Abstract
Objectives: Rapid, cost-effective and objective methods for antimicrobial susceptibility testing of Neisseria gonorrhoeae would greatly enhance surveillance of antimicrobial resistance. Etest, disc diffusion and agar dilution methods are subjective, mostly laborious for large-scale testing and take ∼24 h. We aimed to develop a rapid broth microdilution assay using resazurin (blue), which is converted into resorufin (pink fluorescence) in the presence of viable bacteria.
Methods: The resazurin-based broth microdilution assay was established using 132 N. gonorrhoeae strains and the antimicrobials ceftriaxone, cefixime, azithromycin, spectinomycin, ciprofloxacin, tetracycline and penicillin. A regression model was used to estimate the MICs. Assay results were obtained in ∼7.5 h.
Results: The EC50 of the dose–response curves correlated well with Etest MIC values (Pearson’s r = 0.93). Minor errors resulting from misclassifications of intermediate strains were found for 9% of the samples. Major errors (susceptible strains misclassified as resistant) occurred for ceftriaxone (4.6%), cefixime (3.3%), azithromycin (0.6%) and tetracycline (0.2%). Only one very major error was found (a ceftriaxone-resistant strain misclassified as susceptible). Overall the sensitivity of the assay was 97.1% (95% CI 95.2–98.4) and the specificity 78.5% (95% CI 74.5–82.9).
Conclusions: A rapid, objective, high-throughput, quantitative and cost-effective broth microdilution assay was established for gonococci. For use in routine diagnostics without confirmatory testing, the specificity might remain suboptimal for ceftriaxone and cefixime. However, the assay is an effective low-cost method to evaluate novel antimicrobials and for high-throughput screening, and expands the currently available methodologies for surveillance of antimicrobial resistance in gonococci.
Introduction
Neisseria gonorrhoeae is a very fastidious bacterium that causes the sexually transmitted infection gonorrhoea. Gonorrhoea is a public health concern globally1,2 and N. gonorrhoeae has developed resistance to all antimicrobials introduced for treatment.3 Accordingly, enhanced surveillance of antimicrobial susceptibility in N. gonorrhoeae is imperative globally.1 Ideally, this surveillance should be performed using methods determining the MICs of relevant antimicrobials. MIC-based methods are also valuable to directly inform treatment after laboratory results are available and evaluate in vitro efficacy of novel antimicrobials.
Owing to the lack of any appropriate broth medium for MIC determination, MIC-based susceptibility testing of N. gonorrhoeae has been limited to disc diffusion, Etest and the agar dilution method (gold standard). Essential agreement with the agar dilution method is defined as ±1 doubling dilution and should ideally be >90% for diagnostic purposes where the same resistance breakpoints are applied.4 Etest has shown excellent agreement with the agar dilution method in many settings.4–7 However, discordant results have been found, particularly when different growth media were used.8 A multicentre international study revealed that the categorical agreement between Etest and agar dilution was ≥88%, but was very poor for disc diffusion.9 Unfortunately, all these methods are relatively slow (∼24 h), subjective, require expertise and/or are expensive. Faster methods that allow results to be obtained on the same day have been developed in the past for other bacteria,10,11 but are not available for N. gonorrhoeae.
For many bacterial species, broth microdilution is the reference method due to accuracy, low costs and high versatility.12,13 Several attempts have been made to develop a broth microdilution method also for N. gonorrhoeae, but none of these has been particularly accurate and suitable for routine use.14–16 It is difficult to synchronize the growth of different N. gonorrhoeae strains and effects such as autolysis occur when the bacteria enter the stationary phase.17–19 Chemically defined Graver–Wade (GW) broth20 supports the growth of phylogenetically diverse auxotypes and clinical isolates, and might be a suitable medium for susceptibility testing.21,22
Unfortunately, MIC values based on doubling dilution series are left-, interval- or right-censored discrete data, which makes error statistics challenging.23 The potency of drugs in pharmacology is frequently measured with dose–response curves (Hill models), as this allows the estimation of the effective concentration (EC) at a specified response level.24 Furthermore, EC values on a continuous scale take the variability of the data into account by calculating CIs. In the field of toxicology the lower CI is defined as the non-toxic concentration. This so-called benchmark dose approach has largely replaced methods that rely on dense dose spacing because of its statistical superiority and reduction of animal use.25–28 Furthermore, the shape of the dose–response curve can provide additional valuable information on the compounds being tested.24 The Hill coefficient can provide information about the pharmacodynamic properties of an antimicrobial and has been used in modelling studies of single and dual antimicrobial effects.21,22,29–31 However, the interpretation and significance of the Hill coefficient has been unclear in previous studies and laborious colony counting has limited these studies to a few strains.
