Abstract
The mutant prevention concentration (MPC) is a well-known concept in the chemotherapy of many bacterial infections, but is seldom considered in relation to tuberculosis (TB) treatment, as the required concentrations are generally viewed as unachievable without undue toxicity. Early studies revealed single mutations conferring high MICs of first- and second-line anti-TB agents; however, the growing application of genomics and quantitative drug susceptibility testing in TB suggests a wide range of MICs often determined by specific mutations and strain type. In paediatric TB, pharmacokinetic studies indicate that despite increasing dose recommendations, a proportion of children still do not achieve adult-derived targets. When considering the next stage in anti-TB drug dosing and the introduction of novel therapies for children, we suggest consideration of MPC and its incorporation into pharmacokinetic studies to more accurately determine appropriate concentration targets in children, to restrict the growth of resistant mutants and better manage drug-resistant TB.
Introduction
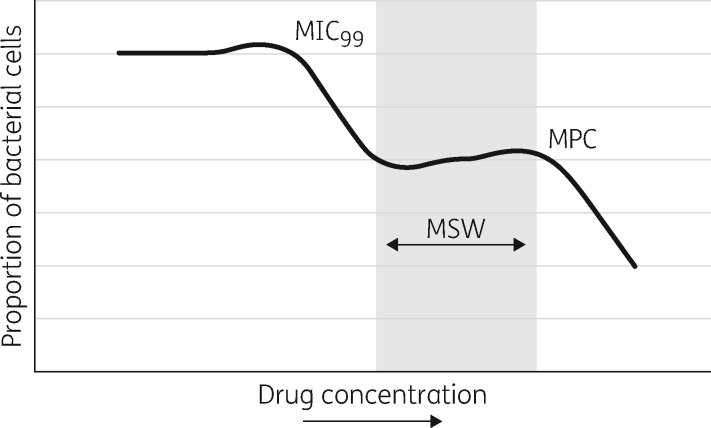
The mutant prevention concentration (MPC) is the minimum concentration restricting the growth of the least susceptible, single-step mutant of a bacterial isolate.1 The mutant selection window (MSW) is the drug concentration between the MIC and MPC, and failure to exceed drug concentrations above the MPC has the potential to allow bacteria with resistance-associated mutations to grow and accumulate further mutations (Figure 1).1,2
Figure 1.
Mutant selection window (MSW) hypothesis. As the concentration of the antibiotic increases, there is an initial drop in bacteria related to the inhibition of growth of 99% of susceptible cells (MIC99). This, however, then leads to a plateau as bacteria with resistance-conferring mutations are selected to grow. The second drop corresponds to the inhibition of resistant growth, termed the mutant prevention concentration (MPC). Adapted from Figure 1 in Drlica and Zhao, 2007.1
This concept is well recognized in relation to other bacterial infections,3–5 but is seldom considered in TB. Although multi-drug therapy is the standard for TB management, variation in pharmacokinetics between drugs can result in periods of relative monotherapy.6 This has been demonstrated during intermittent therapy with isoniazid and a rifamycin, where isoniazid (which has a shorter half-life) failed to protect the accompanying rifamycin, which has a longer half-life.7,8 The penetration of rifampicin into caseous tissue is also not as good as that of isoniazid and this may lead to localized periods of inadvertent monotherapy especially during the continuation phase of therapy.9 Given the rise of MDR-TB (resistance to at least isoniazid and rifampicin), a re-evaluation of MPC in anti-TB drugs is needed to optimize drug dosing and potentially restrict the growth of mutants.
In paediatric TB, the optimal dosing of first- and second-line anti-TB agents in children is still unclear, and is dependent on adult-derived targets. Greater clarity is urgently needed in this respect, given that 2 million children under 5 years old could be infected with MDR-TB; global figures for 2014 indicate that 58 300 children had isoniazid-resistant TB, 24 800 had MDR-TB and 1160 had XDR-TB (MDR plus resistance to fluoroquinolones and a second-line injectable agent).10,11
As dosing for paediatric TB is being re-evaluated in the setting of rising antimicrobial resistance, an opportunity exists to incorporate the MPC in pharmacokinetic studies. Current drug-dosing strategies seek to at least exceed the MIC, which is the lowest drug concentration that will inhibit growth of susceptible strains, but in some instances this is limited by the concentration at which toxicity might emerge (the therapeutic index). However, it is now understood that the MICs of both first- and second-line anti-TB agents are seldom homogeneous, but exist over a range of concentrations, determined in part by the relevant mutation.12 For some drugs, the MIC may be sufficiently low to allow for higher doses above the MPC without undue toxicity. In this paper, we review past studies on the MPC in TB, the range of MICs for mutations associated with resistance in first- and second-line anti-TB drugs, and discuss the possibility of dose adaptation in children.
Methods
We conducted a literature search on Pubmed, EMBASE and Google Scholar. To understand prior work on MPC in TB, we used the following search terms: ‘mutant prevention concentration’, ‘mutant selection window’ and ‘mutant selection window AND TB’. We also paired ‘mutant prevention concentration’ and ‘mutant selection window’ with all first- and second-line anti-TB drugs based on WHO groups 1–5 (as this classification was then used) that are currently approved in children: isoniazid, rifampicin, pyrazinamide, ethambutol, rifabutin, streptomycin, kanamycin, amikacin, capreomycin, fluoroquinolones including levofloxacin, moxifloxacin, and gatifloxacin, para-aminosalicylic acid (PAS), cycloserine, terizidone, ethionamide, prothionamide, clofazimine, linezolid, amoxicillin/clavulanic acid, thiacetazone, carbapenems and clarithromycin. We included original research and review articles based on the search or bibliography that were English language, and specifically discussed in vitro or in vivo work on MPC and Mycobacterium tuberculosis. We excluded results that did not investigate M. tuberculosis, or results related to drugs currently in development or not yet approved in children, including delamanid and bedaquiline. For the second aim of exploring the range of MICs by mutation, we used the following search terms, with and without the above antituberculosis agents: ‘breakpoint tuberculosis’, ‘critical concentration tuberculosis’, ‘pharmacogenomics tuberculosis’, ‘quantitative drug susceptibility testing tuberculosis’, ‘tuberculosis strain mutation resistance’, ‘whole genome sequencing mutation tuberculosis’ and ‘whole genome sequencing resistance tuberculosis’. Original research and review articles in English from the search or bibliography were considered if they included data on MICs of anti-TB drugs stratified by mutation, or discussed the use of quantitative drug susceptibility and whole genome sequencing (WGS) in understanding resistance in M. tuberculosis. For the final aim regarding dosing of antituberculosis agents in children, we included the above list of drugs with the following terms: ‘child AND pharmacokinetics’; and ‘tuberculosis AND pharmacokinetics’. We included English language original research and review articles from the search or bibliography that presented results on the pharmacokinetics of these drugs in children used for active TB disease. We excluded pharmacokinetic data in adults, and did not include studies that focused on latent TB infection management or isoniazid preventative therapy. For Table 1, we included only pharmacokinetic data based on current recommended dosing in children.13 While there are multiple pharmacokinetic parameters, Cmax has been presented because of its role in therapeutic drug monitoring in TB, its predominant use in the paediatric TB literature to determine if dosing is appropriate based on adult-derived targets, and to allow comparison with the MIC and MPC.
