Abstract
Objectives
The slow development of major advances in drug discovery for the treatment of Mycobacterium tuberculosis (Mtb) infection suggests a compelling need for evaluation of more effective drug therapies against TB. New classes of drugs are constantly being evaluated for anti-mycobacterial activity with currently a very limited number of new drugs approved for TB treatment. Minor groove binders (MGBs) have previously revealed promising antimicrobial activity against various infectious agents; however, they have not yet been screened against Mtb.
Methods
The mycobactericidal activity of 96 MGB compounds against Mtb was determined using an H37Rv-GFP microplate assay. MGB hits were screened for their intracellular mycobactericidal efficacy against the clinical Beijing Mtb strain HN878 in bone-marrow-derived macrophages using standard cfu counting. Cell viability was assessed by CellTiter-Blue assays. Selected MGBs were encapsulated into non-ionic surfactant vesicles (NIVs) for drug delivery system evaluation.
Results
H37Rv-GFP screening yielded a hit-list of seven compounds at an MIC99 of between 0.39 and 1.56 μM. MGB-362 and MGB-364 displayed intracellular mycobactericidal activity against Mtb HN878 at an MIC50 of 4.09 and 4.19 μM, respectively, whilst being non-toxic. Subsequent encapsulation into NIVs demonstrated a 1.6- and 2.1-fold increased intracellular mycobacterial activity, similar to that of rifampicin when compared with MGB-alone formulation.
Conclusions
MGB anti-mycobacterial activities together with non-toxic properties indicate that MGB compounds constitute an important new class of drug/chemical entity, which holds promise in future anti-TB therapy. Furthermore, the ability of NIVs to better deliver entrapped MGB compounds to an intracellular Mtb infection suggests further preclinical evaluation is warranted.
Introduction
Mycobacterium tuberculosis (Mtb), the causative agent of TB, has become the top infectious killer worldwide. According to the 2016 WHO Global Tuberculosis Report,1 TB killed approximately 1.8 million people in 2015, up from 1.5 million deaths in 2014.2 The current 6 month treatment regimen for drug-susceptible Mtb, although still effective in most cases, is gradually becoming ineffective due to increasing resistance against the drugs used to treat TB.3 Several advances have been made in the field of TB drug discovery, spearheaded by global partnerships. For example, the Global Alliance for TB Drug Development currently manages the largest array of novel anti-TB drug compounds and novel regimens for MDR and XDR TB.4 Other initiatives to eradicate TB include the STOP TB partnership, which includes an international working group to develop new TB drugs.5 Furthermore, several large consortia of pharmaceutical companies (TB Drug Accelerator) and academia (MM4TB) are driving the discovery of new TB drugs.6 Despite the progress in the pipeline for new diagnostics, drugs, regimens and vaccines, research remains relentlessly underfunded. As a consequence, only a few new drugs have been approved for clinical use, i.e. delamanid, bedaquiline and pretomanid, and only 10 new drugs are in advanced phases of clinical trials as of 2016.7,8 With the slow development of major advances in anti-mycobacterial drug discovery and the emergence of MDR and XDR TB, there is an urgent need for the development of more effective therapies and formulations of existing drugs for the treatment of this disease.8,9 In the area of novel therapeutics discovery, progress has been made in developing new drug classes such as benzothiazinones, which inhibit cell wall arabinan synthesis, and imidazopyridines, which inhibit respiratory chain ATP synthesis.10,11 Minor groove binders (MGBs) have revealed promising antibacterial properties, but have not yet been investigated for their anti-mycobacterial activity against Mtb in vitro.
Derived from the natural product distamycin, MGBs are a class of compounds that selectively bind to the minor groove of bacterial DNA with their helical structure matching that of DNA.12 Most often, proteins binding to bacterial DNA bind to the major groove, leaving the minor groove exposed and, thus, a vacant target for MGBs. Natural forms of MGBs are currently used in clinical treatment of disease. For example, aromatic diamidines, such as pentamidine13,14 and berenil,15 known to bind to the minor groove at adenosine–thymine tracts, have been administered clinically against human African trypanosomiasis and Pneumocystis carinii pneumonia.16–18 MGBs display a wide variety of activity profiles against many infectious organisms evaluated, including Gram-positive bacteria,19Mycobacterium aurum,20 chloroquine-susceptible and -resistant Plasmodium falciparum21 and Trypanosoma brucei brucei.17 In partnership with MGB-Biopharma, one MGB compound has successfully completed Phase I clinical trials for the treatment of Clostridium difficile infections.22 We recently screened a limited number of MGBs for their anti-mycobacterial activity against the laboratory Mtb H37Rv strain with MIC99 reaching 3.1 μM.23 We have now further extended this work by producing more active MGBs with higher MIC99 values for Mtb H37Rv. In addition we examined the anti-mycobacterial activity of MGBs against the intracellular clinical HN878 Beijing strain of Mtb and evaluated the effect of MGB exposure on cell viability in macrophages.