The biological response to a compound can be measured using different readouts. Traditionally the MIC is defined as the concentration of an antimicrobial that inhibits visual growth, but methods to quantify the number of bacterial cells more objectively are available. Methods in which OD (at e.g. OD600 or OD450), resazurin (Alamar blue), MTT, luciferase (ATP levels) and lactate dehydrogenase are measured are widespread, with readouts that correlate with the number of cells.32 Resazurin is a blue dye that is converted into pink-fluorescent resorufin in the presence of metabolically active cells.33,34 Unlike OD, a measure of growth inhibition, it reflects the viability of cells and is potentially suitable for time–kill assays. Resazurin has an excellent signal-to-noise ratio and has been used previously in screening for toxicity testing,35 high-throughput applications,36 biofilm screening37 and MIC testing.33,38–40
The aim of this study was to develop a resazurin-based broth microdilution assay for antimicrobial susceptibility testing of N. gonorrhoeae that is rapid, objective, scalable, quantitative and inexpensive. Three datasets were generated in this study. The 2008 WHO N. gonorrhoeae reference strains (n = 8)41,42 were studied to ensure the reproducibility of the assay and to compare multiple measurement endpoints between 0 and 15 h. Training data consisting of 84 N. gonorrhoeae strains were used to develop a regression model for estimating the MIC from dose–response curves. Finally, a panel of 40 strains with blinded MICs was used for validation.
Materials and methods
Bacterial strains, culture and broth microdilution assay
The variability and reproducibility of the assay were validated in eight WHO reference strains (three replicates).41,42 Additionally, 84 gonococcal strains were used as training data to develop a regression model for estimating the MIC after 6 h of incubation time (one replicate). The assay was finally validated with 40 gonococcal strains with blinded MICs (one replicate). The blinded strains were selected to represent a wide variety of antibiograms. The strains were preserved in glycerol stocks at −80°C. All strains were subsequently cultured on Chocolate Agar PolyViteX (bioMérieux, Marcy-l'Étoile, France) at 37°C in a humid 5% CO2-enriched atmosphere for 16–18 h and then sub-cultured once for 16 h. A McFarland standard of 0.5 was prepared for each strain and 1 mL of bacterial suspension was further diluted to ∼1 × 107 cfu/mL in 15 mL of heated (37°C) GW broth.20 A volume of 90 µL of this suspension was added to 96-well round-bottom microtitre plates (360 µL wells), with each well containing 10 µL of a previously prepared dilution series. Dilution series of the antimicrobials were prepared in GW medium. Positive control (GW medium containing 1% Triton X-100) and negative control (10 µL of GW medium) were added to the first and last well, respectively. The plates were incubated for 6 h at 37°C in a humid 5% CO2-enriched atmosphere. Detailed standard operating procedures, including Figure S1, are available as Supplementary data at JAC Online.
Resazurin readouts
Resazurin powder (Sigma–Aldrich, China) was diluted in PBS (pH 7.4) to a final concentration of 0.1 mg/mL. We ensured that the pH of the highest antimicrobial concentration was neutral in all samples to avoid artefacts. After incubation of the broth microdilution plates, 50 µL of the dye was added to each well and mixed using an electronic multichannel dispenser. The plates were incubated for 75 min at 37°C. Fluorescence was then measured at 560 and 590 nm excitation in a plate reader (Varioskan Flash, Thermo Scientific).
Etest MIC
The Etest MICs (bioMérieux) were determined in accordance with the manufacturer’s instructions, on gonococcal resistance agar plates (GCRAPs) [3.6% Difco GC Medium Base agar (BD Diagnostics, Sparks, MD, USA) supplemented with 1% haemoglobin (BD Diagnostics) and 1% IsoVitalex (BD Diagnostics)].
