Abstract
Background
Giardiasis is the commonest intestinal protozoal infection worldwide. The current first-choice therapy is metronidazole. Recently, other drugs with potentially higher efficacy or with fewer and milder side effects have increased in popularity, but evidence is limited by a scarcity of randomized controlled trials (RCTs) comparing the many treatment options available. Network meta-analysis (NMA) is a useful tool to compare multiple treatments when there is limited or no direct evidence available.
Objectives
To compare the efficacy and side effects of all available drugs for the treatment of giardiasis.
Methods
We selected all RCTs included in systematic reviews and expert reviews of all treatments for giardiasis published until 2014, extended the systematic literature search until 2016, and identified new studies by scanning reference lists for relevant studies. We then conducted an NMA of all available treatments for giardiasis by comparing parasitological cure (efficacy) and side effects.
Results
We identified 60 RCTs from 58 reports (46 from published systematic reviews, 8 from reference lists and 4 from the updated systematic search). Data from 6714 patients, 18 treatments and 42 treatment comparisons were available. Tinidazole was associated with higher parasitological cure than metronidazole [relative risk (RR) 1.23, 95% CI 1.12–1.35] and albendazole (RR 1.35, 95% CI 1.21–1.50). Taking into consideration clinical efficacy, side effects and amount of the evidence, tinidazole was found to be the most effective drug.
Conclusions
We provide additional evidence that single-dose tinidazole is the best available treatment for giardiasis in symptomatic and asymptomatic children and adults.
Introduction
Giardiasis is an infectious disease caused by the parasite Giardia lamblia (also known as Giardia duodenalis)1 that is highly associated with poor hygiene, low water quality and poor sanitation.2 Giardiasis is the commonest gastrointestinal protozoal pathogen worldwide3 and has been identified as a neglected tropical disease.4 Incidence is highest in children, but adults are also affected, especially travellers to endemic countries.5 Every year an average of 280 million people are infected with this parasite, but this may be an underestimate considering the difficulties of diagnosis in endemic countries.6 Giardiasis often presents with symptoms such as diarrhoea, abdominal pain, nausea, dehydration and weight loss.7 Although giardiasis is rarely fatal, WHO disease burden estimates are of 170 000 disability-adjusted life years (DALYs) annually.8 The substantial burden in childhood, with associated weight loss and potentially chronic nature if untreated, contributes to this and highlights the importance of effective treatment. Although not well quantified, treatment is also likely to be important in the control of infection given the importance of the human reservoir for this infection and person-to-person transmission.
Metronidazole (MTZ), a drug of the 5-nitroimidazole (5-NI) class, is the first-choice giardiasis treatment. It is the only giardiasis treatment included as such in the 2015 WHO Essential Medicines List9 or advised by PHE.10 Tinidazole (TNZ) is now also acknowledged by other UK and USA health authorities.11–13 Alternative treatments include nitazoxanide (NTZ), paromomycin (PRM), mepacrine or quinacrine (QC), furazolidone (FZD), albendazole (ABZ) and mebendazole (MBZ).11 These drugs may be useful in cases where metronidazole has failed, as they belong to different chemical families and target different pathways.14
Systematic reviews of randomized controlled trials (RCTs), including two Cochrane reviews, have shown evidence of higher efficacy of metronidazole compared with other drugs,15–18 and to a lesser degree evidence of higher efficacy of other 5-NIs, such as tinidazole, ornidazole (OZN) or secnidazole (SCZ), compared with other drugs15,19 Conversely, another systematic review suggested that albendazole was as effective as metronidazole, but with substantially fewer side effects.20 For the remaining drugs fewer RCTs were available, and the evidence regarding their efficacy and side effects is uncertain.16 Inclusion criteria and search strategies focusing on few single pairwise comparisons or drug classes may have contributed to these inconsistent findings.
Previous systematic reviews only considered pairwise comparisons between treatments. Multiple treatments or network meta-analysis (NMA) offers a solution to situations in which many treatments need to be compared, but there is little to no direct evidence for some of the comparisons.21
We aimed to collect all the available evidence on parasitological cure and side effects from RCTs of drugs for the treatment of giardiasis and combine this evidence using NMA.
Methods
Search strategy and selection criteria
This systematic review and NMA was conducted according to the PRISMA guidelines extension.22 We included all RCTs from previous systematic reviews and meta-analyses15–20 that compared the efficacy of drugs for the treatment of giardiasis. In addition, the literature search from all previously published systematic reviews and meta-analyses was integrated (see search terms and strategy in Appendix 1 and Table S1, available as Supplementary data at JAC Online) and updated from the end date of the broadest systematic review15 (May 2013) to May 2016. Language was restricted to English, Spanish and Portuguese. The references of all included studies and relevant experts’ narrative reviews23–26 were also searched for eligible studies that may have been missed by previous systematic reviews.
Inclusion and exclusion criteria
All RCTs comparing two or more giardiasis treatments or placebo in children and/or adults were included. A study was considered randomized if the article reported that treatments were allocated at random. All settings (outpatient and inpatient) were included. RCTs had to report parasitological cure rates to be included. We did not focus on clinical cure rate because of its potential subjectivity and methodological variability. Studies that only compared a single drug at different doses were excluded.
Data extraction
Two authors (J. M. O. M. and T. R. F.) independently extracted the following data: name of first author, year of publication, country, sample size, age of participants, setting, patients’ characteristics, length of follow-up, treatment/drug assigned, dosage (including dose, daily frequency and duration), number of patients initially assigned to the drug, number treated and number cured at end of follow-up. If a drug was administered at different dosages in different arms of a trial, these arms were combined. Data for the following side effects were extracted: any side effect, metallic taste, abdominal pain, nausea, vomiting, diarrhoea, dizziness, yellow urine, headache (or cephalgia), sickness, loss of appetite (or hyporexia/anorexia), vertigo, somnolence/drowsiness, urticaria (or hives or skin rash), weakness (or fatigue) and jaundice (or yellow skin).
Assessment of risk of bias and quality of the evidence
The reporting quality of included studies was assessed by two investigators (J. M. O. M. and T. R. F.) independently. Disagreements were resolved through discussion. Studies were rated as low, unclear or high risk using the Cochrane Collaboration risk of bias criteria.27
Quality of the evidence was assessed using the GRADE working group approach for network meta-analysis.28
Data analysis
Analyses were conducted in R (version 3.2.3).29 Drug efficacy and side effects within a study arm were calculated in the ITT population to avoid bias due to non-random loss of participants. If ITT data were not provided or could not be estimated, PP data were used.
