Abstract
Objectives: To prevent invasive fungal disease (IFD) in adult patients undergoing remission-induction chemotherapy for newly diagnosed acute lymphoblastic leukaemia (ALL).
Patients and methods: In a double-blind multicentre Phase 3 study, patients received prophylactic liposomal amphotericin B (L-AMB) at 5 mg/kg intravenously or placebo twice weekly in a 2:1 random allocation during remission-induction treatment. The primary endpoint was the development of proven or probable IFD. Secondary endpoints included those focused on the safety and tolerability of prophylactic L-AMB.
Results: Three hundred and fifty-five patients from 86 centres in Europe and South America received at least one dose of L-AMB (n = 237) or placebo (n = 118). Rates of proven and probable IFD assessed independently were 7.9% (18/228) in the L-AMB group and 11.7% (13/111) in the placebo group (P = 0.24). Rates of possible IFD were 4.8% (11/228) in the L-AMB and 5.4% (6/111) in the placebo group (P = 0.82). The remission-induction phase was a median of 22 days for both groups. Overall mortality was similar between the groups: 7.2% (17/237) for L-AMB and 6.8% (8/118) for placebo (P = 1.00). Hypokalaemia and creatinine increase were significantly more frequent with L-AMB.
Conclusions: The IFD rate among adult patients undergoing remission-induction chemotherapy for newly diagnosed ALL was 11.7% in the placebo group, and was not significantly different in patients receiving L-AMB, suggesting that the L-AMB regimen studied is not effective as prophylaxis against IFD. The IFD rate appears higher than previously reported, warranting further investigation. Tolerability of L-AMB was what might be expected. Further studies are needed to determine the optimal antifungal strategy during remission-induction chemotherapy of ALL.
Introduction
Adult patients with acute lymphoblastic leukaemia (ALL) are given intensive multidrug chemotherapy to induce remission based on paediatric protocols of 6–8 weeks duration.1 During this period, patients are at risk of invasive fungal disease (IFD) due to neutropenia and exposure to high-dose glucocorticosteroids.1–3 IFD is challenging to diagnose, particularly in the early stages, when symptoms and signs are absent or non-specific.4 Moreover, IFD has considerable impact on morbidity and mortality, and may interrupt or delay chemotherapy, consequently reducing dose intensity and complete and molecular remission rates.1,2 Antifungal prophylaxis could potentially contribute to treatment efficacy.5,6
An IFD rate of 6.5% has been reported in a retrospective analysis of adult ALL patients, with aspergillosis and candidiasis predominating.2 An IFD rate of 8.8% has been reported in ALL patients with neutropenia,7 and a rate of 6.7% has been identified in patients receiving induction chemotherapy for the treatment of ALL.8 While prophylaxis with azole antifungals has been developed for other patient groups at similar risk, there is currently no approved standard of care for patients with ALL.9,10 The European Working Group for Adult ALL (EWALL) recommends that azole antifungals should be avoided because of drug–drug interactions with vinca alkaloids, a key component of ALL chemotherapy regimens.1 Liposomal amphotericin B (L-AMB) offers an alternative as it is active against a broad variety of fungi, including Aspergillus species, Candida species and agents of mucormycosis.6,11–13 Support for L-AMB use in treatment comes from studies examining its pharmacokinetics in animal models, healthy volunteers and neutropenic patients at doses between 1 and 7.5 mg/kg/day and knowledge that it has a long terminal elimination half-life.14–16 L-AMB is not licensed for prophylaxis, but small prophylaxis studies in neutropenic patients that used different, mostly intermittent, dosing regimens have demonstrated a trend towards a reduced incidence of IFD.17–21 However, doses >5 mg/kg were associated with an increased incidence of infusion-related reactions.18
The medical need for antifungal prophylaxis was identified in close collaboration with EWALL. The AmBiGuard study was designed to determine the efficacy and safety of L-AMB given at 5 mg/kg twice weekly compared with placebo to prevent IFD in adults undergoing remission-induction chemotherapy for newly diagnosed ALL.
Patients and methods
The study was conducted at 86 centres in 13 countries in Europe and South America and registered on clinicaltrials.gov (NCT01259713) and EudraCT (2010-019562-91). The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the institutional review board of each participating site. Written informed consent was obtained from each patient. An independent data safety monitoring board (DSMB) supervised the study and undertook a planned interim data review for futility when half the subjects had completed the study. An independent data review board (IDRB) performed a blinded review of data from all patients to assess the presence of IFD according to the revised consensus definitions.4
Eligibility criteria
Adults ≥18 years were eligible provided they were to receive remission-induction chemotherapy for newly diagnosed ALL that was likely to induce ≥10 days of neutropenia (absolute neutrophil counts <500/μL), and were able to start the study drug within 5 days of the first dose of chemotherapy. Patients were excluded if there was clinical suspicion of IFD including unexplained fever, evidence of pulmonary infiltrates on imaging or if they had been given antifungal treatment in the previous 30 days. Patients were also excluded if their serum creatinine was >2 times the upper limit of normal (ULN), liver function tests were >5 × ULN22 or if there was any severe comorbidity or any hypersensitivity to amphotericin B.
Study drug
The colour and overall appearance of L-AMB and placebo (riboflavin 5′ phosphate formulated in soy phosphatidylcholine as liposomes) did not differ. The two drugs were prepared and given in the same manner. Study personnel were blinded to treatment allocation. A blinded member of the pharmacy staff prepared the solutions and provided blind-labelled infusion bags for administration. L-AMB was given at a dose of 5 mg/kg based on the patient’s weight at screening and dose adjustment to weight changes was allowed. This dose was selected in order to minimize toxicity. Study drug was infused over 2 h twice weekly (3–4 day interval) during the entire remission-induction phase of chemotherapy. The dosing schedule was chosen on practical grounds.