Dose–response modelling
The antimicrobial effect on the different bacterial strains was quantified with dose–response curves. We first subtracted the background fluorescence resulting from dead bacteria in the positive control wells from the resazurin readout. We then fitted a sigmoidal dose–response curve to the fluorescence data of each antimicrobial–strain combination:43,44
| (1) |
where f(x) is the fluorescence, x is the natural logarithm of the antibiotic concentration, and u and l describe the upper and lower asymptote, respectively. The EC50 is the antibiotic concentration at which the effect is half-maximal and H denotes the slope of the sigmoidal function, i.e. the Hill coefficient. Next, the data were divided by u to normalize all dose–response curves to 100% viability. Hill coefficient differences across antimicrobials were tested with pairwise t-tests. Hierarchical complete linkage clustering was used to compare antimicrobial similarity.45
Samples were considered to be above the limit of detection, and therefore categorized as resistant, if the antibiotic, at its highest concentration, reduced viability by <50%. This was the case for six samples in the training data (n = 588) and nine samples in the validation data (n = 280). Excluding samples that were above or below the limit of detection (including Etest MICs beyond the limit of detection) resulted in 571 evaluable samples in the training data and 266 samples in the validation data. Reference strain data were not included to avoid bias from replicate testing of these samples. The relationship between EC50 and Etest was analysed for the training data by log-transforming both values and fitting a linear regression:
| (2) |
where ɛ is a normally distributed error. The slope and intercept of this regression were then used to predict the MIC from the EC50 values for the blinded strains. 95% CIs for each predicted MIC were calculated using 105 bootstrap samples taking into account the uncertainty from the sigmoidal model and the linear regression model. The analysis pipeline, descriptive statistics and raw data are available from GitHub (https://github.com/sunnivas/ResazurinMIC).
Essential agreement with Etest
Essential agreement was defined as the percentage of strains with predicted MICs that did not deviate by more than ±1 doubling dilution from Etest MICs. Deviations from the Etest MICs were calculated as log2 differences from the predicted MIC (837 evaluable samples for training and validation data). Reference strain data were not included to avoid bias from replicate testing of these samples.
Categorical agreement with Etest
The strains were categorized as susceptible (S), intermediate (I) or resistant (R) to each antimicrobial in accordance with the EUCAST 2016 guidelines.46 As previously described,47 minor errors were defined as misclassifications of intermediate strains as susceptible or resistant. Major errors were susceptible strains misclassified as resistant. Very major errors were resistant strains that were misclassified as susceptible. The EC50 values are read on a continuous scale and therefore nearly identical values around a resistance breakpoint (e.g. 0.125 and 0.126) can result in categorical errors. Sensitivity and specificity of the assay were calculated as previously described48 for the resistant strains (true positive samples), intermediate strains (true positive samples) and susceptible strains (true negative samples).