First, exploratory estimates of drug efficacy (i.e. proportion of patients cured) and proportion of patients with side effects were obtained for each treatment separately, using a mixed-effects logistic regression meta-analysis model (the ‘metafor’ R package).30 Heterogeneity was assessed with I2 estimates and Wald P values for drugs tested in five or more RCTs. If drug efficacy was estimated in just one RCT, we used the Wilson formula to derive the exact 95% CI.31
We performed a random-effects NMA using the R package ‘netmeta’ (version 0.9-4).32,33 We estimated a relative risk (RR) and 95% CI, to compare drug efficacies and proportions of patients with side effects for each pair of available treatments. In the case of zero counts, a correction of +0.5 for all arms within the RCT was used. Publication bias was assessed by visual inspection of funnel plots (‘metafor’ R package).30
The assumption of transitivity was tested by looking at the distribution of potential effect modifiers (patients and study characteristics) and treatment allocation in two different subsamples: (i) children and (ii) symptomatic patients. Heterogeneity and inconsistency were assessed by decomposing Cochran’s Q statistic34 and using I2 tests.35 Sources of inconsistency were visualized with net heat plots.34
Results
A flow diagram of the updated literature search is depicted in Figure S1. Briefly, we identified four new RCTs published after the period covered by previous systematic reviews. These were added to the 48 RCTs identified in previous systematic reviews.15–20 We further identified eight additional RCTs by scanning reference lists of included RCTs and experts’ reviews. Thus, 60 RCTs encompassing 6714 patients were eligible. Appendixes 2 and 3 list all included and excluded studies, and sources of identification and reasons for exclusion, respectively.
Table 1 and Table S2 summarize the characteristics of included studies. The majority of RCTs included symptomatic children from outpatient settings. In most studies, the length of follow-up was >2 weeks. Most RCTs were published in the WHO Region of the Americas, particularly in Cuba, followed by the European and South-East Asia regions. The characteristics of RCTs conducted only in children, or only in symptomatic patients, were similar to those of all RCTs.
Table 1.
Summary of characteristics for all included RCTs, for those including children only, and for those including symptomatic patients only
| Characteristic | Overall |
Children only |
Symptomatic only |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total trials (N) | 60 | 100 | 38 | 100 | 37 | 100 |
| Publication year | ||||||
| ≤1979 | 6 | 10 | 3 | 8 | 5 | 14 |
| 1980–89 | 9 | 15 | 2 | 5 | 6 | 16 |
| 1990–99 | 18 | 30 | 16 | 42 | 9 | 24 |
| 2000–09 | 17 | 28 | 13 | 34 | 11 | 30 |
| ≥2010 | 10 | 17 | 4 | 11 | 6 | 16 |
| Participants (n) | ||||||
| <100 | 30 | 50 | 17 | 45 | 18 | 49 |
| ≥100 | 30 | 50 | 21 | 55 | 19 | 51 |
| Age | ||||||
| children | 38 | 63 | 38 | 100 | 23 | 62 |
| children and adults | 13 | 22 | 0 | 0 | 8 | 22 |
| adults | 8 | 13 | 0 | 0 | 5 | 14 |
| unclear | 1 | 2 | 0 | 0 | 1 | 3 |
| Setting | ||||||
| outpatient | 38 | 63 | 27 | 71 | 26 | 70 |
| hospital | 12 | 20 | 7 | 18 | 7 | 19 |
| mixed | 1 | 2 | 0 | 0 | 0 | 0 |
| unclear | 9 | 15 | 4 | 11 | 4 | 11 |
| Patient characteristics | ||||||
| symptomatic | 37 | 62 | 23 | 61 | 37 | 100 |
| asymptomatic | 5 | 8 | 3 | 8 | 0 | 0 |
| mixed | 12 | 20 | 8 | 21 | 0 | 0 |
| unclear | 6 | 10 | 4 | 11 | 0 | 0 |
| WHO regiona | ||||||
| Americas | 26 | 43 | 19 | 50 | 15 | 41 |
| South-East Asia | 12 | 20 | 8 | 21 | 4 | 11 |
| Eastern Mediterranean | 10 | 17 | 6 | 16 | 9 | 24 |
| European | 11 | 18 | 4 | 11 | 9 | 24 |
| African | 1 | 2 | 1 | 3 | 0 | 0 |
| Western Pacific | 0 | 0 | 0 | 0 | 0 | 0 |
| Follow-up (days) | ||||||
| <14 | 22 | 37 | 13 | 34 | 15 | 41 |
| ≥14 | 35 | 58 | 23 | 61 | 19 | 51 |
| unclear | 3 | 5 | 2 | 5 | 3 | 8 |
| Giardiasis treatment | ||||||
| albendazole | 17 | 28 | 14 | 37 | 6 | 16 |
| chloroquine | 2 | 3 | 2 | 5 | 1 | 3 |
| furazolidone | 7 | 12 | 4 | 11 | 7 | 19 |
| mebendazole | 13 | 22 | 8 | 21 | 10 | 27 |
| metronidazole | 39 | 65 | 24 | 63 | 25 | 68 |
| nitazoxanide | 7 | 12 | 6 | 16 | 5 | 14 |
| ornidazole | 6 | 10 | 2 | 5 | 4 | 11 |
| quinacrine | 3 | 5 | 2 | 5 | 3 | 8 |
| secnidazole | 9 | 15 | 6 | 16 | 7 | 19 |
| tinidazole | 18 | 30 | 12 | 32 | 9 | 24 |
| other | 11 | 18 | 4 | 11 | 6 | 16 |
WHO regions included the following countries: Africa: Tanzania (n = 1); Americas: Brazil (n = 5), Cuba (n = 12), Mexico (n = 6), Peru (n = 3); Eastern Mediterranean: Egypt (n = 2), Iraq (n = 2), Iran (n = 6); Europe: Finland (n = 2), Israel (n = 1), Kazakhstan (n = 1), Spain (n = 3), Turkey (n = 4); South-East Asia: Bangladesh (n = 4), India (n = 6), Thailand (n = 2).
Table 2 summarizes the characteristics of drugs administered. We found 18 different treatments for giardiasis, metronidazole being the most tested. Most treatments had an efficacy of >80%, although heterogeneity was high. There was high variability in the drug dose, frequency of administration and duration of treatment for metronidazole, albendazole, mebendazole, furazolidone, nitazoxanide and ornidazole (Table S3). For secnidazole or tinidazole in nearly all cases a single dose was administered.
Table 2.
