Abstract
Background
Bedaquiline has been shown to reduce time to sputum culture conversion (SCC) and increase cure rates in patients with drug-resistant TB, but the influence of drug exposure remains uncharacterized.
Objectives
To investigate whether an exposure–response relationship could be characterized by making better use of the existing information on pharmacokinetics and longitudinal measurements of mycobacterial load.
Methods
Quantitative culture data in the form of time to positivity (TTP) in mycobacterial growth indicator tubes obtained from a randomized placebo-controlled Phase IIb registration trial were examined using non-linear mixed-effects methodology. The link to individual bedaquiline exposures and other patient characteristics was evaluated.
Results
The developed model included three simultaneously fitted components: a longitudinal representation of mycobacterial load in patients, a probabilistic component for bacterial presence in sputum samples, and a time-to-event model for TTP. Data were described adequately, and time to SCC was well predicted. Individual bedaquiline exposure was found to significantly affect the decline in mycobacterial load. Consequently, the proportion of patients without SCC at week 20 is expected to decrease from 25% (95% CI 20%–31%) without bedaquiline to 17% (95% CI 13%–21%), 12% (95% CI 8%–16%) and 7% (95% CI 4%–11%), respectively, with half the median, median and double the median bedaquiline exposure observed in patients with standard dosing. Baseline bacterial load and level of drug resistance were other important predictors.
Conclusions
To our knowledge, this is the first successful description of bedaquiline’s exposure–response relationship and may be used when considering dose optimization. Characterization of this relationship was possible by integrating quantitative information in existing clinical data using novel models.
Introduction
Drug-resistant TB remains a huge public health problem. In 2015, the WHO estimated there were close to half a million new cases of MDR-TB and the global treatment success rate in adults was as low as 50%.1 There is an acute lack of knowledge of how best to select combinations and doses of the available second-line anti-TB drugs, and the WHO classifies the quality of evidence for the currently used recommendations as very low.2 This uncertainty is linked to the poor or absent description of dose exposure–response relationships for the drugs currently in use.
The clinical endpoint for treatment of TB is usually relapse-free cure,3 which is burdensome to study directly due to the long treatment period (typically 20 months for MDR-TB2) and follow-up time needed. A multitude of biomarkers have been proposed as alternative endpoints for earlier assessment of treatment response.4,5 Stable sputum culture conversion (SCC) is commonly defined as the first of two negative samples taken at least 30 days apart with no positive intervening samples.3 In recent clinical trials, time to sputum culture conversion (TSCC) has often been the preferred endpoint.6–8 The metric is based on mycobacterial cultures from sputum samples, either on solid or in liquid media (where the latter is regarded as more sensitive).9–11 TSCC is reported as the average time from start of treatment to SCC and/or as Kaplan–Meier survival curves and hazard ratios. Quantitative information about the bacterial load in a patient, obtained from cultures, either as the number of colony-forming units on solid media or the time to positivity (TTP) in liquid media, is ignored when the results are evaluated only as positive or negative. Assessing treatment effects by serial colony counts,12–15 or serial measures of TTP,14,16,17 is expected to be a more powerful analysis method for differentiating between regimens or defining exposure–response relationships, and enables a more granular evaluation, since the quantitative information then is utilized rather than discarded.
Bedaquiline, a novel diarylquinoline that inhibits the energy metabolism of Mycobacterium tuberculosis,18,19 was granted conditional approval for treatment of MDR-TB based on TSCC and conversion and cure rates 30 months after the start of treatment. In a placebo-controlled Phase IIb trial, addition of 24 weeks bedaquiline treatment to a background regimen shortened the typical TSCC from 125 to 83 days and increased the rate of conversion at follow-up (month 30) from 44% to 62%.6 Only one bedaquiline dosing regimen has been evaluated in long-term trials and no relationship between bedaquiline plasma concentrations and TSCC or conversion status at week 24 was found in previous analyses comparing the outcome between quartiles of static exposure metrics, e.g. AUC0–24.6,20
In this work, we aimed to investigate whether an exposure–response relationship for bedaquiline could be characterized by making better use of the existing information on pharmacokinetics (PK) and quantitative measures of mycobacterial load. A non-linear mixed-effects pharmacodynamic (PD) model was developed and linked to a previously published PK model for a detailed exposure–response analysis.21 PK-PD models can be used to optimize and/or individualize dosing regimens and to evaluate the clinical importance of PK drug–drug interactions known to occur between bedaquiline and antiretroviral drugs or other anti-TB drugs.22–24
Patients and methods
Study design and patient population
Data were obtained from a Phase IIb study (TMC207-C208, ClinicalTrials.gov number NCT00449644) sponsored by Janssen Pharmaceuticals and shared with the authors through the PreDiCT-TB consortium (http://www.predict-tb.eu). Safety outcomes, TSCC and conversion rates at 24 weeks and 30 months have been presented earlier.6,25 The design of the study was randomized, double-blinded and placebo-controlled, enrolling newly diagnosed pulmonary MDR-TB patients between 18 and 65 years old. The study was performed in two stages where the patients in the first group (stage 1) were randomized to receive 8 weeks of either bedaquiline or placebo, while the second group (stage 2) received the randomized intervention for 24 weeks. All patients were also treated with an optimized background regimen (OBR) of five second-line anti-TB drugs (kanamycin, ofloxacin, ethionamide, pyrazinamide and terizidone; a few predefined substitutions were allowed) for 18–24 months. Bedaquiline was administered orally following the recommended regimen of 400 mg daily during the first 2 weeks and thereafter 200 mg three times per week. The intake was supervised with directly observed therapy according to national guidelines. Subjects with TB-HIV coinfection were excluded if they had a CD4+ count <300 cells/mm3 and/or were receiving ART.