Results
Dose–response modelling
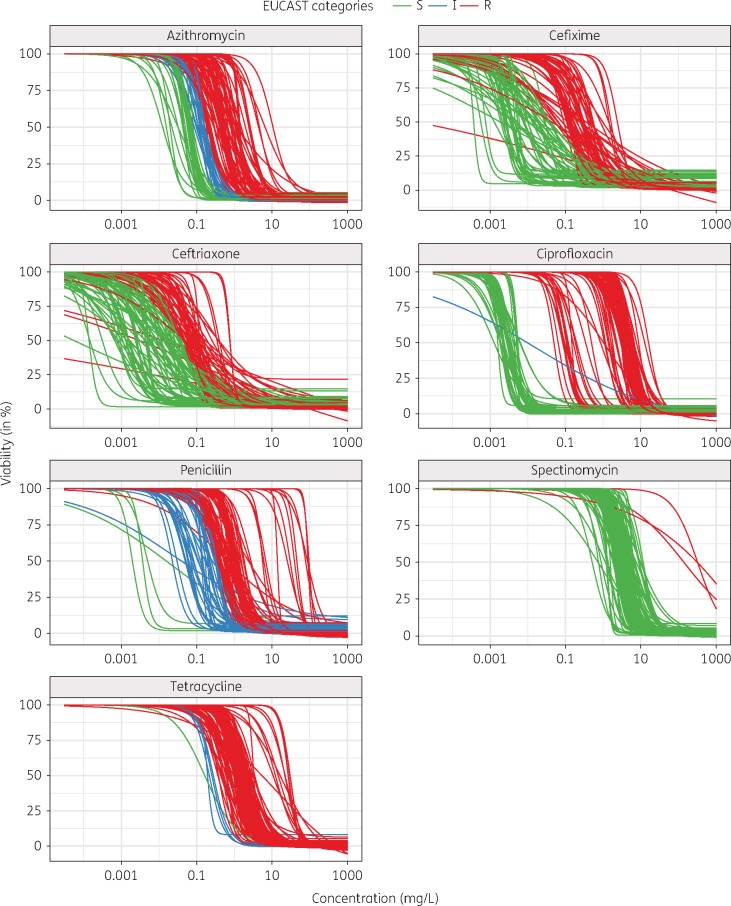
The 2008 WHO reference strains (n = 8) were exposed to ceftriaxone, cefixime, azithromycin, spectinomycin, ciprofloxacin, tetracycline or penicillin for a time course from 0–15 h (Figure S2). After 6 h, the difference between dead and viable gonococcal cells was sufficiently pronounced to fit dose–response curves to the data. For this endpoint of 6 h, the coefficient of variation (CV) was calculated for the EC50 of three independent experiments. The CV ranged from 1.7% to 87% and the intra-assay CV was 29% (n = 56) (Figure S3). Dose–response curves were gradually shifted towards higher concentrations, indicating decreased potency of the antimicrobials against the intermediate and resistant strains compared with susceptible strains (Figure 1). There was a clear separation of susceptible and resistant strains for ciprofloxacin and spectinomycin. For the β-lactam antimicrobials ceftriaxone, cefixime and penicillin the Hill coefficients (slopes) were more heterogeneous than for the other samples (Figure 1). The mean (±SD) of this parameter gradually increased from ceftriaxone (1.6 ± 1.3) to cefixime (1.9 ± 1.5), tetracycline (2.1 ± 0.9), penicillin (2.5 ± 1.7), azithromycin (2.6 ± 1.5), ciprofloxacin (2.7 ± 1.2) and spectinomycin (2.9 ± 1.7). A pairwise t-test showed that the differences between the antimicrobials were significant (P < 0.005) when the distance between the means was >0.5 (Figure S4a). Furthermore, hierarchical clustering showed a high similarity of the Hill coefficient for the β-lactam antimicrobials ceftriaxone, cefixime and penicillin compared with the other antimicrobials (Figure S4b).
Figure 1.
Potency shift of antimicrobials across different strains of N. gonorrhoeae. Dose–response curves for all strains and antimicrobials are shown (except samples above the limit of detection). Strains that were classified as susceptible according to EUCAST 2016 MIC breakpoints46 are coloured green, intermediate strains blue and resistant strains red. The gradual shift of the potencies (EC50) towards higher concentrations can be observed for all antimicrobials.
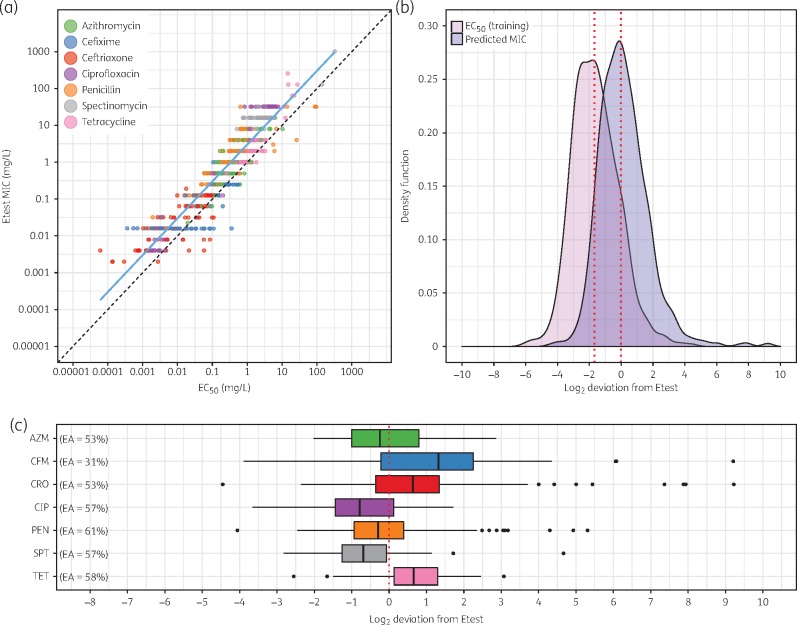
For the training data (84 strains), Pearson’s correlation between the Etest MICs and EC50 values of all antimicrobials was 0.93 (Figure 2a). Compared with the Etest values, the EC50 values were systematically lower, with a median deviation of −1.68 doubling dilutions (Figure 2b). The regression parameters α () and β () of the linear log–log regression were used to predict the 837 MICs of the training and validation data. The deviation of the predicted MIC from Etest followed a normal distribution with a median of −0.015 and 95% of the deviations ranged between −4.45 and 9.22. Outliers were mostly attributed to the β-lactam antimicrobials penicillin (e.g. overestimation in β-lactamase-producing strains) and cefixime and ceftriaxone (e.g. potentially biphasic or triphasic curves with large CIs). One example of a strain with biphasic curves for ceftriaxone and cefixime was studied in detail (Figure S5).49 The 75% quartiles for the deviations were larger for azithromycin, cefixime and ceftriaxone compared with ciprofloxacin, penicillin, spectinomycin and tetracycline (Figure 2c). The essential agreement between the Etest MICs and the predicted MICs was 53% for all antimicrobials, being lowest for cefixime (31%) and highest for penicillin (61%).
