Abstract
Objectives
To investigate the molecular epidemiology, antimicrobial susceptibility and carbapenem resistance determinants of Acinetobacter baumannii isolates from respiratory tract samples of patients diagnosed with ventilator-associated pneumonia (VAP) who were enrolled in the MagicBullet clinical trial.
Methods
A. baumannii isolates were prospectively cultured from respiratory tract samples from 65 patients from 15 hospitals in Greece, Italy and Spain. Susceptibility testing was performed by broth microdilution. Carbapenem resistance determinants were identified by PCR and sequencing. Molecular epidemiology was investigated using rep-PCR (DiversiLab) and international clones (IC) were identified using our in-house database.
Results
Of 65 isolates, all but two isolates (97%) were resistant to imipenem and these were always associated with an acquired carbapenemase, OXA-23 (80%), OXA-40 (4.6%), OXA-58 (1.5%) or OXA-23/58 (1.5%). Resistance to colistin was 47.7%. Twenty-two isolates were XDR, and 20 isolates were pandrug-resistant (PDR). The majority of isolates clustered with IC2 (n = 54) with one major subtype comprising isolates from 12 hospitals in the three countries, which included 19 XDR and 16 PDR isolates.
Conclusions
Carbapenem resistance rates were very high in A. baumannii recovered from patients with VAP. Almost half of the isolates were colistin resistant, and 42 (64.6%) isolates were XDR or PDR. Rep-PCR confirmed IC2 is the predominant clonal lineage in Europe and suggests the presence of an epidemic XDR/PDR A. baumannii clone that has spread in Greece, Italy and Spain. These data highlight the difficulty in empirical treatment of patients with A. baumannii VAP in centres with a high prevalence of carbapenem-resistant A. baumannii.
Introduction
Considered susceptible to major antimicrobial drug classes in the 1970s, the clinical importance of the Gram-negative, nosocomial pathogen Acinetobacter baumannii has increased steadily and today this organism is displaying resistance against all first-line antibiotics.1,2 Owing to its intrinsic antimicrobial resistance and its ability to easily acquire new resistance determinants, MDR, XDR and even pandrug-resistant (PDR) isolates have now been described.1–4A. baumannii has established a niche in healthcare environments where it is involved in a variety of serious infections, particularly in patients with severe comorbidities in the ICU setting.1,2 Infections include ventilator-associated-pneumonia (VAP), bloodstream infection, as well as wound and urinary tract infection, meningitis and soft tissue infection.1,5 Of these, VAP is the most frequent healthcare-associated infection.6 It is associated with excess mortality, excess length of ICU and hospital stay, prolonged mechanical ventilation and therefore higher overall costs.6,7 The predominant organisms in nosocomial pneumonia are Staphylococcus aureus and aerobic Gram-negative bacilli (GNB), predominantly Pseudomonas aeruginosa, Enterobacteriaceae and A. baumannii.6,8 Given the high prevalence of MDR GNB, adequate empirical antimicrobial therapy for VAP is difficult to establish and inappropriate treatment is associated with a poor prognosis in these patients.9 Carbapenem monotherapy or combined with an aminoglycoside or a quinolone has become a widely applied empirical therapy.10,11 However, often this strategy may not be successful as many GNB occurring in ICU settings now exhibit resistance to all three antimicrobial classes.12
Owing to a lack of new antimicrobial agents, the old off-patent drug colistin has come into consideration again, showing good in vitro activity against carbapenem-resistant GNB (CR-GNB).13 To date, the clinical efficacy of colistin has been suggested in experimental models and clinical case series but no clinical trial using colistin as empirical treatment for VAP has been carried out.14,15 Therefore, in 2012 the Phase IV, randomized, open-label, non-inferiority, international clinical trial MagicBullet was initiated to assess the safety and efficacy of colistin versus meropenem in the empirical treatment of VAP.16 The study was conducted over 4 years and included 32 centres in Spain, Italy and Greece, countries with a high incidence of infections caused by CR-GNB. As part of this trial it was the aim of this study to characterize the molecular epidemiology, antimicrobial susceptibility and carbapenem resistance mechanisms of A. baumannii isolates recovered from respiratory tract samples from patients with VAP.