Ethics
The trial was conducted in accordance with Good Clinical Practice standards and received ethics approval from appropriate local authorities. The data were anonymized when shared with the authors.
Microbiological sampling and analysis
Triplicate spot sputum samples were collected the day before the start of treatment, weekly until week 8 and every second week until week 24 after the start of treatment. Ten additional samples were collected over the 96 week follow-up period. Liquid cultures in a mycobacterial growth indicator tube system (MGIT; Becton Dickinson, Sparks, MD, USA) were initiated from each sample and the TTP, i.e. time in hours to a signal indicating presence of M. tuberculosis, was automatically recorded. Samples without a positive signal within 42 days were classified as negative.
PD modelling
The PD model simultaneously included two time-scales: the time after start of treatment (TAST) for patients, and the time after inoculation in the growth tube for each sample. The PD models evaluated consisted of a component describing the mycobacterial load (MBL) in patients over time after the start of treatment linked to a time-to-event component where the positive signal in the MGIT system was defined as an event, either directly as in the model presented by Chigutsa et al.,16 or with a linking probabilistic component describing the risk of bacterial presence in a sputum sample. Parameter values in the sub-models were estimated simultaneously. The decline in MBL in a patient over time after the start of treatment was described by the analytical solutions of either one or two separate compartments, signifying one or more subpopulations with a different half-life for bacterial kill, as described earlier for TB regimens.13,14,16 When included, the probability of bacterial presence was linked to the underlying MBL with either an exponential, Poisson or negative binomial distribution or with an Emax model with or without an estimated slope coefficient. Hazard functions evaluated for the time-to-event component included constant and Weibull models, as well as more complex functions linked to bacterial growth in the MGIT system. The latter included exponential, logistic and Gompertz growth models and models where the growth of the mycobacteria was described with the previously presented multistate TB model.26
Stochastic variability and covariate and PK effects were evaluated on the parameters of the model describing MBL in patients. The considered covariates were: type of drug resistance, presence of lung cavitation, gender, age and ethnicity. Individual estimates of secondary PK metrics were obtained from a previously developed population PK model.21 These included static exposure metrics from different timepoints during the treatment as well as dynamic metrics changing over time, and they were tested with and without adjustment for individual albumin concentrations (expected to impact the protein binding of bedaquiline). For continuous covariates and PK effects the following types of relationships were considered: linear with and without estimated intercept, power, and Emax with and without estimated slope coefficient. For patients missing PK, the metrics were imputed to the median of observed values. A description of the software used is included in the Supplementary data (available at JAC Online).
Model selection and evaluation
The model development was guided by the fit of the model to the observations, quantified by the objective function value (OFV, equal to − 2 log likelihood). Graphical evaluation primarily utilized categorical visual predictive checks (VPCs) of the probability of a positive sample (i.e. bacterial presence) over time after start of treatment and Kaplan–Meier VPCs of TTP stratified on week after start of treatment. Statistical testing of model extensions was conducted with likelihood ratio tests and a significance level of 5% (ΔOFV < −3.84 for 1 degree of freedom). Parameter precision was obtained by the SIR procedure, and a detailed description of the settings used can be found in the Supplementary data.27 Posterior predictive checks of TSCC calculated based on observed and model simulated datasets (n = 100) were performed.28 TSCC was defined the same way as in the primary analysis, namely as the time between the start of treatment and the first of two consecutive sampling occasions with only negative cultures obtained at least 25 days apart.6 The predicted clinical importance of detected covariate effects was assessed through repeated simulations including parameter uncertainty (n = 100) of a large dataset of patients (n = 2000) with a selected set of characteristics and sampled weekly until week 24. The metrics evaluated for each scenario were the time to 50% of patients reaching SCC (median TSCC) and proportion of patients without SCC at week 20.
