Abstract
Objectives: In the UK, patients who require intravenous antimicrobial (IVA) treatment may receive this in the community through outpatient parenteral antimicrobial therapy (OPAT) services. Services include: IVA administration at a hospital outpatient clinic (HO); IVA administration at home by a general nurse (GN) or a specialist nurse (SN); or patient self-administered (SA) IVA administration following training. There is uncertainty regarding which OPAT services represent value for money; this study aimed to estimate their cost-effectiveness.
Methods: A cost-effectiveness decision-analytic model was developed using a simulation technique utilizing data from hospital records and a systematic review of the literature. The model estimates cost per QALY gained from the National Health Service (NHS) perspective for short- and long-term treatment of infections and service combinations across these.
Results: In short-term treatments, HO was estimated as the most effective (0.7239 QALYs), but at the highest cost (£973). SN was the least costly (£710), producing 0.7228 QALYs. The combination between SN and HO was estimated to produce 0.7235 QALYs at a cost of £841. For long-term treatments, SN was the most effective (0.677 QALYs), costing £2379, while SA was the least costly at £1883, producing 0.666 QALYs. A combination of SA and SN was estimated to produce 0.672 QALYs at a cost of £2128.
Conclusions: SN and SA are cost-effective for short- and long-term treatment of infections, while combining services may represent the second-best alternative for OPAT in the UK.
Introduction
There is increased interest in the UK in offering patients who require intravenous antimicrobials (IVAs) outpatient or community-based services rather than inpatient care.1–3 Such outpatient parenteral antimicrobial therapy (OPAT) services are most often used to treat skin and soft-tissue infections. However, facilitated by newer antibiotics with longer half-lives, a number of other diseases (e.g. joint and bone infections, bacteraemia, osteomyelitis, diabetic foot and TB) can be treated safely in an outpatient or home setting.4
Some authors have estimated that an OPAT service could reduce treatment costs by reducing bed days. A UK study by Chapman et al.3 found that OPAT reduced inpatient costs by 47%, while another study in the UK reported that 7394 bed days were saved over a period of 44 months; assuming National Health Service (NHS) bed day costs of £208 (2015 prices) the associated potential savings would be over £1.5 million.5,6
In terms of safety, evidence has shown that OPAT has been associated with a low risk of adverse events such as hospital re-admissions and line complications including infections and minor episodes of redness in the application site.1,7–9 In addition, no difference in time to heal between OPAT and inpatient care has been observed; however, there are no randomized control trials (RCTs) comparing the different OPAT services on offer.1,10 A systematic review of the literature on cost-effectiveness analyses of OPAT services found a number of cost-effectiveness analyses, but none that would meet the technology appraisal reference case criteria set out by the National Institute for Health and Care Excellence (NICE)11 in the UK.
Despite the benefits of OPAT, service provision in the UK has been limited. It is possible that the evidence-gap on the models of care in the area has hindered investment from decision-makers and service commissioners. In the absence of RCT evidence and robust economic evaluations to commend one OPAT service over another, commissioning decision-making in the area is fraught with uncertainty and barriers to the wider adoption of services remain. It is clear that further research is required to inform decision making. However, given that research resources are scarce and RCTs are often expensive and relatively slow to yield results, it is imperative that they are streamlined to answer the important questions and include the comparators most likely to be cost-effective. This is especially true in OPAT services where a number of service configurations are possible.
There are a number of different OPAT service models currently in use in the UK that can be classified as follows: daily IVA delivery at a hospital or clinic in an outpatient visit (HO); daily IVA delivery at home by a general nurse (GN) or a specialist nurse (SN); and daily patient self-administered IVAs at home following receipt of training (SA).
The aim of the current study was to develop a decision-analytic model to estimate the cost-effectiveness of the four OPAT services offered in the UK (HO, GN, SN and SA) to provide evidence for decision-making.
Methods
A decision-analytic model employing a discrete event simulation (DES) approach was developed. Decision modelling is an analytical approach to performing an economic evaluation of at least two alternative courses of action and determines which offers best value for money. Such a model is created to reflect the healthcare process or pathway, capturing the events that occur to the patient or health system during care and estimating the expected costs and (dis)benefits of the treatment options.
Many modelling methods exist,12 but with DES it is possible to follow individual patients through the duration of their treatment, explicitly accounting for time and treatment history. Within the context of IVAs it allows assessment of time-related risks and by recording their treatment history we were able to easily add events such as relapses that are difficult to account for when using other types of methods. This allows for a better estimation of the overall impact of the services being evaluated. The DES model followed the methods outlined by Caro et al.13
Two economic evaluations, for short- and long-term IVA treatments, were performed. The evaluations followed established methods, adhered to the NICE ‘reference case’11,14 and therefore adopt the NHS perspective (costs considered were those incurred by the NHS only). Patient pathways were modelled over a 12 month period.