Table 1.
Summary of maximum concentrations and mutant prevention concentrations (MPC) among children with TB receiving WHO-recommended dosing
| WHO group15 | Drug | Current WHO dosing recommendationa daily mg/kg (range), max13,62 | Mean age range (years) |
Cmax (mg/L) |
||||
|---|---|---|---|---|---|---|---|---|
| mean range | references | target54 | Critical concentrationb (mg/L)62,63 | MPC, mg/L (references) | ||||
| First-line drugs | ||||||||
| isoniazid | 10 (7 –15), max 300 mg | 0.28 –15 | 2.5 –11.2 | 40–43, 64–69 | 3.0 –6.0 | 0.1 | 2.4, 20 (14, 19) | |
| rifampicin | 15 (10 –20); max 600 mg | 0.6 –8.9 | 2.9 –11.7 | 40, 41, 68, 69 | 8.0 –24 | 1.0 | 2.0, >80 (14, 19) | |
| pyrazinamide | 35 (30 –40) | 1.1 –14 | 22.5 –49.4 | 40–43, 65, 66, 69–73 | 20 –60 | 100 | ||
| ethambutol | 15 (15 –25) | 0.2 –17 | 0.78 –6.6 | 41, 42, 68, 69, 72, 74–76 | 2.0 –6.0 | 5.0 | ||
| rifabutin | 5 –10 | 2.3 | 0.39 | 77 | 0.45 –0.90 | 0.5 | 0.4 (19) | |
| Second-line drugs | ||||||||
| A. Fluoroquinolones | ||||||||
| levofloxacin | 7.5 –10, max 750 mg | 3.1 | 6.8 | 44 | 8.0 –13 | 1.5 | 1.8 (20) | |
| moxifloxacin | 7.5 –10, max 400 mg | 11.1 | 3.1 | 45 | 3.0 –5.0 | 2.0 | 0.9 –8 (14, 16, 20) | |
| gatifloxacin | 1.0 Middlebrook 7H10 | 1.0, 1.5 (14, 20) | ||||||
| B. Second-line injectable agents | ||||||||
| amikacin | 15 –22.5, max 1000 mg | 35 –45 | 1.0 | |||||
| capreomycin | 15 –30, max 1000 mg | 35 –45 | 2.5 | 160 (14) | ||||
| kanamycin | 15 –30, max 1000 mg | 25 –35 | 2.5 | >800 (14) | ||||
| streptomycin | 20 –40, max 1000 mg | 1.0 | >320 (14) | |||||
| C. Other core second-line agents | ||||||||
| ethionamide | 15 –20 in two doses, max 1000 mg | 0.25 –12 | 1.4 –4.4 | 46, 78 | 2.0 –5.0 | 5.0 | ||
| prothionamide | 15 –20 in two doses, max 1000 mg | 2.5 | ||||||
| cycloserine | 10 –20 in 1 or 2 doses, max 1000 mg | 20 –35 | 30 (Lowenstein-Jensen) | 70 (14) | ||||
| terizidone | 10 –20 in 1 or 2 doses, max 1000 mg | |||||||
| linezolid | 10 –12 twice daily, max 300 mg | 12 –26 | 1.0 | 1.2 (14) | ||||
| D. Add-on agents | ||||||||
| high-dose INH | 15 –20, max 400 mg | |||||||
| meropenem | 20 –40 every 8 h, max 2 g/dose | |||||||
| amoxicillin/ clavulanate | 80 in two doses, max 3000 mg | |||||||
| PAS | 150 in 2 or 3 doses, max 12 000 mg | 1.0 –12 | 56.5 (granular slow release) | 79 | 20 –60 | 4.0 | ||
| thiacetazone | 3 –4, max 150 mg | |||||||
Past studies on MPC in Mycobacterium tuberculosis
Early studies on M. tuberculosis concluded that drug concentrations above the MPC were not possible for the first-line and several second-line antituberculosis agents. In 2000, Dong et al.14 presented the MPC of several first- and second-line drugs (Table 1). They compared the peak concentration (Cmax) of first-line options rifampicin and isoniazid, and second-line drugs including streptomycin, capreomycin, kanamycin, and cycloserine to the determined MIC and MPC. The authors found that the MPC ranged from 2 to >38 times greater than the Cmax, and concluded that these concentrations could not be reached without considerable toxicity.