Materials and methods
Bacterial isolates
One hundred and twenty-four A. baumannii isolates were collected from respiratory tract samples (bronchoalveolar lavage or bronchial aspirates) from 65 patients diagnosed with VAP from seven hospitals in Greece (70 isolates), five hospitals in Spain (28 isolates) and three hospitals in Italy (26 isolates) between May 2012 and September 2015 as part of the clinical trial MagicBullet (ClinicalTrials.gov NCT01292031). Samples were taken before initiation of any empirical treatment for VAP, and thereafter if available at 72 h, at day 8, at the end of antibiotic treatment, and at day 28 of follow-up. Species identification and susceptibility testing were performed according to local standards. Acinetobacter isolates were then sent to the central laboratory for further investigation. Species identification was confirmed by gyrB multiplex PCR as described previously.17
Antimicrobial susceptibility testing
The following antibacterial agents were tested (respective concentration ranges are given in parentheses): amikacin (0.125–256 mg/L); ampicillin/sulbactam (0.25/0.125–256/128 mg/L); cefepime (0.125–256 mg/L); colistin (0.03–256 mg/L); cotrimoxazole (0.06–32 mg/L); doxycycline (0.03–32 mg/L); imipenem (0.125–256 mg/L); levofloxacin (0.03–64 mg/L); meropenem (0.125–256 mg/L); minocycline (0.06–128 mg/L); sulbactam (0.06–128 mg/L); tigecycline (0.03–64 mg/L); and tobramycin (0.06–128 mg/L). Antimicrobial susceptibility testing was performed by standard broth microdilution in CAMHB according to the international standard ISO 20776-1.18 Microtitre plates containing dehydrated antibacterial agents were purchased from Merlin Diagnostica (Bornheim-Hersel, Germany). Plates were incubated at 35 ± 1 °C for 18 ± 2 h and read visually. Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control strains. MICs were interpreted according to the species-specific clinical breakpoints approved by EUCAST (Version 7.0, January 2017).19 As no susceptibility breakpoint for tigecycline is available for A. baumannii, the EUCAST breakpoint for Enterobacteriaceae was used instead (susceptible ≤1 mg/L; resistant ≥4 mg/L). Classification of MDR, XDR and PDR was based on current EUCAST breakpoints for Acinetobacter spp., i.e. not including tigecycline using the following ad-hoc definition; MDR, resistant to at least one antibiotic from three classes tested; XDR, resistant to all antibiotics tested except one; PDR, resistant to all antibiotics tested.
PCR and sequencing of blaOXA genes
Detection of intrinsic and acquired OXA-type carbapenemases and presence of IS elements adjacent to genes encoding OXA-51-like, OXA-23-like and OXA-58-like was performed by PCR and blaOXA genes were sequenced using primers as previously described.20,21
Molecular epidemiology
The molecular epidemiology of the isolates was determined by repetitive sequence-based PCR (DiversiLabTM System; bioMérieux).17 Clonal relatedness was analysed with the DiversiLab software using the Pearson correlation statistical method. Isolates showing ≥98% similarity in their rep-PCR pattern were considered identical. Using the modified Kullback–Leibler statistical method, isolates were assigned to international clones (IC) using our in-house database and isolates that clustered at >95% were considered related to the IC.17
Results
Bacterial isolates
Of the total 124 Acinetobacter isolates that were confirmed as A. baumannii using the gyrB multiplex PCR, 65 were selected based on being the first isolate per patient and analysed. Among these 65 isolates, the majority were collected before initiation of empirical antimicrobial treatment (DT0, n = 26) or within 72 h of treatment (DT3; n = 16). The remaining isolates were recovered at 8 days of treatment (DT8; n = 11), at the end of antibiotic treatment (DT-F; n = 8) and at 28 days of follow-up (DT28; n = 4). All following data are based on the first isolate per patient, i.e. 65 isolates.
Antimicrobial susceptibility
Antimicrobial susceptibility results are summarized in Table 1. The vast majority of isolates (96.9%) were determined to be resistant to imipenem, with the exception of two isolates, one each from Italy and Greece. Thirty-one isolates (47.7%) were resistant to colistin and 34 isolates were susceptible, with an MIC50 of 2 mg/L and MIC90 of 256 mg/L. Isolates from Greece exhibited the highest rate of colistin resistance (56.8%), whereas isolates from Italy and Spain had resistance rates of 42.9% and 28.6%, respectively. Furthermore, four, two and one isolates from Greece, Italy and Spain had colistin MICs ≥256 mg/L, respectively. Regarding tigecycline, five isolates exhibited a resistant phenotype (7.7%), 23 were intermediate (35.4%) and 37 isolates (56.9%) were considered susceptible. Tigecycline MIC50 and MIC90 values were 1 and 2 mg/L, respectively.