IFD monitoring and diagnostic-driven antifungal treatment
Patients underwent prospective monitoring for the development of IFD according to defined study algorithms throughout the duration of the study and for the 30 days after stopping the study drug (Figures S1–S3, available as Supplementary data at JAC Online). Serum samples were drawn twice weekly and tested for galactomannan (GM) and 1,3-β-d-glucan (BDG) centrally in Belgium, Argentina or Brazil. The results were reported to the site within 48–72 h. A thoracic CT scan was recommended when the mycological criteria were met for a proven or probable IFD, and the study algorithm was followed according to the result (Figure S2).23 When no mycological criteria were met,4 a work-up was undertaken when patients had unexplained fever (temperature ≥38°C for ≥72 h) despite antibiotic treatment, relapsing fever, new pulmonary infiltrates on chest X-ray or other potential signs and symptoms of IFD (Figure S3).
Systemically active antifungal agents were not permitted except for patients who developed oropharyngeal candidiasis despite topical measures; they were allowed to receive concomitant oral fluconazole at 100–200 mg for 10 days.
Study treatment was replaced for up to 5 days with an antifungal other than amphotericin B when there were findings suggestive of IFD to allow further diagnostic tests to be done. Treatment was stopped and prophylaxis restarted when these tests proved negative. Protocol-specified criteria to stop prophylaxis and start broad-spectrum antifungals were defined.
Study procedures
All concomitant medications, including chemotherapy and other antimicrobials, were recorded from 30 days before recruitment and until study completion. All adverse events (AEs) were recorded up to 30 days from last dose, regardless of their potential relationship to the study drug. Laboratory assessments (haematology and chemistry) and complete or symptom-directed physical examinations, including vital signs and temperature, were done at scheduled times.
Study treatment ended for the patient when one of the following occurred: (i) diagnosis of a proven or probable IFD; (ii) remission-induction chemotherapy was completed, neutropenia resolved and the patient was well enough to be discharged from hospital; or (iii) consolidation or salvage chemotherapy was started.
Pharmacokinetic sampling
Amphotericin B serum trough concentrations were determined in a subset of patients.
Efficacy and safety endpoints
The primary efficacy endpoint was the development of proven or probable IFD during remission-induction chemotherapy, measured as the proportion of patients who met this endpoint as assessed by the IDRB. Secondary endpoints included: the proportion in complete remission at last follow-up (e.g. 30 days after end of study treatment); the time to diagnosis of proven or probable IFD; the proportion that required antifungal treatment according to protocol guidance; the proportion of patients who developed pulmonary infiltrates; the proportion of patients with proven IFD and probable IFD;4 and the number of deaths due to IFD as assessed by the IDRB. Additionally, the development of AEs and abnormal clinical laboratory results were examined.
Randomization, sample size and statistical methods
Patients who met the eligibility criteria were randomized, using an interactive voice or web response system, to receive either 5 mg/kg L-AMB or matched placebo twice weekly. Randomization was done centrally and stratified by geographical region: Europe versus South America using permuted blocks (Table S1).
The intended sample size (n = 354) was based on having 81% power to detect a 75% relative reduction in IFD in the L-AMB group, assuming a rate of IFD of 10% in the placebo group using a Cochran–Mantel–Haenszel (CMH) test with a 0.05 two-sided significance level and 2:1 allocation. The assumed IFD rate of 10% for the placebo group is in line with previously reported prophylaxis studies in other patient populations at high risk of IFD.10,24,25 A 0.05 two-sided test was chosen because the interim boundaries were based around a two-sided test. We selected 2:1 randomization of L-AMB to placebo rather than 1:1 randomization due to ethical considerations and its potential to provide more comprehensive safety data for the L-AMB group.
The primary efficacy analysis used the ITT population, i.e. all subjects who were randomly allocated to study treatment, who received at least one dose of study drug and met the major eligibility criteria (newly diagnosed with ALL and had not received systemically active antifungals within the previous 30 days). An ITT analysis excluding patients who were never neutropenic was done as was a posthoc per-protocol analysis set excluding patients receiving antifungals not permitted by the protocol. The safety analysis population included all patients who received at least one dose of the randomly allocated study drug.
All statistical tests were performed at the 0.05 level (unless otherwise stated) without consideration for multiplicity. The CMH test was used for the primary analysis after adjusting for region.
A planned interim analysis for futility was undertaken when 50% of patients had completed the study, so α for the primary analysis was adjusted by 0.0003.
Categorical secondary efficacy endpoints including the proportion of patients diagnosed with proven or probable IFD were analysed using a non-stratum-adjusted relative risk reduction. The time to diagnosis of proven or probable IFD and the duration of remission-induction chemotherapy were analysed using Kaplan–Meier estimates and the log rank test, after stratifying by region. A P value of <0.05 was considered statistically significant.