Summary of drug efficacy
| Druga | No. of RCTs | Patients cured | Patients treated | Pooled efficacy (95% CI) | Heterogeneity |
||
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| Albendazole | 17 | 757 | 983 | 0.79 | (0.69–0.86) | 89 | <0.001 |
| A. graveolens | 1 | 14 | 14 | 1.00 | (0.78–1.00) | NA | NA |
| Chloroquine | 2 | 95 | 111 | 0.86 | (0.78–0.91) | NA | NA |
| Furazolidone | 7 | 149 | 180 | 0.86 | (0.75–0.92) | 53 | 0.05 |
| Mebendazole | 13 | 348 | 493 | 0.66 | (0.51–0.79) | 88 | <0.001 |
| Mentha crispa | 1 | 22 | 50 | 0.44 | (0.31–0.58) | NA | NA |
| Metronidazole | 39 | 1284 | 1532 | 0.88 | (0.83–0.91) | 84 | <0.001 |
| Nitazoxanide | 7 | 214 | 300 | 0.71 | (0.63–0.78) | 51 | 0.05 |
| Oleozon | 1 | 63 | 112 | 0.56 | (0.47–0.65) | NA | NA |
| Ornidazole | 6 | 223 | 351 | 0.80 | (0.60–0.92) | 91 | <0.001 |
| Placebo | 3 | 13 | 64 | 0.01 | (0.00–0.88) | NA | NA |
| Propolis | 2 | 102 | 153 | 0.63 | (0.45–0.79) | NA | NA |
| Paromomycin | 1 | 54 | 59 | 0.92 | (0.82–0.96) | NA | NA |
| Praziquantel | 1 | 17 | 30 | 0.57 | (0.39–0.73) | NA | NA |
| Quinacrine | 3 | 140 | 161 | 0.87 | (0.81–0.91) | NA | NA |
| Sausalin | 1 | 107 | 125 | 0.86 | (0.78–0.91) | NA | NA |
| Secnidazole | 9 | 523 | 595 | 0.88 | (0.83–0.91) | 45 | 0.08 |
| Tinidazole | 18 | 791 | 957 | 0.85 | (0.79–0.89) | 78 | <0.001 |
NA, not assessed.
In addition, albendazole was administered in combination with nitazoxanide in one trial, and in combination with praziquantel in another trial.
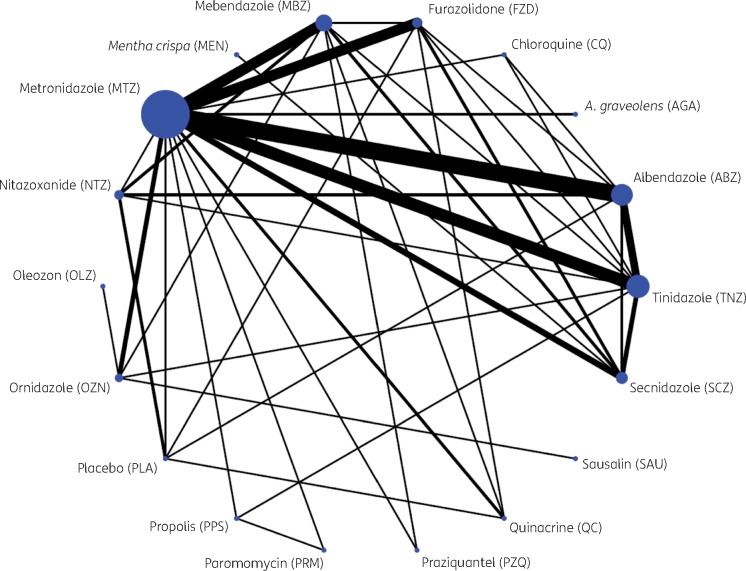
Table 3 and Figure 1 give an overview of the different treatment comparisons. There were 33 different combinations of treatment comparison. The majority of RCTs (n = 51) compared just two arms, with the most common study design being albendazole:metronidazole (n = 10), followed by metronidazole:tinidazole (n = 7). Nine RCTs had a multi-arm design.
Table 3.
Clinical trial design including number of treated and cured patients (sorted by descending number of arms and by treatment in alphabetical order)
| First author, year | Design (no. of trials) | Arms | ABZ | AGA | CQ | FZD | MBZ | MEN | MTZ | NTZ | OLZ | OZN | PLA | PPS | PRM | PZQ | QC | SAU | SCZ | TNZ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan del Pino, 1999 | ABZ/FZD/MTZ/SCZ/TNZ (1) | 5 | 11/17 | 14/15 | 13/17 | 11/15 | 13/15 | |||||||||||||
| Bassily, 1970 | FZD/MTZ/PLA/QC (1) | 4 | 16/20 | 19/20 | 0/20 | 20/20 | ||||||||||||||
| Escobedo, 2003a | ABZ/CQ/TNZ (1) | 3 | 37/60 | 43/50 | 50/55 | |||||||||||||||
| Speich, 2013 | ABZ/NTZ/PLA (1) | 3 | 15/25 | 12/21 | 13/25 | |||||||||||||||
| Kalayci, 1995 | FZD/MBZ/MTZ (1) | 3 | 12/15 | 11/15 | 14/15 | |||||||||||||||
| Chacon, 1991 | FZD/MTZ/SCZ (1) | 3 | 19/20 | 18/20 | 12/17 | |||||||||||||||
| Bulut, 1996 | MBZ/MTZ/OZN (1) | 3 | 12/34 | 13/15 | 10/11 | |||||||||||||||
| Imani, 2004 | MBZ/MTZ/PZQ (1) | 3 | 15/30 | 28/30 | 17/30 | |||||||||||||||
| Nunez, 2004 | MTZ/PPS/PRM (1) | 3 | 71/89 | 80/108 | 54/59 | |||||||||||||||
| Alizadeh, 2006 | ABZ/MTZ (10) | 2 | 54/60 | 46/60 | ||||||||||||||||
| Cañete, 2012 | 62/75 | 64/75 | ||||||||||||||||||
| Dutta, 1994 | 73/75 | 73/75 | ||||||||||||||||||
| Hall, 1993a | 112/144 | 77/78 | ||||||||||||||||||
| Hall, 1993b | 122/141 | 63/63 | ||||||||||||||||||
| Karabay, 2004 | 27/33 | 29/34 | ||||||||||||||||||
| Misra, 1995 | 28/32 | 29/32 | ||||||||||||||||||
| Rodriguez-Garcia, 1996 | 21/27 | 16/22 | ||||||||||||||||||
| Romero-Cabello, 1995 | 47/50 | 49/50 | ||||||||||||||||||
