Abstract
Background
The simian malaria parasite Plasmodium knowlesi is now a well-recognized pathogen of humans in South-East Asia. Clinical infections appear adequately treated with existing drug regimens, but the evidence base for this practice remains weak. The availability of P. knowlesi cultures adapted to continuous propagation in human erythrocytes enables specific studies of in vitro susceptibility of the species to antimalarial agents, and could provide a surrogate system for testing investigational compounds against Plasmodium vivax and other non-Plasmodium falciparum infections that cannot currently be propagated in vitro.
Objectives
We sought to optimize protocols for in vitro susceptibility testing of P. knowlesi and to contrast outputs with those obtained for P. falciparum under comparable test conditions.
Methods
Growth monitoring of P. knowlesi in vitro was by DNA quantification using a SYBR Green fluorescent assay or by colorimetric detection of the lactate dehydrogenase enzyme. For comparison, P. falciparum was tested under conditions identical to those used for P. knowlesi.
Results
The SYBR Green I assay proved the most robust format over one (27 h) or two (54 h) P. knowlesi life cycles. Unexpectedly, P. knowlesi displays significantly greater susceptibility to the dihydrofolate reductase inhibitors pyrimethamine, cycloguanil and trimethoprim than does P. falciparum, but is less susceptible to the selective agents blasticidin and DSM1 used in parasite transfections. Inhibitors of dihydroorotate dehydrogenase also demonstrate lower activity against P. knowlesi.
Conclusions
The fluorescent assay system validated here identified species-specific P. knowlesi drug susceptibility profiles and can be used for testing investigational compounds for activity against non-P. falciparum malaria.
Introduction
One of six species of Plasmodium that infect humans, the zoonotic parasite Plasmodium knowlesi is increasingly recognized as an important contributor to malaria infection in South-East Asia, including Malaysia, Myanmar and Indonesia.1–3 Infections are characterized by the rapid (24 h) schizogonic cycle, can be severe and are occasionally lethal. A thorough understanding of P. knowlesi susceptibility to both existing and pipeline antimalarial therapies is critical.
Thus far, in vitro screening of newly developed antimalarial drugs has been limited to Plasmodium falciparum—the only tractable human malaria species in vitro until the recent adaptation of P. knowlesi to continuous culture in human erythrocytes.4–6 Originally isolated from a Malaysian macaque in the 1960s, the culture-adapted isolate has no history of exposure to antimalarial drugs and provides an unselected genetic background on which to screen new antimalarials by assessment of parasite susceptibility in vitro. As early as 2004, incorporation of [3H]hypoxanthine was used to monitor growth of P. knowlesi cultured in rhesus erythrocytes following exposure to selective agents used for transfection,7 and in human erythrocyte-adapted P. knowlesi cultures.8Ex vivo drug susceptibility has been investigated using the microscopy-based WHO microtest and the colorimetric lactate dehydrogenase (LDH) assay.9 All studies thus far have failed to address key differences in P. knowlesi biology that may reduce applicability of standard assays developed for P. falciparum. These include albumin content of growth media, differences in life cycle length and contrasting multiplication rates. Thus meaningful, adequately controlled comparisons of in vitro drug susceptibility in the two parasite species have yet to be reported.
We assess the susceptibility of P. knowlesi cultured in human red cells against a panel of current and experimental antimalarial agents, in comparison with drug-susceptible P. falciparum (3D7). We evaluate assays using the DNA intercalating fluorescent dye SYBR Green I, and the LDH-based colorimetric assay, to measure parasite growth inhibition in vitro, and in so doing elucidate detailed susceptibility profiles for several compound classes.
Materials and methods
Drugs
Antimalarial compounds were provided by the Medicines for Malaria Venture, Geneva, Switzerland. Drug stocks were prepared in DMSO except chloroquine and blasticidin, which were prepared in sterile distilled water.
Parasite culture
P. knowlesi (A1-H.1 clone) was cultured as described previously with minor modifications.10 Briefly, parasites were maintained at 2% haematocrit in RPMI 1640 supplemented with 25 mM HEPES, 25 mM Na2HCO3, 10 mM d-glucose, 2 mM l-glutamine, 25 mg/L gentamicin sulphate, 50 mg/L hypoxanthine, 5 g/L Albumax II and 10% (v/v) equine serum (Thermo Fisher Scientific, 26050-070). For routine culturing P. falciparum (3D7 clone) was maintained in identical growth medium, supplemented with 2% heat-inactivated human serum (Sigma–Aldrich, H4522) in place of the equine serum. For drug assays, unless stated, both parasite species were grown in the P. knowlesi growth medium/serum mix. Both P. knowlesi and P. falciparum parasites were grown in human A+ blood (National Health Blood and Transplant, UK). Some experiments were performed in blood from Macaca fascicularis, provided by NIBSC (UK) in K2EDTA vacutainers (Becton Dickinson). Parasites were incubated at 37 °C under a culture gas mixture of 93% N2, 4% CO2 and 3% O2.