Table 1.
Susceptibility data of A. baumannii isolates (first isolates only, n = 65) collected from patients with VAP in Greece, Italy and Spain
| Antimicrobial agent | MIC50a | MIC90 | Range | %Sb | %I | %R |
|---|---|---|---|---|---|---|
| Amikacin | >256 | >256 | 1–>256 | 6.2 | 7.7 | 86.1 |
| Ampicillin/sulbactam | 32/16 | 64/32 | 1/0.5–128/64 | – | – | – |
| Cefepime | 64 | 128 | 2–>256 | – | – | – |
| Colistin | 2 | 256 | 0.25–>256 | 52.3 | – | 47.7 |
| Cotrimoxazole | 32 | >32 | 0.125–>32 | 26.2 | 1.5 | 72.3 |
| Doxycycline | >32 | >32 | 0.125–>32 | – | – | – |
| Imipenem | 32 | 64 | 0.25–128 | 1.5 | 1.5 | 96.9 |
| Levofloxacin | 16 | 64 | 0.125–>64 | 1.5 | – | 98.5 |
| Meropenem | 64 | 64 | 0.25–128 | 3.1 | 9.2 | 87.7 |
| Minocycline | 8 | 16 | 0.125–16 | – | – | – |
| Sulbactam | 16 | 32 | 1–64 | – | – | – |
| Tigecyclinec | 1 | 2 | 0.125–8 | 56.9 | 35.4 | 7.7 |
| Tobramycin | >128 | >128 | 0.125–>128 | 21.5 | – | 78.5 |
MIC values are given in mg/L.
S, susceptible; I, intermediate; R, resistant; –, no EUCAST breakpoint available.
Enterobacteriaceae breakpoints were applied for tigecycline MIC interpretation; S ≤ 1 mg/L; R ≥ 4 mg/L.
Susceptibility testing against the other antibiotics revealed high overall resistance rates to the aminoglycosides and levofloxacin (Table 1). One isolate was fully susceptible to all antibiotics tested, 21 were considered MDR, 22 were XDR and 20 were PDR. There was no difference in resistance rates by country (data not shown).
Detection of carbapenemases
The A. baumannii isolates were investigated for the presence of intrinsic and acquired OXA-type carbapenemases (Table 2). Among the intrinsic OXA-51 subclass, OXA-66 was predominant, present in 54 of 65 isolates (83%) collected from all three countries. Five isolates from Greece had OXA-69, whereas three isolates from Italy carried OXA-82, which was associated with ISAba1 encoded upstream. Furthermore, two isolates from one hospital in Spain harboured OXA-387. A single carbapenem-susceptible isolate from Italy was found to carry OXA-391, and no further acquired OXA-type carbapenemases were detected in this isolate. Similarly, one of the OXA-69 harbouring isolates did not carry any additional OXA, and was susceptible to the carbapenems.
Table 2.
Percentage of isolates (n = 65) with the following intrinsic and acquired OXA-type carbapenemases
| OXA-type | Percentage of isolates |
|---|---|
| Intrinsic carbapenemases | |
| OXA-66 | 83.1 |
| OXA-69 | 7.7 |
| OXA-82a | 4.6 |
| OXA-387 | 3.1 |
| OXA-391 | 1.5 |
| Acquired carbapenemases | |
| OXA-23a | 80.0 |
| OXA-40 | 4.6 |
| OXA-58b | 10.8 |
| OXA-23a/OXA-58b | 1.5 |
| none | 3.1 |
Associated with ISAba1.
Associated with ISAba3.
Among the acquired carbapenem resistance genes, OXA-23 associated with ISAba1 encoded upstream was found in 80% (n = 52) of isolates. OXA-58 associated with ISAba3 (n = 6) was found in Spain and Italy, and OXA-40 (n = 3) was only detected in isolates from Spain; only one isolate from Italy harboured OXA-58. Furthermore, one isolate from Athens, Greece harboured both OXA-23 and OXA-58.