| Yereli, 2004 | 47/52 | 49/55 | ||||||||||||||||||
| Bances-Garcia, 2013 | ABZ/NTZ (1) | 2 | 40/49 | 43/49 | ||||||||||||||||
| Mendoza, 2003 | ABZ/TNZ (3) | 2 | 17/49 | 31/43 | ||||||||||||||||
| Pengsaa, 1999 | 31/68 | 49/63 | ||||||||||||||||||
| Pengsaa, 2002 | 13/26 | 25/27 | ||||||||||||||||||
| Sahib, 2014 | AGA/MTZ (1) | 2 | 14/14 | 14/14 | ||||||||||||||||
| Canete, 2010 | CQ/MTZ (1) | 2 | 52/61 | 45/61 | ||||||||||||||||
| Garg, 1972 | FZD/MTZ (3) | 2 | 38/40 | 38/40 | ||||||||||||||||
| Nair, 1979 | 16/20 | 16/19 | ||||||||||||||||||
| Quiros-Buelna, 1989 | 34/50 | 43/50 | ||||||||||||||||||
| Al-Waili, 1992 | MBZ/MTZ (4) | 2 | 21/23 | 18/21 | ||||||||||||||||
| Gascon, 1989 | 2/14 | 8/9 | ||||||||||||||||||
| Gascon, 1990 | 1/8 | 10/11 | ||||||||||||||||||
| Sadjjadi, 2001 | 43/50 | 45/50 | ||||||||||||||||||
| Davila-Gutierrez, 2002 | MBZ/NTZ (2) | 2 | 11/19 | 18/32 | ||||||||||||||||
| Rodriguez-Garcia, 1999 | 33/41 | 32/41 | ||||||||||||||||||
| Canete, 2006a | MBZ/QC (1) | 2 | 48/61 | 51/61 | ||||||||||||||||
| Almirall, 2011 | MBZ/SCZ (2) | 2 | 55/64 | 56/62 | ||||||||||||||||
| Escobedo, 2003b | 57/73 | 58/73 | ||||||||||||||||||
| Canete, 2006b | MBZ/TNZ (1) | 2 | 39/61 | 50/61 | ||||||||||||||||
| Teles, 2011 | MEN/SCZ (1) | 2 | 22/50 | 42/50 | ||||||||||||||||
| Ortiz, 2001 | MTZ/NTZ (1) | 2 | 41/55 | 39/55 | ||||||||||||||||
| Leite, 1976 | MTZ/OZN (2) | 2 | 12/15 | 13/15 | ||||||||||||||||
| Oren, 1991 | 37/37 | 35/38 | ||||||||||||||||||
| Kavousi, 1979 | MTZ/QC (1) | 2 | 75/80 | 69/80 | ||||||||||||||||
| Cimerman, 1988 | MTZ/SCZ (2) | 2 | 52/60 | 57/62 | ||||||||||||||||
| Rastegar-Lari, 1996 | 24/25 | 27/27 | ||||||||||||||||||
| Fallah, 2007 | MTZ/TNZ (7) | 2 | 43/64 | 37/42 | ||||||||||||||||
| Gazder, 1977 | 19/50 | 40/50 | ||||||||||||||||||
| Kyronseppa, 1981 | 19/25 | 22/25 | ||||||||||||||||||
| Nigam, 1991 | 19/35 | 39/40 | ||||||||||||||||||
| Perez-Choliz, 1989 | 12/27 | 23/25 | ||||||||||||||||||
| Speelman, 1985a | 9/17 | 16/18 | ||||||||||||||||||
| Speelman, 1985b | 14/17 | 15/18 | ||||||||||||||||||
| Rossignol, 2001 | NTZ/PLA (1) | 2 | 12/17 | 0/19 | ||||||||||||||||
| Escobedo, 2008 | NTZ/TNZ (1) | 2 | 58/85 | 57/81 | ||||||||||||||||
| Amoroto, 2002 | OLZ/OZN (1) | 2 | 63/112 | 67/112 | ||||||||||||||||
| Begaydarova, 2014 | OZN/SAU (1) | 2 | 53/125 | 107/125 | ||||||||||||||||
| Jokipii, 1982 | OZN/TNZ (1) | 2 | 45/50 | 45/50 | ||||||||||||||||
| Miyares, 1988 | PPS/TNZ (1) | 2 | 22/45 | 17/45 | ||||||||||||||||
| Cimerman, 1997 | SCZ/TNZ (2) | 2 | 116/129 | 120/138 | ||||||||||||||||
| Cimerman, 1999 | 144/160 | 142/161 |
ABZ, albendazole; AGA, Anethum graveolens; CQ, chloroquine; FZD, furazolidone; MBZ, mebendazole; MEN, Mentha crispa; MTZ, metronidazole; NTZ, nitazoxanide; OLZ, oleozon; OZN, ornidazole; PLA, placebo; PPS, propolis; PRM, paromomycin; PZQ, praziquantel; QC, quinacrine; SAU, sausalin; SCZ, secnidazole; TNZ, tinidazole.
Figure 1.
Network graph. The sizes of the nodes and edges are proportional to the number of patients receiving the drug and number of trials comparing these two drugs, respectively. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
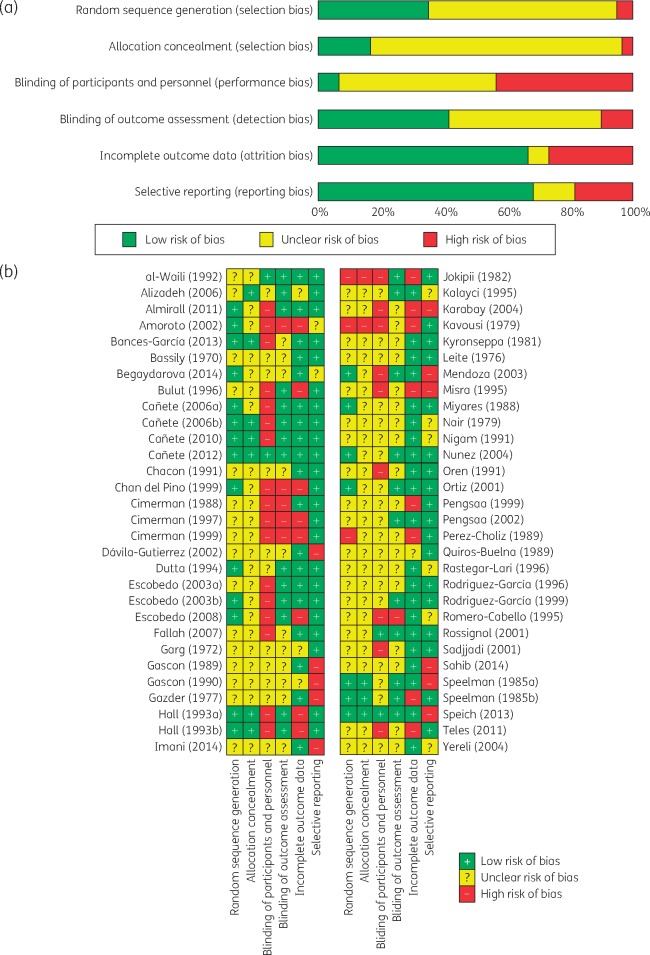
Figure 2(a and b) summarizes the assessment of risk of bias. For random sequence generation, allocation concealment and blinding, the majority of studies were judged as having high or unclear risk of bias. For the attrition and reporting bias domains, >66% of RCTs were at low risk.
Figure 2.