Results
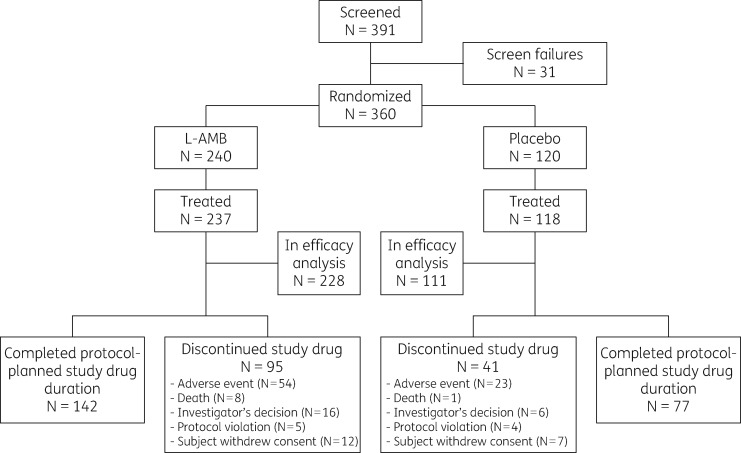
From April 2011 to November 2013, 391 patients were screened and 360 were enrolled. Five patients were not treated and 16 failed to meet the entry criteria, i.e. 15 received protocol-prohibited systemic antifungal treatment in the 30 days prior to first dose of study drug and 1 did not have ALL. Hence, 339 patients were included in the efficacy analysis. The safety analysis comprised 355 patients who received at least one dose of study drug (Figure 1), of whom 237 were randomly allocated to receive L-AMB and 118 to receive placebo.
Figure 1.
CONSORT flow diagram. Of 360 subjects randomized, 5 were never dosed, and 16 (9 L-AMB, 7 placebo) were excluded from the efficacy analysis, 1 due to misdiagnosis (AML) and 15 due to protocol-prohibited antifungal treatment either concomitant with or within the previous 30 days.
Demographic and baseline characteristics were similar across the treatment groups (Table 1). The median age was 46 (IQR 30–58) years. Commonly used constituents of chemotherapy for remission induction included cytarabine, cyclophosphamide, vincristine and anthracyclines, particularly daunorubicin and idarubicin. Systemic glucocorticosteroids were part of the chemotherapy in the majority of patients [L-AMB 90.4% (206/228), placebo 91.0% (101/111)]. Over 90% of subjects received twice-weekly infusions of study drug as specified in the protocol.
Table 1.
Patient baseline characteristics
| Characteristic | L-AMB (N = 237) | Placebo (N = 118) | P valuea |
|---|---|---|---|
| Age (years), median (IQR) | 45 (32–57) | 47 (28–60) | 0.82 |
| Male, n (%) | 139 (59) | 60 (51) | 0.16 |
| White, n (%) | 211 (89) | 100 (85) | 0.25 |
| Weight (kg), median (IQR) | 74.0 (66.0–84.0) | 74.7 (62.3–87.9) | 0.92 |
| Height (cm), median (IQR) | 170.0 (164.0–178.0) | 170.0 (164.0–179.0) | 0.94 |
| BMI (kg/m2), median (IQR) | 25.2 (22.7–28.7) | 25.0 (22.0–28.7) | 0.76 |
| Baseline ANC (cells/μL), median (IQR) | 820 (270–1880) | 560 (210–1610) | 0.27 |
| Patient ANC distribution, n (%) | |||
| <200 cells/μL | 49 (21.2) | 26 (22.8) | |
| 200 to < 500 cells/μL | 34 (14.7) | 28 (24.6) | |
| 500 to < 1500 cells/μL | 79 (34.2) | 26 (22.8) | |
| ≥1500 cells/μL | 69 (29.9) | 34 (29.8) | |
| Median days between start of remission induction and first dose of study drug (IQR) | 3 (2–4) | 3 (2–4) | 0.55 |
ANC, absolute neutrophil count.
Cochran–Mantel–Haenszel test for categorical variables and Wilcoxon rank-sum test for continuous variables.
Efficacy analyses
Rates of proven/probable IFD as assessed by the IDRB (primary endpoint) were 7.9% (18/228) in the L-AMB and 11.7% (13/111) in the placebo group (P = 0.24), suggesting that L-AMB is not effective as prophylaxis against IFD in these patients. The remission-induction phase was a median of 22 days for both groups. Additionally, rates of possible IFD were 4.8% (11/228) in the L-AMB and 5.4% (6/111) in the placebo group (P = 0.82) (Table 2, Table S2).
Table 2.
Invasive fungal disease rates
| Patient population | L-AMB | Placebo | P valuea |
|---|---|---|---|
| IFD assessment by IDRB | N = 228 | N = 111 | |
| Proven or probable IFD (primary endpoint) | 18 (7.9%) | 13 (11.7%) | 0.24 |
| proven candidaemia | 1 (0.4%)b | 3 (2.7%)c | 0.07 |
| proven filamentous IFD | 0 | 0 | |
| probable IFD | 17 (7.5%) | 10 (9.0%) | 0.60 |
| Possible IFD | 11 (4.8%) | 6 (5.4%) | 0.82 |
| Pulmonary infiltrates | 46 (20.2%) | 30 (27.0%) | 0.15 |
| Deaths due to IFD | 2 (0.9%)d | 0 | 0.32 |
| IFD assessment by investigator | N = 228 | N = 111 | |
| Proven or probable IFD | 25 (11.0%) | 12 (10.8%) | 0.97 |
| Requirement for antifungal treatment | 37 (16.2%) | 24 (21.6%) | 0.22 |
| Deaths due to IFD | 2 (0.9%) | 0 | 0.32 |
| Post hoc analysis on patients without major protocol deviationse | N = 184 | N = 90 | |
| Proven or probable IFD | 14 (7.6%) | 13 (14.4%) | 0.07 |
| Neutropenic for ≥10 days (ANC <500 cells/μL) | N = 174 | N = 84 | |
| Proven or probable IFD | 12 (6.9%) | 11 (13.1%) | 0.10 |
ANC, absolute neutrophil count.
Three subjects (one L-AMB, two placebo) had Pneumocystis spp. in bronchoalveolar lavage (BAL) and were not considered to have IFD as it is not part of the EORTC/MSG criteria.
Stratum-adjusted (stratified by region) CMH test.