Results
Demographics and TTP data
TTP data were available for 47 subjects (23 in the bedaquiline arm) from stage 1 and for 159 subjects (79 in the bedaquiline arm) from stage 2. Individual pharmacokinetic data were available for all patients on bedaquiline except four. Demographic characteristics and mean TTP at baseline are summarized in Table 1. Samples collected during the period up to week 8 for the bedaquiline arm of stage 1 or up to week 24 otherwise, and as long as the patient had not dropped out of the study, were considered in the analysis. In total, the dataset included 7385 MGIT evaluations, of which 3900 samples resulted in a positive signal before 42 days. The data are shown in Figure S1. A detailed description of which patients and observations that were excluded from the model development process can be found in the Supplementary data. Baseline TTP values from before initiation of treatment were used as a covariate and not as observations. The dataset used for model building included 5833 TTP observations (56.6% positive) from 189 individuals (98 in the placebo arm and 91 in the bedaquiline arm).
Table 1.
Summary of patient characteristics at start of treatment
| Variable (unit) | Value [median (range) or n (%)] |
|---|---|
| Age (years) | 33 (18–63) |
| Weight (kg) | 54 (35–83) |
| Female sex | 71 (34.5) |
| HIV-positive | 30 (14.6) |
| Presence of lung cavitation | 188 (91.7) |
| Race | |
| Caucasian | 21 (10.2) |
| Black | 82 (39.8) |
| Hispanic | 28 (13.6) |
| Asian | 15 (7.3) |
| other | 59 (28.6) |
| missing | 1 (0.5) |
| TB type | |
| drug susceptible | 8 (3.86) |
| MDR | 96 (46.4) |
| pre-XDR | 52 (25.1) |
| XDR | 11 (5.31) |
| missing | 39 (18.9) |
| Mean TTP MGITa (n = 191) (days) | 6.8 (2.3–42) |
The average of the three TTP observations from MGIT.
PD model
The developed model included three simultaneously fitted components: a longitudinal representation of MBL in patients, a probabilistic component for bacterial presence in sputum samples, and a time-to-event model for TTP. The MBL in patients over TAST was described by a mono-exponential decline as expressed in Equation (1). MBL0 is the estimated number of bacteria per sample inoculum at the start of treatment and was informed by each individual’s observed TTP at baseline (mTTP0; mean of triplicate), normalized to the population median value and with an estimated effect-size (COVTTP). Stochastic inter-individual variability with a Box–Cox transformed distribution29 was included on the half-life (HL) of the decline in MBL. The typical half-life was estimated to be 0.8 weeks; i and p denote individual and population values, respectively.
| (1) |
The probability of bacterial presence (Ppos) given the MBL was described by an Emax model including the maximal risk of bacterial presence (Pmax) and the MBL value corresponding to 50% of Pmax (MBL50) as described by Equation (2). Inter-occasion variability in the sputum sampling procedure was included in the MBL with log-normal distribution (IOVsputum, identifiable thanks to the triplicate sampling).
| (2) |
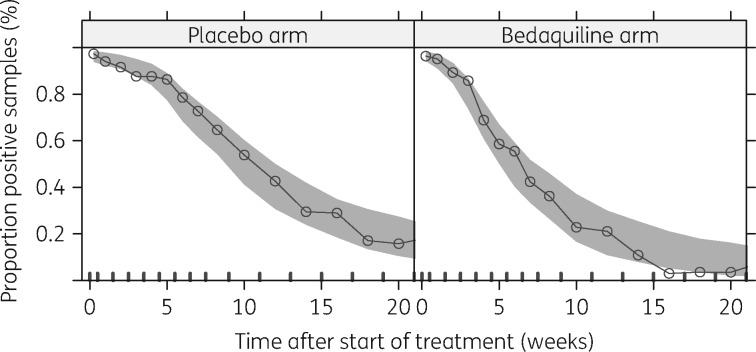
Reflecting the sensitivity of MGIT, Pmax was fixed to the proportion of positive samples observed at baseline before the start of treatment: 96.9%. MBL50 was fixed to 0.5 bacteria/inoculum. The fit of the model to the observed proportion of positive samples over time after the start of treatment is shown in Figure 1.
Figure 1.
VPC (n = 1000) of final logistic model describing the probability of having a positive sample given the estimated underlying mycobacterial load over time on treatment for the placebo and bedaquiline arms.