(a) Summary risk of bias assessment. (b) Risk of bias assessment across individual studies. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The results of traditional pairwise meta-analysis and NMA are combined in Table 4. Both metronidazole (RR 1.10, 95% CI 1.01–1.19) and tinidazole (RR 1.35, 95% CI 1.21–1.50) were associated with higher cure rates than albendazole. Tinidazole was associated with a higher cure rate than metronidazole (RR 1.23, 95% CI 1.12–1.35), the WHO-recommended treatment. Tinidazole was associated with significantly or non-significantly higher cure rates on NMA than all other drugs apart from sausalin. Sausalin was statistically significantly associated with higher cure rates than all other drugs, but this was based on data from a single trial against ornidazole, whose efficacy appeared lower in this trial than in other RCTs.
Table 4.
RRs and 95% CI for the comparative efficacy of drugs for the treatment of giardiasisa
| ABZ | 1.39 | 1.44 | 1.04 | 1.05 | 0.87 | 1.13 | 1.63 | ||||||||||
| (1.00–1.95) | (0.92–2.26) | (0.96–1.14) | (0.80–1.36) | (0.50–1.51) | (0.67–1.92) | (1.36–1.97) | |||||||||||
| 1.10 | AGA | 1.00 | |||||||||||||||
| (0.82–1.47) | (0.75–1.33) | ||||||||||||||||
| 1.30 | 1.18 | CQ | 0.87 | 1.06 | |||||||||||||
| (1.05–1.60) | (0.83–1.67) | (0.64–1.18) | |||||||||||||||
| (0.80–1.41) | |||||||||||||||||
| 1.10 | 1.00 | 0.85 | FZD | 0.92 | 1.05 | 0.03 | 1.24 | 0.76 | 0.93 | ||||||||
| (0.95–1.26) | (0.74–1.36) | (0.67–1.07) | (0.57–1.46) | (0.92–1.19) | (0.00–0.48) | (0.88–1.75) | (0.57–1.02) | (0.66–1.31) | |||||||||
| 0.99 | 0.90 | 0.76 | 0.90 | MBZ | 1.28 | 0.97 | 2.58 | 1.13 | 1.06 | 1.04 | 1.28 | ||||||
| (0.86–1.13) | (0.66–1.22) | (0.61–0.96) | (0.77–1.05) | (1.09–1.51) | (0.73–1.29) | (1.48–4.47) | (0.66–1.94) | (0.79–1.44) | (0.84–1.27) | (0.92–1.79) | |||||||
| 0.60 | 0.55 | 0.46 | 0.55 | 0.61 | MEN | 1.91 | |||||||||||
| (0.39–0.93) | (0.33–0.92) | (0.29–0.74) | (0.35–0.85) | (0.39–0.94) | (1.26–2.9) | ||||||||||||
| 1.10 | 1.00 | 0.85 | 1.00 | 1.11 | 1.83 | MTZ | 0.95 | 0.99 | 0.03 | 0.93 | 1.15 | 0.61 | 0.98 | 0.99 | 1.41 | ||
| (1.01–1.19) | (0.75–1.33) | (0.69–1.04) | (0.89–1.12) | (0.99–1.24) | (1.19–2.82) | (0.68–1.33) | (0.82–1.2) | (0.00–0.40) | (0.69–1.24) | (0.87–1.52) | (0.40–0.92) | (0.81–1.19) | (0.84–1.17) | (1.22–1.63) | |||
| 1.07 | 0.98 | 0.83 | 0.98 | 1.08 | 1.79 | 0.98 | NTZ | 0.79 | 1.03 | ||||||||
| (0.91–1.26) | (0.71–1.35) | (0.64–1.06) | (0.80–1.18) | (0.91–1.29) | (1.13–2.82) | (0.83–1.14) | (0.45–1.40) | (0.75–1.42) | |||||||||
| 1.14 | 1.03 | 0.88 | 1.03 | 1.15 | 1.89 | 1.03 | 1.06 | OLZ | 1.06 | ||||||||
| (0.78–1.66) | (0.65–1.65) | (0.58–1.33) | (0.70–1.52) | (0.78–1.69) | (1.07–3.33) | (0.71–1.50) | (0.71–1.58) | (0.76–1.48) | |||||||||
| 1.21 | 1.10 | 0.93 | 1.10 | 1.22 | 2.01 | 1.10 | 1.13 | 1.06 | OZN | 2.02 | 1.00 | ||||||
| (1.01–1.44) | (0.80–1.52) | (0.72–1.20) | (0.90–1.34) | (1.01–1.48) | (1.27–3.18) | (0.94–1.29) | (0.91–1.4) | (0.76–1.48) | (1.45–2.81) | (0.76–1.32) | |||||||
| 0.75 | 0.68 | 0.58 | 0.68 | 0.75 | 1.24 | 0.68 | 0.70 | 0.66 | 0.62 | PLA | 41.0 | ||||||
| (0.46–1.21) | (0.39–1.20) | (0.34–0.97) | (0.41–1.12) | (0.46–1.24) | (0.65–2.38) | (0.42–1.11) | (0.43–1.14) | (0.36–1.21) | (0.37–1.03) | (2.62–642) | |||||||
| 1.15 | 1.05 | 0.89 | 1.05 | 1.16 | 1.92 | 1.05 | 1.07 | 1.01 | 0.95 | 1.54 | PPS | 1.24 | 0.77 | ||||
| (0.88–1.50) | (0.72–1.54) | (0.64–1.23) | (0.79–1.39) | (0.88–1.54) | (1.16–3.17) | (0.81–1.36) | (0.80–1.45) | (0.65–1.59) | (0.71–1.29) | (0.89–2.68) | (0.93–1.64) | (0.45–1.33) | |||||
| 1.34 | 1.22 | 1.03 | 1.22 | 1.35 | 2.23 | 1.22 | 1.25 | 1.18 | 1.11 | 1.79 | 1.16 | PRM | |||||
| (1.01–1.77) | (0.82–1.80) | (0.74–1.45) | (0.90–1.64) | (1.01–1.82) | (1.34–3.71) | (0.93–1.60) | (0.91–1.71) | (0.74–1.86) | (0.81–1.52) | (1.02–3.14) | (0.88–1.53) | ||||||
| 0.74 | 0.67 | 0.57 | 0.67 | 0.75 | 1.23 | 0.67 | 0.69 | 0.65 | 0.61 | 0.99 | 0.64 | 0.55 | PZQ | ||||
| (0.49–1.11) | (0.41–1.10) | (0.36–0.89) | (0.44–1.02) | (0.50–1.13) | (0.68–2.22) | (0.45–1.01) | (0.45–1.06) | (0.38–1.12) | (0.40–0.94) | (0.53–1.87) | (0.4–1.04) | (0.34–0.90) | |||||
| 1.10 | 1.01 | 0.85 | 1.01 | 1.12 | 1.84 | 1.01 | 1.03 | 0.97 | 0.91 | 1.48 | 0.96 | 0.83 | 1.49 | QC | |||
| (0.92–1.32) | (0.73–1.39) | (0.66–1.10) | (0.83–1.21) | (0.94–1.34) | (1.16–2.91) | (0.86–1.18) | (0.83–1.28) | (0.65–1.46) | (0.73–1.15) | (0.89–2.48) | (0.71–1.30) | (0.60–1.13) | (0.97–2.30) | ||||
| 2.44 | 2.22 | 1.88 | 2.22 | 2.47 | 4.06 | 2.22 | 2.27 | 2.15 | 2.02 | 3.27 | 2.12 | 1.82 | 3.30 | 2.21 | SAU | ||
| (1.68–3.54) | (1.40–3.52) | (1.24–2.85) | (1.51–3.26) | (1.69–3.61) | (2.31–7.14) | (1.54–3.20) | (1.53–3.38) | (1.34–3.43) | (1.45–2.81) | (1.78–6.02) | (1.36–3.31) | (1.16–2.88) | (1.92–5.67) | (1.48–3.29) | |||
| 1.15 | 1.04 | 0.88 | 1.04 | 1.16 | 1.91 | 1.04 | 1.07 | 1.01 | 0.95 | 1.54 | 1.00 | 0.86 | 1.55 | 1.04 | 0.47 | SCZ | 1.00 |