Synchronization
P. knowlesi schizont culture was adjusted to 50% haematocrit in RPMI medium; 2 mL was layered on top of 5 mL of 55% Nycodenz solution in 10 mM HEPES (pH 7.0) and centrifuged at 900 g for 12 min. The pigmented interphase containing mature parasites was removed and washed in RPMI then returned to culture with fresh red cells.6P. falciparum parasites were synchronized with 5% (w/v) d-sorbitol as described previously.11
Growth inhibition assays
Drug susceptibility of P. knowlesi and P. falciparum was assayed using 96-well flat-bottomed microplates, with 100 μL of parasite stock added to 100 μL of drug dilution in medium per well. Drug-free control wells were included in each experiment and background fluorescence determined in parasite-seeded wells containing a supralethal concentration of chloroquine (10 μM). The plates were incubated at 37 °C in an incubation chamber (Billups-Rothenburg Inc.) under culture gas, and then stored at –20 °C overnight.
Microplates were thawed and incubated with 100 μL of SYBR Green lysis buffer [1:5000 SYBR Green I (Thermo Fisher Scientific, S7563), diluted in 20 mM Tris, 5 mM EDTA, 0.008% (w/v) saponin, 0.08% (v/v) Triton X-100, pH 7.5] in the dark for 1 h, before fluorescence was read in a Spectramax M3 microplate reader (Molecular Devices) at 490 nm excitation and 520 nm emission.
The colorimetric LDH assay was performed as described for P. falciparum.12–15 Briefly, 100 μL of LDH lysis buffer [100 mM Tris–HCl, 200 mM l-lactic acid, 0.2% (v/v) Triton X-100, 125 μM 3-acetylpyridine adenine dinucleotide], 20 μL of nitroblue tetrazolium (1.6 mg/mL) plus phenazine ethosulphate (80 μg/mL) solution and 20 μL of the resuspended parasite preparation were added to each well of a duplicate plate. The plate was developed in the dark for 30–60 min until a clear difference between drug-free controls and background controls was apparent. Parasite growth is measured by accumulation of a blue formazan salt, giving absorbance at 650 nm.9,14,16
Time course
To test for the effect of parasite synchrony on drug responses, we initiated a time course of drug susceptibility assays at 6 or 12 h intervals across the P. knowlesi and P. falciparum life cycles of 27 and 48 h, respectively. Late-stage parasites were synchronized with a 2 h window using sequential Nycodenz purification as described previously.6 New ring stages (0–2 h post-invasion) were diluted to 1% parasitaemia and exposed to drugs (as described above) for one or two life cycles (27 or 54 h for P. knowlesi A1-H.16 and 48 or 96 h for P. falciparum 3D7). From this parasite stock, subsequent drug assays on P. knowlesi were initiated every 6 h for 24 h and on P. falciparum every 12 h for 36 h.
Statistics
Z′ factors were calculated to measure the assay quality as described previously,17 using assay plates containing six negative control wells and six positive control wells. Assays with Z′ values lying between 0.5 and 1.0 are considered indicative of a robust assay performance. P values were calculated using Student’s two-tailed t-test for unpaired or paired samples.
Results and discussion
Effect of starting parasitaemia and haematocrit on non-isotopic growth assays
Although previously used for parasite growth assay in P. knowlesi,7,8,18 the requirement for radiolabelled hypoxanthine and specialized equipment prevent the [3H]hypoxanthine incorporation assay from being widely implemented. We therefore focused our attention on optimization of two non-isotopic methods, namely the fluorometric SYBR Green I assay and the colorimetric LDH enzyme assay, to measure and compare in vitro drug susceptibility between P. knowlesi and P. falciparum.
P. knowlesi and P. falciparum parasites were diluted to a series of starting parasitaemia at 1% haematocrit (Figure 1) or 2% haematocrit (Figure S1, available as Supplementary data at JAC Online). Whilst the P. knowlesi life cycle in vivo is 24 h, the life cycle in vitro takes longer at 27 h, and incubation times were modified accordingly. Cultures were therefore incubated in the presence or absence of drugs for one, two or three complete life cycles: 27, 54 and 81 h for P. knowlesi; 48 and 96 h for P. falciparum.