The hazard function in the time-to-event model describing observed TTP was linked to a model of mycobacterial growth in the MGIT system. The number of bacteria in the tube over time after inoculation [B(t)] was described by logistic growth and an inoculum size corresponding to MBL with the above-mentioned additional inter-occasion variability representing the week-to-week randomness in the sampling. The model was defined by Equation (3) where kg is the growth rate and Bmax is the maximal bacteria-carrying capacity of the system.
| (3) |
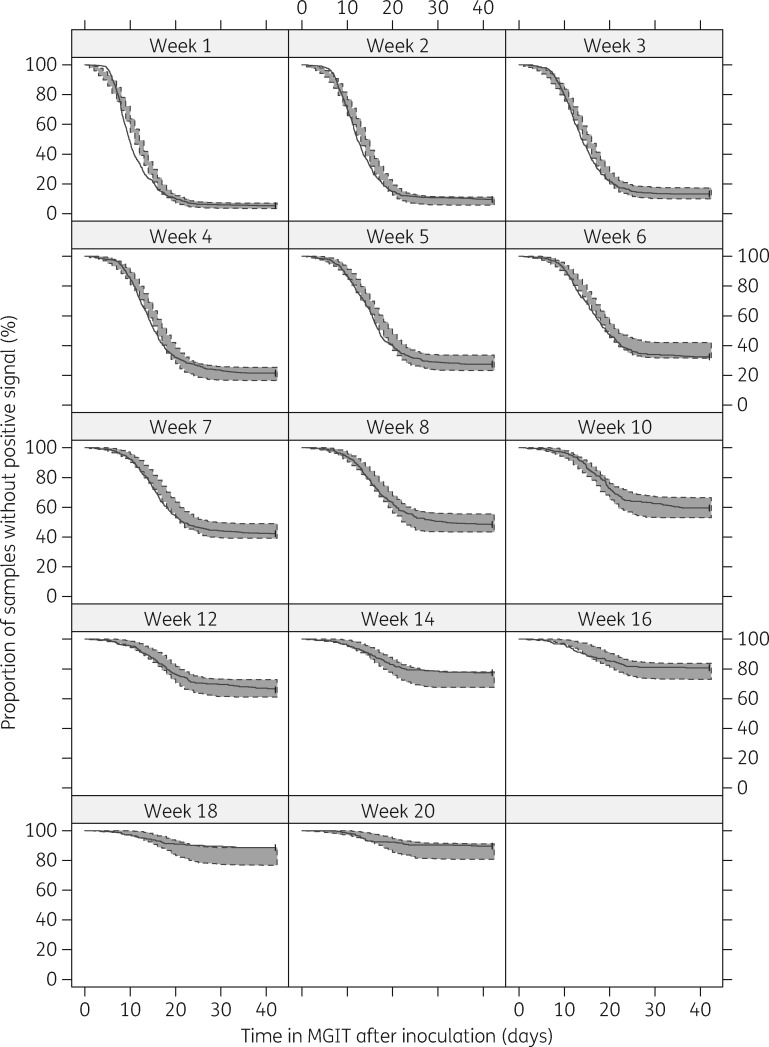
The hazard of a positive signal was directly proportional to the amount of bacteria in the tube, scaled by an estimated factor (). The fit of the final model to the observed TTP per week is shown in Figure 2.
Figure 2.
VPC (n = 100) of time to positivity in MGIT per week on treatment. The solid lines represent the observed time to positivity and the shaded areas the 95% prediction intervals based on model simulations of time to positivity.
Covariate and PK effects
Patients with pre-XDR-TB were typically found to have a 28.1% (95% CI 9.1–51.6) longer half-life of the MBL. Patients with missing information about TB-type (19%) were assigned as MDR-TB. The assumption was tested by estimating a separate effect for the group with missing information, which showed that this group was not significantly different from the patients with MDR-TB. Patients in the bedaquiline arm generally had a shorter half-life of the MBL compared with patients in the placebo arm. The magnitude of the bedaquiline effect was significantly connected to the patients’ exposure levels. Weekly average concentration (which changed dynamically, reflecting the impact of loading dose and the accumulation over the treatment period) was the best predictor of the evaluated metrics. An Emax model with maximum effect on the half-life fixed to –100% was selected to describe the relationship. The estimated EC50 was 1.42 mg/L (95% CI 1.00–2.05), which is higher than the median observed average concentration in the continuation phase of bedaquiline treatment, but falls within the observed range. For a patient with bedaquiline exposure corresponding to the 5th, 50th or 95th percentile of observed exposures at week 2 of treatment, the half-life of bacterial clearance would typically be 32.8% (25.9%–41.0%), 51.6% (43.2%–60.3%) or 64.2% (56.2%–71.9%) shorter than for a typical patient in the placebo arm, respectively. All parameter estimates of the final model and their precision are presented in Table 2; the model code detailing the parameterization is provided in the online Supplementary data.
Table 2.