C. kefyr.
C. albicans (2×), C. tropicalis bloodstream infections.
Probable invasive pulmonary aspergillosis, proven candidaemia.
Major protocol deviations were protocol-prohibited systemic antifungals, baseline IFD or chemotherapy not expected to induce sufficient neutropenia.
The IFD incidence rates for patients who were neutropenic for ≥10 days (n = 258) were 6.9% in the L-AMB group versus 13.1% in the placebo group (P = 0.10) (Table 2; Table S3).
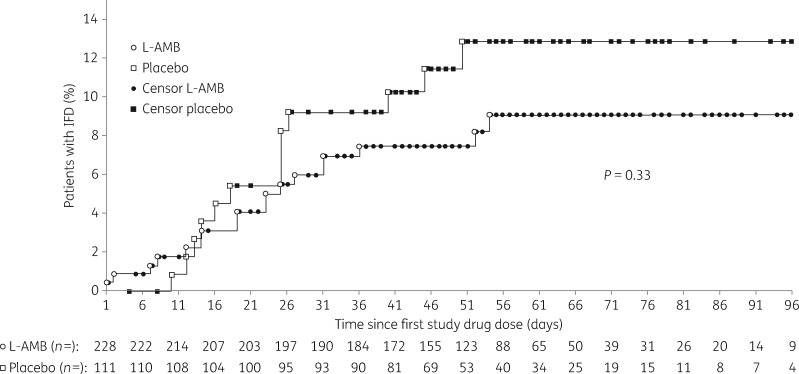
Subgroup analyses of the primary endpoint showed that the proportion of patients with proven or probable IFD was consistently lower in the L-AMB group than in the placebo group (Figure S4). Time to diagnosis of IFD was not significantly different between the two study groups (P = 0.33) (Figure 2).
Figure 2.
Time to diagnosis of invasive fungal disease as assessed by the independent data review board.
Chest CT scans were done for 82 (36.0%) patients in the L-AMB group and 51 (45.9%) patients in the placebo group. Antifungal treatment was given during remission-induction chemotherapy to 16.2% (37/228) patients in the L-AMB group and 21.6% (24/111) in the placebo group (P = 0.22). These treatments were considered protocol violations in eight patients in each group.
Overall mortality was similar between the groups, being 7.2% (17/237) in the L-AMB group and 6.8% (8/118) in the placebo group (P = 1.00) (Figure S5). Two deaths in the L-AMB group were attributed to IFD by the IDRB (probable invasive pulmonary aspergillosis and proven candidaemia), but none in the placebo group.
Most patients in each group were neutropenic (<500/μL) during remission-induction chemotherapy (L-AMB 90.8%; placebo 92.8%), and 76.3% and 75.7% were neutropenic for ≥10 days. Median duration of neutropenia <500/μL was 18 days for both groups (IQR L-AMB 13.0–26.0, placebo 11.0–25.0 days). The absolute neutrophil count nadir was <100/μL in 167 (73.2%) patients in the L-AMB group and 82 (73.9%) patients in the placebo group.
There was no significant difference in the median duration of neutropenia.
Complete remission rates were lower in the L-AMB group [72.8% (166/228)] than in the placebo group [79.3% (88/111)], although not significantly so (P = 0.20).
Post hoc efficacy analysis on patients without major protocol deviations
For this analysis, a total of 44 patients in the L-AMB group and 21 in the placebo group were excluded from the ITT analysis set because of at least one protocol deviation that could have influenced the primary endpoint. This included 41 patients who received systemic antifungals in violation of the protocol (24 L-AMB versus 17 placebo), 2 patients in the L-AMB group with baseline IFD and 28 patients who had not been given regimens expected to induce ≥10 days of neutropenia (21 L-AMB versus 7 placebo). IFD rates in the remaining 274 patients were 7.6% (14/184) in the L-AMB group and 14.4% (13/90) in the placebo group (P = 0.07).
Pharmacokinetic analyses
A total of 175 trough pharmacokinetic samples were collected from 27 patients given L-AMB between 1.4 and 6.4 days after the previous dose of study drug. Mean ± SD trough serum concentration of free amphotericin B ranged from 0.89 ± 0.59 to 1.58 ± 1.55 μg/mL.
Safety analyses
Of 355 patients treated, 61.7% (219/355) completed study drug treatment for the planned duration: L-AMB 59.9% (142/237) and placebo 65.3% (77/118) (P = 0.36) (Table 3). Patients in both treatment groups remained on study drug for a median 22 days (IQR L-AMB 15–32, placebo 15–36 days). The reasons for premature discontinuation of study drug were similar in the two groups, most commonly because of an AE, the investigator’s discretion or withdrawal of consent. Most patients had at least one AE and a similar proportion of patients in each group had AEs of grade 3 or higher.16,20 Most patients in each group had AEs considered by the investigator to be related to ALL or to chemotherapy. A similar proportion of patients in each group had AEs considered to be related to IFD or AEs that resulted in premature discontinuation of the study drug. More patients in the L-AMB group had an AE that resulted in interruption of treatment with the study drug (20.3% versus 7.6%; P = 0.002), and experienced serious AEs (Table S4) considered related to study drug by the investigator (8.4% versus 1.7%; P = 0.02). Statistically significantly more patients were affected by hypokalaemia and elevated creatinine in the L-AMB group (Table 3).
Table 3.