Oral drug administration has various limitations, including drug inefficiency resulting from drug insolubility caused by low gastric pH, or poor absorbance in the gastrointestinal tract. However, an effective drug delivery system can improve drug retention at the site of infection. Therefore, the ability to deliver a drug to the site of infection may provide a sustained drug concentration, enabling increased effectiveness of a drug against its target. In the case of pulmonary TB treatment, oral drug administration leads to high systemic concentrations of the drugs with associated side effects such as liver toxicity and cytotoxicity, amongst others.24 Ultimately, the drawbacks associated with the oral administration of antibiotics laid the foundation for the development of innovative drug delivery approaches. The use of liposomes as a drug delivery system has been previously reported to reduce microbial drug resistance through faster drug delivery and increasing the antimicrobial drug concentration, thereby preventing microbial drug efflux pump activity.25 Liposome-encapsulated drugs kill microbes faster, before microbial mutations can develop. For example, the incorporation of the antibiotic levofloxacin into liposomes improved the anti-mycobacterial activity to kill Mtb strains resistant to levofloxacin.26 Other drug delivery systems, such as non-ionic surfactant vesicles (NIVs), have the ability to encapsulate both hydrophobic and hydrophilic drugs for direct delivery to the site of infection.27 NIVs are small colloidal particles made of a non-aqueous, non-ionic surfactant bilayer that surrounds a central aqueous compartment. They are thermodynamically stable, easily manufactured and do not require special storage conditions. One of the major advantages of NIVs is that they are able to entrap different types of drug substances and can have their size altered. Their capacity to improve the delivery of small molecules is an important trait that allows for precise targeting of deposition of particles within the respiratory tract. Previous studies have shown NIVs to be a promising inhalable drug delivery system against pulmonary aspergillosis with aerosolized amphotericin B/NIV administration reducing fungal lung burden when compared with amphotericin B solution only.28 More recent studies have demonstrated the antibacterial action of moxifloxacin29 and cefixime30 and the antiviral action of nevirapine31 in NIV formulations. Although many different drug delivery systems have been utilized to entrap first-line TB drugs,32 only a few have systematically explored their anti-mycobacterial activity against Mtb and against intracellular Mtb in infected primary macrophages. Thus, we have investigated the use of NIVs as a drug delivery system for improved delivery and efficacy of novel MGB compounds active against Mtb-infected macrophages.
Materials and methods
MGB compounds
MGB compounds were synthesized using distamycin template, a natural product with known anti-infective properties, as previously reported.17,23,33 Alterations of the head, tail, side chains and body resulted in a number of diverse compounds, with later synthesis driven by acquired screening data (Table S1, available as Supplementary data at JAC Online). MGBs were resuspended in DMSO to a concentration of 1.25 mM and were stored at −80 °C.
Preparation of compounds and NIVs
MGB compounds (stock: 1.25 mM) and rifampicin (stock: 20 mM) were diluted to a starting concentration of 50 μM followed by 2-fold dilutions in 7H9 broth medium or DMEM to yield the required screening range. Freeze-dried NIVs were prepared as previously described28 and rehydrated in DMEM + 10% FCS (Gibco, Thermofisher Scientific, USA) to an NIV concentration range of 23–5000 μM (empty NIVs) and subsequently added to bone-marrow-derived macrophages (BMDMs) in order to assess cell viability through CellTiter-Blue (Promega, WI, USA) assay with fluorescence detection at (544ex/590em nm). Subsequently, drug/NIV solutions were prepared in DMEM + 10% FCS at a 2:5 molar ratio (MGB:NIVs) at a compound 2-fold serial dilution range from 1.56 to 12.5 μM (3.91–31.25 μM NIVs) to assess cell viability and intracellular anti-mycobacterial activity. Two-fold serial drug dilution was performed as previously reported in other drug screening studies.34
H37Rv-GFP microplate screening assay
MGB compounds were screened for their anti-mycobacterial activity using 96-well, black, clear, flat-bottom microplates (Greiner Bio-One, Germany) as previously reported.35,36 Single-cell suspension of H37Rv-GFP from frozen stock with a working concentration of 1 × 106 cfu/mL was prepared in Middlebrook 7H9 supplemented with 25 mg/L kanamycin, 10% Middlebrook OADC (v/v) and 0.05% Tween 80 (w/v). Next, 100 μL of H37Rv-GFP at a concentration of 1 × 105 cfu/well was added to each experimental well, and then 100 μL of drug compounds prepared in 7H9 broth supplemented with 25 mg/L kanamycin to generate 0.195–50 μM screening range was added to wells containing H37Rv-GFP for a final screening range of 0.0977–25 μM. Wells containing compound only at the highest screening concentration were used to detect autofluorescence of compounds and broth (vehicle control). Fluorescence (485ex/520em nm) was measured at designated timepoints, namely days 0, 4, 8, 10 and 12, with a BMG Labtech Omega Plate Reader (Germany). The addition of sterile water to the outer wells of each plate served to minimize the evaporation. Time intervals were selected as previously reported in other drug screening studies.36
BMDM generation and Mtb infection
BMDMs were generated from 8–12 week old C57BL/6 mice as previously reported.37 After differentiation, BMDMs were plated into 96-well plates (Nunc, Denmark) at 2 × 105 cells per well. Following overnight adherence, BMDMs were then infected with Mtb HN878 (moi = 5) and cultured at 37 °C under 5% CO2 for 4 h. BMDMs were washed once with pre-warmed culture medium to remove extracellular bacteria or lysed and lysates plated on 7H10 agar plates supplemented with 10% OADC and 0.5% glycerol for cfu counting to determine bacilli uptake. Drug compounds prepared in DMEM supplemented with 10% FCS at defined concentrations were added to infected BMDMs to determine anti-mycobacterial activity and cell viability. After 5 days of culture, cells were lysed for cfu plating or assessed for cell viability by CellTiter-Blue assay.