Parameter estimates of final model including uncertainty
| Sub-model/parameter (unit) | Value | 95% CI |
|---|---|---|
| MBL in patients | ||
| MBL0 (n bacteria/inoculum) | 2.14×103 | 1.39×103–3.46×103 |
| half-life MBL (weeks) | 0.81 | 0.71–0.93 |
| IIV half-life MBL (variance) | 0.33 | 0.25–0.45 |
| Box–Cox transformation IIV half-life MBL | 0.66 | 0.34–1.05 |
| bedaquiline maximal effect on half-life MBL | –1 FIX | — |
| EC50 bedaquiline effect on half-life MBL (mg/L) | 1.42 | 1.00–2.05 |
| (pre-) XDR effect on half-life MBL (%) | 28.1 | 9.1–51.5 |
| baseline TTP effect on MBL0 | –3.69 | −4.15 to − 3.30 |
| IOV sputum sampling MBL (variance) | 3.71 | 3.29–4.38 |
| Probability of bacterial presence | ||
| PMAX bacterial presence | 0.969 FIX | — |
| MBL50 (n bacteria/inoculum) | 0.5 FIX | — |
| Growth in MGIT (hazard) | ||
| kg [1/(day × bacteria)] | 1.38×10−6 | 7.77×10−5–2.24×10−6 |
| Bmax (n bacteria) | 4.76×105 | 2.79×105–8.88×105 |
| scaling of hazard | 9.52×10−5 | 5.08×10−5–1.64×10−6 |
Abbreviations: RSE, relative standard error; MLB, mycobacterial load; IIV, inter-individual variability; IOV, inter-occasion variability; Pmax, maximal probability; MBL50, MBL value corresponding to 50% of Pmax; kg, growth rate in MGIT; Bmax, maximal bacteria carrying capacity in MGIT.
Posterior predictive check: TSCC
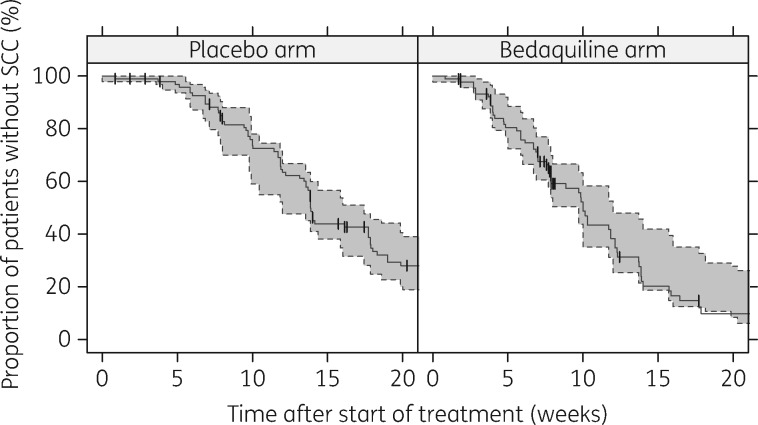
The validity of the model was further confirmed with a posterior predictive check of TSCC per study arm. The 95% prediction interval for TSCC according to the model is compared with the observed TSCC in Figure 3 and demonstrated good agreement.
Figure 3.
Posterior predictive check of time to sputum culture conversion (SCC). The solid lines represent the observed time to SCC and the shaded areas the 95% prediction intervals based on time to SCC calculated from model simulations of time to positivity. The vertical dashes represent censoring events.
Impact of covariates
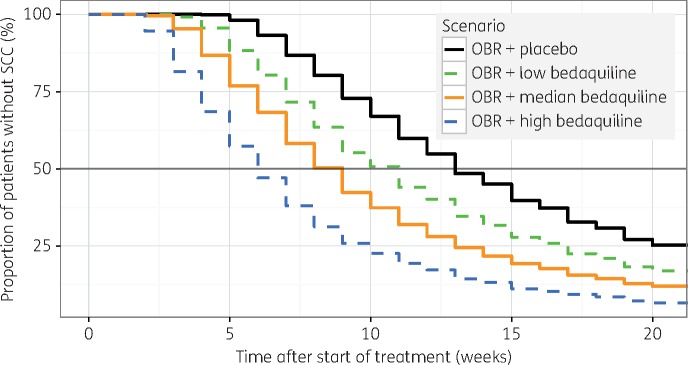
The following scenarios were evaluated for patients with MDR- and (pre-) XDR-TB patients, respectively: placebo and bedaquiline with low, median or high exposure. The low and high exposures evaluated were half and double the median observed weekly average concentration, both falling within the observed range of exposures. The median TSCC and proportion of patients without SCC at week 20 for the different scenarios are listed in Table 3. The confidence intervals primarily reflect parameter uncertainty since the random variability is expected to be small for the simulated study size. Kaplan–Meier TSCC profiles for MDR-TB patients are shown in Figure 4.
Table 3.