To incorporate the impact of treatment on the health-related quality of life of the patients and the length of time in a condition, the model uses QALYs as an outcome measure. The QALY is a measure that encapsulates both quality and length of life and is widely used in health economics.15 By estimating the incremental costs (costs of intervention A minus costs of intervention B) and dividing them by the incremental outcomes (outcomes of intervention A minus outcomes of intervention B), we generate the incremental effectiveness ratio (ICER), which is used to assess cost-effectiveness.16 In line with current UK guidelines, services with an ICER <£20 000 per QALY gained were considered cost-effective. To allow a linear comparison between interventions only in monetary terms the net monetary benefit (NMB) was also calculated (QALY × £20 000 − cost). A cost-effective strategy will have the highest NMB. Costs were not discounted as the evaluation period was of 12 months only. QALYs gained during treatment were not discounted; however, QALYs lost due to premature death were discounted at a 3.5% rate. All prices are presented in pounds sterling 2015.
Population
We defined short-term treatment as that required for skin and soft-tissue infections or similar infections, usually taking between 4 and 7 days (depending on the service) of IVAs to heal or transition to oral antibiotics. We defined long-term treatment as that required for bone infections, infective exacerbations of cystic fibrosis and other infections taking an average of more than 7 days to heal.
Interventions
In the HO service, patients attend a hospital outpatient clinic to receive treatment on a daily basis, while in the GN and SN services, nurses administer the IVAs at the patient’s home every day. In contrast to a GN, an SN only delivers IVAs. Only HO, GN and SN were compared when analysing patients requiring short-term treatments, as the SA model was unlikely to be offered to (or demanded by) this patient group. The evaluation for long-term treatment compares the four service strategies (HO, GN, SN and SA).
The HO service was considered the ‘standard’ OPAT care in the UK even though there is geographical variation in service provision. Interventions were initially compared against HO to evaluate its cost-effectiveness. If this analysis showed that it was not cost-effective, an incremental analysis (ordering interventions from the least costly) was carried out.
An additional analysis combining the most cost-effective service with a relevant second-best strategy was carried out; this assumes that 50% of the patients in a particular clinic would receive one service and 50% would receive the other. This combined setting was compared against the most cost-effective single service intervention.
Model structure
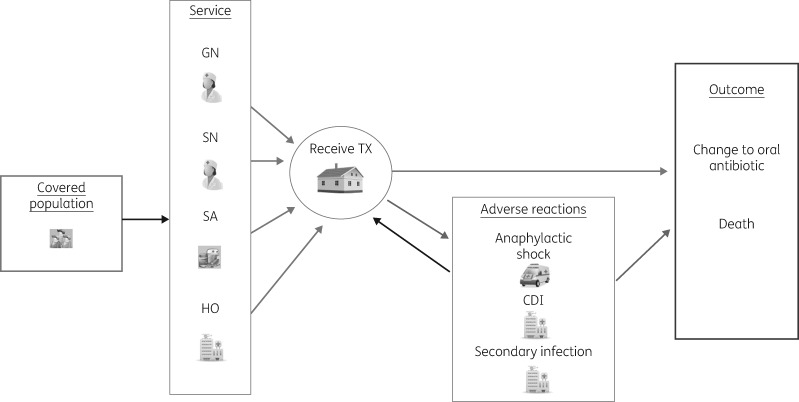
Model structure was informed by a rapid review of published decision models and through discussions with patients and clinicians. Patients enter the simulation after having been referred to an OPAT service and they are followed from this point in time on a daily basis until they are healed (or switched to oral antibiotics) or die. Although patients can experience a variety of severe adverse events, we chose to include only three in the model, due to their use of medical resources: anaphylactic shock, Clostridium difficile infection (CDI) and intravenous line infection. Patients who experience any of these were subject to a mortality risk. Patients were also exposed to a daily risk of a mild adverse event (rash, nausea, vomiting, dizziness, fever and line obstructions or leaking, phlebitis, redness, swelling, pain at the site of access or minor line events). These incurred additional costs, but no quality of life decrement or increase in healing time, as they are both mild and transient in nature. Some patients were assumed to ‘relapse’ and begin IVA treatment again (Figure 1).
Figure 1.
Simulation model structure. Model constructed using SIMUL8®.17 TX, treatment.