The one exception was the fluoroquinolones, a core drug class in MDR-TB regimens in adults and children.15 Dong and colleagues examined the growth curves of 14 clinical M. tuberculosis isolates from three different genetic strains exposed to increasing concentrations of various fluoroquinolones.14 They found a more achievable MPC, ranging from 1.0–4.0 mg/L, compared with a Cmax of 4.4 mg/L with 750 mg of ciprofloxacin. They further found that newer fluoroquinolones that added a methoxy group to C8, such as moxifloxacin, were associated with an even lower MPC, narrower MSW and left shift of the growth inhibition curve. For moxifloxacin, the MPC was estimated at 2.5 mg/L, corresponding to 0.55 times the Cmax achieved in adults with a 400 mg daily dose. This suggested that fluoroquinolones might be a useful antibiotic to combine with other antituberculosis drugs to exceed levels above the MPC and restrict mutant growth. This was further supported in an in vivo TB mouse model, which showed that maintaining moxifloxacin serum concentrations above the MPC effectively prevented the amplification of mutations.16 However, this group determined the MPC to be 8.0 mg/L, compared with the Cmax of 2.2 mg/L they found with an equivalent 400 mg dose.16 A hollow fibre model compared 400, 600 and 800 mg doses of moxifloxacin and then, with mathematical modelling, concluded that 800 mg was associated with 93% likelihood of suppressing resistance.17 Of note, this is twice the current recommended daily maximum dose in children and adults, but an 800 mg dose has been considered safe in adults and has been recommended by several groups.18
There have been few efforts to further examine MPC in TB. One study examined the MPC of isoniazid, rifampicin and rifabutin among 224 clinical isolates in Spain over an 11 year period (Table 1).19 In contrast to the findings of Dong et al.,14 the authors found that 90% of the strains had an MPC below 2.4 mg/L for isoniazid, 0.4 mg/L for rifabutin, and 2.2 mg/L for rifampicin, as compared with 20 mg/L for isoniazid and >80 mg/L for rifampicin reported by Dong et al.14 The same group found the MPC of fluoroquinolones and linezolid to be 1.0–2.0 mg/L and 1.2 mg/L, respectively, and generally similar to the findings of Dong et al.,14 except for ciprofloxacin, which was 8.0 mg/L.20 These findings, coupled with the discussion below on variance in MIC, raise questions regarding the possible heterogeneity of MPC in TB.
Variation in MIC of antituberculosis drugs
In determining the MPC of first- and second-line drugs against M. tuberculosis, a single strain was primarily used, and MPC was not generally stratified by mutation.14 However, for fluoroquinolones, it was shown that increasing concentrations selected for different mutations in gyrA or gyrB in M. tuberculosis.21 This suggested that the MPC could be lower depending on the mutation. The increasing use of genomics in TB has revealed a wide variety of mutations that are associated with resistance to first- and second-line agents (Table 2). For example, WGS studies in South Africa and Pakistan have been able to identify resistance-conferring mutations not normally seen by traditional assays, as well as compensatory mutations that help to maintain fitness.22–24
Table 2.
Common resistance mutations in M. tuberculosis and associated MIC range
| WHO group15 | Antibiotic | Mutation | Range of MICs (mg/L) | References |
|---|---|---|---|---|
| First-line drugs | ||||
| isoniazid | katG | 1 –125 | 55, 80 | |
| inhA | ≥0.1 –8.0 | 81, 82 | ||
| rifampicin | rpoB | 0.5 –≥160 | 81, 83, 84 | |
| L511P | 0.125 –0.5 | 27 | ||
| pyrazinamide | pncA | 12.5 –>1024 | 83, 85, 86 | |
| ethambutol | embC | 16 –32 | 28 | |
| embB | ≥2.5 –≤50 | 87 | ||
| rifabutin | rpoB | <0.25 –≥5 | 84 | |
| Second-line drugs | ||||
| A. Fluoroquinolones | gyrA | |||
| levofloxacin | Ala90Val (A90V) | 0.25 –8.0 | 88–90 | |
| Ser91Pro (S91P) | 1.5 –3.0 | |||
| Asp94Ala (D94A) | 1.5 –6.0 | |||
| Asp94Gly (D94G) | 2.0 –16 | |||
| Asp94Asn/Tyr (D94N) | 3.0 –12 | |||
| Asp94His (D94H) | 3.0 –6.0 | |||
| moxifloxacin | Ala90Val (A90V) | 0.12 –8.0 | 89, 91–93 | |
| Ser91Pro (S91P) | 1.0 –4.0 | |||
| Asp94Ala (D94A) | 0.25 –>8.0 | |||
| Asp94Gly (D94G) | 0.12 –8.0 | |||
| Asp94Asn/Tyr (D94N) | 0.5 –>8 | |||
| Asp94His (D94H) | 0.25 –4 | |||
| gatifloxacin | Ala90Val (A90V) | ≤0.125 –2.0 | 89, 93, 94 | |
| Ser91Pro (S91P) | 0.25 –0.5 | |||
| Asp94Ala (D94A) | 0.25 –1.0 | |||
| Asp94Gly (D94G) | 0.25 –4.0 | |||
| Asp94Asn/Tyr (D94N) | 0.5 –4.0 | |||
| Asp94His (D94H) | 0.25 –2.0 | |||
| B. Second-line injectable agents | ||||
| amikacin | rrs | 95, 96 | ||
| A1401G | >120 | |||
| G1484T | 16 –80 | |||
| C1402T | 2.0 –4.0 | |||
| thyA | ≤4.0 | 97 | ||
| eis | 0.5 –<4.0 | 98 | ||
| capreomycin | rrs | 96, 97 | ||
| G1484T | 160 –>320 | |||
| C1402T | 80 –>160 | |||
| A1401G | 20 –>160 | |||
| thyA | 20 –160 | |||
| kanamycin | rrs | 96, 97 | ||
| A1401G | >160 | |||
| G1484T | 80 –160 | |||
| C1402T | 10 –20 | |||
| eis | 5.0 –80 | 98 | ||
| thyA | ≤5.0 –40 | |||
| streptomycin | rpsL | 0.5 –>1000 | 81, 83, 99 | |
| rrs | 12.5 –50 | |||
| gidB | 0.5 –16 | 100 | ||
| C. Other core second-line agents | ||||
| ethionamide | ethA | 50 –>200 | 82 | |
| inhA | ≥1.25 –≥200 | 81, 82 | ||
| PAS | thyA | <32 –>128 | 101 | |
| folC | 0.125 –8.0 | 102 | ||
At the same time, there is a growing understanding that the presence of a mutation in M. tuberculosis does not always indicate a high MIC. Rather, for a given drug, there can be a wide range of MICs depending on the mutation conferring resistance (Table 2), strain type, accumulation of further mutations and use of concomitant drugs. For example, the presence of the rpoB gene is associated with rifampicin resistance, but a WGS study in Haiti found rare mutations within the Rifampicin Resistance-Determining Region (RRDR) that confer low MICs.25 In an in vitro study of M. tuberculosis isolates with mutations associated with isoniazid resistance, the MIC varied by lineage.26 In addition to the fluoroquinolone class, stepwise escalation of MIC with accumulation of mutations has been found with both rifampicin and ethambutol.27,28 The synergistic effects of multi-drug therapy can also impact MIC. The addition of clarithromycin and its metabolite 14-hydroxyclarithromycin was associated with a reduction in the MIC of first-line agents isoniazid, rifampicin and ethambutol by 4- to 32-fold.29 Thus, while identifying a mutation is important, there is a greater appreciation of the complex relationships that influence phenotypic resistance.