| (1.00–1.31) | (0.77–1.41) | (0.70–1.11) | (0.89–1.22) | (1.02–1.32) | (1.26–2.90) | (0.93–1.17) | (0.89–1.28) | (0.69–1.48) | (0.79–1.15) | (0.93–2.53) | (0.75–1.32) | (0.64–1.15) | (1.02–2.35) | (0.86–1.25) | (0.32–0.69) | (0.85–1.19) | |
| 1.35 | 1.23 | 1.04 | 1.23 | 1.37 | 2.25 | 1.23 | 1.26 | 1.19 | 1.12 | 1.81 | 1.17 | 1.01 | 1.82 | 1.22 | 0.55 | 1.18 | TNZ |
| (1.21–1.50) | (0.91–1.65) | (0.85–1.28) | (1.06–1.42) | (1.20–1.56) | (1.46–3.47) | (1.12–1.35) | (1.07–1.49) | (0.82–1.73) | (0.94–1.32) | (1.10–2.97) | (0.90–1.53) | (0.76–1.34) | (1.21–2.75) | (1.02–1.47) | (0.38–0.80) | (1.04–1.33) |
Numbers in bold are statistically significant.
ABZ, albendazole; AGA, Anethum graveolens; CQ, chloroquine; FZD, furazolidone; MBZ, mebendazole; MEN, Mentha crispa; MTZ, metronidazole; NTZ, nitazoxanide; OLZ, oleozon; OZN, ornidazole; PLA, placebo; PPS, propolis; PRM, paromomycin; PZQ, praziquantel; QC, quinacrine; SAU, sausalin; SCZ, secnidazole; TNZ, tinidazole.
The lower triangle (in grey) shows summary RRs (95% CIs) derived in NMA (taking into account both the direct and indirect evidence) for the comparison of the drug in the row versus the drug in the column as reference. In contrast, the upper triangle (in white) shows summary RRs (95% CIs) derived in traditional pairwise meta-analysis (taking into account direct evidence only) for the comparison of the drug in the column versus the drug in the row as reference. White spaces indicate lack of direct evidence for the given comparison. Thus, for example, for the comparison of TNZ with MTZ, the RRs and 95% CIs were 1.23 (1.12–1.35) and 1.41 (1.22–1.63) according to the results from network and traditional pairwise meta-analyses, respectively.
The total variability in effect sizes was substantial (Q = 176, df = 55, I2 = 68%, P < 0.0001). Heterogeneity (variability within designs) was also substantial and statistically significant (Q = 69, df = 27, I2 = 61%, P < 0.0001). The main contributors to the total heterogeneity were the comparisons albendazole:metronidazole (Q = 34, df = 9, I2 = 74%, P < 0.0001), mebendazole:metronidazole (Q = 12, df = 3, I2 = 76%, P = 0.0062) and metronidazole:tinidazole (Q = 17, df = 6, I2 = 65%, P = 0.0080). Inconsistency (variability between designs) estimated by the full design-by-treatment interaction model was also high (Q = 71, df = 27, I2 = 61%, P < 0.0001). The designs contributing most to the total inconsistency were albendazole:tinidazole and metronidazole:tinidazole. Detaching these designs did not result in a loss of statistical significance for the total inconsistency, but increased the inconsistency for many other designs (see net heat plot in Figure S2). There was no evidence of publication bias for designs involving albendazole, metronidazole and tinidazole (Figure S4).
The GRADE assessment of the quality of the evidence for the comparisons metronidazole:albendazole and tinidazole:metronidazole was low, and moderate for tinidazole:albendazole (Table S17).
The number and percentage of side effects reported are shown in Table S4. Of all the RCTs, 85% (n = 51) reported some information on side effects. The summary proportion of side effects for each drug is reported in Table S5. The drugs with the highest proportion of patients with any side effects were chloroquine and Mentha crispa, although these were assessed in a single RCT for each drug. Heterogeneity was high for metronidazole, ornidazole, quinacrine, secnidazole and tinidazole.
The results of traditional pairwise meta-analysis and NMA for any side effects as a composite outcome are shown in Table S6. The network graph is available in Figure S3. In NMA, metronidazole was not associated with higher risk of any side effects than all other drugs. Tinidazole, ornidazole and nitazoxanide were statistically significantly associated with more side effects than albendazole and mebendazole but not than metronidazole. The total variability in the effect sizes was moderate but statistically significant (Q = 59, df = 35, I2 = 40%, P = 0.0073) and could be explained by substantial heterogeneity (Q = 40, df = 16, I2 = 60%, P = 0.0008), particularly in the designs albendazole:metronidazole (Q = 25, df = 5, I2 = 80%, P = 0.0001) and mebendazole:secnidazole (Q = 6.6, df = 1, I2 = 85%, P = 0.0102). Total inconsistency for this NMA was estimated as zero.
With regard to specific side-effects (Tables S7–S16), tinidazole was associated with higher incidence of metallic taste, nausea and vomiting than albendazole, mebendazole and secnidazole. Compared with metronidazole, tinidazole was only significantly associated with higher incidence of nausea.
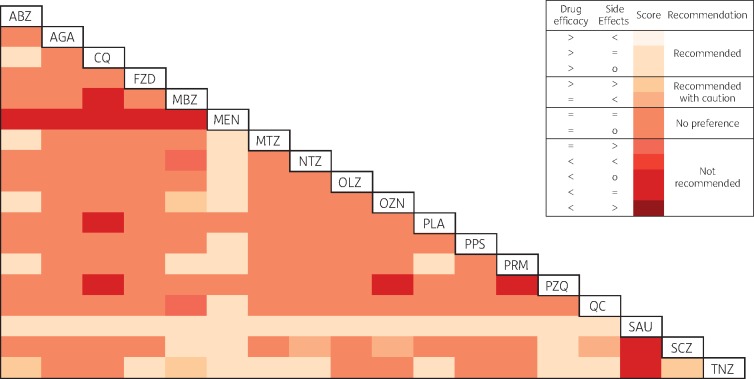
A joint evaluation of drug efficacy and side effects is shown in Figure 3. Sausalin ranked higher than all drugs as it was associated with higher efficacy and was generally not associated with higher or lower side effects. Tinidazole ranked higher than albendazole and mebendazole for efficacy but was associated with more side effects. Metronidazole was preferred to albendazole and Mentha crispa only.