Parameter values – probabilities
The patient’s transition through the treatment pathway depends on a series of probabilities. These, along with the costs and effects, were taken from a number of sources including a systematic review,9,18–24 expert clinical opinion and hospital records of a group of patients (n = 465) who had recently received OPAT (sample characteristics are given in Table S1, available as Supplementary data at JAC Online). Patients were recruited from six centres in England (Bradford, Huddersfield, Hull, Leeds, Oxford and Sheffield) which between them provided all the models of service studied; some offered more than one model.
The measure of the service ‘effectiveness’ was defined as the number of days of IVA treatment required. We derived these values from the hospital record data and applied adjusted ‘time-to-heal’ values for the base case analysis with sensitivity analyses exploring the same heal time across services. Not-healed patients could travel to the CDI state according to a daily probability based on the time they spent in a hospital environment or in contact with a GN or SN. It was assumed that HO patients had a greater chance of developing CDI compared with those treated at home. The smallest risk was for the SA service as they have less contact with a healthcare setting.
An anaphylactic shock was assumed to require 1 day of in-hospital treatment after which the patient resumed treatment. The daily risk of such episodes was assumed to be equal across services. Risks of secondary infection of intravenous lines were related to the duration of treatment irrespective of the type of service received.
A differential risk of mild adverse events was added for each service (from hospital record data). The base case analysis assumed that the relapse rate was zero and equivalent between services. However, a sensitivity analysis was conducted where heal time was assumed equivalent, but a differential relapse rate was adopted.
Mortality
Risk of death was only considered for those patients who had a severe adverse event. The daily mortality rate for patients with CDI was obtained from Wiegand et al.22 and was assumed the same for all services. The associated mortality risk for patients who had an anaphylactic shock was obtained from Hopf et al.19 This risk was assumed to be double for the home-based services (SN, GN and SA) compared with HO, since patients experiencing anaphylactic shock in hospital would receive more rapid access to intensive care. Lastly, the mortality risk for patients who had a secondary infection of intravenous lines was obtained from Thwaites et al.23 and was assumed the same for all services.
Parameter values – costs
The costs of the services included: antimicrobials; additional expenses required for self-administration (training and equipment); nurse (including paperwork and travel) and hospital visit for IVA delivery and reviews; additional healthcare resources used by the patient (e.g. general practitioner visits); and costs associated with mild and severe adverse events (e.g. hospitalization following secondary infection). Unit costs were obtained from the NHS reference cost resource, Personal Social Services Research Unit (PSSRU) report and drug and pharmaceutical electronic market information tool (eMit).6,25,26
The base case scenario assumes that one of the outpatient visits in the HO service will be led by an infection specialist, who will undertake an initial, review or discharge session. Patients in the GN, SN and SA groups, however, will have a discharge and a two-weekly review consultation with an infectious disease specialist (only for long-term treatments). Only one infectious disease consultation was included for HO as it was assumed that they were being more closely monitored by attending the outpatient unit on a daily basis. To test the impact of these assumptions, a sensitivity analysis assuming that all services had an initial and discharge consultation with an infectious disease specialist was conducted.
Parameter values – utility/quality of life
Utility values were similar for short- and long-term treatments when healed, but during the infection the long-term patients experienced a much larger utility drop.27–30 Since the mortality risk linked to adverse events presents a risk of reduced length of life, a lifetime QALY loss value (16.6) was estimated. This represented the discounted (at 0.035% per annum) total QALYs lost for individuals who died during the model horizon using an average starting age of 50 years, survival estimates from life tables and ‘healed’ utility values. Given the rarity of mortality, it was not considered worthwhile including extensive survival analysis. The probability, cost and utility parameter values can be found in Table S2.
Uncertainty
A number of deterministic one-way and scenario sensitivity analyses were conducted: same healing times for all services; increased number of IVAs per day; changes in risk, mortality rates and healing time from adverse events; changes to the per hour nurse visit rate; bed day costs and changes in the utility values for the heal/not heal state as well as for the utility losses due to adverse events. We also tested a scenario in which all patients, irrespective of the service model, received an initial and a discharge consultation led by an infection specialist.