Model-predicted median time to sputum culture conversion (TSCC) and proportion without SCC at week 20 for MDR- and pre-XDR- or XDR-TB patients given different bedaquiline treatment scenarios (all scenarios include an optimized background regimen)
| Scenario | Median TSCC [weeks (95% CI)] |
Percentage without SCC at week 20 (95% CI) |
||
|---|---|---|---|---|
| MDR | pre-XDR or XDR | MDR | pre-XDR or XDR | |
| Placebo | 13 (12–15) | 17 (15–19) | 25.2 (20.3–30.9) | 38.9 (28.8–45.1) |
| Low exposurea | 10 (9–11) | 14 (12–15) | 17.1 (12.6–21.1) | 26.3 (19.9–32.9) |
| Median exposureb | 8 (8–9) | 11 (10–13) | 12.3 (8.3–16.3) | 19.3 (14.6–25.1) |
| High exposurec | 6 (5–7) | 8 (7–9) | 6.6 (4.2–11.0) | 11.3 (6.8–16.4) |
Half median bedaquiline weekly average concentration.
Median bedaquiline weekly average concentration.
Double median bedaquiline weekly average concentration.
Figure 4.
Typical impact of bedaquiline exposure on sputum culture conversion (SCC) in MDR-TB patients based on simulations from the final model. OBR, optimized baseline regimen. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
To our knowledge, we present here the first analysis successfully characterizing bedaquiline’s exposure–response relationship in patients with drug-resistant TB. The characterization was made possible by application of an analysis method that was able to utilize quantitative information in microbiological load data from a Phase IIb registration study. Earlier analyses of the same study focused on secondary metrics derived from the culture data, such as the proportion of patients with SCC6 or TSCC.20 These analyses could not identify any relationship with exposure, probably because of the lower statistical power related to the depleted information in such derived metrics. The model presented here analyses the quantitative observations directly, but can be translated to different types of secondary metrics, such as TSCC (Figure 3), or average change in (log) TTP between given days.
Higher bedaquiline exposure was found to have a beneficial effect on treatment response during the first 20 weeks. This is not the same as showing an effect on the clinical outcome variable, namely relapse-free cure. However, for this dataset it has previously been shown that the bedaquiline arm with shorter mean TSCC also achieved higher cure rates at month 30, but without any exposure–response relationship.6 Therefore, we anticipate that faster microbiological response with bedaquiline treatment is correlated to the clinical endpoint of relapse-free cure.
Patients with pre-XDR- or XDR-TB were typically found to have slower clearance of bacteria compared with patients with MDR-TB, resulting in 2–4 weeks longer median TSCC and a notably lower conversion rate at week 20 (Table 3). The baseline bacterial load was found to be strongly influential: the estimated effect predicted a four times longer median TSCC in patients with the lowest observed mTTP0 compared with those with the highest. Degree of cavitation and HIV infection, factors known to influence the outcome of TB treatment,7,30,31 were not significant factors in this analysis, perhaps due to the low numbers of patients without cavitating disease or with HIV infection. Addition of bedaquiline clearly accelerated bacterial clearance and the magnitude of the effect was exposure driven. The best exposure metric was dynamic, but several static PK metrics early during treatment (e.g. AUC0–24,day14 and Cmin,day14) were also significantly correlated to the response. Predicted steady-state exposure could not be linked to efficacy, possibly due to correlations between time-varying covariates, such as body weight and albumin concentrations,21 affecting PK and the treatment response. Adjustment of the PK metrics with individual albumin levels—an attempt to mimic unbound concentration which is expected to correlate better with the pharmacological effect—did not improve the fit. This could imply either that in this case albumin concentrations are a poor marker for protein binding, that unbound plasma concentration is not a better predictor of effect than total concentration, or that albumin levels are influenced by disease progression, which obscures the relation. The simulations performed to investigate different exposure levels (Figure 4, Table 3) suggest that an increased bedaquiline dose should be considered, if the safety of higher exposures can be confirmed.