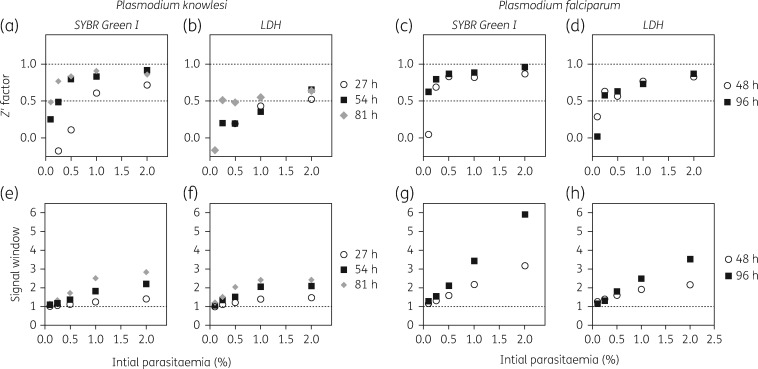
Figure 1.
Influence of starting parasitaemia of P. knowlesi (A1-H.1) and P. falciparum (3D7) on assay quality for both the fluorescent and colorimetric methods. Parasites set to 1% haematocrit and varying parasitaemia (0.1%–2%) were cultured in the presence or absence of a supralethal concentration of chloroquine for 27 h (circles), 54 h (squares) or 81 h (diamonds) for P. knowlesi, and 48 h (cirlces) or 96 h (squares) for P. falciparum. Upon termination of the assay, the plates were read using either the SYBR Green I fluorescence assay (a, c, e and g) or the LDH assay (b, d, f and h). The signal window and Z′ factor were calculated for each assay. The signal window was calculated by dividing the average reading for the drug-free control by the average reading for the high chloroquine concentration (background) control. The assay quality was assessed by determining the Z′ factor using the formula described in Zhang et al.17
For P. knowlesi, the SYBR Green I assay produced high-quality results for a single life cycle exposure (27 h) using a starting parasitaemia of 1% and 1% haematocrit (Figure 1a). Lower starting parasitaemia also generated good-quality assays if exposed for two (54 h) or three (81 h) life cycles. LDH assays starting at 1% parasitaemia/1% haematocrit yielded assays of only borderline quality and parasitaemia below 1% gave unsatisfactory results—thus initiating assays at 2% parasitaemia is preferable for this method (Figure 1b). The signal window improved with longer exposures at all starting parasitaemia for the SYBR Green assay (Figure 1e) and the LDH assay (Figure 1f) but remained <3.0 for both assay methods.
For P. falciparum, good-quality assays were obtained at starting parasitaemia of 0.25% and 1% haematocrit for a single life cycle (48 h) and at 0.1% parasitaemia/1% haematocrit for two life cycles (96 h) using the SYBR Green I method (Figure 1c). Similarly, the LDH assay performed better with P. falciparum down to 0.25% starting parasitaemia (Figure 1d). Again, the signal window improved with higher initial parasitaemia and with longer exposures for both the SYBR Green (Figure 1g) and LDH assays (Figure 1h). The species-specific difference in assay quality for both formats is partly explained by the lower multiplication rate per life cycle for P. knowlesi (3- to 4-fold) compared with P. falciparum (6- to 8-fold). Furthermore, the activity of the LDH enzyme is poorly characterized in P. knowlesi relative to P. falciparum.
Effect of synchrony on drug susceptibility measured across the life cycle
Drug susceptibility testing of P. falciparum is usually initiated using sorbitol-synchronized ring-stage parasites. P. knowlesi is less amenable to sorbitol synchronization, requiring density gradient synchronization instead, and also loses synchrony rapidly in vitro. To examine the effect of synchrony on susceptibility to antimalarial agents, a time course was initiated with synchronized P. knowlesi or P. falciparum exposed to chloroquine, dihydroartemisinin or pyrimethamine for one and two complete life cycles, and results compared between the SYBR Green I fluorescence method (Figure 2) and the colorimetric method (Figure S2).
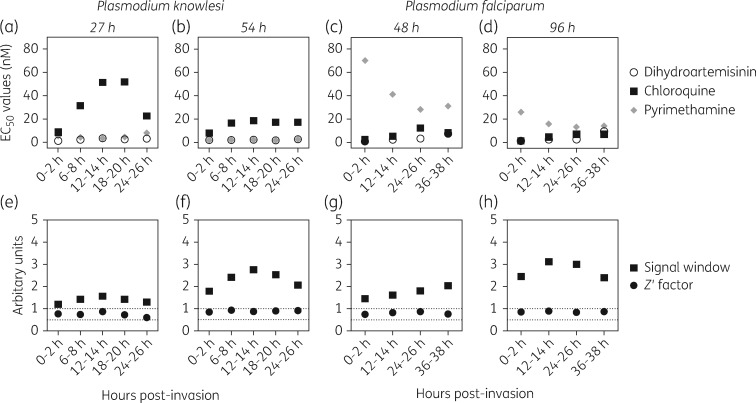
Figure 2.