Figure 2.
Correlation and deviations between Etest MICs and predicted MICs. (a) Linear regression between EC50 and Etest MIC for the training data (n = 571). Pearson's correlation coefficient for the linear regression (blue line) was 0.93. Slope and intercept for a perfect correlation are drawn as a dashed black line for comparison. (b) The kernel density function of the EC50 values for the training data (n = 571) is shown in pink (median −1.68). The kernel density of the predicted MICs for the training and validation data (n = 837) is shown in purple (median −0.015). (c) Deviations of predicted MICs from Etest MIC for each antimicrobial (n = 837). Boxplots show the median and IQR. Whiskers span the range from the bottom 5% to the highest 95% of the data. Essential agreement (EA) is indicated next to the boxplots. AZM, azithromycin; CFM, cefixime; CRO, ceftriaxone; CIP, ciprofloxacin; PEN, penicillin; SPT, spectinomycin; TET, tetracycline.
Categorical agreement
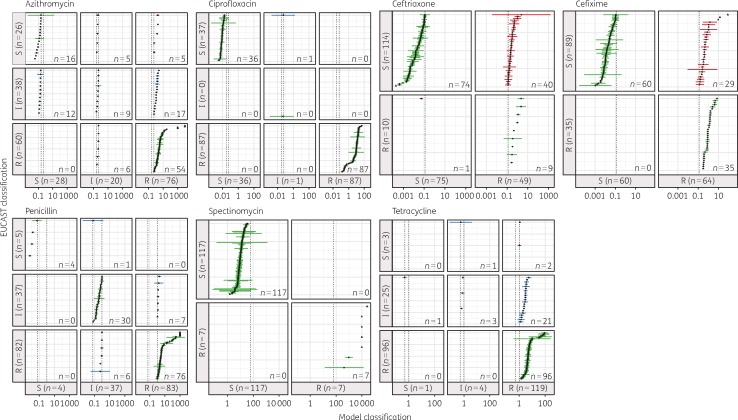
The Etest and predicted MICs (n = 868) were classified as susceptible, intermediate resistant and resistant according to the EUCAST 2016 resistance breakpoints46 (Figure 3). The sensitivity of the assay was 97.1% (95% CI 95.2–98.4). Minor errors resulting from misclassifications of intermediate resistant strains were found for 9% of the data. For penicillin, spectinomycin and ciprofloxacin no major errors were identified. False-positive misclassifications (S misclassified as R), i.e. major errors, occurred for tetracycline (0.2%), azithromycin (0.6%), cefixime (3.3%) and ceftriaxone (4.6%) for a total of 9% of the data. One very major error (R misclassified as S) occurred for ceftriaxone (Etest MIC 0.19 versus 0.053 mg/L). Many predicted MIC values (16.5%) had 95% CIs spanning two categories. The overall specificity of the assay was 78.5% (95% CI 74.5–82.9).
Figure 3.
Contingency table with categorical errors of model-predicted MICs. Etest MIC data were classified into the categories R, I and S according to the EUCAST 2016 criteria.46 The cut-off values (mg/L) are shown as dashed black lines. Predicted MIC values (n = 868) are shown as point estimates (black dots) with 95% CI (coloured dashes). For some estimates no CI could be calculated (limit of detection); these are drawn as triangles. Correctly classified strains are drawn in green. Minor errors resulting from misclassifications of intermediate strains are shown in blue. Major errors (S misclassified as R) were found for ceftriaxone (n = 40), cefixime (n = 29), azithromycin (n = 5) and tetracycline (n = 2) and are shown in red. One very major error (R misclassified as S) was found for ceftriaxone (red). A high number of estimates (n = 138) have CIs spanning two categories.