Statistical analysis
All data were analysed using R, a Student’s t-test (two-tailed with equal variance) was used, unless otherwise stated in figure legends. A P value <0.05 was considered significant.
Results
MIC99 of MGB compounds for H37Rv-GFP
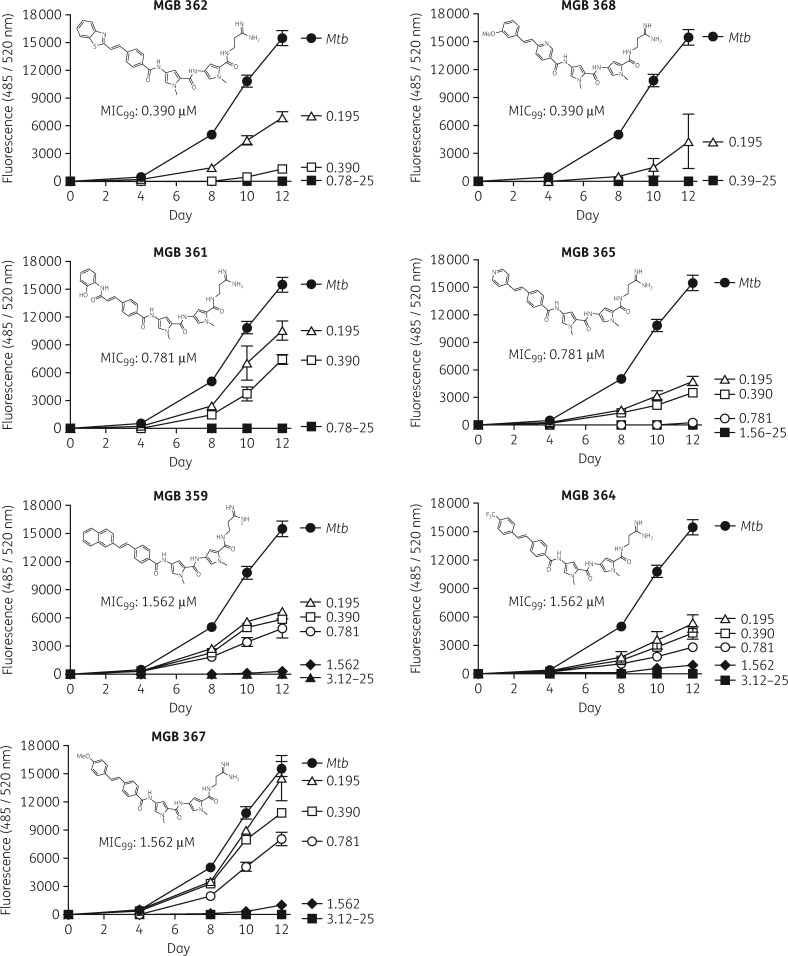
We screened 96 MGBs for their anti-mycobacterial activity against GFP-labelled H37Rv Mtb in liquid broth culture using a 96-well plate assay (Table 1). Relative fluorescence was measured at 0, 4, 8, 10 and 12 days in broth culture of MGBs (serially diluted from 25 to 0.19 μM) to determine the MIC99 of MGBs required to eradicate 99% of Mtb (Figure 1). Hit compounds, defined as previously reported,38 were identified as drugs that were active at or below the threshold concentration of 3.12 μM. A hit-list of seven compounds with an MIC99 of 1.56 μM or less was identified (Figure 1 and Table 1). Rifampicin, which had an MIC of 0.0977 μM, was used as the positive control. The selected hit compounds were MGBs 362, 368, 361, 365, 359, 364 and 367 with an MIC99 range of 0.391–1.56 μM, and were therefore identified for subsequent intracellular anti-mycobactericidal activity screening.
Table 1.