Quantitative drug susceptibility testing (DST) in TB is an example of how understanding variation in MIC can aid management decisions. The Sensititre MYCOTB MIC plate (Trek Diagnostic Systems, Cleveland, OH, USA) determines first- and second-line drug MICs, and creates a borderline category between 1 dilution less and 2 dilutions greater than the critical concentration.30,31 A 2 year implementation of this system in Bangladesh found a considerable proportion of borderline isolates, and the authors suggest this can help providers to determine whether they can give a higher dose to exceed the MIC.32 Additionally, the MGIT960™ (Mycobacteria Growth Indicator Tube, Becton Dickinson, Franklin Lakes, NJ, USA) platform with the EpiCentre™ TBeXiST (Extended Individual Susceptibility Testing, Becton Dickinson, Franklin Lakes, NJ, USA) module, first screens for resistance based on the epidemiological cut-off (ECOFF), and then exposes resistant isolates to higher concentrations to ultimately categorize them as low, intermediate or high.33 Cambau et al.34 applied this to first- and second-line TB drugs, and found that while rifampicin, rifabutin and amikacin generally were in the high group, they noted a wide range among isoniazid, fluoroquinolones, streptomycin, capreomycin, PAS and ethionamide.
The heterogeneity of MIC may suggest a range of MPC depending on the mutation that one seeks to restrict. The concept that the presence of an M. tuberculosis mutation ‘reduces susceptibility so much that no tolerable concentration of drug can block mutant growth’ is now unclear.35 At the same time, changes in MIC have been shown to correlate poorly with MPC.36 Consequently, MPC needs to be stratified by mutation and strain in order to understand if there are particular mutant subpopulations that can be feasibly restricted at higher dose concentrations.
Challenges of increasing the dosage of anti-TB drugs in children
Anti-TB drug dosing in children can be difficult, as growth and development influence absorption (changes in motility, gastric acid secretion), distribution (variability in body composition, protein binding), metabolism (liver size relative to body, maturation of hepatic enzymes) and excretion (changes in renal clearance).37,38 Yet, there is a significant need to determine appropriate dosing, and MPC could serve as an important additional consideration to restrict the growth of mutant subpopulations. The heterogeneity of MIC in TB suggests that higher drug concentrations may be able to exceed the MPC depending on the mutation. Table 1 outlines the range of maximum concentrations found in children receiving currently recommended standard dosing of anti-TB drugs. In comparison to Table 2, every drug with available data can potentially achieve a Cmax greater than a mutant’s MIC to suppress growth, depending on the mutation. However, there are two key challenges to increasing the doses of anti-TB agents in children to exceed the MPC. First, there are no clear pharmacokinetic targets in children with TB, and efforts to achieve concentrations based on various adult-derived goals have been difficult.39 Second, more safety data are needed to determine whether an increase in dose will be tolerated. This highlights the need for more pharmacokinetic studies in children with TB in order to correlate outcomes and adverse effects, and the incorporation of the MPC may guide goals for therapeutic doses that also suppress mutant growth.
Pharmacokinetics goals of anti-TB agents in paediatric TB
The revised WHO dosing guidelines for TB in children recommend increased doses of isoniazid, rifampicin, pyrazinamide and ethambutol, following a number of studies demonstrating that the previously recommended doses were too low to achieve target Cmax based on standard adult dosing.13,40 However, these targets are derived from adult trials, and several updated paediatric pharmacokinetic studies suggest that children are still unable to reach some of these goals and raise questions about what the target should be (Table 1). For example, among 39 infants in South Africa, none met concentration targets for rifampicin and only 6% met target concentrations for ethambutol at the currently recommended WHO doses according to weight bands. It should be noted that the liquid formulation of rifampicin was changed mid-study; although the new formulation was given at a higher mg/kg dose, it had a lower Cmax, raising concerns regarding its bioavailability.41 Beyond infancy, of 31 children in KwaZulu-Natal, South Africa, many of whom received the new WHO-recommended doses, only 6% given rifampicin, 65% given isoniazid, 55% given pyrazinamide and 15% given ethambutol reached the target Cmax.42 Among HIV-positive children in India who received intermittent dosing based on weight bands, 97% did not achieve target Cmax when treated with rifampicin, 28% with isoniazid and 33% with pyrazinamide.43
As seen in Table 1, second-line anti-TB drug pharmacokinetic studies are lacking in children, but also suggest that current doses may lead to Cmax below adult-derived targets. A pharmacokinetic study in South Africa among children under 8 years old found that the median Cmax of levofloxacin was 6.79 mg/L, below the target of 8–13 mg/L.44 Moxifloxacin at 10 mg/kg was evaluated among children 7–15 years, and median Cmax was less than the Cmax found in adult studies with 400 mg dosing, with a lower trend if HIV-positive.45 A pharmacokinetic study of ethionamide use among 31 children aged 0–2, 2–5 and 6–12 years showed that standard dosing of 15–20 mg/kg achieved appropriate concentration (Cmax > 2.5 mg/L) for most children (with significant variation), but was lower among younger and HIV-positive children.46
Potential toxicity associated with increased doses
The main concern with higher doses of anti-TB agents is greater toxicity. Increasing the isoniazid dose from 4–6 to 8–10 mg/kg has not yet been associated with greater hepatotoxicity in children.47 Early studies that evaluated pyrazinamide dosing at 50 mg/kg (instead of the current recommendation of 30–40 mg/kg) experienced a high incidence of hepatotoxicity.48 A review of ocular toxicity with ethambutol found a low prevalence among children (2/3811, 0.05%) receiving standard dosing, though the authors note a dose-dependent toxicity in adults.49 Fluoroquinolones carry a risk of osteoarticular adverse events and QT-interval prolongation; two pharmacokinetic studies in South Africa did not find that ofloxacin, levofloxacin or moxifloxacin were associated with significant QT-prolongation in children,44,45 but the effect on osteoarticular adverse effects at higher doses needs to be evaluated. For aminoglycosides, standard dosing of amikacin, capreomycin or kanamycin for treatment of children with MDR-TB in South Africa was associated with 24% developing hearing loss.50 Intolerance with the currently available enteric-coated PAS is not severe,51 and intolerance may be similar regardless of once-daily versus intermittent treatment; single daily dosing will also lead to a higher Cmax that exceeds the MIC in more than half of the documented PAS-resistant isolates.52,53 Unfortunately, drug serum concentration and association with toxicity is not well documented in TB, with the exception of neuropsychiatric adverse effects with cycloserine >35 mg/L.54 Any attempts to increase the dose would need to be carefully monitored to avoid unwanted toxicity.