Molecular epidemiology
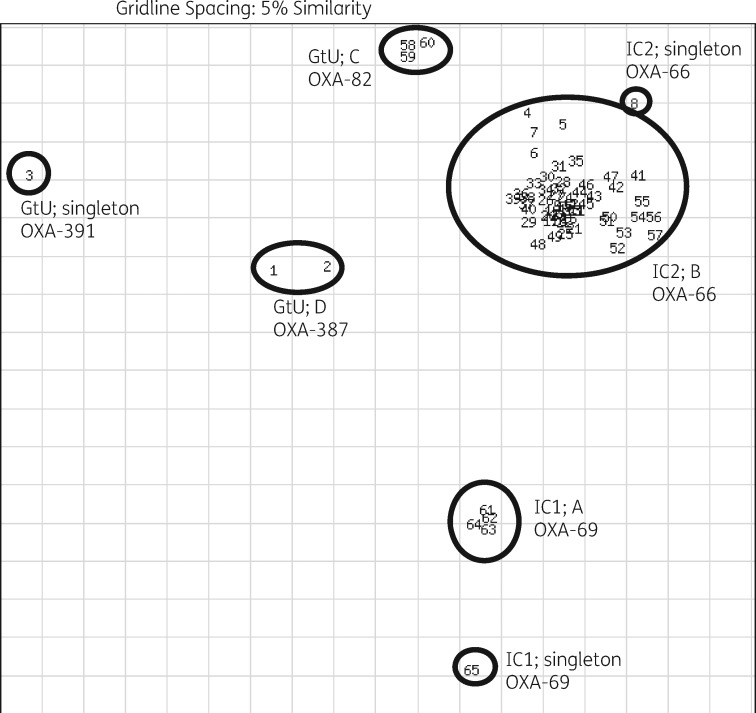
The majority of the isolates clustered with international clonal lineages IC1 and IC2 (Table 3). Five isolates from Greece clustered with IC1 strains from our in-house database, four of which were from one hospital in Greece and showed ≥98% similarity (cluster A; Figure 1). Eighty-three per cent of isolates (n = 54) exhibited a rep-PCR pattern characteristic for IC2 strains, 53 of which clustered with ≥98% similarity (cluster B) and comprised isolates from all three countries, and one isolate displayed a unique rep-PCR pattern. Sixteen of 20 PDR isolates and 19 of 22 of the XDR isolates were from cluster B. Furthermore, six isolates from Italy and Spain could not be assigned to any of the eight previously described ICs and were considered genotypically unique. Of these, three isolates from two hospitals in Italy formed cluster C (all were PDR), two isolates from one hospital in Spain represented cluster D, and one isolate from Italy had a unique rep-PCR pattern.
Table 3.
Molecular typing and clustering of 65 A. baumannii isolates
| Lineage | rep-PCR cluster | No. of isolates | Country (no. of hospitals) |
|---|---|---|---|
| IC1 | A | 4 | Greece (1) |
| singleton | 1 | Greece (1) | |
| IC2 | B | 53 | Spain (4), Italy (2), Greece (6) |
| singleton | 1 | Spain (1) | |
| Genotypically unique | C | 3 | Italy (2) |
| D | 2 | Spain (1) | |
| singleton | 1 | Italy (1) |
Figure 1.
DiversiLab scatterplot of 65 A. baumannii isolates with their respective DiversiLab clustering and intrinsic blaOXA. GtU, genotypically unique; A–D, clusters, based on rep-PCR typing.
All IC1 isolates had the OXA-69 variant encoded on the chromosome, whereas all IC2 isolates had the OXA-66 variant. Isolates harbouring OXA-82, OXA-387 or OXA-391 each had a genotypically unique rep-PCR pattern.
Thirty patients were treated with colistin, of which 18 had strains resistant to colistin, compared with 13 of 35 colistin-resistant strains in patients who were treated with meropenem. Typing showed that there was sometimes more than one colistin-resistant strain within a hospital, suggesting that resistance was developing independently. Furthermore, we observed in some hospitals that colistin-resistant clones persisted over time and were recovered from patients irrespective of the patient receiving meropenem or colistin. We also observed colistin-resistant isolates first appearing in meropenem-treated patients and the strain spreading to other patients.