Discussion
The developed resazurin-based broth microdilution assay was able to discriminate between resistant and susceptible strains relatively reliably, was faster (∼7.5 h for results) than currently available MIC methods for N. gonorrhoeae and had an excellent sensitivity of 97.1% (95% CI 95.2–98.4). The gold standard MIC-based method agar dilution and the Etest method are both based on subjective, visual readouts and are therefore limited to a relatively low throughput. Dose–response modelling allows the precise estimation of the EC50 of antimicrobials from a continuous scale and provides CIs rather than having the precision limited by doubling dilutions. It is inherently difficult to apply resistance breakpoints that were designed for doubling dilution-based methods to dose–response curve-based MICs. This was reflected by many categorical errors resulting from estimates that had CIs overlapping two S/I/R categories. The performance of the assay was excellent for ciprofloxacin, penicillin and spectinomycin (no major errors) and acceptable for azithromycin (0.6% major errors) and tetracycline (0.2% major errors).
The deviations of resazurin-based MICs from Etest MICs followed a normal distribution. Outliers were mainly attributed to the β-lactam antimicrobials and contributed to the suboptimal essential agreement and assay specificity of only 78.5% (95% CI 74.5–82.9). For penicillin, substantially higher MICs were measured with the resazurin assay, e.g. for β-lactamase-producing strains. For cefixime and ceftriaxone there were many false-positive results and consequently an overestimation of resistance was measured. The complex mechanism of action and evolution of resistance to these antimicrobials is not fully understood and involves several resistance determinants in multifaceted interactions (penA, penB, mtrR, factor X).3,50 The correlation of EC50 and MIC has been previously shown to be not strictly linear and was largely influenced by different PBPs in Streptococcus pneumoniae.51 The binding kinetics of a mechanism involving several targets and/or resistance determinants can result in dose–response curves that are biphasic and potentially triphasic (Figure S5).22,49 In these cases the correlation between EC50 and Etest MIC differs from those in dose–response curves with only one inflection point and can result in false-positive results.
Performing the regression analysis for the different antimicrobials separately might improve the assay specificity, particularly for the β-lactam antimicrobials. An endpoint of 6 h provided only a snapshot of the antimicrobial properties and examining many more timepoints, more starting inocula and a very large number of strains, covering a wide range of MICs and ideally including in vitro-selected resistant strains, might also provide valuable data for improvements. Scaling up the assay to a robotic platform might be necessary for appropriate examination of all these parameters and strains.
Despite these limitations, the developed rapid resazurin-based broth microdilution assay was highly objective (avoiding visual subjective readout) and employs a standardized algorithm reducing operator bias, which can be especially valuable in multicentre studies. These properties, and the low price of resazurin, are especially valuable when screening large libraries of new compounds, antimicrobials or antimicrobial combinations. Frequently, the question that needs to be answered is the potency of antimicrobials relative to each other rather than absolute numbers. The β-lactam antimicrobials cefixime, ceftriaxone and penicillin displayed significantly lower Hill coefficients than the other antimicrobials. Information about this parameter is useful for research questions beyond susceptibility testing, such as combination therapy and pharmacodynamic modelling.
In summary, the developed resazurin-based broth microdilution assay is a rapid, objective, high-throughput, quantitative and cost-effective new tool for studying N. gonorrhoeae in liquid culture. The Hill coefficient could be compared for a large number of strains, highlighting differences between antimicrobials. The new assay opens up avenues for high-throughput synergy testing, evaluation of novel antimicrobials and surveillance of resistance.
Funding
This study was funded through an Interdisciplinary PhD (IPhD) project from SystemsX.ch (The Swiss Initiative for Systems Biology), RaDAR-Go (RApid Diagnosis of Antibiotic Resistance in Gonorrhoea; funded by the Swiss Platform for Translational Medicine), and the Örebro County Council Research Committee and the Foundation for Medical Research at Örebro University Hospital, Sweden.
Transparency declarations
None to declare.
Supplementary data
The standard operating procedures and Figures S1–S5 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1. WHO. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria gonorrhoeae 2012. http://apps.who.int/iris/bitstream/10665/44863/1/9789241503501_eng.pdf.
