Gen Pract 2014; 64: 60–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pouwels KB, Dolk FCK, Smith DRM. et al. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother 2018; 73Suppl 2: ii19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smieszek T, Pouwels KB, Dolk FCK. et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother 2018; 73Suppl 2: ii36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spinks A, Glasziou PP, Del Mar CB.. Antibiotics for sore throat. Cochrane Database Syst Rev 2013; issue 11: CD000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Venekamp RP, Sanders SL, Glasziou PP. et al. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015; issue 6: CD000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rovers MM, Glasziou PP, Appelman CL. et al. Predictors of pain and/or fever at 3 to 7 days for children with acute otitis media not treated initially with antibiotics: a meta-analysis of individual patient data. Pediatrics 2007; 119: 579–85. [DOI] [PubMed] [Google Scholar]
- 33. Rovers MM, Glasziou PP, Appelman CL. et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet 2006; 368: 1429–35. [DOI] [PubMed] [Google Scholar]
- 34. Bent S, Nallamothu BK, Simel DL. et al. Does this woman have an acute uncomplicated urinary tract infection? JAMA 2002; 287: 2701–10. [DOI] [PubMed] [Google Scholar]
- 35. Hay AD, Fahey T.. Clinical diagnosis of urinary tract infection. JAMA 2002; 288: 1229. [PubMed] [Google Scholar]
- 36. Sutlieff PA. A comparison between a 3-day and a 5-day course of pivmecillinam as a treatment for acute lower urinary tract infections in general practice. Curr Med Res Opin 1982; 7: 563–8. [Google Scholar]
- 37. Fahey T, Webb E, Montgomery AA. et al. Clinical management of urinary tract infection in women: a prospective cohort study. Fam Pract 2003; 20: 1–6. [DOI] [PubMed] [Google Scholar]
- 38. Vellinga A, Cormican M, Hanahoe B. et al. Antimicrobial management and appropriateness of treatment of urinary tract infection in general practice in Ireland. BMC Fam Pract 2011; 12: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zalmanovici Trestioreanu A, Lador A, Sauerbrun-Cutler MT. et al. Antibiotics for asymptomatic bacteriuria. Cochrane Database Syst Rev 2015; issue 4: CD009534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. St John A, Boyd JC, Lowes AJ. et al. The use of urinary dipsticks to exclude urinary tract infection: a systematic review of the literature. Am J Clin Pathol 2006; 126: 428–36. [DOI] [PubMed] [Google Scholar]
- 41. Schmiemann G, Kniehl E, Gebhardt K. et al. The diagnosis of urinary tract infection. Dtsch Arztebl Int 2010; 107: 361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thomas ST, Heneghan C, Price CP. et al. Point-of-Care Testing for Urinary Tract Infections NIHR Diagnostic Evidence Co-Operative Oxford. https://www.oxford.dec.nihr.ac.uk/reports-and-resources/horizon-scanning-reports/point-of-care-testing-for-urinary-tract-infections.
- 43. Scottish Intercollegiate Guidelines Network. Management of Suspected Bacterial Urinary Tract Infection in Adults. Healthcare Improvement Scotland; http://www.sign.ac.uk/assets/sign88.pdf. [Google Scholar]
- 44. Farthing M, Feldman R, Finch R. et al. The management of infective gastroenteritis in adults: a consensus statement by an expert panel convened by the British Society for the study of infection. J Infect 1996; 33: 143–52. [DOI] [PubMed] [Google Scholar]
- 45. Wood J, Butler CC, Hood K. et al. Antibiotic prescribing for adults with acute cough/lower respiratory tract infection: congruence with guidelines. Eur Respir J 2011; 38: 112–8. [DOI] [PubMed] [Google Scholar]
- 46. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV. et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev 2014; issue 2: CD000243. [DOI] [PubMed] [Google Scholar]
- 47. Lemiengre MB, van Driel ML, Merenstein D. et al. Antibiotics for clinically diagnosed acute rhinosinusitis in adults. Cochrane Database Syst Rev 2012; issue 10: CD006089. [DOI] [PubMed] [Google Scholar]
- 48. Young J, De Sutter A, Merenstein D. et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet 2008; 371: 908–14. [DOI] [PubMed] [Google Scholar]
- 49. Smith SM, Fahey T, Smucny J. et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2014; issue 3: CD000245. [DOI] [PubMed] [Google Scholar]
- 50. Vollenweider DJ, Jarrett H, Steurer-Stey CA. et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; issue 12: CD010257. [DOI] [PubMed] [Google Scholar]
- 51. Williams HC, Dellavalle RP, Garner S.. Acne vulgaris. Lancet 2012; 379: 361–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.