Discussion
The prevalence of MDR, XDR and in particular PDR A. baumannii is increasing worldwide, limiting the treatment options for infected patients. As part of the MagicBullet clinical trial, this study characterized A. baumannii isolates recovered from respiratory tract samples from patients with VAP to gain insights into the current clinical epidemiology of A. baumannii isolates from southern European countries (Greece, Italy and Spain), which are notorious for a high incidence of MDR and XDR A. baumannii.
In our A. baumannii isolates, we found a clear predominance of IC2, which is widely distributed around the world.17 Furthermore, in longitudinal studies carried out over a 10 year period in Greece and Spain, a shift from other lineages, in particular from IC1 to IC2 has been observed,22,23 and only five isolates from two hospitals in Greece belonged to IC1 in our study.
IC1 and IC2 isolates encoded the intrinsic OXA-type carbapenemase genes blaOXA-69 or blaOXA-66, respectively, as previously described.24 It has been suggested that the emergence of these two lineages may play a role in the spread of carbapenem resistance, due to the acquired carbapenemases they harbour,25 and we found with only one exception, all isolates assigned to IC1 or IC2 were resistant to carbapenems. The resistant phenotype was always associated with acquired carbapenemases, with OXA-23 being the most prevalent. OXA-23 was first described in Italy, Greece and Spain in 2009, 2011 and 2013, respectively, and has now almost completely replaced OXA-58 in these countries, a phenomenon that has also been described in other countries.25–28
Overall, carbapenem resistance was almost universal in the isolates from our study confirming or even exceeding the resistance rates reported previously in the three countries.29–31 This spread and high incidence of carbapenem-resistant isolates has been attributed to the extensive use of carbapenems for the treatment of infections caused by MDR Gram-negative bacteria, including A. baumannii. In addition, carbapenem-resistant isolates within the present study also exhibited high resistance rates to other β-lactams, aminoglycosides, fluoroquinolones, tetracyclines (with the exception of tigecycline) and cotrimoxazole as previously reported.29 For these reasons, tigecycline and, in particular, colistin, were considered last resort antimicrobials for the treatment of patients infected with CR A. baumannii.29,30 However, over the course of this study, colistin resistance rates were already initially high and increased from 32% in 2012–13, to 62% in 2014–15, respectively. Colistin resistance is still relatively rare in A. baumannii although this might be due to the difficulties in susceptibility testing. A recent study suggests that colistin resistance is greatly underestimated using commercial susceptibility testing methods.32 We do not have the original laboratory-generated colistin susceptibility data for all the strains in this study. Where we have these data, of 38 samples, 5 recorded higher MICs in the local laboratories compared with broth microdilution, and these 5 were shown to be falsely reported as resistant. Thirty isolates recorded lower MICs in the local laboratories compared with broth microdilution and of these, 18 were shown to be falsely reported as susceptible. Four isolates recorded the same MICs as reported by the local laboratory and broth microdilution. These data reinforce the idea that colistin resistance is probably underreported. We found that the highest incidence of colistin resistance was from Greece, and in a recent study 27% of A. baumannii from Greece were colistin resistant, while in Germany, a PDR strain was isolated from a patient who had been hospitalized in Greece.4,33
Although there is a published definition for PDR,34 we did not apply this to the present study because many of the antibiotics listed have no EUCAST breakpoint values set for Acinetobacter spp. The XDR and PDR isolates we found in all three countries had very similar rep-PCR patterns suggesting clonal relatedness. Previously we have witnessed the importation of a PDR strain from Greece into Germany, which highlights the danger these organisms pose not only in their country of origin, but globally.4
In conclusion, among A. baumannii isolates recovered from patients with VAP in southern Europe, carbapenem resistance was almost universal. Alarmingly, 32% (n = 21) of isolates were MDR, 34% (n = 22) were XDR and 31% (n = 20) PDR, highlighting the difficulty in selecting antimicrobial treatment regimens for patients with A. baumannii VAP. Rep-PCR revealed IC2 as the predominant lineage with 82% of isolates grouping in one large cluster, which included the majority of the PDR isolates, suggesting the presence of an epidemic PDR A. baumannii clone that has spread within Greece, Italy and Spain.
Acknowledgements
We thank the clinical and laboratory staff of the participating hospitals for obtaining clinical samples and initial identification of microorganisms.