MIC99s (μM) of all screened MGBs for H37Rv-GFP
| Compound | MIC99 | Compound | MIC99 | Compound | MIC99 |
|---|---|---|---|---|---|
| Rifampicin | 0.0977 | 371 | 25 | 235 | >25 |
| 362 (hit) | 0.391 | 372 | 25 | 245 | >25 |
| 368 (hit) | 0.391 | 373 | 25 | 246 | >25 |
| 361 (hit) | 0.781 | 374 | 25 | 247 | >25 |
| 365 (hit) | 0.781 | 381 | 25 | 248 | >25 |
| 359 (hit) | 1.56 | 1 | >25 | 270 | >25 |
| 364 (hit) | 1.56 | 2 | >25 | 271 | >25 |
| 367 (hit) | 1.56 | 9 | >25 | 283 | >25 |
| 353* | 3.12 | 12 | >25 | 286 | >25 |
| 354* | 3.12 | 74* | >25 | 287 | >25 |
| 391 | 3.12 | 85 | >25 | 288 | >25 |
| 263 | 6.25 | 92 | >25 | 289 | >25 |
| 343 | 6.25 | 114 | >25 | 300 | >25 |
| 385 | 6.25 | 121 | >25 | 303 | >25 |
| 386 | 6.25 | 122 | >25 | 304 | >25 |
| 351* | 12.5 | 123 | >25 | 305 | >25 |
| 352* | 12.5 | 124 | >25 | 306 | >25 |
| 376 | 12.5 | 131 | >25 | 322 | >25 |
| 377 | 12.5 | 134 | >25 | 323 | >25 |
| 378 | 12.5 | 147 | >25 | 324* | >25 |
| 379 | 12.5 | 154 | >25 | 325 | >25 |
| 380 | 12.5 | 176 | >25 | 329* | >25 |
| 383 | 12.5 | 185 | >25 | 331* | >25 |
| 387 | 12.5 | 187 | >25 | 332* | >25 |
| 390 | 12.5 | 188 | >25 | 333* | >25 |
| 282 | 12.5–25 | 192 | >25 | 334* | >25 |
| 4* | 25 | 210 | >25 | 335* | >25 |
| 116 | 25 | 212 | >25 | 336* | >25 |
| 164 | 25 | 213 | >25 | 338* | >25 |
| 292 | 25 | 214 | >25 | 356 | >25 |
| 317* | 25 | 222 | >25 | 357 | >25 |
| 330* | 25 | 234 | >25 | 358 | >25 |
| 337 | 25 |
Seven hits were identified out of 96 MGBs screened.
MGBs previously screened as reported.23
Figure 1.
Screening of anti-mycobacterial activity of MGB compounds against H37Rv-GFP. Direct antimicrobial activity of MGB compounds at the drug concentration range of 0.195–25 μM was tested against H37Rv-GFP (1 × 105 cfu/well) in 7H9 liquid broth culture using a microplate assay. The anti-mycobacterial activity of MGB compounds against H37Rv-GFP was determined in a concentration-dependent manner by measuring fluorescence (485ex/520em nm) on days 0, 4, 8, 10 and 12. Data were corrected for background 7H9 fluorescence. Data are shown as mean ± SEM of duplicates.
Intracellular drug activity against clinical Mtb and macrophage cell viability
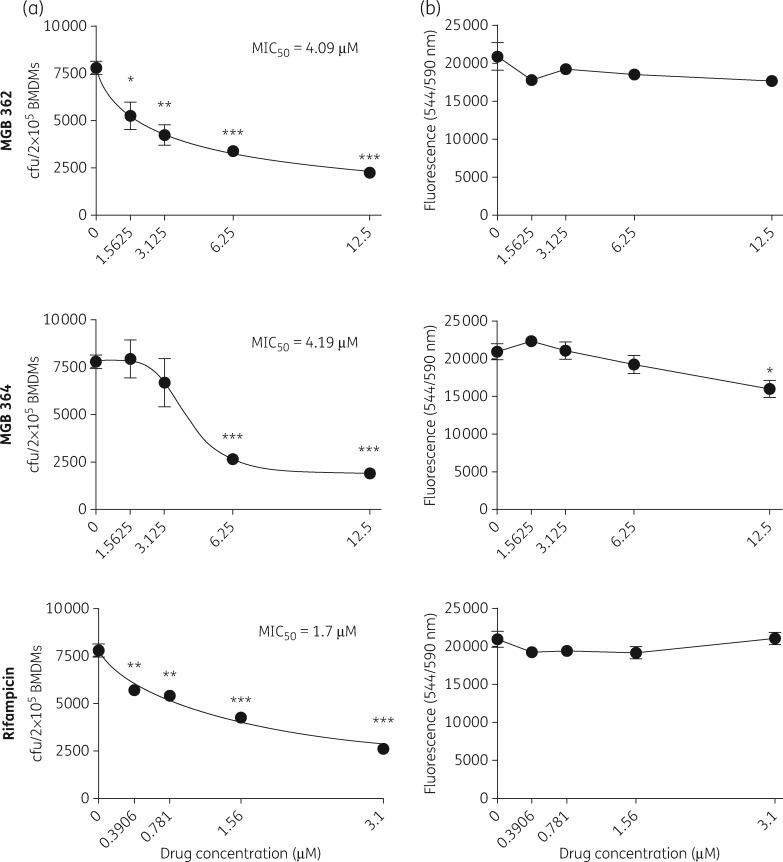
The ability of anti-TB drug compounds to penetrate macrophages and induce mycobactericidal activity, while being non-toxic to the macrophages, is a salient property sought after in TB drug development. Hence, BMDMs were exposed to serial MGB drug concentrations from 1.56 to 12.5 μM to evaluate their anti-mycobacterial activity against the clinical Mtb strain HN878, after 5 days of infection. Compounds were screened for the concentration that eradicated 50% of bacilli (MIC50; Figure 2a). Two of the seven hit compounds