Additionally, a probabilistic sensitivity analysis (PSA) was performed (2000 Monte Carlo simulation runs) to allow for random changes in all parameter values at the same time based on pre-specified value distributions. Only 2000 simulation runs were performed due to the computationally intensive nature of the simulation. To overcome the latter, Jackknife CIs were estimated around the ICERs to determine if the number of iterations was sufficient to produce a robust answer. Jackknife is a tool to assess non-parametric estimates of bias.31
These simulated analyses results were plotted on a cost-effectiveness plane, where the vertical axis represents the simulated incremental costs and the horizontal axis represents the incremental QALYs. The plane indicates the general spread of values and thus indicates the level of uncertainty in the results. The probability that services were cost-effective given a range of willingness to pay thresholds, however, was represented on a cost-effectiveness acceptability curve (CEAC).32
Results
Short-term treatment
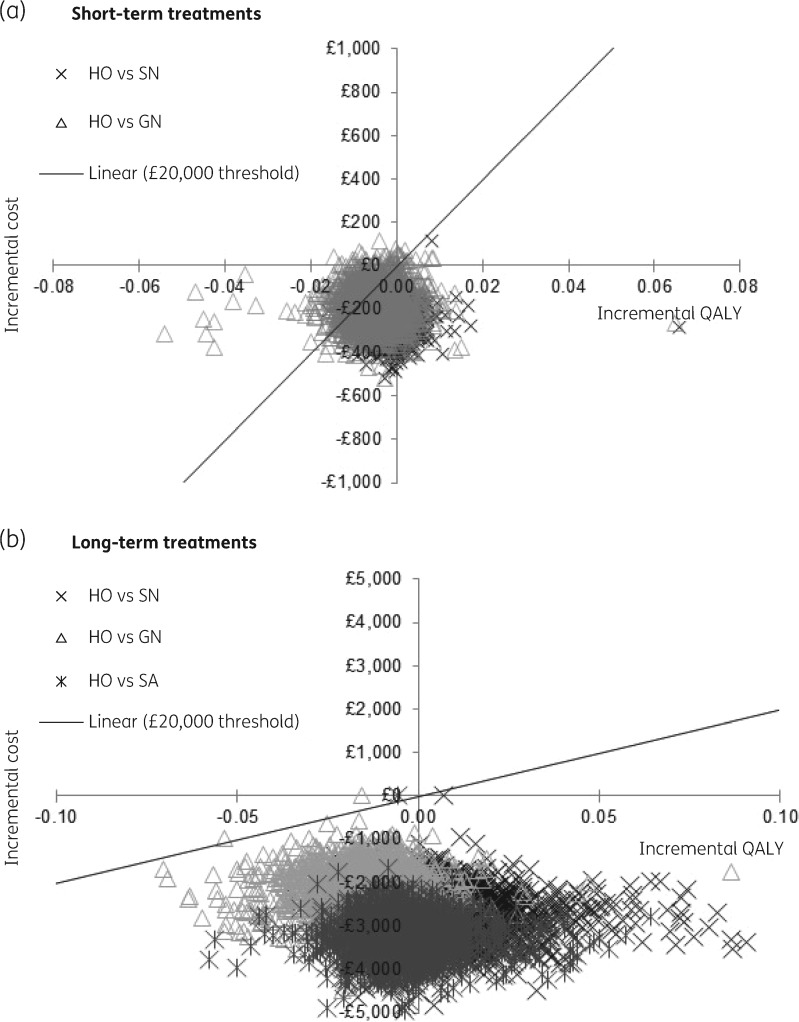
The deterministic base case analysis shows that HO (£973) is more expensive than GN (£788) and SN (£710). HO is also less effective than SN (−0.001 QALYs), but more effective than GN (0.005 QALYs). This results in HO being dominated by SN (as SN is less costly and more effective) and in an ICER close to £40 000 per QALY gained when compared against GN. This suggests that both SN and GN are cost-effective when compared with HO individually (Table 1). The scatterplot comparing all interventions against HO confirms the results as most of the iterations from the PSA fall below the horizontal axis, suggesting that SN and GN are generally cost-saving (Figure 2a).
Table 1.
Probabilistic cost-effectiveness analysis for short-term treatment
| Intervention | Costs | QALYs | ICERa | Jackknife 95% CIb |
NMB | Result | |
|---|---|---|---|---|---|---|---|
| lower boundary | upper boundary | ||||||
| SN | £710 | 0.7228 | £13 745 | cost-effective | |||
| GN | £788 | 0.7193 | — | — | — | £13 597 | dominated |
| SN 50%; HO 50% | £841 | 0.7235 | £182 493 | £157 046 | £206 302 | £13 628 | not cost-effective |
| HO | £973 | 0.7239 | £233 034 | £196 077 | £267 269 | £13 505 | not cost-effective |
ICERs and Jackknife 95% CIs were not estimated for dominated strategies.
Incremental analysis versus the next-best strategy.
Jackknifing was undertaken to assess the uncertainty in the mean value to determine if the number of iterations was sufficient for non-dominated strategies. The 95% CI shows that this was the case.31
Figure 2.
Scatterplot of short- and long-term treatments: all strategies versus HO. Please note different y-axis scales to account for the difference in the costs between short- and long-term treatments.