Future directions
High-dose isoniazid provides an example of how understanding TB genomics, its relationship with MIC, and pharmacokinetics allows customization of therapy and restriction of mutant growth. Genetics studies found prevalent mutations associated with isoniazid resistance in two genes, katG and inhA (Table 2).55 However, katG mutations are mainly associated with high MICs, and inhA mutations are associated with lower MICs. Pharmacokinetic studies with isoniazid also noted that a Cmax of 5 mg/L, on the higher range (Table 1), was associated with greater sputum culture negativity following monotherapy for 1 year,56 and that children <2 years, regardless of acetylator status, were able to reach a Cmax of 5 mg/L.40 Ultimately, the integration of these ideas suggested that if an inhA mutation is present, a higher dose (15–20 mg/kg) could be used to exceed the MIC, and studies in adults have suggested improved clinical outcomes.57 Currently, the WHO-recommended 9 month MDR-TB regimen utilizes high-dose isoniazid as well as higher than standard doses of fluoroquinolones.58
It is in this context that we seek to revisit the MPC in TB. If the MPC for isoniazid in isolates that contain subpopulations of inhA or katG mutations were known, as well as the N-acetyltransferase genotype, dosing could be customized to not only kill susceptible cells, but also restrict the growth of the mutants.59 Early studies did not support the use of the MPC given the high concentrations required, but our wider understanding of the genomics of M. tuberculosis and of the host suggests that the specific mutation can play an important role in the MIC, and thus perhaps the MPC. This redefines resistance beyond the presence of the mutation, and allows clinicians to continue to utilize core anti-TB agents in the setting of limited options. In paediatrics, we have shown that dosing remains unclear for both first- and second-line agents in terms of efficacy and safety. This creates an opportunity to incorporate the MPC in pharmacokinetic studies in children to determine the Cmax/MPC, T >MPC and AUC/MPC, and potential toxicity at these concentrations. Before the MPC can be utilized clinically, a number of areas still need to be explored (Table 3).
Table 3.
Areas of future research in the mutant prevention concentration in M. tuberculosis
| In vitro | WGS and quantitative drug susceptibility testing to correlate mutations with phenotypic resistance |
| determine MPC for current and experimental therapies | |
| stratify MPC by resistance mutation and strain | |
| In vivo | validation of the MSW for all TB drugs |
| pharmacokinetics and pharmacodynamics at MPC | |
| safety and tolerability at MPC | |
| Clinical | pharmacokinetics of current dosing of second-line therapies in children, and relationship to clinical outcome |
| development of targets based on Cmax/MPC and AUC/MPC | |
| correlate adverse effects with serum drug concentrations in children |
MSW, mutation selection window.
In addition to current therapy, the MPC may have a role in future drug development. Piperine is an efflux pump inhibitor that has been shown to reduce the MIC of rifampicin by 4- to 8-fold in both drug-susceptible and drug-resistant isolates, and reduced the MPC in laboratory strains from immeasurable to 2 mg/L, well below the target Cmax of rifampicin.60,61 As new drugs are developed, incorporating studies on the MPC may inform ways to restrict the emergence of new resistant isolates and/or increase the potency of current therapy when this can be achieved without undue toxicity.
We are entering challenging times where resistance will place increasing stress on current choices to treat TB. Fortunately, advances in our understanding of resistance and response to therapy provide an opportunity to examine new dosing strategies to customize and improve treatment. The MPC is a concept that should be further explored to determine how best to treat TB and restrict the growth of mutations associated with resistance. The gaps in knowledge around dosing in paediatric TB provide a unique opportunity to reintroduce the MPC to guide the identification of appropriate pharmacokinetic targets.
Acknowledgements
We thank Dr Elizabeth Harausz for her assistance in conceiving the review, and Dr Jennifer Furin for her critical review of the article.
Transparency declarations
H. S. S. is involved in studies of pharmacokinetics of second-line anti-TB drugs in children for which Stellenbosch University is receiving two grants from the United States National Institutes of Health (NIH) and in pharmacokinetic and safety studies of delamanid in children, for which Stellenbosch University receives a grant from Otsuka Pharmaceuticals. All other authors: none to declare.