Figure 3.
Joint assessment of drug efficacy and side effects (drug in the row compared with drug in the column). Symbols: >, higher drug efficacy, or more side effects than comparator drug; <, lower drug efficacy, or fewer side effects than comparator drug; =, no evidence of higher or lower drug efficacy, or of more or fewer side effects than comparator drug; o, side effects were not assessed for this comparison. ABZ, albendazole; AGA, Anethum graveolens; CQ, chloroquine; FZD, furazolidone; MBZ, mebendazole; MEN, Mentha crispa; MTZ, metronidazole; NTZ, nitazoxanide; OLZ, oleozon; OZN, ornidazole; PLA, placebo; PPS, propolis; PRM, paromomycin; PZQ, praziquantel; QC, quinacrine; SAU, sausalin; SCZ, secnidazole; TNZ, tinidazole. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
In this large and comprehensive investigation we have applied NMA to all available treatments of giardiasis to evaluate all available trial evidence simultaneously. Previous systematic reviews and traditional meta-analysis have not investigated all treatments, nor provided estimates of the absolute and comparative efficacy of all drugs. Furthermore, we provide a joint assessment of drug efficacy and side effects for each available treatment relative to the others.
Balancing the evidence for drug efficacy and side effects, tinidazole appears to be the best available treatment for giardiasis. It is the only 5-NI associated with a higher parasitological cure rate than metronidazole and it also has a higher cure rate than albendazole. Our estimate of the efficacy of tinidazole relative to albendazole is lower than that from a previous meta-analysis,19 but our estimate is based on a larger number of trials and total sample size, and is strengthened by indirect evidence from the comparisons tinidazole:metronidazole and metronidazole:albendazole. Tinidazole was associated with a higher incidence of side effects than albendazole, mebendazole and secnidazole, but these are largely mild in nature (metallic taste, nausea and vomiting) and are unlikely to affect compliance as they occur after treatment completion.
Based on its combined performance in the assessment of efficacy and side effects, tinidazole may be an appropriate first-choice treatment for giardiasis. The better adherence possible with a single-dose regimen, which is usual for tinidazole, is likely to be even more important in clinical and public health practice than in the more controlled settings of RCTs. We used a measure of efficacy based on parasitological cure of the originally infected individual. Transmission to close contacts and potential reinfection, not generally reported in RCT results, may additionally occur. Greater efficacy of treatments is therefore likely to support better public health control than a single estimate of parasitological cure might suggest.
With regard to costs, in the UK36 the price per gram of tinidazole (£1.38, Fasigyn 16 × 500 mg, Pfizer) is slightly higher than that of metronidazole (£1.13, Flagyl 14 × 400 mg, Zentiva). However, considering the average recommended dosage (400 mg three times a day for 7 days for metronidazole and 1500 mg single dose for tinidazole), a course of tinidazole treatment is likely to be the cheaper. Moreover, since both compounds are out of patent, cost is unlikely to be a barrier to considering tinidazole as a first-line treatment globally. Thus, inclusion of tinidazole in the next update of the WHO Essential Medicines List for the treatment of giardiasis should be considered.
Sausalin was associated with higher cure rates than all other drugs, although CIs were wider than those for tinidazole. Only one RCT used sausalin as a treatment.37 Although it did not qualify as high risk for any of the biases assessed, risk was judged as unclear for some domains. However, in this RCT the comparator treatment (ornidazole) had a strikingly low efficacy compared with the performance of ornidazole in other trials. This combination of effects thus produces weak evidence for the very high observed relative efficacy of sausalin in indirect comparisons. Additional RCTs are needed to test sausalin in other populations and in direct comparison with front-line treatments. This may be important to guide treatment for those failing 5-NI first-line treatment or to give an evidence base for first-line treatments in areas where NI resistance is common.3
Previous systematic reviews and meta-analyses have argued that albendazole is as effective as metronidazole but causes fewer side effects.16,20 We found that metronidazole was statistically significantly associated with a higher cure rate than albendazole in both traditional and network meta-analysis. Other drugs that were found to be statistically significantly better in treating giardiasis than albendazole are chloroquine, ornidazole, paromomycin, sausalin and tinidazole. Our work does not therefore support a place for albendazole in the treatment of giardiasis.
Our study has many advantages compared with previous systematic reviews, including completeness, broad criteria for inclusion and minimal exclusion criteria, external validity, wider range of treatments, and assessment of efficacy and proportion of patients with side effects for all drugs.
Our investigation has several limitations. Heterogeneity and inconsistency were substantial around designs including metronidazole, which may be a result of the high variability in the dose and duration of metronidazole. Other potential explanatory variables such as patient characteristics or study setting have been previously tested as moderators but they were not found to explain the variability in effect sizes.15 Owing to the limited number of RCTs within each design we could not explore quantitatively the sources of heterogeneity. Overall, the quality of RCTs was moderate, the biggest problem being the lack of blinding of participants and personnel due to the different regimens (e.g. single dose versus multiple doses) being administered. Also, because of heterogeneity and inconsistency the quality of the evidence and thus our confidence in the estimates is low to moderate. Drug efficacy is likely to be related to length of follow-up, which varied between studies. We used data from the latest timepoint available, but estimates of drug efficacy may have been influenced by cases who became cured after the end of follow-up or by instances of re-infection after initial cure. We decided a priori to combine treatments with different dosages to simplify the network, and so did not assess any dose-related efficacy effects. Nearly every included RCT assessed parasitological cure with microscopy methods, which are considered less sensitive than immunoassay and polymerase chain reaction methods in a single stool specimen, but highly sensitive in three or more faecal samples,38,39 which was the methodology used by most RCTs in this review. Finally, we were not able to estimate the impact that the different drugs could have on the global public health burden or the impact on the emerging problem of metronidazole-resistant giardiasis owing to the lack of data in the included RCTs.
In this comprehensive investigation of all giardiasis treatments we provide strong evidence in favour of the adoption of tinidazole as a first-line treatment, given its higher efficacy and comparable side effects to the current mainstay therapy, metronidazole. Thus, inclusion of tinidazole in the WHO Essential Medicines List for the treatment of giardiasis should be considered. Further research is needed to clarify whether newer therapies such as sausalin may be more effective, particularly given the evidence of emerging nitroimidazole resistance.3
Supplementary Material
Acknowledgements
We thank Julie McLellan for her assistance finding some of the articles included in this review.