Effect of synchrony on drug susceptibility measured across the life cycle using the SYBR Green I method after one or two life cycles. EC50 values for chloroquine (squares), dihydroartemisinin (circles) or pyrimethamine (diamonds) were determined from experiments initiated at the times shown on the y-axis using synchronized parasites at 1% parasitemia and 1% haematocrit that were incubated for either 27 h (a) or 54 h (b) for P. knowlesi, and 48 h (c) or 96 h (d) for P. falciparum. Signal window (squares) and assay quality (Z′ factor, circles) (e–h) were determined as in Figure 1.
In the fluorescence assay after one cycle with P. knowlesi, the initial life cycle stage had little effect on the EC50 for either the endoperoxide dihydroartemisinin or the antifolate pyrimethamine, but the EC50 varied dramatically with chloroquine (Figure 2a). For P. falciparum there was relatively little variability in EC50 values for chloroquine and dihydroartemisinin but large differences for pyrimethamine after a single 48 h exposure (Figure 2c). For both species, variability between EC50 values at different initial life cycle stages was markedly reduced when samples were read after two cycles (Figure 2b and d).
The fluorescence method yielded good Z′ factors of between 0.6 and 0.91 for P. knowlesi (Figure 2e and f) and between 0.75 and 0.89 for P. falciparum (Figure 2g and h), supporting the use of the SYBR Green I method for assays initiated at 1% parasitaemia and 1% haematocrit on parasites of varying synchrony. For both species, timing of initiation of the experiment and use of double life cycle exposure were important determinants of quality (Figure 2f versus e and Figure 2h versus g).
Synchronized assays read by the LDH method (Figure S2) showed a similar pattern to those read for the fluorescence assay after one cycle. Highly variable EC50 estimates were obtained with chloroquine in P. knowlesi, and pyrimethamine in P. falciparum, depending on the initial life cycle stage. For P. knowlesi, EC50 values could not be obtained for pyrimethamine in 27 h experiments initiated in early trophozoites (Figure S2A), even though estimates were readily obtained using the SYBR Green I method (Figure 2a). This suggests that short exposures of pyrimethamine were able to inhibit DNA replication but not LDH activity in P. knowlesi. By the second life cycle exposure LDH activity was inhibited at higher drug concentrations and all assays yielded EC50 values (Figure S2B). Similarly, one of the P. falciparum curves for pyrimethamine failed to yield an EC50 estimate after 48 h exposure but was able to generate data after 96 h exposure. This is clearly a weakness of enzyme-based assays for measuring parasite growth and may lead to otherwise active, potent compounds being incorrectly rejected if the timing or duration of exposure is non-optimal. As in the previous assays, two-cycle experiments greatly reduced any variation in EC50 caused by altering the initial life cycle stage.
Whilst the LDH assays performed on P. falciparum were of good quality (Figure S2G and H), the assays on P. knowlesi performed poorly with a small signal window and low Z′ factor after a single life cycle (<2; Figure S2E). Signal and assay quality improved with double life cycle exposure (Z′ range = 0.51–0.77; Figure S2F). This suggests that, for P. knowlesi, the LDH assay is not ideal for short-exposure drug assays initiated at 1% parasitaemia and 1% haematocrit. The LDH assay is suitable for P. falciparum drug assays but caution is needed when examining the effect of antifolates such as pyrimethamine. Although EC50 results for synchronous single-cycle experiments varied dramatically depending on the initial life cycle stage in P. knowlesi (Figure 2a), the mean EC50 obtained from these synchronized assay data closely approximated the EC50 estimates from experiments on non-synchronous parasites (Figure S3). Thus, in addition to being logistically simpler, the non-synchronous experiments can ameliorate the variation observed due to stage-specific effects in synchronous experiments. Considering this, and the variable performance of the LDH platform, all subsequent susceptibility testing in P. knowlesi deployed the non-synchronous fluorescent SYBR Green I method.
Activity of antimalarial agents
Using starting conditions of 1% parasitaemia and 1% haematocrit, we compared the drug susceptibility of P. knowlesi and P. falciparum (3D7) exposed for one complete life cycle. As P. knowlesi requires media heavily supplemented with Albumax/serum, all EC50 experiments were carried out in the P. knowlesi media, which readily supports growth of both parasite species. This removes the confounding effect of serum protein levels on EC50 estimates for certain drugs (e.g. atovaquone; Table S1).
The susceptibility of P. knowlesi to the 4-aminoquinolines and amino-alcohols was similar to that of P. falciparum (Table 1). All EC50 estimates for P. knowlesi fell below 100 nM and within 2.5-fold of the EC50 reported for P. falciparum (Table 1). Although the EC50 differences were not large between species, several were statistically significant (P ≤ 0.0424). Ferroquine, currently in Phase II trials, was highly potent against P. knowlesi (12.2 nM; Table 1).
Table 1.