References
- 1. Drlica K, Zhao X.. Mutant selection window hypothesis updated. Clin Infect Dis 2007; 44: 681–8. [DOI] [PubMed] [Google Scholar]
- 2. Zhao X, Drlica K.. A unified anti-mutant dosing strategy. J Antimicrob Chemother 2008; 62: 434–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blondeau JM, Shebelski SD, Hesje CK.. Killing of Streptococcus pneumoniae by azithromycin, clarithromycin, erythromycin, telithromycin and gemifloxacin using drug minimum inhibitory concentrations and mutant prevention concentrations. Int J Antimicrob Agents 2015; 45: 594–9. [DOI] [PubMed] [Google Scholar]
- 4. Ni W, Song X, Cui J.. Testing the mutant selection window hypothesis with Escherichia coli exposed to levofloxacin in a rabbit tissue cage infection model. Eur J Clin Microbiol Infect Dis 2014; 33: 385–9. [DOI] [PubMed] [Google Scholar]
- 5. Mei Q, Ye Y, Zhu YL. et al. Testing the mutant selection window hypothesis in vitro and in vivo with Staphylococcus aureus exposed to fosfomycin. Eur J Clin Microbiol Infect Dis 2015; 34: 737–44. [DOI] [PubMed] [Google Scholar]
- 6. Zhao X, Drlica K.. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin Infect Dis 2001; 33: S147–56. [DOI] [PubMed] [Google Scholar]
- 7. Weiner M, Benator D, Burman W. et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 2005; 40: 1481–91. [DOI] [PubMed] [Google Scholar]
- 8. Weiner M, Burman W, Vernon A. et al. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 2003; 167: 1341–7. [DOI] [PubMed] [Google Scholar]
- 9. Kenny MT, Strates B.. Metabolism and pharmacokinetics of the antibiotic rifampin. Drug Metab Rev 1981; 12: 159–218. [DOI] [PubMed] [Google Scholar]
- 10. Dodd PJ, Sismanidis C, Seddon JA.. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis 2016; 16: 1193–201. [DOI] [PubMed] [Google Scholar]
- 11. Seddon JA, Hesseling AC, Finlayson H. et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis 2013; 57: 1676–84. [DOI] [PubMed] [Google Scholar]
- 12. Böttger EC. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin Microbiol Infect 2011; 17: 1128–34. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization (WHO). Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children http://apps.who.int/medicinedocs/documents/s21535en/s21535en.pdf. [PubMed]
- 14. Dong Y, Zhao X, Kreiswirth BN. et al. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2000; 44: 2581–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization (WHO). WHO Treatment Guidelines for Drug-Resistant Tuberculosis: 2016 Update http://www.who.int/tb/MDRTBguidelines2016.pdf. [PubMed]
- 16. Almeida D, Nuermberger E, Tyagi S. et al. In vivo validation of the mutant selection window hypothesis with moxifloxacin in a murine model of tuberculosis. Antimicrob Agents Chemother 2007; 51: 4261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gumbo T, Louie A, Deziel MR. et al. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 2004; 190: 1642–51. [DOI] [PubMed] [Google Scholar]
- 18. Alffenaar JW, Gumbo T, Aarnoutse R.. Shorter moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2015; 372: 576.. [DOI] [PubMed] [Google Scholar]
- 19. Rodríguez JC, Cebrian L, Ruiz M. et al. Mutant prevention concentration of isoniazid, rifampicin and rifabutin against Mycobacterium tuberculosis. Chemotherapy 2005; 51: 76–9. [DOI] [PubMed] [Google Scholar]
- 20. Rodríguez J, Cebrian L, Lopez M. et al. Mutant prevention concentration: comparison of fluoroquinolones and linezolid with Mycobacterium tuberculosis. J Antimicrob Chemother 2004; 53: 441–4. [DOI] [PubMed] [Google Scholar]
- 21. Zhou J, Dong Y, Zhao X. et al. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J Infect Dis 2000; 182: 517–25. [DOI] [PubMed] [Google Scholar]
- 22. Ali A, Hasan Z, McNerney R. et al. Whole genome sequencing based characterization of extensively drug-resistant Mycobacterium tuberculosis isolates from Pakistan. PLoS One 2015; 10: e0117771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ioerger TR, Koo S, No E-G. et al. Genome analysis of multi-and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PLoS One 2009; 4: e7778.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen KA, Abeel T, McGuire AM. et al. Evolution of extensively drug-resistant tuberculosis over four decades: whole genome sequencing and dating analysis of Mycobacterium tuberculosis isolates from KwaZulu-Natal. PLoS Med 2015; 12: e1001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ocheretina O, Escuyer VE, Mabou MM. et al. Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: investigation of cases with discrepant susceptibility results. PLoS One 2014; 9: e90569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fenner L, Egger M, Bodmer T. et al. Effect of mutation and genetic background on drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2012; 56: 3047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ocheretina O, Shen L, Escuyer VE. et al. Whole genome sequencing investigation of a tuberculosis outbreak in Port-au-Prince, Haiti caused by a strain with a “low-level” rpoB mutation L511P—insights into a mechanism of resistance escalation. PLoS One 2015; 10: e0129207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Safi H, Lingaraju S, Amin A. et al. Evolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-β-d-arabinose biosynthetic and utilization pathway genes. Nat Genet 2013; 45: 1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cavalieri SJ, Biehle JR, Sanders W.. Synergistic activities of clarithromycin and antituberculous drugs against multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 1995; 39: 1542–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abuali MM, Katariwala R, LaBombardi VJ.. A comparison of the Sensititre(R) MYCOTB panel and the agar proportion method for the susceptibility testing of Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis 2012; 31: 835–9. [DOI] [PubMed] [Google Scholar]
- 31. Heysell SK, Pholwat S, Mpagama SG. et al. Sensititre MycoTB plate compared to Bactec MGIT 960 for first- and second-line antituberculosis drug susceptibility testing in Tanzania: a call to operationalize MICs. Antimicrob Agents Chemother 2015; 59: 7104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heysell SK, Ahmed S, Ferdous SS. et al. Quantitative drug-susceptibility in patients treated for multidrug-resistant tuberculosis in Bangladesh: implications for regimen choice. PLoS One 2015; 10: e0116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Springer B, Lucke K, Calligaris-Maibach R. et al. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J Clin Microbiol 2009; 47: 1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cambau E, Viveiros M, Machado D. et al. Revisiting susceptibility testing in MDR-TB by a standardized quantitative phenotypic assessment in a European multicentre study. J Antimicrob Chemother 2015; 70: 686–96. [DOI] [PubMed] [Google Scholar]
- 35. Drlica K. The mutant selection window and antimicrobial resistance. J Antimicrob Chemother 2003; 52: 11–7. [DOI] [PubMed] [Google Scholar]