identified from screening studies against Mtb in Figure 1 had good intracellular mycobacterial killing efficacy against Mtb-infected macrophages, with MIC50 values of 4.09 μM (MGB 362) and 4.19 μM (MGB 364). Rifampicin, selected as a positive control, had an MIC50 of 1.7 μM. CellTiter-Blue cell viability assay was performed to assess for macrophage cell viability in MGBs-treated BMDMs after 5 days of exposure (Figure 2b). MGB 362 and 364 and rifampicin had no significant effect on macrophage viability at the respective intracellular drug activity MIC50 concentrations (Figure 2b). These data suggest that MGB 362 and 364 have an efficient intracellular anti-mycobacterial activity against Mtb while being non-toxic to the host cells.
Figure 2.
MIC50s of MGB compounds for HN878 Mtb-infected BMDMs and cell viability. (a) The intracellular anti-mycobacterial activities of MGBs (1.5625–12.5 μM) and rifampicin (0.3906–3.125 μM) were assessed by counting cfu at the respective concentration at 5 days post-Mtb HN878 infection. MIC50 values of each drug compound were identified in GraphPad Prism by non-linear regression analysis. (b) Macrophage cell viability was determined at 5 days of MGB compound exposure and measured by CellTiter-Blue assay with fluorescence detection at 544ex/590em nm. Data were corrected for background culture medium fluorescence and are shown as mean ± SEM, representative of triplicates. Two-tailed Student’s t-test: *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control.
MBG/NIV encapsulation increased intracellular drug activity against a clinical strain of Mtb
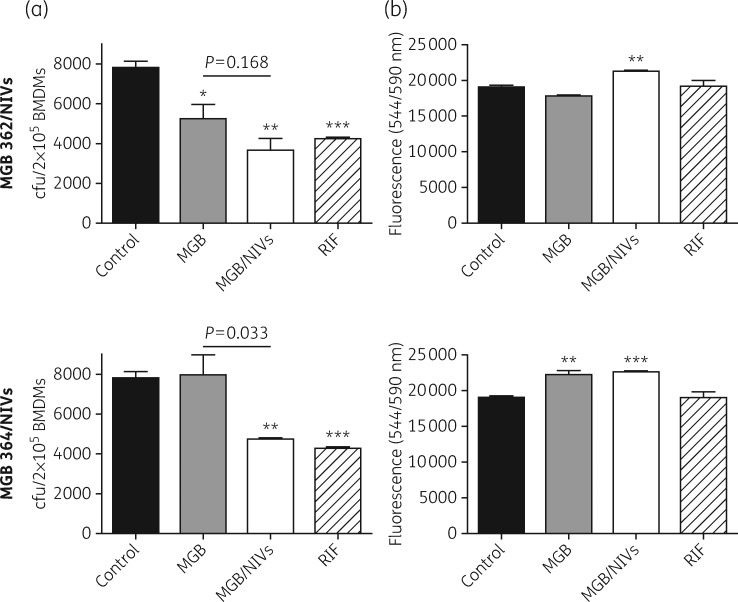
We next investigated whether encapsulating our hit MGB compounds into NIVs, a drug delivery system previously reported to improve drug delivery of amphotericin B to macrophages,28 would improve MGB drug efficacy against the intracellular clinical HN878 Mtb strain. We demonstrated that encapsulating MGBs into NIVs improved the intracellular anti-mycobacterial abilities by 2.1-fold for MGB 362 and 1.6-fold for MGB 364 in Mtb HN878-infected BMDMs, displaying a significant cfu reduction (P < 0.01) compared with controls (Figure 3a). The anti-mycobacterial killing activities of MGB 362/NIVs and MGB 364/NIVs were similar to that of rifampicin. MGB 364/NIVs displayed significantly decreased cfu counts (P < 0.033) when compared with MGB alone. Furthermore, Mtb-infected macrophages were viable following MGB/NIV treatment (Figure 3b). Treatment with NIVs alone also had no significant effect on macrophage viability (data not shown). These results demonstrate that NIVs can act as a suitable delivery system by transporting MGB inside macrophages, the target cells for Mtb.
Figure 3.