- 2. Newman L, Rowley J, Hoorn SV. et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10: e0143304.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unemo M, Shafer WM.. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27: 587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biedenbach DJ, Jones RN.. Comparative assessment of Etest for testing susceptibilities of Neisseria gonorrhoeae to penicillin, tetracycline, ceftriaxone, cefotaxime, and ciprofloxacin: investigation using 510(k) review criteria, recommended by the Food and Drug Administration. J Clin Microbiol 1996; 34: 3214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu H, Taylor TH, Pettus K. et al. Assessment of Etest as an alternative to agar dilution for antimicrobial susceptibility testing of Neisseria gonorrhoeae. J Clin Microbiol 2014; 52: 1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh V, Bala M, Kakran M. et al. Comparative assessment of CDS, CLSI disc diffusion and Etest techniques for antimicrobial susceptibility testing of Neisseria gonorrhoeae: a 6-year study. BMJ Open 2012; 2: e000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gose S, Kong CJ, Lee Y. et al. Comparison of Neisseria gonorrhoeae MICs obtained by Etest and agar dilution for ceftriaxone, cefpodoxime, cefixime and azithromycin. J Microbiol Methods 2013; 95: 379–80. [PubMed] [Google Scholar]
- 8. Liao C-H, Lai C-C, Hsu M-S. et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates determined by the agar dilution, disk diffusion and Etest methods: comparison of results using GC agar and chocolate agar. Int J Antimicrob Agents 2010; 35: 457–60. [DOI] [PubMed] [Google Scholar]
- 9. Ison CA, Martin IMC, Lowndes CM. et al. Comparability of laboratory diagnosis and antimicrobial susceptibility testing of Neisseria gonorrhoeae from reference laboratories in Western Europe. J Antimicrob Chemother 2006; 58: 580–6. [DOI] [PubMed] [Google Scholar]
- 10. Kelly MT, Leicester C.. Evaluation of the Autoscan Walkaway system for rapid identification and susceptibility testing of gram-negative bacilli. J Clin Microbiol 1992; 30: 1568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Godsey JH, Bascomb S, Bonnette T. et al. Rapid antimicrobial susceptibility testing of gram-negative bacilli using Baxter MicroScan rapid fluorogenic panels and autoSCAN-W/A. Pathol Biol (Paris) 1991; 39: 461–5. [PubMed] [Google Scholar]
- 12. Reller LB, Weinstein M, Jorgensen JH. et al. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 2009; 49: 1749–55. [DOI] [PubMed] [Google Scholar]
- 13. Wiegand I, Hilpert K, Hancock REW.. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008; 3: 163–75. [DOI] [PubMed] [Google Scholar]
- 14. Takei M, Yamaguchi Y, Fukuda H. et al. Cultivation of Neisseria gonorrhoeae in liquid media and determination of its in vitro susceptibilities to quinolones. J Clin Microbiol 2005; 43: 4321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geers TA, Donabedian AM.. Comparison of broth microdilution and agar dilution for susceptibility testing of Neisseria gonorrhoeae. Antimicrob Agents Chemother 1989; 33: 233–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shapiro MA, Heifetz CL, Sesnie JC.. Comparison of microdilution and agar dilution procedures for testing antibiotic susceptibility of Neisseria gonorrhoeae. J Clin Microbiol 1984; 20: 828–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dillard JP, Seifert HS.. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol Microbiol 1997; 25: 893–901. [DOI] [PubMed] [Google Scholar]
- 18. Elmros T, Burman LG, Bloom GD.. Autolysis of Neisseria gonorrhoeae. J Bacteriol 1976; 126: 969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan YA, Hackett KT, Dillard JP.. The lytic transglycosylases of Neisseria gonorrhoeae. Microb Drug Resist 2012; 18: 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wade JJ, Graver MA.. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol Lett 2007; 273: 35–7. [DOI] [PubMed] [Google Scholar]
- 21. Foerster S, Golparian D, Jacobsson S. et al. Genetic resistance determinants, in vitro time–kill curve analysis and pharmacodynamic functions for the novel topoisomerase II inhibitor ETX0914 (AZD0914) in Neisseria gonorrhoeae. Front Microbiol 2015; 6: 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foerster S, Unemo M, Hathaway LJ. et al. Time–kill curve analysis and pharmacodynamic functions for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol 2016; 16: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van de Kassteele J, van Santen-Verheuvel MG, Koedijk FDH. et al. New statistical technique for analyzing MIC-based susceptibility data. Antimicrob Agents Chemother 2012; 56: 1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prinz H. Hill coefficients, dose–response curves and allosteric mechanisms. J Chem Biol 2009; 3: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slob W. Benchmark dose and the three Rs. Part I. Getting more information from the same number of animals. Crit Rev Toxicol 2014; 44: 557–67. [DOI] [PubMed] [Google Scholar]