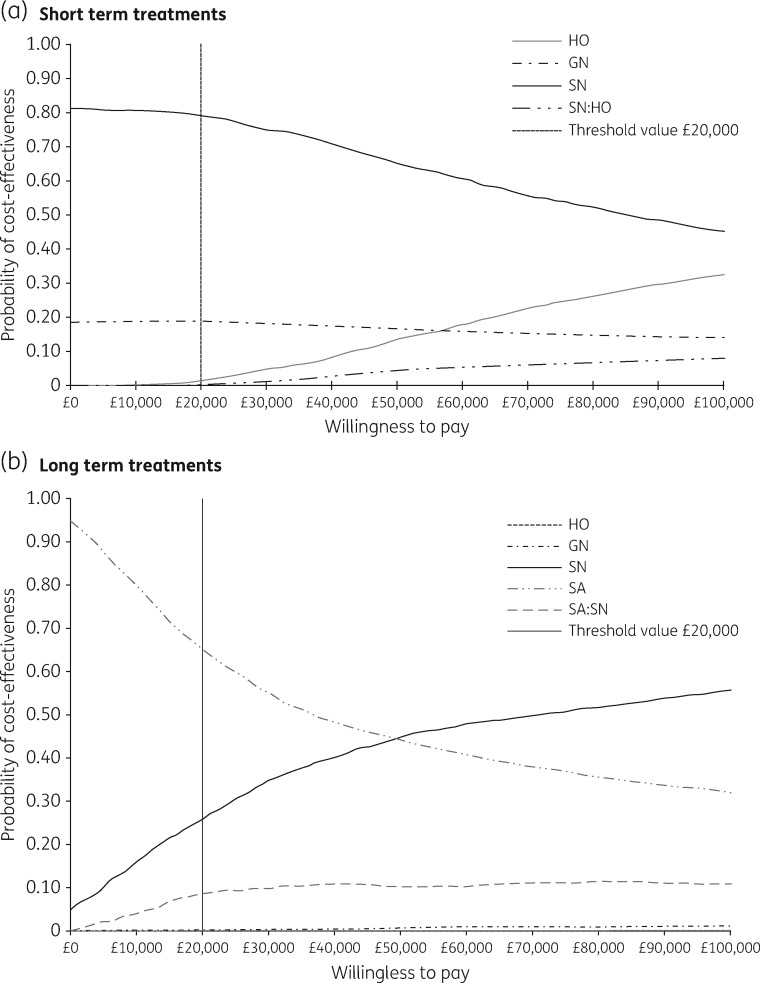
An incremental analysis (ranking the interventions from the least to the most costly) based on the PSA was carried out. Given a £20 000 threshold, SN had the highest NMB, and therefore was considered the most cost-effective strategy for short-term treatments. The PSA estimated that in 79% of the iterations the NMB of SN was the highest of the three interventions (CEAC) (Figure 3a). A service providing both SN and HO is a more effective, but more costly, intervention than providing SN alone, but less costly and effective than providing HO alone (Table 1).
Figure 3.
Short- and long-term CEAC. Please note that in part (b) the HO line is not apparent as its probability is zero for all threshold values.
Long-term treatment
The deterministic analysis estimated that HO was the most costly (£5135) strategy followed by GN (£2957) and SN (£2379) while SA (£1883) was the cheapest, suggesting that all strategies were cost-saving compared with HO. This was confirmed in the scatterplot from the PSA since comparing all interventions against HO shows that all iterations fall below the horizontal axis (Figure 2b). In terms of the effects, SN had the highest QALYs gained (0.678) followed by HO (0.667) and SA (0.666) while GN had the lowest (0.655) (Table 2).
Table 2.
Probabilistic cost-effective analysis for long-term treatment
| Intervention | Costs | QALYs | ICERa | Jackknife 95% CIb |
NMB | Result | |
|---|---|---|---|---|---|---|---|
| lower boundary | upper boundary | ||||||
| SA | £1883 | 0.6660 | £11 436 | cost-effective | |||
| SA 50%; SN 50% | £2128 | 0.6721 | £39 819 | £35 277 | £44 136 | £11 314 | not cost-effective |
| SN | £2379 | 0.6767 | £54 364 | £46 059 | £62 117 | £11 155 | not cost-effective |
| GN | £2957 | 0.6552 | — | — | — | £10 147 | dominated |
| HO | £5135 | 0.6698 | — | — | — | £8261 | dominated |
ICERs and Jackknife 95% CIs were not estimated for dominated strategies.
Incremental analysis versus the next-best strategy.