Intracellular mycobacterial activity of MGB/NIV formulations in HN878 Mtb-infected BMDMs and cell viability. (a) The intracellular anti-mycobacterial activity of MGBs only (1.56 μM), MGB (1.56 μM)/NIV (3.96 μM) formulations and rifampicin (1.56 μM) were determined in comparison with control (no drug treatment). cfu was determined at 5 days post-Mtb HN878 infection. (b) Macrophage cell viability was determined at 5 days post-Mtb HN878 infection and measured by CellTiter-Blue assay with fluorescence detection at 544ex/590em nm. Data were corrected for background culture medium fluorescence and are shown as mean ± SD, representative of triplicates. Two-tailed Student’s t-test: *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control. RIF, rifampicin.
Discussion
MGB compounds have shown great potential as antibacterial therapeutic agents.33 However, their activity against Mtb remains unknown. Here, we demonstrated the anti-mycobacterial (MIC99) properties of MGBs against Mtb (H37Rv-GFP) with a reliable screening method that enables the detection of most active compounds,39 using rifampicin as a positive control. All of the active MGB compounds belong to the well-established alkene-linked MGB family discovered at the University of Strathclyde, with high killing activities against different pathogens as previously reported.17,19–21,23,33 Since the primary binding sites of all of these MGBs in the DNA minor groove are AT-rich regions it is unlikely that target sequence specificity is responsible for the selectivity observed. This is true also for the active compounds against Mtb described here. However, it is more likely that activity and selectivity against a particular pathogen are caused by differential access to cells caused by differing cell wall and cell membrane structures in a way that with the current state of knowledge is idiosyncratic and unpredictable.33 What can be reliably stated is that the alkene-linked compounds are significantly the most biologically active of the Strathclyde MGB family. In general, MGBs with the most significant antibacterial activity possess a range of different tail groups, all of which are exemplified within the set in our screen. However, all of the most active MGBs identified in this study possess an amidine-containing tail group, which perhaps suggests an important role of tail group pKa in targeting mycobacteria.
Screening of MGB compounds in the context of their cell viability and anti-mycobacterial activity against the intracellular clinical Mtb strain HN878 has identified two compounds with promising results, giving a hit rate of 2.1% (2/96). In most studies the hit rate for hit compounds is of the order of 1%, in line with previous studies.40 These findings, however, warrant in vivo testing, which aims to allow for better clinical therapeutic translation of the findings. The use of NIVs has been demonstrated repeatedly in the literature before and constitutes a prominent focus within current Mtb research in order to combat the infection.27,41 NIVs given by nebulization delivered amphotericin B to the lungs and liver with significantly improved treatment outcome when compared with amphotericin B solution against pulmonary aspergillosis and visceral leishmaniasis.28 Our investigation of NIVs as a delivery device indeed demonstrates that NIVs can be used to enhance the efficacy of MGB compounds against HN878 in infected BMDMs, whilst not increasing the toxicity of the drug to BMDMs. MGBs contain hydrophobic head groups,12 enabling encapsulation into NIVs. Liposomes have previously been reported to encapsulate an alkyl derivative of distamycin A,42 and are naturally occurring backbones for MGB compound synthesis.
The ability of NIVs to trap the drug within their hydrophilic/hydrophobic compartment allows the drug to be taken up by phagocytosis by the infected macrophage, thereby transporting the drug to the site of infection. Using NIV drug formulations resulted in higher drug levels compared with similar treatment with drug solution at the site of infection after treatment by the pulmonary or intravenous routes for water-soluble43,44 and lipid-soluble drugs.28 Studies in dogs treated by the intravenous route with a sodium stibogluconate (SSG)/dextran/NIV formulation increased the elimination half-life and the volume of distribution at steady state compared with SSG/dextran solution.45 Therefore MGB/NIV formulations can be a feasible pulmonary treatment for Mtb.
In conclusion, this study showed that MGBs constitute an important new class of drug/chemical entity with favourable anti-mycobacterial activity and hold promise in future anti-TB therapy. Furthermore, we demonstrate that NIVs (i) contribute to better delivery of drugs to an intracellular infection, (ii) act as a delivery device for entrapped MGB compounds and (iii) serve as the initial step into future research of targeted delivery of entrapped drugs to Mtb-infected cells.
Supplementary Material
Acknowledgements
We thank Ms Fadwah Booley, Ms Lorna Gcanga and Mr George Jacobs for their excellent technical assistance.
Funding
This work was supported by the Knowledge Exchange Development Fund (KEDF) from the University of Strathclyde to K. C. C., the International Centre for Genetic Engineering & Biotechnology (ICGEB) Arturo Falaschi PhD Fellowship to M. O. and S. K., the Claude Leon Foundation and CIDRI, Wellcome Trust (Grant No. 084323) post-doctoral fellowships to S. P. P., the South African Medical Research Council (SAMRC) doctoral fellowship to L. H., grants from the South African National Research Foundation (NRF) and from the Department of Science and Technology, South African Research Chair Initiative (SARCHi) and South Africa Medical Research Council (SAMRC) to F. B. and grants from the Department of Science and Technology (DST)/South African National Research Foundation (NRF) Collaborative Postgraduate Training Programme to R. G.