Other members of the MagicBullet Study Group Working Group WP4
José Garnacho-Montero, Clara Rosso-Fernández, Mª Eugenia Pachon-Ibañez, Marina Pulido and Patricia Moreno from the University Hospital Virgen del Rocío, Sevilla, Spain; Massimo Antonelli, Policlinico Universitario A Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy; George Dimopoulos, University Hospital ATTIKO, Athens, Greece; German Bou, Complejo Hospitalario Universitario A Coruña, La Coruña, Spain; Laurent Poirel and Patrice Nordmann, Medical and Molecular Microbiology, Department of Medicine, University of Fribourg, Fribourg, Switzerland; Thierry Naas, Hôpital Bicêtre, Le Kremlin-Bicêtre, France.
Funding
This work was supported by the MagicBullet study. MagicBullet is a project funded by the European Union Directorate-General for Research and Innovation through the Seventh Framework Program for Research and Development (grant agreement 278232) and has been running since 1 January 2012 (duration, 48 months). Paul G. Higgins was supported by grant FOR2251 from the German Research Council (DFG) (www.acinetobacter.de).
Transparency declarations
H. S. has received grants or research support from the German Centre for Infection Research (DZIF), German Research Foundation, Accelerate and Novartis, has been a consultant for Astellas, Basilea, Cubist, Durata, Roche Pharma and Tetraphase, and has received payments for lectures from Astellas, Cubist, Gilead, MSD and Infectopharm. P. G. H. received research support from the German Centre for Infection Research (DZIF), the German Research Foundation, and was the recipient of a Pfizer Investigator Initiated Research grant. J. M. C. has received payments for lectures from Astellas, Gilead, MSD, Pfizer and AstraZeneca. All other authors have no financial relationships relevant to this article to disclose and have no conflicts of interest in connection with this article.
References
- 1. Peleg AY, Seifert H, Paterson DL.. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008; 21: 538–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dijkshoorn L, Nemec A, Seifert H.. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 2007; 5: 939–51. [DOI] [PubMed] [Google Scholar]
- 3. Kuo SC, Chang SC, Wang HY. et al. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis 2012; 12: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Göttig S, Gruber TM, Higgins PG. et al. Detection of pan drug-resistant Acinetobacter baumannii in Germany. J Antimicrob Chemother 2014; 69: 2578. [DOI] [PubMed] [Google Scholar]
- 5. Garnacho-Montero J, Ortiz-Leyba C, Fernández-Hinojosa E. et al. Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med 2005; 31: 649–55. [DOI] [PubMed] [Google Scholar]
- 6. Vincent JL, Rello J, Marshall J. et al. ; EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302: 2323–9. [DOI] [PubMed] [Google Scholar]
- 7. Garnacho J, Sole-Violan J, Sa-Borges M. et al. Clinical impact of pneumonia caused by Acinetobacter baumannii in intubated patients: a matched cohort study. Crit Care Med 2003; 31: 2478–82. [DOI] [PubMed] [Google Scholar]
- 8. Sievert DM, Ricks P, Edwards JR. et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34: 1–14. [DOI] [PubMed] [Google Scholar]
- 9. Kuti EL, Patel AA, Coleman CI.. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care 2008; 23: 91–100. [DOI] [PubMed] [Google Scholar]
- 10. Kalil AC, Metersky ML, Klompas M. et al. Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and The American Thoracic Society. Clin Infect Dis 201663: 575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heyland DK, Dodek P, Muscedere J. et al. Canadian Critical Care Trials Group. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med 2008; 36: 737–44. [DOI] [PubMed] [Google Scholar]
- 12. Enne VI, Personne Y, Grgic L. et al. Aetiology of hospital-acquired pneumonia and trends in antimicrobial resistance. Curr Opin Pulm Med 2014; 20: 252–8. [DOI] [PubMed] [Google Scholar]
- 13. Falagas ME, Kasiakou SK.. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005; 40: 1333–41. [DOI] [PubMed] [Google Scholar]
- 14. Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ. et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis 2003; 36: 1111–8. [DOI] [PubMed] [Google Scholar]
- 15. Kallel H, Hergafi L, Bahloul M. et al. Safety and efficacy of colistin compared with imipenem in the treatment of ventilator-associated pneumonia: a matched case-control study. Intensive Care Med 2007; 33: 1162.. [DOI] [PubMed] [Google Scholar]
- 16. Rosso-Fernández C, Garnacho-Montero J, Antonelli M. et al. ; MagicBullet study group. Safety and efficacy of colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia as part of a macro-project funded by the Seventh Framework Program of the European Commission studying off-patent antibiotics: study protocol for a randomized controlled trial. Trials 2015; 16: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins PG, Dammhayn C, Hackel M. et al. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 2010; 65: 233–8. [DOI] [PubMed] [Google Scholar]
- 18. International Organization for Standardization. ISO 20776-1: 2006. Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases https://www.iso.org/obp/ui/#iso:std:iso:20776:-1:ed-1:v1:en.