Adverse events as assessed by the investigators21
| Adverse events, n (%) | L-AMB (N = 237) | Placebo (N = 118) | P valuea |
|---|---|---|---|
| Any treatment-emergentb AE | 237 (100) | 115 (97.5) | 0.036 |
| pyrexia | 67 (28.3) | 40 (33.9) | 0.33 |
| hypotension | 18 (7.6) | 15 (12.7) | 0.12 |
| Treatment-emergentb renal AEs | |||
| hypokalaemia, any grade | 83 (35.0) | 21 (17.8) | <0.001 |
| <LLN (3.0 mmol/L) | 45 (19.0) | 12 (10.2) | 0.03 |
| <LLN (3.0 mmol/L); symptomatic, intervention indicated | 25 (10.5) | 6 (5.1) | 0.11 |
| <3.0–2.5 mmol/L | 10 (4.2) | 2 (1.7) | 0.35 |
| <2.5 mmol/L | 3 (1.3) | 1 (0.8) | 1.00 |
| creatinine increase, any grade | 22 (9.3) | 0 | <0.001 |
| >1 to 1.5× baseline; >ULN to 1.5 × ULN | 7 (3.0) | 0 | 0.10 |
| >1.5 to 3.0× baseline; >1.5 to 3.0 × ULN | 15 (6.3) | 0 | 0.003 |
| >3.0× baseline; >3.0 to 6.0 × ULN | 0 | 0 | 1.00 |
| Other AE categories | |||
| serious adverse events (SAEs) | 79 (33.3) | 38 (32.2) | 0.90 |
| SAE related to study drug, any grade | 20 (8.4) | 2 (1.7) | 0.02 |
| AE leading to study drug interruption | 48 (20.3) | 9 (7.6) | 0.002 |
| AE leading to study drug discontinuation | 63 (26.6) | 26 (22.0) | 0.37 |
| any grade 3 AE | 102 (43.0) | 43 (36.4) | 0.25 |
| any grade 4 AE | 69 (29.1) | 40 (33.9) | 0.39 |
| AE related to death | 17 (7.2) | 8 (6.8) | 1.00 |
| AE related to ALL or ALL treatments | 226 (95.4) | 110 (93.2) | 0.45 |
| AE related to IFD | 17 (7.2) | 10 (8.5) | 0.67 |
LLN, lower limit of normal; ULN, upper limit of normal.
Fisher’s exact test.
Treatment emergent is defined as started on or after first dose and up to 30 days after last dose of study drug.
Discussion
This large, randomized, double-blind, placebo-controlled study, using a pre-specified diagnostic algorithm, showed that almost 1 in 11 patients receiving remission-induction chemotherapy for ALL developed a probable or proven IFD. This is almost twice the rate reported in a retrospective cohort study2 and is of the same order of magnitude as reported for patients receiving remission-induction therapy for AML without mould-directed antifungal prophylaxis.10 The observed incidence in this study is also towards the upper end of IFD rates reported in other retrospective case collections in patients with ALL of between 6.5% and 8.8%.2,7,8 It is therefore important to consider the potential factors behind this possibly high observed incidence, which resulted from a prospective trial with a well-designed diagnostic algorithm.
Neutropenia and prolonged treatment with glucocorticosteroids are considered host factors that may contribute to the high incidence of IFD observed.4 Another factor may be the inclusion of non-culture-based methods in our diagnostic strategy, which may have led to a higher number of cases of IFD being identified. In any event, the high incidence observed clearly warrants further investigation and consideration of appropriate preventative measures, including antifungal prophylaxis.
Our study failed to show that twice-weekly dosing of L-AMB 5 mg/kg significantly reduced the IFD rate, which is difficult to explain since L-AMB is effective in treating IFD.11–13 The dose used was chosen to minimize toxicity but may not have been high enough to be effective, representing a major limitation of the study. However, despite the low dose (half the standard treatment dose of 3 mg/kg/day), the number of withdrawals due to AEs and the number of serious AEs were both much higher with L-AMB than with placebo. The dosing interval was chosen on practical grounds and may have been too long, although pharmacokinetic analysis showed that L-AMB trough concentrations were adequate.26,27 Better understanding of the pharmacokinetics of L-AMB is therefore necessary to identify the most appropriate dosage for prophylaxis.
Other factors to consider include the point that the 10% estimate of the IFD rate for the placebo group was exceeded, but expecting a 75% reduction in IFD incidence in the sample size calculation may have been too optimistic.10,24,25 Attrition of statistical power due to protocol deviations may provide a further explanation.
Some patients were included although they received regimens that did not induce severe neutropenia, and others received systemic antifungal agents in violation of the protocol despite detailed management algorithms for suspected IFD that may have impacted the assessment of the primary endpoint. This early antifungal treatment may have caused false negativity of the diagnostic-driven strategy applied. Posthoc analyses of the group of patients without substantial protocol deviations showed that the difference between treatment groups was more pronounced, though this did not reach statistical significance. The lack of neutropenia in subjects was not explained by the use of growth factors.
Complete remission rates were lower than expected, but this is likely to be due to evaluation of subjects before the end of induction/beginning of consolidation and to some subjects being lost to follow-up (Figure 1).
Mortality was very similar in both treatment groups (7.2% versus 6.8%), and deaths in the placebo group were not attributed to IFD by the investigators. The diagnostic-driven strategy may pick up IFD earlier at a more effectively treatable stage. Because the trial did not reveal a difference in overall mortality or in mortality attributable to IFD, a diagnostic-driven treatment approach may be a more suitable strategy in this specific patient population. While prophylaxis is beneficial for patients receiving remission-induction chemotherapy for AML,10 heightened clinical awareness combined with intensive microbiological screening may be appropriate for those undergoing similar treatment for ALL.