Comparison of the antiplasmodial activity against P. knowlesi or P. falciparum, assessed using the SYBR Green I assay, for a set of clinical and experimental antimalarials exposed over one complete life cycle
| Compound | EC50 values (nM) |
Fold difference (P. falciparum/P. knowlesi) | Pa | |
|---|---|---|---|---|
| P. knowlesi (A1-H.1), 27 h exposure | P. falciparum (3D7), 48 h exposure | |||
| 4-Aminoquinolines and amino-alcohols | ||||
| chloroquine | 29.3 ± 4.7 | 15.9 ± 3.0 | 0.54 | 0.0303 |
| amodiaquine | 9.3 ± 1.7 | 5.9 ± 0.6 | 0.63 | 0.0662 |
| desethylamodiaquine | 12.4 ± 1.4 | 12.4 ± 3.1 | 1.00 | 0.9973 |
| quinine | 54.8 ± 3.0 | 57.9 ± 6.9 | 1.06 | 0.7177 |
| mefloquine | 10.9 ± 1.1 | 26.2 ± 4.2 | 2.40 | 0.0090 |
| lumefantrine | 90.4 ± 13 | 152 ± 26 | 1.68 | 0.0424 |
| piperaquine | 21.0 ± 3.1 | 39.8 ± 4.9 | 1.90 | 0.0115 |
| pyronaridine | 10.7 ± 1.6 | 4.4 ± 1.6 | 0.41 | 0.0268 |
| ferroquineb | 12.2 ± 1.6 | 4.7 ± 0.6 | 0.39 | 0.0068 |
| Endoperoxides | ||||
| dihydroartemisinin | 2.0 ± 0.3 | 4.2 ± 0.5 | 2.10 | 0.0098 |
| artesunate | 10.9 ± 1.7 | 9.0 ± 1.5 | 0.83 | 0.4280 |
| OZ439b | 6.6 ± 1.4 | 7.4 ± 1.2 | 1.12 | 0.6750 |
| DHFR inhibitors | ||||
| pyrimethamine | 5.1 ± 0.8 | 54.0 ± 5.0 | 10.6 | <0.0001 |
| cycloguanil | 1.3 ± 0.3 | 11.8 ± 0.6 | 9.08 | <0.0001 |
| trimethoprim | 265±47 | 3098 ± 229 | 11.7 | <0.0001 |
| P218b | 4.1±0.7 | 3.5 ± 0.2 | 0.85 | 0.4884 |
| Transfection reagents | ||||
| WR99210c | 0.16 ± 0.04 | 0.43 ± 0.03 | 2.69 | 0.0003 |
| blasticidinc | 31684 ± 3485 | 1413 ± 190 | 0.04 | <0.0001 |
| DSM1c | 509 ± 11 | 149 ± 5 | 0.29 | <0.0001 |
| Other | ||||
| primaquine | 3871 ± 887 | 5627 ± 1195 | 1.45 | 0.2847 |
| atovaquone | 2.6 ± 0.4 | 2.3 ± 0.5 | 0.88 | 0.6366 |
EC50 values are reported as mean ± SEM from at least three experiments, and some up to eight repeats, each performed in duplicate.
P values are calculated by comparing EC50 values for P. falciparum versus P. knowlesi using Student’s two-tailed unpaired t-test.
These agents are undergoing development for their potential use as antimalarial agents (http://www.mmv.org/research-development/interactive-rd-portfolio).
These compounds are used in transfection studies with P. falciparum to select for parasites that harbour plasmids carrying drug resistance cassettes.
Presently, artemisinin-based combination therapy is recommended for the treatment of uncomplicated P. knowlesi malaria.19 Artesunate, dihydroartemisinin and a synthetic endoperoxide, OZ439, were all highly potent against both parasite species, with P. knowlesi significantly more susceptible to dihydroartemisinin than P. falciparum (Table 1; P = 0.0098).
Interestingly, we found P. knowlesi parasites to be highly susceptible to dihydrofolate reductase (DHFR) inhibitors, being more than 9-fold more susceptible to pyrimethamine, cycloguanil and trimethoprim than the drug-susceptible P. falciparum line tested here. However, both species showed similar susceptibility (∼4 nM) to the new DHFR inhibitor P218, designed to overcome resistant forms of the P. falciparum enzyme.20 Thus existing medicines such as sulfadoxine/pyrimethamine may prove to be very effective agents against P. knowlesi, both for treatment and prophylaxis. Future studies should explore the impact of both DHFR and dihydropteroate synthase inhibitors on P. knowlesi metabolism in depth, the latter requiring specialized growth media sufficiently depleted of folate and para-aminobenzoic acid,21,22 and so not tested here.