- 26. Slob W. Benchmark dose and the three Rs. Part II. Consequences for study design and animal use. Crit Rev Toxicol 2014; 44: 568–80. [DOI] [PubMed] [Google Scholar]
- 27. Davis JA, Gift JS, Zhao QJ.. Introduction to benchmark dose methods and U.S. EPA’s benchmark dose software (BMDS) version 2.1.1. Toxicol Appl Pharmacol 2011; 254: 181–91. [DOI] [PubMed] [Google Scholar]
- 28. Filipsson AF, Sand S, Nilsson J. et al. The benchmark dose method—review of available models, and recommendations for application in health risk assessment. Crit Rev Toxicol 2003; 33: 505–42. [PubMed] [Google Scholar]
- 29. Regoes RR, Wiuff C, Zappala RM. et al. Pharmacodynamic functions: a multiparameter approach to the design of antibiotic treatment regimens. Antimicrob Agents Chemother 2004; 48: 3670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foucquier J, Guedj M.. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 2015; 3: e00149.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu G, Baeder DY, Regoes RR. et al. Combination effects of antimicrobial peptides. Antimicrob Agents Chemother 2016; 60: 1717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rampersad SN. Multiple applications of Alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012; 12: 12347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khalifa RA, Nasser MS, Gomaa AA. et al. Resazurin microtiter assay plate method for detection of susceptibility of multidrug resistant Mycobacterium tuberculosis to second-line anti-tuberculous drugs. Egypt J Chest Dis Tuberc 2013; 62: 241–7. [Google Scholar]
- 34. Palomino J-C, Martin A, Camacho M. et al. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2002; 46: 2720–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zimmer B, Pallocca G, Dreser N. et al. Profiling of drugs and environmental chemicals for functional impairment of neural crest migration in a novel stem cell-based test battery. Arch Toxicol 2014; 88: 1109–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim KT, Zahari Z, Amanah A. et al. Development of resazurin-based assay in 384-well format for high throughput whole cell screening of Trypanosoma brucei rhodesiense strain STIB 900 for the identification of potential anti-trypanosomal agents. Exp Parasitol 2016; 162: 49–56. [DOI] [PubMed] [Google Scholar]
- 37. Pettit RK, Weber CA, Pettit GR.. Application of a high throughput Alamar blue biofilm susceptibility assay to Staphylococcus aureus biofilms. Ann Clin Microbiol Antimicrob 2009; 8: 28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmitt DM, Connolly KL, Jerse AE. et al. Antibacterial activity of resazurin-based compounds against Neisseria gonorrhoeae in vitro and in vivo. Int J Antimicrob Agents 2016; 48: 367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elshikh M, Ahmed S, Funston S. et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett 2016; 38: 1015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mann C, Markham J.. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 1998; 84: 538–44. [DOI] [PubMed] [Google Scholar]
- 41. Unemo M, Fasth O, Fredlund H. et al. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J Antimicrob Chemother 2009; 63: 1142–51. [DOI] [PubMed] [Google Scholar]
- 42. Unemo M, Golparian D, Sánchez-Busó L. et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 2016; 71: 3096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ritz C, Streibig J.. Bioassay analysis using R. J Stat Soft 2005; 12: 1–22. [Google Scholar]
- 44. Ritz C, Baty F, Streibig J. et al. Dose-response analysis using R. PLoS One 2015; 10: e0146021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gaujoux R, Seoighe C.. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics 2010; 11: 367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters 2016. http://www.eucast.org/clinical_breakpoints/.
- 47. National Committee for Clinical Laboratory Standards. Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters—Second Edition: Approved Guideline M23-A2. NCCLS, Wayne, PA, USA, 2001. [Google Scholar]
- 48. Parikh R, Mathai A, Parikh S. et al. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol 2008; 56: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Di Veroli GY, Fornari C, Goldlust I. et al. An automated fitting procedure and software for dose–response curves with multiphasic features. Sci Rep 2015; 5: 14701.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Unemo M, Golparian D, Nicholas R. et al. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012; 56: 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kocaoglu O, Tsui H-CT, Winkler ME. et al. Profiling of β-lactam selectivity for penicillin-binding proteins in Streptococcus pneumoniae D39. Antimicrob Agents Chemother 2015; 59: 3548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.