Jackknifing was undertaken to assess the uncertainty in the mean value to determine if the number of iterations was sufficient for non-dominated strategies. The 95% CI shows that this was the case.31
The incremental analysis indicated HO and GN were more costly and less effective than (and consequently dominated by) SN. When SA (cheapest option) was compared with SN, the estimated ICER was higher than the £20 000 per QALY gained threshold. Furthermore, the estimated NMB for SA was the highest of the four interventions. These results suggest that SA is the most cost-effective strategy for long-term treatments. The PSA estimated that in 70% of the iterations the NMBs of SA were the highest of the four interventions (Figure 3b).
Combining SA and SN services was cheaper, but less effective than SN alone. The ICER showed that SA remained the most cost-effective strategy. Several SA–SN combination strategies (55:45, 60:40 and 40:60 ratios) were analysed, but none was more cost-effective than SA alone (Table 2).
Sensitivity analysis
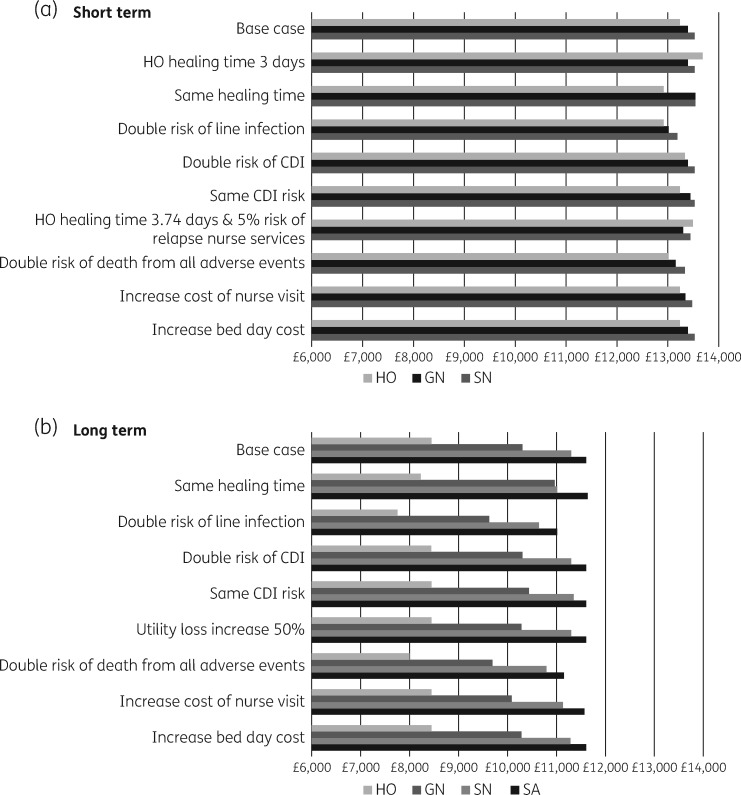
Several one-way sensitivity and scenario analyses were run. In both the short and the long term, the results remained the same: SN and SA had the highest NMB for short- and long-term treatments, respectively.
In the short treatment model, however, when healing time was assumed the same for all services, the NMB of GN and SN was almost identical. Furthermore, in one particular scenario where the healing time for HO was set to 3.74 days (reduced by 1 day from the base case) and a 5% risk of relapse introduced for the nurse-led services (0% in the base case), HO had the highest NMB and was therefore the most cost-effective strategy. When only the relapse rate was changed, the results remained unchanged. However, when only the healing time is reduced further (from 4.74 to 3 days) HO is cost-effective. In the long treatment model, however, all one-way and scenario analysis results suggested that SA was the most cost-effective strategy. Lastly, as expected, adding one initial and one discharge consultation with an infection specialist to all service models added the same net cost to all services (£237.68), and so maintained the base case results (Figure 4).
Figure 4.
Short- and long-term models: NMB estimation of the one-way and scenario sensitivity analysis.
Discussion
The aim of the study was to develop a decision-analytic model to estimate the cost-effectiveness of the four OPAT services offered in the UK for infections requiring short- or long-term treatment.
The deterministic and PSA analysis in both models indicated that HO was not the optimal strategy. The incremental analysis results showed that SN and SA were the most cost-effective strategies for short- and long-term treatments, respectively. The results were mainly driven by costs as the QALY difference observed was negligible (less than 0.01 QALY gained). The explanation for this is that the time horizon employed (12 months for both models) was relatively short and for many, the health event of interest is transient in nature with a very low risk of mortality. In contrast, there were significant cost differentials between the services which drove the cost-effectiveness results. The shorter healing time reported by HO and SN showed that these services can benefit from their ability to initiate intravenous to oral switch quicker than the GN or SA services. However, for HO in particular costs seem to outweigh this advantage. This is the case unless a significant reduction in healing time for short-term treatments is observed (more than 30%). In general, results were robust to changes in the parameter values. Only a substantive reduction in average healing time or a particular combination of circumstances appear to change the decisions.