Funding
This work was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at the University of Liverpool in partnership with Public Health England (PHE), University of East Anglia, University of Oxford and the Institute of Food Research. The corresponding and senior authors had full access to the data and had final responsibility for the decision to submit for publication.
Transparency declarations
None to declare.
Disclaimer
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Supplementary data
Appendixes 1 to 3, Tables S1 to S17 and Figures S1 to S4 are available as Supplementary data at JAC Online.
References
- 1. Minetti C, Chalmers RM, Beeching NJ. et al. Giardiasis. BMJ 2016; 355: i5369.. [DOI] [PubMed] [Google Scholar]
- 2. Speich B, Croll D, Furst T. et al. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 87–99. [DOI] [PubMed] [Google Scholar]
- 3. Nabarro LEB, Lever RA, Armstrong M. et al. Increased incidence of nitroimidazole-refractory giardiasis at the Hospital for Tropical Diseases, London: 2008–2013. Clin Microbiol Infect 2015; 21: 791–6. [DOI] [PubMed] [Google Scholar]
- 4. Savioli L, Smith H, Thompson A.. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 2006; 22: 203–8. [DOI] [PubMed] [Google Scholar]
- 5. Schlagenhauf P, Weld L, Goorhuis A. et al. Travel-associated infection presenting in Europe (2008-12): an analysis of EuroTravNet longitudinal, surveillance data, and evaluation of the effect of the pre-travel consultation. Lancet Infect Dis 2015; 15: 55–64. [DOI] [PubMed] [Google Scholar]
- 6. Canete R, Rodriguez P, Mesa L. et al. Albendazole versus metronidazole in the treatment of adult giardiasis: a randomized, double-blind, clinical trial. Curr Med Res Opin 2012; 28: 149–54. [DOI] [PubMed] [Google Scholar]
- 7. Robertson LJ, Hanevik K, Escobedo AA. et al. Giardiasis–why do the symptoms sometimes never stop? Trends Parasitol 2010; 26: 75–82. [DOI] [PubMed] [Google Scholar]
- 8. Torgerson PR, Devleesschauwer B, Praet N. et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med 2015; 12: e1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO/EMP. WHO Model List of Essential Medicines, 19th List (April 2015)—Amended November 2015. Geneva: World Health Organization (WHO; ), 2015. [Google Scholar]
- 10. Public Health England (PHE). Managing Suspected Infectious Diarrhoea Quick Reference Guidance For Primary Care. London, UK, 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/409768/Managing_Suspected_Infectious_Diarrhoea_7_CMCN29_01_15_KB_FINAL.pdf [Google Scholar]
- 11. Centers for Disease Control and Prevention (CDC). Parasites—Giardia—Treatment http://www.cdc.gov/parasites/giardia/treatment.html.
- 12. National Health Service (NHS). NHS Choices. Giardiasis http://www.nhs.uk/conditions/giardiasis/Pages/Introduction.aspx.
- 13. Joint Formulary Committee. British National Formularly. 5 Infections. 5.4 Antiprotozoal Drugs. 5.4.4 Antigiardial Drugs (online) https://bnf.nice.org.uk/treatment-summary/antiprotozoal-drugs.html.
- 14. Canete R, Escobedo AA, Gonzalez ME. et al. Randomized clinical study of five days apostrophe therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol 2006; 12: 6366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasupuleti V, Escobedo AA, Deshpande A. et al. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: a systematic review of randomized controlled trials. PLoS Negl Trop Dis 2014; 8: e2733.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granados CE, Reveiz L, Uribe LG. et al. Drugs for treating giardiasis. Cochrane Database Syst Rev 2012; issue 12: CD007787.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaat JOM, Mank T, Assendelft WJJ.. Drugs for treating giardiasis. Cochrane Database Syst Rev 2007; issue 2: CD000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaat JOM, Mank TG, Assendelft WJJ.. A systematic review on the treatment of giardiasis. Trop Med Int Health 1997; 2: 63–82. [DOI] [PubMed] [Google Scholar]
- 19. Escobedo AA, Ballesteros J, González-Fraile E. et al. A meta-analysis of the efficacy of albendazole compared with tinidazole as treatments for Giardia infections in children. Acta Trop 2016; 153: 120–7. [DOI] [PubMed] [Google Scholar]
- 20. Solaymani-Mohammadi S, Genkinger JM, Loffredo CA. et al. A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis. PLoS Negl Trop Dis 2010; 4: e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cipriani A, Higgins JT, Geddes JR. et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013; 159: 130–7. [DOI] [PubMed] [Google Scholar]
- 22. Hutton B, Salanti G, Caldwell DM. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–84. [DOI] [PubMed] [Google Scholar]
- 23. Busatti HG, Santos JF, Gomes MA.. The old and new therapeutic approaches to the treatment of giardiasis: where are we? Biologics 2009; 3: 273–87. [PMC free article] [PubMed] [Google Scholar]
- 24. Escobedo AA, Cimerman S.. Giardiasis: a pharmacotherapy review. Expert Opin Pharmacother 2007; 8: 1885–902. [DOI] [PubMed] [Google Scholar]
- 25. Gardner TB, Hill DR.. Treatment of giardiasis. Clin Microbiol Rev 2001; 14: 114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wright JM, Dunn LA, Upcroft P. et al. Efficacy of antigiardial drugs. Expert Opin Drug Saf 2003; 2: 529–41. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Altman DG, Gotzsche PC. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puhan MA, Schunemann HJ, Murad MH. et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014; 349: g5630.. [DOI] [PubMed] [Google Scholar]
- 29. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2010. [Google Scholar]
- 30. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 48. [Google Scholar]
- 31. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17: 857–72. [DOI] [PubMed] [Google Scholar]
- 32. Rücker G, Schwarzer G, Krahn U. et al. netmeta: Network Meta-Analysis Using Frequentist Methods R package version 0.8-0, 2015.
- 33. Rucker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012; 3: 312–24. [DOI] [PubMed] [Google Scholar]
- 34. Krahn U, Binder H, Konig J.. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol 2013; 13: 35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins JP, Jackson D, Barrett JK. et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012; 3: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joint Formulary Committee. British National Formulary (Online). London, UK: BMJ Group and Pharmaceutical Press, 2017. [Google Scholar]
- 37. Begaydorova R, Nasakaeva GE, Tabagari SI. et al. Clinical and diagnostic features and treatment of giardiasis. Georgian Med News 2014; 55–61. [PubMed] [Google Scholar]
- 38. Jahan N, Khatoon R, Ahmad S.. A comparison of microscopy and enzyme linked immunosorbent assay for diagnosis of Giardia lamblia in human faecal specimens. J Clin Diagn Res 2014; 8: Dc04–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Behr MA, Kokoskin E, Gyorkos TW. et al. Laboratory diagnosis for Giardia lamblia infection: a comparison of microscopy, coprodiagnosis and serology. Can J Infect Dis 1997; 8: 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.