The foundation of the developed model is similar to the model presented by Chigutsa et al.:16 an underlying and unobserved dynamic variable describing the bacterial load in TB patients which drives a time-to-event model of TTP observations from MGIT. We found that MBL could be represented by a mono-exponential decline when individual baseline TTP informed the starting point, and inter-individual variability in the half-life was included. A model with bi-exponential decline is expected to perform better for other datasets with more frequent sampling during the first treatment weeks, but was not supported in this dataset. The estimated number of bacteria/inoculum at the start of treatment was of the magnitude of 103 which is somewhat low compared with the values commonly seen with other quantification methods, e.g. cfu. This may partly be because an initial fast decline in MBL, commonly observed in early bactericidal activity studies,13,15 went unnoticed due to the lack of samples during the first treatment week. There are several novel aspects of our model, such as the probability component describing the risk of bacterial presence in a sample. The probability model is mechanistically justified as a means to handle the fact that the number of bacteria in the sputum sample can be zero, while an exponentially declining function could never take on that value. The model handles the increasing portion of negative samples over time on treatment and contributes to the description of the characteristic shape of the TTP Kaplan–Meier curves reaching a plateau after about 25 days in the MGIT system (see Figure 2). Note that even a single viable bacterium in the inoculum is expected to give rise to a positive MGIT signal around this time. With the probability model in place, it was possible to have the inoculum size as the driver of TTP without incorporating large and hard-to-justify changes in the bacterial growth processes in the MGIT system over time on treatment as previously needed.16 The logistic bacterial growth model used is parsimonious and probably does not reflect all the processes in the MGIT system, but use of a multistate TB model requires multiple assumptions to enable separation of bacterial sub-states and did not improve the fit to data in this case.26 The estimated growth rate and Bmax in the final model corresponds to an initial doubling time of 1.1 days in the MGIT system, which agrees well with observed growth rates for M. tuberculosis in liquid in vitro cultures (doubling time of 0.67–2.2 days).32,33 Further discussion regarding the interpretation of model parameters and a figure of the probability of a positive signal in MGIT over time is included in the online supplementary information (Figure S2).
There are several limitations to the model presented here. The maximal effect of bedaquiline could not be estimated due to the limited range of observed exposures. Extrapolation of the effect far outside the range of exposures included in the estimation of the relationship will be uncertain; this uncertainty may limit accuracy when using the model to simulate novel regimens with markedly higher bedaquiline doses. It is also uncertain how well the model will be able to describe the effect of varying durations of bedaquiline treatment. The model does not account for any inter-individual variability in bedaquiline effectiveness caused by variability in susceptibility between bacterial strains since MIC measurements were not available. Lastly, the present analysis characterizes the response during treatment, but cannot describe recurrence of disease.
In conclusion, a model with three linked components, namely (i) a longitudinal representation of mycobacterial load in patients, (ii) a model describing the probability of bacterial presence in a sputum sample, and (iii) a time-to-event model describing TTP in MGIT, was developed and fitted the observations well. In contrast to simpler analyses of secondary metrics calculated from the same data, this model could detect and describe an exposure–response relationship for bedaquiline, a property that is crucial for evaluation of dosing regimens and assessment of drug–drug interactions. The model developed here could be used for analysis of other Phase II and III studies of novel anti-TB regimens and provides a more powerful tool for characterization of exposure–response relationships than commonly used derived metrics.
Supplementary Material
Acknowledgements
We thank the patients, investigators and site staff participating in the study, Anne-Gaëlle Dosne for her generous assistance with the SIR methodology and Thomas Dorlo for constructively reviewing this manuscript. An earlier version of the model was described in E. M. Svensson’s PhD thesis defended at Uppsala University May 2016, and presented at the 25th PAGE conference in Lisbon, June 2016 (abstract number 5937). Simulations describing the impact of pharmacokinetic drug–drug interactions using the developed model were presented at the 47th Union World Conference on Lung Health in Liverpool, October 2016 (abstract number PD-1069–29), and at the 9th International Workshop on Clinical Pharmacology of TB drugs (abstract number O_1) in Liverpool, October 2016.
Funding
This work was supported by the Swedish Research Council (grant number 521-2011-3442) and the Innovative Medicines Initiative Joint Undertaking (www.imi.europa.eu) for the PreDiCT-TB consortium (grant agreement 115337), resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in-kind contribution.
Transparency declarations
E. M. S. has no conflict of interest to declare. M. O. K. has received research grants from Janssen Pharmaceuticals for unrelated projects.
Author contributions
Both authors designed the research and interpreted the results, E. M. S. performed the model-based analysis and wrote the manuscript, and M. O. K. reviewed and edited the manuscript.
Supplementary data
Figures S1 and S2, plus information on software, model development, etc., are available as Supplementary data at JAC Online.
References
- 1. World Health Organization. Global Tuberculosis Report 2016 WHO/HTM/TB/2016.13. Geneva, Switzerland: WHO, 2016.