A combination of services for both short- and long-term treatment was tested to acknowledge that more than one service model will often be provided. However, none of the combinations was shown to be cost-effective. Despite the latter, this analysis found that they were second best in terms of NMB.
To our knowledge, this is the first study to compare the four analysed services following the NICE economic evaluation reference case. We found a study based in Canada, but it only compared home IVA treatment against hospital inpatient-based services.33 Chapman et al.3 did a complete cost-effectiveness analysis of OPAT for the UK; however, the study was based on one health centre and it compared standard hospital inpatient care with daily attendances at a hospital facility.
The employed technique, DES, allows us the possibility to simulate the operation of an OPAT service keeping track of the progress and timing of the patients throughout their disease (i.e. account for side effects or complications), and therefore the measurement of costs and QALYs produced was more accurate than that provided by cohort models.
This work has some limitations. We were constrained to some extent by the available data. There was a paucity of useful comparative UK data on the effectiveness and safety of the OPAT services. We chose to use a hospital record dataset to derive our measure of ‘effectiveness’ (time to heal) as the systematic review could only identify effectiveness and risk values presented in observational studies. These were of limited value as the figures were likely to be biased; for example, some departments may only have considered certain patients (e.g. less severe or more independent) for particular OPAT services. The dataset, however, permitted adjustment for patient heterogeneity between services and did indicate differences in time to heal (or switch to oral antimicrobials).
Antimicrobial stewardship is currently a key concern, but we chose not to model antimicrobial resistance. We believe the differential rate of resistance between the service models would have been negligible and did not warrant the additional layer of complexity in the models.
After discussing with clinicians it was apparent that dividing patients into those requiring short- and long-term treatments was necessary as these two groups had distinct characteristics. For instance, it was not practical to train patients to self-administer antibiotics for short treatment courses while patients with long-term treatments are more at risk of acquiring CDI or a secondary intravenous line infection. In terms of the adverse events considered, we focused on those reported by the participants and which were expected to have a higher impact in terms of costs and quality of life. For example, we have not included deep venous thrombosis as none of the participants in the study suffered such an event.
Future research on the cost-effectiveness of the OPAT service using a DES model could explore the need and use of resources (such as number of nurses needed) to provide information for commissioners on the requirements to establish an OPAT service in the UK. The findings of this paper can also be used to inform future RCTs as they suggest that efforts should be focused on SN and HO for short-term treatments and SA and SN for long-term treatments.
Supplementary Material
Acknowledgements
The CIVAS team would like to formally acknowledge the support of the Steering Group: Professor Jenny Hewison (Chair), University of Leeds; Dr Barbara Summers, University of Leeds; Dr Claire McKenna and Ms Ada Keding, University of York; Dr Jonathan Sandoe and Dr Philip Howard, Leeds Teaching Hospitals NHS Trust; Dr Richard Bellamy, South Tees NHS Trust; and Mrs Heather Gent (patient). We would also like to thank the independent members of our Expert Panel: Professor Ann Jacklin (Chair), Dr Gavin Barlow, Ms Elizabeth Beech, Dr David Cairns, Dr Paul Chadwick, Ms Sue O’Hanlon, Mr Gerry Richardson, Ms Fiona Robb and Mr Chris Townley (patient representative).
Funding
This paper is part of the Community IntraVenous Antibiotic Study (CIVAS) project, which is funded by the National Institute for Health Research (NIHR) under its Health Services and Delivery Research Programme (11/2003/60).
Transparency declarations
None to declare.
Disclaimer
This article presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. MacKenzie M, Rae N, Nathwani D.. Outcomes from global adult outpatient parenteral antimicrobial therapy programmes: a review of the last decade. Int J Antimicrob Agents 2014; 43: 7–16. [DOI] [PubMed] [Google Scholar]
- 2. Department of Health. Our Health, Our Care, Our Say: A New Direction for Community Services 2006. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/272238/6737.pdf.
- 3. Chapman ALN, Dixon S, Andrews D. et al. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother 2009; 64: 1316–24. [DOI] [PubMed] [Google Scholar]
- 4. Seaton RA, Barr DA.. Outpatient parenteral antibiotic therapy: principles and practice. Eur J Intern Med 2013; 24: 617–23. [DOI] [PubMed] [Google Scholar]
- 5. Hitchcock J, Jepson AP, Main J. et al. Establishment of an outpatient and home parenteral antimicrobial therapy service at a London teaching hospital: a case series. J Antimicrob Chemother 2009; 64: 630–4. [DOI] [PubMed] [Google Scholar]
- 6. Department of Health. NHS Reference Costs 2013-14 2014. https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014.