L-AMB was not effective in preventing IFD in patients receiving remission-induction chemotherapy for ALL. Tolerability of L-AMB was what might be expected but further studies are clearly needed to determine whether a change in dosing might have yielded better results. Nonetheless, the rate of IFD found in this study was higher than that reported in previous studies; however, whether prophylaxis is the best antifungal strategy during remission-induction chemotherapy of ALL, or whether the use of a diagnostic-driven approach combining intensive clinical and microbiological screening to detect IFD at an early stage would be more appropriate, remains to be seen. Finally, even though L-AMB given as prophylaxis did not prove effective in our study, this should not deter others from exploring this subject further, given the high incidence of IFD in this patient population.
Supplementary Material
Acknowledgements
We extend our thanks to the participating patients, the investigators and their support staff. We also acknowledge the contribution of the Data Safety Monitoring Board (DSMB; A. Pagliuca, A. J. Ullmann, C. Viscoli and D. Wang), and the continuous reliable support of S. Abella MD, R. Hargreaves, E. Harvey, S. Sanchez and C. Simcock.
The Steering Committee members were S. Agrawal, O. A. Cornely, J. P. Donnelly and N. Goekbuget (Chair). The Independent Data Review Board members were C. Cordonnier, C. P. Heussel and C. O. Morrissey.
The results were presented in part at the 56th ASH Annual Meeting, 6–9 December 2014, San Francisco, CA, USA (Blood 2014; 24: 3646).
Members of the AmBiGuard Study Group
Argentina: G. Jarchum, Sanatorio Allende Cerro, Córdoba (1 patient); M. Dictar, Instituto Alexander Fleming, Buenos Aires (2); S. Ramirez Borga, Hospital Italiano de La Plata, La Plata (2); A. Valledor, Hospital Italiano de Buenos Aires, Buenos Aires (3). Austria: P. Knoebl, Medizinische Univ. Wien, Wien (2); R. Greil, Paracelsus Medical Univ. Salzburg, Salzburg (3); W. Linkesch, H. Sill, Medizinische Univ., Graz (4). Belgium: B. De Prijck, CHU Sart-Tilman, Liege (1); A. Sonet, CH Mont-Godinne, Yvoir (4); K. Theunissen, Jessa Ziekenhuis, Hasselt (6); D. Selleslag, AZ Sint-Jan Brugge-Oostende AV, Brugge (7); J. Maertens, UZ Leuven, Leuven (13). Brazil: A. Vargas Schwarzbold, Hospital Univ. de Santa Maria, Santa Maria (1); M. L. M. Nucci, Hospital Univ. Clementino Fraga Filho, UFRJ, Rio de Janeiro (2); C. Lopes de Castro Lobo, HEMORIO, Rio de Janeiro (5); L. Fogliatto, Hospital de Clínicas de Porto Alegre, Porto Alegre (7). France: C. Bonmati, CHU de Nancy, Hôpitaux de Brabois, Vandoeuvre les Nancy (1); P. Turlure, CHU de Limoges, Limoges (2); R. Herbrecht, Hôpital de Hautepierre, Strasbourg (3); A. Thiebaut, Hôpital A. Michallon, Grenoble (7); M. Michallet, Centre Hospitalier Lyon Sud (8); T. Leguay, CHU Bordeaux Haut Leveque, Pessac (31). Germany: G. Egerer, Univ. Hospital Heidelberg, Heidelberg (1); G. Silling, Univ. Hospital Münster, Münster (1); M. Pfreundschuh, Univ. Hospital Saarland, Homburg/Saar (1); J. Hasenkamp, Universitätsmedizin Göttingen, Göttingen (2); D. M. Kraemer, Klinikum Oldenburg GmbH, Oldenburg (2); M. Topp, W. Heinz, Univ. Würzburg, Würzburg (2); C. Junghanss, Univ. Hospital Rostock, Rostock (2); M. A. Schaich, S. Parmentier, C. Roellig, Univ. Hospital Carl Gustav Carus, Dresden (3); H. J. Beck, Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Mainz (4); A. Hüttmann, Univ. Hospital Essen, Essen (4); S. Mousset, U. N. Duenzinger, Univ. Hospital Frankfurt, Frankfurt am Main (4); S. Schwartz, Charite Universitaetsmedizin Berlin, Berlin (4); G. Haerter, Comprehensive Infectious Diseases Center (CIDC) Ulm (9); M. Kondakci, Univ. Hospital Düsseldorf, Düsseldorf (8); H. Ostermann, C. Rieger, Klinikum der Universität München, München (10); O. A. Cornely, M. J. G. T. Vehreschild, Univ. Hospital Köln, Köln (18). Greece: P. Tsirigotis, General Univ. Hospital Attikon, Athens (1); P. Matsouka, Univ. General Hospital of Larissa, Larissa (3); M. K. Angelopoulou, Laiko General Hospital of Athens, Athens (4); M. Karakantza, A. Spyridonidis, General Univ. Hospital of Patras, Rio (7); A. Anagnostopoulos, General Hospital of Thessaloniki G. Papanikolaou, Thessaloniki (12). Israel: A. Kolomansky, Tel Aviv Medical Center, Tel Aviv (1); A. Moses, Hadassah Medical Center, Jerusalem (1); N. Horowitz, Rambam Health Care Campus, Haifa (3); G. Rahav, Chaim Sheba Medical Center, Ramat Gan (4). Italy: F. Aversa, A. Velardi, Ospedale Santa Maria della Misericordia, Perugia (1); L. Pagano, Policlinico Universitario Agostino Gemelli, Rome (1); G. Gentile, Università La Sapienza di Roma, Rome (2); M. Gobbi, Università degli studi di Genova-Azienda Ospedaliero-Universitaria (AOU) S. Martino, Genova (3); M. Luppi, A. M. Nosari, Ospedaliero-Universitaria Policlinico di Modena, Modena (5); A. Rambaldi, Ospedali Riuniti di Bergamo, Bergamo (3); A. Candoni, Santa Maria della