- 19. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.xls.
- 20. Heritier C, Poirel L, Fournier PE. et al. Characterization of the naturally occurring oxacillinase of Acinetobacter baumannii. Antimicrob Agents Chemother 2005; 49: 4174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zander E, Fernández-González A, Schleicher X. et al. Worldwide dissemination of acquired carbapenem-hydrolysing class D β-lactamases in Acinetobacter spp. other than Acinetobacter baumannii. Int J Antimicrob Agents 2014; 43: 375–7. [DOI] [PubMed] [Google Scholar]
- 22. Villalón P, Valdezate S, Medina-Pascual MJ. et al. Clonal diversity of nosocomial epidemic Acinetobacter baumannii strains isolated in Spain. J Clin Microbiol 2011; 49: 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gogou V, Pournaras S, Giannouli M. et al. Evolution of multidrug-resistant Acinetobacter baumannii clonal lineages: a 10 year study in Greece (2000-09). J Antimicrob Chemother 2011; 66: 2767–72. [DOI] [PubMed] [Google Scholar]
- 24. Zander E, Nemec A, Seifert H. et al. Association between β-lactamase-encoding bla(OXA-51) variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J Clin Microbiol 2012; 50: 1900–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schleicher X, Higgins PG, Wisplinghoff H. et al. Molecular epidemiology of Acinetobacter baumannii and Acinetobacter nosocomialis in Germany over a 5-year period (2005-2009). Clin Microbiol Infect 2013; 19: 737–42. [DOI] [PubMed] [Google Scholar]
- 26. Liakopoulos A, Miriagou V, Katsifas EA. et al. Identification of OXA-23-producing Acinetobacter baumannii in Greece, 2010 to 2011. Euro Surveill 2012; 17: pii=20117. [PubMed] [Google Scholar]
- 27. Mendes RE, Spanu T, Deshpande L. et al. Clonal dissemination of two clusters of Acinetobacter baumannii producing OXA-23 or OXA-58 in Rome, Italy. Clin Microbiol Infect 2009; 15: 588–92. [DOI] [PubMed] [Google Scholar]
- 28. Espinal P, Macià MD, Roca I. et al. First report of an OXA-23 carbapenemase-producing Acinetobacter baumannii clinical isolate related to Tn2006 in Spain. Antimicrob Agents Chemother 2013; 57: 589–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pournaras S, Markogiannakis A, Ikonomidis A. et al. Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit. J Antimicrob Chemother 2006; 57: 557–61. [DOI] [PubMed] [Google Scholar]
- 30. Esposito S, Gioia R, De Simone G. et al. Bacterial epidemiology and antimicrobial resistance in the surgery wards of a large teaching hospital in Southern Italy. Mediterr J Hematol Infect Dis 2015; 7: e2015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marco F, Dowzicky MJ.. Antimicrobial susceptibility among important pathogens collected as part of the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.) in Spain, 2004-2014. J Glob Antimicrob Resist 2016; 6: 50–6. [DOI] [PubMed] [Google Scholar]
- 32. Vourli S, Dafopoulou K, Vrioni G. et al. Evaluation of two automated systems for colistin susceptibility testing of carbapenem-resistant. Acinetobacter baumannii clinical isolates. J Antimicrob Chemother 2017; 72: 2528–30. [DOI] [PubMed] [Google Scholar]
- 33. Pournaras S, Dafopoulou K, Del Franco M. et al. Predominance of international clone 2 OXA-23-producing-Acinetobacter baumannii clinical isolates in Greece, 2015: results of a nationwide study. Int J Antimicrob Agents 2017; 49: 749–53. [DOI] [PubMed] [Google Scholar]
- 34. Magiorakos AP, Srinivasan A, Carey RB. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. [DOI] [PubMed] [Google Scholar]