Transparency declarations
None to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. WHO. Global TuberculosisReport 2016. Geneva, Switzerland: WHO; http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2. WHO. Global Tuberculosis Report 2015. Geneva, Switzerland: WHO; http://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf. [Google Scholar]
- 3. Zumla A, Abubakar I, Raviglione M. et al. Drug-resistant tuberculosis—current dilemmas, unanswered questions, challenges, and priority needs. J Infect Dis 2012; 205 Suppl 2: S228–40. [DOI] [PubMed] [Google Scholar]
- 4. Murray S, Mendel C, Spigelman M.. TB Alliance regimen development for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2016; 20: 38–41. [DOI] [PubMed] [Google Scholar]
- 5. Mwaba P, McNerney R, Grobusch MP. et al. Achieving STOP TB Partnership goals: perspectives on development of new diagnostics, drugs and vaccines for tuberculosis. Trop Med Int Health 2011; 16: 819–27. [DOI] [PubMed] [Google Scholar]
- 6. Zuniga ES, Early J, Parish T.. The future for early-stage tuberculosis drug discovery. Future Microbiol 2015; 10: 217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pai M, Behr MA, Dowdy D. et al. Tuberculosis. Nat Rev Dis Primers 2016; 2: 16076.. [DOI] [PubMed] [Google Scholar]
- 8. Mdluli K, Kaneko T, Upton A.. The tuberculosis drug discovery and development pipeline and emerging drug targets. Cold Spring Harb Perspect Med 2015; 5: pii: a021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brigden G, Hewison C, Varaine F.. New developments in the treatment of drug-resistant tuberculosis: clinical utility of bedaquiline and delamanid. Infect Drug Resist 2015; 8: 367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pethe K, Bifani P, Jang J. et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 2013; 19: 1157–60. [DOI] [PubMed] [Google Scholar]
- 11. Makarov V, Manina G, Mikusova K. et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 2009; 324: 801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suckling CJ. Molecular recognition and physicochemical properties in the discovery of selective antibacterial minor groove binders. J Phys Org Chem 2008; 21: 575–83. [Google Scholar]
- 13. Edwards KJ, Jenkins TC, Neidle S.. Crystal structure of a pentamidine-oligonucleotide complex: implications for DNA-binding properties. Biochemistry 1992; 31: 7104–9. [DOI] [PubMed] [Google Scholar]
- 14. Fox KR, Sansom CE, Stevens MFG.. Footprinting studies on the sequence-selective binding of pentamidine to DNA. FEBS Lett 1990; 266: 150–4. [DOI] [PubMed] [Google Scholar]
- 15. Brown DG, Sanderson MR, Garman E. et al. Crystal structure of a berenil-d(CGCAAATTTGCG) complex. J Mol Biol 1992; 226: 481–90. [DOI] [PubMed] [Google Scholar]
- 16. Paine MF, Wang MZ, Generaux CN. et al. Diamidines for human African trypanosomiasis. Curr Opin Investig Drugs 2010; 11: 876–83. [PubMed] [Google Scholar]
- 17. Scott FJ, Khalaf AI, Giordani F. et al. An evaluation of minor groove binders as anti-Trypanosoma brucei brucei therapeutics. Eur J Med Chem 2016; 116: 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tao B, Huang TL, Zhang Q. et al. Synthesis and anti-Pneumocystis carinii activity of conformationally restricted analogues of pentamidine. Eur J Med Chem 1999; 34: 531–8. [Google Scholar]
- 19. Khalaf AI, Bourdin C, Breen D. et al. Design, synthesis and antibacterial activity of minor groove binders: the role of non-cationic tail groups. Eur J Med Chem 2012; 56: 39–47. [DOI] [PubMed] [Google Scholar]
- 20. Khalaf AI, Anthony N, Breen D. et al. Amide isosteres in structure-activity studies of antibacterial minor groove binders. Eur J Med Chem 2011; 46: 5343–55. [DOI] [PubMed] [Google Scholar]
- 21. Scott FJ, Khalaf AI, Duffy S. et al. Selective anti-malarial minor groove binders. Bioorg Med Chem Lett 2016; 26: 3326–9. [DOI] [PubMed] [Google Scholar]
- 22. Ravic M, Firmin D, Sahgal O. et al. A single-centre, double-blind, placebo-controlled study in healthy men to assess the safety and tolerability of single and repeated ascending doses of MGB-BP-3, a new class of antibacterial agent. In: Abstracts of the American Society of Microbiology Microbe Meeting 2016, Boston, MA, USA. Abstract: Monday-524.