Misericordia, Udine (5); L. Marbello, Ospedale Niguarda, Milano (4); G. Rossi, Spedali Civili di Brescia, Brescia (9); E. Pogliani, L. Verga, Ospedale San Gerardo, Monza (10); B. Allione, AOU San Giovanni Battista di Torino (11); C. Castagnola, Fondazione IRCCS Policlinico San Matteo, Pavia (12). Portugal: I. Moreira, Instituto Português de Oncologia do Porto, EPE, Porto (5); A. Nunes, Instituto Portugues de Oncologia (IPO), Lisboa (5); A. Botelho de Sousa, Hospital Santo António dos Capuchos, CHLC, Lisboa (7). Spain: A. I. Rubio Tejero, Hospital Universitario Virgen de la Arrixaca, Murcia (1); C. Vallejo, Hospital Universitario Central de Asturias (HUCA), Oviedo (1); L. Vazquez, Hospital Universitario de Salamanca (1); J. Besalduch Vidal, Hospital Universitari Son Espases, Palma de Mallorca (1); V. Gomez-Garcia de Soria, Hospital Universitario de La Princesa (HULP), Madrid (1); M. Jurado Chacon, Hospital Virgen De Las Nieves, Granada (1); J. González Campos, Hospital Universitario Virgen del Rocío, Sevilla (3); E. Olavarria, Complejo Hospitalario de Navarra, Pamplona (3); P. Barba, Hospital Vall d'Hebron, Barcelona (4); J. de la Serna Torroba, Hospital Universitario 12 de Octubre, Madrid (4); R. Duarte, Duran i Reynals Hospital, Barcelona (8). Switzerland: D. Heim, Universitätsspital Basel (1); S. Zimmerli, Univ. Hospital Inselspital, Bern (2); B. Gerber, Universitätsspital Zurich (4). Turkey: M. Akova, Liv Hospital, Istanbul (1); A. Z. Bolaman, School of Medicine, Aydin (1); F. Tabak, Istanbul University Hospital, Istanbul (1); H. Akan, Cebeci Hospital, Cebeci (2); E. Senol, Gazi University Hospital, Besevler (6).
Funding
This study was funded by Gilead Sciences, Inc. M. B., W. G. and M. J. H. are employees of Gilead Sciences. All other authors or their institutions have received compensation for study participation from Gilead Sciences International Ltd.
Transparency declarations
Note: The use of liposomal amphotericin B (AmBisome®) in this study is off-label.
O. A. C. reports research grants from Actelion, Aramis Pharma, Astellas, AstraZeneca, Basilea, Bayer, Cidara, Duke University (NIH UM1AI104681), F2G, Gilead, GSK, Leeds University, MedPace, Melinta Therapeutics, Merck/MSD, Miltenyi, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, Seres Therapeutics, The Medicine Company, is a consultant to Achaogen, Anacor, Amplyx, Actelion, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen Pharmaceuticals, Matinas, Menarini Ricerche, Merck/MSD, Paratek Pharmaceuticals, Scynexis, Seres, Summit, Tetraphase, Vical, Vifor, and received lecture honoraria from Astellas, Basilea, Gilead and Merck/MSD, outside the submitted work. T. L. has no conflicts of interest to disclose. J. M. reports grants, personal fees and non-financial support from Gilead Sciences, MSD and Pfizer, and personal fees and non-financial support from Astellas. M. J. G. T. V. has served at the speakers’ bureau of Pfizer, Merck, Gilead Sciences, and Astellas Pharma, received research funding from 3 M, Astellas Pharma and Gilead Sciences and is a consultant to Berlin Chemie. A. A. reports grants from Gilead Pharmaceutical outside the submitted work. C. Costagliola has no conflicts of interest to disclose. C. R. reports personal fees from Gilead Sciences, Pfizer International and MSD Sharp & Dohme GmbH outside the submitted work. R. F. D. reports personal fees from Astellas, Gilead Science and Pfizer, grants and personal fees from Merck Sharp & Dohme outside the submitted work. M. B. reports stock ownership and employment at Gilead Sciences, Inc. outside the submitted work. W. G. and M. J. H. report stock ownership and employment at Gilead Sciences, Inc. outside the submitted work. C. Cordonnier reports personal fees from Gilead Sciences during the conduct of the study and has received honoraria for participating in Gilead Sciences advisory boards. C. P. H. reports personal fees from Gilead during the conduct of the study, grants and personal fees from Astellas, AstraZeneca, Basilea, Boehringer Ingelheim, Bracco, Chiesi, Covidien, Essex, Fresenius, Grifols, Intermune, Lilly, MEDA Pharma, MeVis, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Schering-Plough and Siemens, and stock ownership in Stada and GSK outside the submitted work. C. O. M. reports grants from Gilead Sciences during the conduct of the study, grants and lecture honoraria from Merck, Sharp and Dohme and Pfizer Australia outside the submitted work. All funds were paid to C. O. M.’s institution. S. G. A. reports personal fees and non-financial support from Gilead Sciences Ltd. during the conduct of the study, grants, personal fees and non-financial support from Astellas, other from MSD outside the submitted work. J. P. D. reports grants and personal fees from Gilead Sciences, MSD and Pfizer outside the submitted work. N. G. reports honoraria from Gilead during the conduct of the study. L. V., M. K., G. H. and B. A. have none to declare.