Transfection reagents
We tested P. knowlesi susceptibility to three common selective agents used to favour growth of transfected P. falciparum parasites harbouring exogenous DNA. The DHFR inhibitor WR99210 was highly potent against P. knowlesi with an EC50 value of 0.16 ± 0.04 nM. Similar to other DHFR inhibitors tested, WR99210 was significantly more potent against P. knowlesi than against P. falciparum (0.43 ± 0.03 nM; P = 0.0003). Blasticidin was 22-fold less potent against P. knowlesi when compared with P. falciparum (Table 1) over a single life cycle, which is consistent with a previous report,7 in which P. knowlesi H strain was grown in rhesus erythrocytes. Reduced susceptibility of P. knowlesi to blasticidin prevents its use as a selectable marker at the concentrations generally used for transfection studies. Similarly, P. knowlesi was also 3-fold less susceptible than 3D7 to DSM1 (Table 1); dihydroorotate dehydrogenase (DHODH)-containing plasmid selection with DSM1 needs to be conducted at higher concentrations for this species.
P. knowlesi and P. falciparum were both highly susceptible to the mitochondrial cytochrome b inhibitor atovaquone (Table 1), but both poorly susceptible to primaquine in vitro with EC50 values at micromolar concentrations.
DHODH inhibitors
The DHODH enzyme is a newly validated antimalarial target.18,23–25 Several inhibitors of this enzyme have been identified and the two most advanced, DSM421 and DSM265, are currently in preclinical and Phase II trials.23,25 Considering the reduced potency of the transfection reagent DSM1 against P. knowlesi versus P. falciparum, we tested other DHODH inhibitors against P. knowlesi (Table 2). All compounds were ≥2.7-fold more potent against P. falciparum than against P. knowlesi whether tested over one or two life cycles. In particular, DSM265 was 8-fold more potent against P. falciparum (Table 2). This supports previous observations suggesting that P. knowlesi was less susceptible to DHODH inhibitors than P. falciparum.18 However, in that study the two species were tested under completely discordant assay conditions. Whether P. knowlesi is less susceptible than P. falciparum to the effects of DHODH inhibitors because of differences in enzyme activity, access to the enzyme or fundamental differences in the biology of these parasites remains to be established. Of note, DSM265 was shown to be about 5-fold more active against P. falciparum field isolates than Plasmodium vivax field isolates ex vivo, while DSM421 was equipotent against both species,25 reinforcing the relevance of using P. knowlesi drug susceptibility testing to inform drug development for P. vivax.4
Table 2.
Susceptibility of P. knowlesi and P. falciparum to three DHODH inhibitors assessed using the SYBR Green I assay
| DHODH inhibitor | EC50 (nM), single life cycle |
EC50 (nM), two life cycles |
||||
|---|---|---|---|---|---|---|
| P. knowlesi, 27 h | P. falciparum, 48 h | fold difference | P. knowlesi, 54 h | P. falciparum, 96 h | fold difference | |
| DSM1 | 509 ± 11 | 149 ± 5 | 3.4 | 417 ± 2 | 91 ± 10 | 4.6 |
| DSM265 | 303 ± 15 | 37 ± 3 | 8.2 | 186 ± 11 | 21 ± 1 | 8.9 |
| DSM421 | 194 ± 23 | 72 ± 5 | 2.7 | 123 ± 10 | 42 ± 4 | 2.9 |
All inhibitors were tested in duplicate on three separate occasions from a starting parasitaemia and haematocrit of 1%. The EC50 values are reported as the mean ± SEM. The fold difference is calculated by dividing the P. knowlesi EC50 value by the P. falciparum EC50 value. For each DSM compound, the mean P. falciparum EC50 value was significantly lower than the mean P. knowlesi EC50 value when compared over either a single parasite life cycle or over two life cycles (P ≤ 0.0018).
Delayed death effect
Antibacterial agents, such as azithromycin and clindamycin, have been shown to exert potent activity against P. falciparum in vitro but only after two complete asexual life cycles (96 h).26 This phenomenon is referred to as the delayed death effect, and has also been reported for clindamycin against P. knowlesi in vitro.8 In our experiments, a delayed death effect in P. knowlesi is confirmed for clindamycin, doxycycline and azithromycin (Table 3). EC50 values for P. knowlesi were measured over three life cycles (81 h), as additional time was required to resolve the full delayed drug effect in our experiments using unsynchronized cultures. For P. falciparum parasites the assay used synchronized parasites, and therefore, two cycles (96 h) were sufficient to detect the delayed death effect. Azithromycin was equally potent between species over a single life cycle (P = 0.4397) and not significantly different in its delayed death effect (P = 0.2514). Similarly, clindamycin had no measurable effect over a single life cycle in either species but was very potent against P. knowlesi (15.9 nM) and P. falciparum (7.0 nM) over 81 and 96 h, respectively. For doxycycline the delayed death potency for P. knowlesi was much reduced (2061 nM) relative to P. falciparum (623 nM). We noted that the delayed death curves did not level out to 0% viability but were asymptotic at about 25% viability, presumably due to the greater amount of residual DNA from parasites surviving the first cycle of growth compared with the chloroquine control wells in which parasites die in the first cycle. This was corrected for clindamycin and doxycycline, but not azithromycin, by using a background control generated for the second cycle only (Figure S4).