- 7. Wong KK, Fraser TG, Shrestha NK. et al. Low incidence of Clostridium difficile infection (CDI) in patients treated with outpatient parenteral antimicrobial therapy (OPAT). Infect Control Hosp Epidemiol 2015; 36: 110–2. [DOI] [PubMed] [Google Scholar]
- 8. Seaton RA, Gonzalez-Ramallo VJ, Prisco V. et al. Daptomycin for outpatient parenteral antibiotic therapy: a European registry experience. Int J Antimicrob Agents 2013; 41: 468–72. [DOI] [PubMed] [Google Scholar]
- 9. Barr DA, Semple L, Seaton RA.. Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2012; 31: 2611–9. [DOI] [PubMed] [Google Scholar]
- 10. Minton J, Murray CC, Meads D. et al. The Community IntraVenous Antibiotic Study (CIVAS): a mixed-methods evaluation of patient preferences for and cost-effectiveness of different service models for delivering outpatient parenteral antimicrobial therapy. Health Serv Deliv Res 2017; no. 5.6. [PubMed] [Google Scholar]
- 11. NICE. Guide to the Methods of Health Technology Appraisal London, 2013.
- 12. Briggs A, Claxton K, Sculpher M.. Decision Modelling for Health Economic Evaluation. Oxford University Press, 2006. [Google Scholar]
- 13. Caro J, Moller J, Karnon J. et al. Discrete Event Simulation for Health Technology Assessment. CRC Press, 2016. [Google Scholar]
- 14. Caro J, Briggs A, Siebert U. et al. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–1. Med Decis Making 2012; 32: 667–77. [DOI] [PubMed] [Google Scholar]
- 15. Whitehead SJ, Ali S.. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull 2010; 96: 5–21. [DOI] [PubMed] [Google Scholar]
- 16. Drummond MF, Sculpher MJ, Claxton K. et al. Methods for the Economic Evaluation of Health Care Programmes, 4th edn.Oxford University Press, 2015. [Google Scholar]
- 17. SIMUL8 Corporation. SIMUL8 Software 2016. https://www.simul8.com/.
- 18. Matthews PC, Conlon CP, Berendt AR. et al. Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother 2007; 60: 356–62. [DOI] [PubMed] [Google Scholar]
- 19. Hopf Y, Watson M, Williams D.. Adverse-drug-reaction related admissions to a hospital in Scotland. Pharm World Sci 2008; 30: 854–62. [DOI] [PubMed] [Google Scholar]
- 20. Ryan M, Gerard K, Amaya-Amaya M.. Using Discrete Choice Experiments to Value Health and Health Care. Springer, 2007. [Google Scholar]
- 21. Forster AJ, Taljaard M, Oake N. et al. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. Can Med Assoc J 2012; 184: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiegand PN, Nathwani D, Wilcox MH. et al. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 2012; 81: 1–14. [DOI] [PubMed] [Google Scholar]
- 23. Thwaites GE, United Kingdom Clinical Infection Research Group. The management of Staphylococcus aureus bacteremia in the United Kingdom and Vietnam: a multi-centre evaluation. PLoS One 2010; 5: e14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lillie PJ, Andrews D, Eaves K. et al. Baseline factors predicting the duration of intravenous antibiotic therapy for cellulitis in an outpatient setting. Eur J Clin Microbiol Infect Dis 2010; 29: 347–9. [DOI] [PubMed] [Google Scholar]
- 25. Curtis LA. Unit Costs of Health and Social Care 2014 2014. http://www.pssru.ac.uk/project-pages/unit-costs/2014/.
- 26. Department of Health. Drugs and Pharmaceutical Electronic Market Information (eMit) 2011. https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit.
- 27. Mason JM, Thomas KS, Crook AM. et al. Prophylactic antibiotics to prevent cellulitis of the leg: economic analysis of the PATCH I & II trials. PLoS One 2014; 9: e82694.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernard L, Dinh A, Ghout I. et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015; 385: 875–82. [DOI] [PubMed] [Google Scholar]
- 29. Konijeti GG, Sauk J, Shrime MG. et al. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis 2014; 58: 1507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lloyd A, Price D, Brown R.. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007; 16: 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iglehart D. Simulating stable stochastic systems, V: comparison of ratio estimators. Nav Res Logist Q 1975; 22: 553–65. [Google Scholar]
- 32. Fenwick E, Byford S.. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005; 187: 106–8. [DOI] [PubMed] [Google Scholar]
- 33. Teuffel O, Amir E, Alibhai S. et al. Cost effectiveness of outpatient treatment for febrile neutropaenia in adult cancer patients. Br J Cancer 2011; 104: 1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.