Author contributions
O. A. C. designed and performed the research, analysed the data and wrote the paper. T. L. performed the research and contributed to the writing of the paper. J. M. performed the research, analysed the data and wrote the paper. M. J. G. T. V., A. A., C. Costagliola, L. V., C. R., M. K., G. H. and B. A. performed the research and contributed to the writing of the paper. R. F. D. wrote the paper. M. B. designed the research, analysed the data and contributed to the writing of the paper. W. G. designed the research, analysed the data and wrote the paper. M. J. H. designed the research, analysed the data and contributed to the writing of the paper. C. Cordonnier analysed the data and contributed to the writing of the paper. C. P. H. analysed the data and wrote the paper. C. O. M. analysed the data and wrote the paper. S. G. A., J. P. D and N. G. designed the research, analysed the data and wrote the paper.
Supplementary data
Tables S1 to S4 and Figures S1 to S5 are available as Supplementary data at JAC Online.
References
- 1. Bassan R, Hoelzer D.. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol 2011; 29: 532–43. [DOI] [PubMed] [Google Scholar]
- 2. Pagano L, Caira M, Candoni A. et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91: 1068–75. [PubMed] [Google Scholar]
- 3. Teng JC, Slavin MA, Teh BW. et al. Epidemiology of invasive fungal disease in lymphoproliferative disorders. Haematologica 2015; 100: e462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Pauw B, Walsh TJ, Donnelly JP. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rüping MJ, Vehreschild JJ, Cornely OA.. Patients at high risk of invasive fungal infections: when and how to treat. Drugs 2008; 68: 1941–62. [DOI] [PubMed] [Google Scholar]
- 6. Doan TN, Kirkpatrick CM, Walker P. et al. Primary antifungal prophylaxis in adult patients with acute lymphoblastic leukaemia: a multicentre audit. J Antimicrob Chemother 2015; 71: 497–505. [DOI] [PubMed] [Google Scholar]
- 7. Nicolato A, Nouér SA, Garnica M. et al. Invasive fungal diseases in patients with acute lymphoid leukemia. Leuk Lymphoma 2016; 57: 2014–9. [DOI] [PubMed] [Google Scholar]
- 8. Mariette C, Tavernier E, Hocquet D. et al. Epidemiology of invasive fungal infections during induction therapy in adults with acute lymphoblastic leukemia: a GRAALL-2005 study. Leuk Lymphoma 2016; 58: 586–93. [DOI] [PubMed] [Google Scholar]
- 9. Tacke D, Buchheidt D, Karthaus M. et al. Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol 2014; 93: 1449–56. [DOI] [PubMed] [Google Scholar]
- 10. Cornely OA, Maertens J, Winston DJ. et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356: 348–59. [DOI] [PubMed] [Google Scholar]
- 11. Kuse ER, Chetchotisakd P, da Cunha CA. et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369: 1519–27. [DOI] [PubMed] [Google Scholar]
- 12. Cornely OA, Maertens J, Bresnik M. et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 2007; 44: 1289–97. [DOI] [PubMed] [Google Scholar]
- 13. Cornely OA, Arikan-Akdagli S, Dannaoui E. et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014; 20 Suppl 3: 5–26. [DOI] [PubMed] [Google Scholar]
- 14. Adler-Moore J, Proffitt RT.. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother 2002; 49 Suppl 1: 21–30. [DOI] [PubMed] [Google Scholar]
- 15. Bekersky I, Fielding RM, Dressler DE. et al. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother 2002; 46: 828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walsh TJ, Yeldandi V, McEvoy M. et al. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother 1998; 42: 2391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penack O, Schwartz S, Martus P. et al. Low-dose liposomal amphotericin B in the prevention of invasive fungal infections in patients with prolonged neutropenia: results from a randomized, single-center trial. Ann Oncol 2006; 17: 1306–12. [DOI] [PubMed] [Google Scholar]
- 18. Cordonnier C, Mohty M, Faucher C. et al. Safety of a weekly high dose of liposomal amphotericin B for prophylaxis of invasive fungal infection in immunocompromised patients: PROPHYSOME Study. Int J Antimicrob Agents 2008; 31: 135–41. [DOI] [PubMed] [Google Scholar]
- 19. Mattiuzzi GN, Estey E, Raad I. et al. Liposomal amphotericin B versus the combination of fluconazole and itraconazole as prophylaxis for invasive fungal infections during induction chemotherapy for patients with acute myelogenous leukemia and myelodysplastic syndrome. Cancer 2003; 97: 450–6. [DOI] [PubMed] [Google Scholar]
- 20. Kelsey SM, Goldman JM, McCann S. et al. Liposomal amphotericin (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled study. Bone Marrow Transplant 1999; 23: 163–8. [DOI] [PubMed] [Google Scholar]
- 21. Tollemar J, Ringden O, Andersson S. et al. Randomized double-blind study of liposomal amphotericin B (Ambisome) prophylaxis of invasive fungal infections in bone marrow transplant recipients. Bone Marrow Transplant 1993; 12: 577–82. [PubMed] [Google Scholar]
- 22. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. 2010. NIH Publication No. 09-5410; USA. [Google Scholar]
- 23. Ruhnke M, Böhme A, Buchheidt D. et al. Diagnosis of invasive fungal infections in hematology and oncology—guidelines from the Infectious Diseases Working Party in Haematology and Oncology of the German Society for Haematology and Oncology (AGIHO). Ann Oncol 2012; 23: 823–33. [DOI] [PubMed] [Google Scholar]
- 24. Goodman JL, Winston DJ, Greenfield RA. et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326: 845–51. [DOI] [PubMed] [Google Scholar]
- 25. Slavin MA, Osborne B, Adams R. et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis 1995; 171: 1545–52. [DOI] [PubMed] [Google Scholar]
- 26. Würthwein G, Young C, Lanvers-Kaminsky C. et al. Population pharmacokinetics of liposomal amphotericin B and caspofungin in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 2012; 56: 536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A. et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob Agents Chemother 2011; 55: 5150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.