Table 3.
Delayed death effect of three antibacterial agents against P. knowlesi and P. falciparum assessed using the SYBR Green I assay
| Antibacterial |
P. knowlesi EC50 (nM) |
P. falciparum EC50 (nM) |
||||
|---|---|---|---|---|---|---|
| 27 h | 81 h | fold difference | 48 h | 96 h | fold difference | |
| Azithromycin | 5662 ± 725 | 31.9 ± 10 | 177 | 6003 ± 323 | 19.2 ± 4 | 313 |
| Doxycycline | >10000 | 2061 ± 343 | >4.9 | >10000 | 623 ± 148 | >16 |
| Clindamycin | >10000 | 15.9 ± 4 | >629 | >10000 | 7.0 ± 1.0 | >1429 |
All antibacterial agents were screened in duplicate on at least three separate occasions from a starting parasitaemia and haematocrit of 1%. The EC50 values are reported as the mean ± SEM. The fold difference for each compound is calculated by dividing the EC50 value after three life cycles (for P. knowlesi) or two life cycles (for P. falciparum) by the EC50 value measured after a single life cycle exposure.
Drug susceptibility of P. knowlesi grown in human versus macaque blood
We assessed the effect of culturing P. knowlesi parasites in human versus macaque erythrocytes on susceptibility to a subset of antimalarials (Table 4). No significant host-specific differences in potency were observed, although it was evident that the EC50 values were generally higher in parasites grown in macaque erythrocytes (Table 4). This could be related to higher growth rates of P. knowlesi parasites in macaque cells, estimated at 5- to 7-fold compared with 3- to 4-fold in human erythrocytes.6
Table 4.
Comparison of the susceptibility of P. knowlesi grown in either human or macaque red blood cells to selected antimalarial agents assessed using the SYBR Green I assay
| Antimalarial | EC50 (nM) |
Fold difference | P | |
|---|---|---|---|---|
| human blood | macaque blood | |||
| Chloroquine | 24.6 ± 3.3 | 42.0 ± 8.1 | 0.59 | 0.2114 |
| Dihydroartemisinin | 1.9 ± 0.2 | 4.1 ± 0.7 | 0.46 | 0.1250 |
| Quinine | 48.0 ± 6.7 | 54.7 ± 1.4 | 0.88 | 0.4303 |
| Mefloquine | 11.0 ± 1.5 | 18.3 ± 0.2 | 0.60 | 0.0502 |
| Pyrimethamine | 8.9 ± 0.8 | 13.2 ± 2.7 | 0.67 | 0.2940 |
Antimalarial agents were screened in duplicate on three separate occasions from a starting parasitaemia and haematocrit of 1%. The EC50 values are reported as the mean ± SEM. The fold difference for each compound is calculated by dividing the EC50 in human blood by the EC50 value in macaque blood measured after a single life cycle exposure (27 h).
The P. knowlesi A1-H.1 is descended from a 1964 macaque isolate and is assumed to be drug susceptible.27,28 Using identical growth media and viability readouts, we expected to find EC50 estimates very similar to those for P. falciparum 3D7 for most, if not all, antimalarials tested. The unexpected differences in susceptibility to DHFR inhibitors (pyrimethamine, cycloguanil and trimethoprim) and DHODH inhibitors suggest that important species-specific differences in drug responses exist. A recent study reported the in vitro activity of the 400 compound Malaria Box against P. falciparum 3D7 and showed that 90% were also active against P. knowlesi yH-1 strain.29 Closer examination of those data show that EC50 estimates for 52 compounds were at least 3-fold higher or lower for P. knowlesi than for P. falciparum.
Conclusions
We have provided detailed validation of a fluorescent assay system for drug susceptibility testing in P. knowlesi. This provides an important new tool for in vitro drug studies in non-P. falciparum malaria. Significant species-specific differences in susceptibility to certain compound classes was observed, highlighting the added value of in vitro screens against additional human malaria pathogens. The generalizability of our findings should now be tested in recent P. knowlesi field isolates from geographically distinct regions of South-East Asia.
Supplementary Material
Acknowledgements
We wish to thank Eloise Walker and Franziska Mohring for assistance and advice with parasite cultures and Dr Jeremy Burrows (MMV) for his critical review of the manuscript prior to submission. We would also like to thank Dr Neil Almond and Jo Hall (NIBSC, Potters Bar, UK) for the supply of normal blood samples from M. fascicularis used in these studies.
Funding
This project was funded by the Medicines for Malaria Venture, grant MMV RD/15/0017. R. W. M. is supported by a Medical Research Council (MRC) Career Development Award jointly funded by the UK MRC and UK Department for International Development. C. J. S. is supported by Public Health England.