- 36. Drlica K, Zhao X, Blondeau JM. et al. Low correlation between MIC and mutant prevention concentration. Antimicrob Agents Chemother 2006; 50: 403–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson BJ, Holford NH.. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child 2013; 98: 737–44. [DOI] [PubMed] [Google Scholar]
- 38. Ramachandran G, Kumar AKH, Swaminathan S.. Pharmacokinetics of anti-tuberculosis drugs in children. Indian J Pediatr 2011; 78: 435–42. [DOI] [PubMed] [Google Scholar]
- 39. Wilby KJ, Shabana S, Ensom MH. et al. A critical review of the current evidence for measuring drug concentrations of first-line agents used to treat tuberculosis in children. Clin Pharmacokinet 2016; 55: 17–31. [DOI] [PubMed] [Google Scholar]
- 40. Thee S, Seddon JA, Donald PR. et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother 2011; 55: 5560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bekker A, Schaaf HS, Draper H. et al. The pharmacokinetics of rifampicin, isoniazid, pyrazinamide and ethambutol in infants dosed at revised WHO-recommended treatment guidelines. Antimicrob Agents Chemother 2016; 60: 2171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hiruy H, Rogers Z, Mbowane C. et al. Subtherapeutic concentrations of first-line anti-TB drugs in South African children treated according to current guidelines: the PHATISA study. J Antimicrob Chemother 2015; 70: 1115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramachandran G, Kumar AH, Kannan T. et al. Low serum concentrations of rifampicin and pyrazinamide associated with poor treatment outcomes in children with tuberculosis related to HIV status. Pediatr Infect Dis J 2016; 35: 530–4. [DOI] [PubMed] [Google Scholar]
- 44. Thee S, Garcia-Prats AJ, McIlleron HM. et al. Pharmacokinetics of ofloxacin and levofloxacin for prevention and treatment of multidrug-resistant tuberculosis in children. Antimicrob Agents Chemother 2014; 58: 2948–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thee S, Garcia-Prats AJ, Draper HR. et al. Pharmacokinetics and safety of moxifloxacin in children with multidrug-resistant tuberculosis. Clin Infect Dis 2015; 60: 549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thee S, Seifart HI, Rosenkranz B. et al. Pharmacokinetics of ethionamide in children. Antimicrob Agents Chemother 2011; 55: 4594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McIlleron H, Willemse M, Werely CJ. et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis 2009; 48: 1547–53. [DOI] [PubMed] [Google Scholar]
- 48. Donald PR, Maritz JS, Diacon AH.. Pyrazinamide pharmacokinetics and efficacy in adults and children. Tuberculosis (Edinb) 2012; 92: 1–8. [DOI] [PubMed] [Google Scholar]
- 49. Donald PR, Maher D, Maritz JS. et al. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis 2006; 10: 1318–30. [PubMed] [Google Scholar]
- 50. Seddon JA, Thee S, Jacobs K. et al. Hearing loss in children treated for multidrug-resistant tuberculosis. J Infect 2013; 66: 320–9. [DOI] [PubMed] [Google Scholar]
- 51. Peloquin CA, Zhu M, Adam RD. et al. Pharmacokinetics of para-aminosalicylic acid granules under four dosing conditions. Ann Pharmacother 2001; 35: 1332–8. [DOI] [PubMed] [Google Scholar]
- 52. Sy SKB, de Kock L, Diacon AH. et al. N-Acetyltransferase genotypes and the pharmacokinetics and tolerability of para-aminosalicylic acid in patients with drug-resistant pulmonary tuberculosis. Antimicrob Agents Chemother 2015; 59: 4129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Donald PR, Diacon AH.. Para-aminosalicylic acid: the return of an old friend. Lancet Infect Dis 2015; 15: 1091–9. [DOI] [PubMed] [Google Scholar]
- 54. Alsultan A, Peloquin CA.. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74: 839–54. [DOI] [PubMed] [Google Scholar]
- 55. Rouse DA, Li Z, Bai G-H. et al. Characterization of the katG and inhA genes of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother 1995; 39: 2472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitchison DA. Plasma concentrations of isoniazid in the treatment of tuberculosis In: Prichard BNC Davies DS.eds. Biological Effects of Drugs in Relation to Their Plasma Concentration. London: British Pharmacological Society, Macmillan, 1973; 169–82. [Google Scholar]
- 57. Katiyar SK, Bihari S, Prakash S. et al. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2008; 12: 139–45. [PubMed] [Google Scholar]
- 58. Moodley R, Godec TR.. Short-course treatment for multidrug-resistant tuberculosis: the STREAM trials. Eur Respir Rev 2016; 25: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kinzig-Schippers M, Tomalik-Scharte D, Jetter A. et al. Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses? Antimicrob Agents Chemother 2005; 49: 1733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sharma S, Kumar M, Sharma S. et al. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother 2010; 65: 1694–701. [DOI] [PubMed] [Google Scholar]
- 61. Pule CM, Sampson SL, Warren RM. et al. Efflux pump inhibitors: targeting mycobacterial efflux systems to enhance TB therapy. J Antimicrob Chemother 2015; 15: 17–26. [DOI] [PubMed] [Google Scholar]
- 62. World Health Organization (WHO). Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf. [PubMed]
- 63. World Health Organization (WHO). Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis-2011 Update http://apps.who.int/iris/bitstream/10665/44597/1/9789241501583_eng.pdf. [PubMed]
- 64. Kiser JJ, Zhu R, D’Argenio DZ. et al. Isoniazid pharmacokinetics, pharmacodynamics, and dosing in South African infants. Ther Drug Monit 2012; 34: 446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ramachandran G, Hemanth Kumar AK, Bhavani PK. et al. Age, nutritional status and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis 2013; 17: 800–6. [DOI] [PubMed] [Google Scholar]
- 66. Ramachandran G, Kumar AK, Bhavani PK. et al. Pharmacokinetics of first-line antituberculosis drugs in HIV-infected children with tuberculosis treated with intermittent regimens in India. Antimicrob Agents Chemother 2015; 59: 1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bekker A, Schaaf HS, Seifart HI. et al. Pharmacokinetics of isoniazid in low-birth-weight and premature infants. Antimicrob Agents Chemother 2014; 58: 2229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kwara A, Enimil A, Gillani FS. et al. Pharmacokinetics of first-line antituberculosis drugs using WHO revised dosage in children with tuberculosis with and without HIV coinfection. J Ped Infect Dis 2016; 5: 356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mukherjee A, Velpandian T, Singla M. et al. Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in Indian children. BMC Infect Dis 2015; 15: 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roy V, Tekur U, Chopra K.. Pharmacokinetics of pyrazinamide in children suffering from pulmonary tuberculosis. Int J Tuberc Lung Dis 1999; 3: 133–7. [PubMed] [Google Scholar]
- 71. Thee S, Detjen A, Wahn U. et al. Pyrazinamide serum levels in childhood tuberculosis. Int J Tuberc Lung Dis 2008; 12: 1099–101. [PubMed] [Google Scholar]