- 23. Scott FJ, Nichol RJ, Khalaf AI. et al. An evaluation of minor groove binders as anti-fungal and anti-mycobacterial therapeutics. Eur J Med Chem 2017; 136: 561–72. [DOI] [PubMed] [Google Scholar]
- 24. Gulbay BE, Gurkan OU, Yildiz OA. et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med 2006; 100: 1834–42. [DOI] [PubMed] [Google Scholar]
- 25. Pelgrift RY, Friedman AJ.. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev 2013; 65: 1803–15. [DOI] [PubMed] [Google Scholar]
- 26. Gaidukevich SK, Mikulovich YL, Smirnova TG. et al. Antibacterial effects of liposomes containing phospholipid cardiolipin and fluoroquinolone levofloxacin on Mycobacterium tuberculosis with extensive drug resistance. Bull Exp Biol Med 2016; 160: 675–8. [DOI] [PubMed] [Google Scholar]
- 27. Kumar GP, Rajeshwarrao P.. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharmaceutica Sinica B 2011; 1: 208–19. [Google Scholar]
- 28. Alsaadi M, Italia JL, Mullen AB. et al. The efficacy of aerosol treatment with non-ionic surfactant vesicles containing amphotericin B in rodent models of leishmaniasis and pulmonary aspergillosis infection. J Control Release 2012; 160: 685–91. [DOI] [PubMed] [Google Scholar]
- 29. Sohrabi S, Haeri A, Mahboubi A. et al. Chitosan gel-embedded moxifloxacin niosomes: an efficient antimicrobial hybrid system for burn infection. Int J Biol Macromol 2016; 85: 625–33. [DOI] [PubMed] [Google Scholar]
- 30. Imran M, Shah MR, Ullah F. et al. Glycoside-based niosomal nanocarrier for enhanced in-vivo performance of cefixime. Int J Pharm 2016; 505: 122–32. [DOI] [PubMed] [Google Scholar]
- 31. Mehta SK, Jindal N.. Tyloxapol niosomes as prospective drug delivery module for antiretroviral drug nevirapine. AAPS PharmSciTech 2015; 16: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hari BN, Chitra KP, Bhimavarapu R. et al. Novel technologies: a weapon against tuberculosis. Indian J Pharmacol 2010; 42: 338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barrett MP, Gemmell CG, Suckling CJ.. Minor groove binders as anti-infective agents. Pharmacol Ther 2013; 139: 12–23. [DOI] [PubMed] [Google Scholar]
- 34. Andreu N, Fletcher T, Krishnan N. et al. Rapid measurement of antituberculosis drug activity in vitro and in macrophages using bioluminescence. J Antimicrob Chemother 2011; 67: 404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Collins LA, Torrero MN, Franzblau SG.. Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother 1998; 42: 344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salie S, Hsu NJ, Semenya D. et al. Novel non-neuroleptic phenothiazines inhibit Mycobacterium tuberculosis replication. J Antimicrob Chemother 2014; 69: 1551–8. [DOI] [PubMed] [Google Scholar]
- 37. Schwegmann A, Guler R, Cutler AJ. et al. Protein kinase C δ is essential for optimal macrophage-mediated phagosomal containment of Listeria monocytogenes. Proc Natl Acad Sci U S A 2007; 104: 16251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hughes JP, Rees S, Kalindjian SB. et al. Principles of early drug discovery. Br J Pharmacol 2011; 162: 1239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Changsen C, Franzblau SG, Palittapongarnpim P.. Improved green fluorescent protein reporter gene-based microplate screening for antituberculosis compounds by utilizing an acetamidase promoter. Antimicrob Agents Chemother 2003; 47: 3682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fuchs JE, Spitzer GM, Javed A. et al. Minor groove binders and drugs targeting proteins cover complementary regions in chemical shape space. J Chem Inf Model 2011; 51: 2223–32. [DOI] [PubMed] [Google Scholar]
- 41. Rajera R, Nagpal K, Singh SK. et al. Niosomes: a controlled and novel drug delivery system. Biol Pharm Bull 2011; 34: 945–53. [DOI] [PubMed] [Google Scholar]
- 42. Cortesi R, Romagnoli R, Menegatti E. et al. Liposomes containing distamycins: preparation, characterization and antiproliferative activity. Drug Deliv 2004; 11: 83–8. [DOI] [PubMed] [Google Scholar]
- 43. Carter KC, Mullen AB, Sundar S. et al. Efficacies of vesicular and free sodium stibogluconate formulations against clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 2001; 45: 3555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williams D, Mullen AB, Baillie AJ. et al. Comparison of the efficacy of free and non-ionic-surfactant vesicular formulations of paromomycin in a murine model of visceral leishmaniasis. J Pharm Pharmacol 1998; 50: 1351–6. [DOI] [PubMed] [Google Scholar]
- 45. Nieto J, Alvar J, Mullen AB. et al. Pharmacokinetics, toxicities, and efficacies of sodium stibogluconate formulations after intravenous administration in animals. Antimicrob Agents Chemother 2003; 47: 2781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.