- 2. World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant TB—2011 Update WHO/HTM/TB/2011.6. Geneva, Switzerland: WHO, 2011. [PubMed]
- 3. Laserson KF, Thorpe LE, Leimane V. et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9: 640–5. [PubMed] [Google Scholar]
- 4. Perrin FMR, Lipman MCI, McHugh TD. et al. Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis 2007; 7: 481–90. [DOI] [PubMed] [Google Scholar]
- 5. Wallis RS, Doherty TM, Onyebujoh P. et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 2009; 9: 162–72. [DOI] [PubMed] [Google Scholar]
- 6. Diacon AH, Pym A, Grobusch MP. et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014; 371: 723–32. [DOI] [PubMed] [Google Scholar]
- 7. Holtz TH, Sternberg M, Kammerer S. et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med 2006; 144: 650–9. [DOI] [PubMed] [Google Scholar]
- 8. Gillespie SH, Crook AM, McHugh TD. et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014; 371: 1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abe C, Hosojima S, Fukasawa Y. et al. Comparison of MB-Check, BACTEC, and egg-based media for recovery of mycobacteria. J Clin Microbiol 1992; 30: 878–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pheiffer C, Carroll NM, Beyers N. et al. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis 2008; 12: 792–8. [PubMed] [Google Scholar]
- 11. Chihota VN, Grant AD, Fielding K. et al. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis 2010; 14: 1024–31. [PubMed] [Google Scholar]
- 12. Gillespie SH, Charalambous BM.. A novel method for evaluating the antimicrobial activity of tuberculosis treatment regimens. Int J Tuberc Lung Dis 2003; 7: 684–9. [PubMed] [Google Scholar]
- 13. Davies GR, Brindle R, Khoo SH. et al. Use of nonlinear mixed-effects analysis for improved precision of early pharmacodynamic measures in tuberculosis treatment. Antimicrob Agents Chemother 2006; 50: 3154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sloan DJ, Mwandumba HC, Garton NJ. et al. Pharmacodynamic modeling of bacillary elimination rates and detection of bacterial lipid bodies in sputum to predict and understand outcomes in treatment of pulmonary tuberculosis. Clin Infect Dis 2015; 61: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burger DA, Schall R.. A Bayesian nonlinear mixed-effects regression model for the characterization of early bactericidal activity of tuberculosis drugs. J Biopharm Stat 2015; 25: 1247–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chigutsa E, Patel K, Denti P. et al. A time-to-event pharmacodynamic model describing treatment response in patients with pulmonary tuberculosis using days to positivity in automated liquid mycobacterial culture. Antimicrob Agents Chemother 2013; 57: 789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Epstein MD, Schluger NW, Davidow AL. et al. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest 1998; 113: 379–86. [DOI] [PubMed] [Google Scholar]
- 18. Andries K, Verhasselt P, Guillemont J. et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005; 307: 223–7. [DOI] [PubMed] [Google Scholar]
- 19. Koul A, Dendouga N, Vergauwen K. et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol 2007; 3: 323–4. [DOI] [PubMed] [Google Scholar]
- 20. FDA. Center for Drug Evaluation and Research. Application number 204384Orig1s000, Clinical Pharmacology and Biopharmaceutics review(s). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/204384Orig?>1s000ClinPharmR.pdf.
- 21. Svensson EM, Dosne A-G, Karlsson MO.. Population pharmacokinetics of bedaquiline and metabolite M2 in drug-resistant tuberculosis patients—the effect of time-varying weight and albumin. CPT Pharmacometrics Syst Pharmacol 2016; 5: 682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Svensson EM, Aweeka F, Park J-G. et al. Model-based estimates of the effects of efavirenz on bedaquiline pharmacokinetics and suggested dose adjustments for patients coinfected with HIV and tuberculosis. Antimicrob Agents Chemother 2013; 57: 2780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Svensson EM, Dooley KE, Karlsson MO.. Impact of lopinavir-ritonavir or nevirapine on bedaquiline exposures and potential implications for patients with tuberculosis-HIV coinfection. Antimicrob Agents Chemother 2014; 58: 6406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Svensson EM, Murray S, Karlsson MO. et al. Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug. J Antimicrob Chemother 2015; 70: 1106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diacon AH, Donald PR, Pym A. et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 2012; 56: 3271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clewe O, Aulin L, Hu Y. et al. A multistate tuberculosis pharmacometric model: a framework for studying anti-tubercular drug effects in vitro. J Antimicrob Chemother 2015; 74: 964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dosne A-G, Bergstrand M, Harling K. et al. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn 2016; 43: 583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yano Y, Beal SL, Sheiner LB.. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 2001; 28: 171–92. [DOI] [PubMed] [Google Scholar]
- 29. Petersson KJF, Hanze E, Savic RM. et al. Semiparametric distributions with estimated shape parameters. Pharm Res 2009; 26: 2174–85. [DOI] [PubMed] [Google Scholar]
- 30. Kliiman K, Altraja A.. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. Eur Respir J 2009; 33: 1085–94. [DOI] [PubMed] [Google Scholar]
- 31. Ahmad N, Javaid A, Basit A. et al. Management and treatment outcomes of MDR-TB: results from a setting with high rates of drug resistance. Int J Tuberc Lung Dis 2015; 19: 1109–14, i–ii. [DOI] [PubMed] [Google Scholar]
- 32. Gill WP, Harik NS, Whiddon MR. et al. A replication clock for Mycobacterium tuberculosis. Nat Med 2009; 15: 211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beste DJV, Espasa M, Bonde B. et al. The genetic requirements for fast and slow growth in mycobacteria. PloS One 2009; 4: e5349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.