- 72. Graham SM, Bell DJ, Nyirongo S. et al. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother 2006; 50: 407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roy V, Sahni P, Gupta P. et al. Blood levels of pyrazinamide in children at doses administered under the Revised National Tuberculosis Control Program. Indian Pediatr 2012; 49: 721–5. [DOI] [PubMed] [Google Scholar]
- 74. Zhu M, Burman WJ, Starke JR. et al. Pharmacokinetics of ethambutol in children and adults with tuberculosis. Int J Tuberc Lung Dis 2004; 8: 1360–7. [PubMed] [Google Scholar]
- 75. Thee S, Detjen A, Quarcoo D. et al. Ethambutol in paediatric tuberculosis: aspects of ethambutol serum concentration, efficacy and toxicity in children. Int J Tuberc Lung Dis 2007; 11: 965–71. [PubMed] [Google Scholar]
- 76. Verhagen L, Lopez D, Hermans P. et al. Pharmacokinetics of anti-tuberculosis drugs in Venezuelan children younger than 16 years of age: supportive evidence for the implementation of revised WHO dosing recommendations. Trop Med Int Health 2012; 17: 1449–56. [DOI] [PubMed] [Google Scholar]
- 77. Moultrie H, McIlleron H, Sawry S. et al. Pharmacokinetics and safety of rifabutin in young HIV-infected children receiving rifabutin and lopinavir/ritonavir. J Antimicrob Chemother 2015; 70: 543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhu M, Namdar R, Stambaugh JJ. et al. Population pharmacokinetics of ethionamide in patients with tuberculosis. Tuberculosis (Edinb) 2002; 82: 91–6. [DOI] [PubMed] [Google Scholar]
- 79. Liwa AC, Schaaf HS, Rosenkranz B. et al. Para-aminosalicylic acid plasma concentrations in children in comparison with adults after receiving a granular slow-release preparation. J Trop Pediatr 2013; 59: 90–4. [DOI] [PubMed] [Google Scholar]
- 80. Clemente WT, Lima SSS, Palaci M. et al. Phenotypic and genotypic characterization of drug-resistant Mycobacterium tuberculosis strains. Diagn Microbiol Infect Dis 2008; 62: 199–204. [DOI] [PubMed] [Google Scholar]
- 81. Springer B, Calligaris-Maibach RC, Ritter C. et al. Tuberculosis drug resistance in an area of low endemicity in 2004 to 2006: semiquantitative drug susceptibility testing and genotyping. J Clin Microbiol 2008; 46: 4064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morlock GP, Metchock B, Sikes D. et al. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 2003; 47: 3799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Feuerriegel S, Oberhauser B, George AG. et al. Sequence analysis for detection of first-line drug resistance in Mycobacterium tuberculosis strains from a high-incidence setting. BMC Microbiol 2012; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jamieson F, Guthrie J, Neemuchwala A. et al. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol 2014; 52: 2157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Whitfield MG, Warren RM, Streicher EM. et al. Mycobacterium tuberculosis pncA polymorphisms that do not confer pyrazinamide resistance at a breakpoint concentration of 100 micrograms per milliliter in MGIT. J Clin Microbiol 2015; 53: 3633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Werngren J, Sturegård E, Juréen P. et al. Reevaluation of the critical concentration for drug susceptibility testing of Mycobacterium tuberculosis against pyrazinamide using wild-type MIC distributions and pncA gene sequencing. Antimicrob Agents Chemother 2012; 56: 1253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sirgel FA, Warren RM, Streicher EM. et al. embB306 mutations as molecular indicators to predict ethambutol susceptibility in Mycobacterium tuberculosis. Chemotherapy 2012; 58: 358–63. [DOI] [PubMed] [Google Scholar]
- 88. Kambli P, Ajbani K, Nikam C. et al. Determination of MICs of levofloxacin for Mycobacterium tuberculosis with gyrA mutations. Int J Tuberc Lung Dis 2015; 19: 1227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nosova EY, Bukatina AA, Isaeva YD. et al. Analysis of mutations in the gyrA and gyrB genes and their association with the resistance of Mycobacterium tuberculosis to levofloxacin, moxifloxacin and gatifloxacin. J Med Microbiol 2013; 62: 108–13. [DOI] [PubMed] [Google Scholar]
- 90. Niward K, Ängeby K, Chryssanthou E. et al. Susceptibility testing breakpoints for Mycobacterium tuberculosis categorize isolates with resistance mutations in gyrA as susceptible to fluoroquinolones: implications for MDR-TB treatment and the definition of XDR-TB. J Antimicrob Chemother 2016; 71: 333–8. [DOI] [PubMed] [Google Scholar]
- 91. Chien J-Y, Chiu W-Y, Chien S-T. et al. Mutations in gyrA and gyrB among fluoroquinolone-and multidrug-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 2016; 60: 2090–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Farhat MR, Jacobson KR, Franke MF. et al. Gyrase mutations are associated with variable levels of fluoroquinolone resistance in Mycobacterium tuberculosis. J Clin Microbiol 2016; 54: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li J, Gao X, Luo T. et al. Association of gyrA/B mutations and resistance levels to fluoroquinolones in clinical isolates of Mycobacterium tuberculosis. Emerg Microbes Infect 2014; 3: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang Z, Lu J, Wang Y. et al. Prevalence and molecular characterization of fluoroquinolone-resistant Mycobacterium tuberculosis isolates in China. Antimicrob Agents Chemother 2014; 58: 364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Georghiou SB, Magana M, Garfein RS. et al. Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to amikacin, kanamycin and capreomycin: a systematic review. PLoS One 2012; 7: e33275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reeves AZ, Campbell PJ, Willby MJ. et al. Disparities in capreomycin resistance levels associated with the rrs A1401G mutation in clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2015; 59: 444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Maus CE, Plikaytis BB, Shinnick TM.. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2005; 49: 3192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zaunbrecher MA, Sikes RD, Metchock B. et al. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2009; 106: 20004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Meier A, Sander P, Schaper KJ. et al. Correlation of molecular resistance mechanisms and phenotypic resistance levels in streptomycin-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 1996; 40: 2452–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wong SY, Lee JS, Kwak HK. et al. Mutations in gidB confer low-level streptomycin resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2011; 55: 2515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mathys V, Wintjens R, Lefevre P. et al. Molecular genetics of para-aminosalicylic acid resistance in clinical isolates and spontaneous mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2009; 53: 2100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhao F, Wang X-D, Erber LN. et al. Binding pocket alterations in dihydrofolate synthase confer resistance to para-aminosalicylic acid in clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2014; 58: 1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]