Transparency declarations
B. B. is an employee of the funder, MMV. All other authors: none to declare.
Author contributions
C. J. S. and R. W. M. conceived and designed the study. D. A. v. S. performed the experiments. D. A. v. S., R. W. M., B. B. and C. J. S. analysed the data and wrote the paper. All authors read and approved the final manuscript.
Supplemetary data
Figures S1–S4 and Table S1 are available as Supplementary data at JAC Online.
References
- 1. William T, Jelip J, Menon J. et al. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J 2014; 13: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghinai I, Cook J, Hla TT. et al. Malaria epidemiology in central Myanmar: identification of a multi-species asymptomatic reservoir of infection. Malar J 2017; 16: 16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lubis IN, Wijaya H, Lubis M. et al. Contribution of Plasmodium knowlesi to multi-species human malaria infections in North Sumatera, Indonesia. J Infect Dis 2017; 215: 1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gruring C, Moon RW, Lim C. et al. Human red blood cell-adapted Plasmodium knowlesi parasites: a new model system for malaria research. Cell Microbiol 2014; 16: 612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim C, Hansen E, DeSimone TM. et al. Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nat Commun 2013; 4: 1638.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moon RW, Hall J, Rangkuti F. et al. Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc Natl Acad Sci USA 2013; 110: 531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wel A, Kocken CH, Pronk TC. et al. New selectable markers and single crossover integration for the highly versatile Plasmodium knowlesi transfection system. Mol Biochem Parasitol 2004; 134: 97–104. [DOI] [PubMed] [Google Scholar]
- 8. Arnold MS, Engel JA, Chua MJ. et al. Adaptation of the [3H]hypoxanthine uptake assay for in vitro-cultured Plasmodium knowlesi malaria parasites. Antimicrob Agents Chemother 2016; 60: 4361–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fatih FA, Staines HM, Siner A. et al. Susceptibility of human Plasmodium knowlesi infections to anti-malarials. Malar J 2013; 12: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moon RW, Sharaf H, Hastings CH. et al. Normocyte-binding protein required for human erythrocyte invasion by the zoonotic malaria parasite Plasmodium knowlesi. Proc Natl Acad Sci USA 2016; 113: 7231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 1979; 65: 418–20. [PubMed] [Google Scholar]
- 12. Bennett TN, Paguio M, Gligorijevic B. et al. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother 2004; 48: 1807–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corbett Y, Herrera L, Gonzalez J. et al. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am J Trop Med Hyg 2004; 70: 119–24. [PubMed] [Google Scholar]
- 14. Makler MT, Ries JM, Williams JA. et al. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg 1993; 48: 739–41. [DOI] [PubMed] [Google Scholar]
- 15. Smilkstein M, Sriwilaijaroen N, Kelly JX. et al. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 2004; 48: 1803–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg 1993; 48: 205–10. [DOI] [PubMed] [Google Scholar]
- 17. Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 1999; 4: 67–73. [DOI] [PubMed] [Google Scholar]
- 18. Booker ML, Bastos CM, Kramer ML. et al. Novel inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase with anti-malarial activity in the mouse model. J Biol Chem 2010; 285: 33054–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO. Guidelines for the Treatment of Malaria 3rd edn. 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/.
- 20. Yuthavong Y, Tarnchompoo B, Vilaivan T. et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci USA 2012; 109: 16823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milhous WK, Weatherly NF, Bowdre JH. et al. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob Agents Chemother 1985; 27: 525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang P, Sims PF, Hyde JE. A modified in vitro sulfadoxine susceptibility assay for Plasmodium falciparum suitable for investigating Fansidar resistance. Parasitology 1997; 115: 223–30. [DOI] [PubMed] [Google Scholar]
- 23. Phillips MA, Lotharius J, Marsh K. et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci Transl Med 2015; 7: 296ra111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phillips MA, Rathod PK. Plasmodium dihydroorotate dehydrogenase: a promising target for novel anti-malarial chemotherapy. Infect Disord Drug Targets 2010; 10: 226–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phillips MA, White KL, Kokkonda S. et al. A triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with improved drug-like properties for treatment and prevention of malaria. ACS Infect Dis 2016; 2: 945–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dahl EL, Rosenthal PJ. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother 2007; 51: 3485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Assefa S, Lim C, Preston MD. et al. Population genomic structure and adaptation in the zoonotic malaria parasite Plasmodium knowlesi. Proc Natl Acad Sci USA 2015; 112: 13027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins WE, Contacos PG, Skinner JC. et al. Fluorescent antibody studies on simian malaria. I. Development of antibodies to Plasmodium knowlesi. Am J Trop Med Hyg 1967; 16: 1–6. [DOI] [PubMed] [Google Scholar]
- 29. Paul AS, Moreira CK, Elsworth B. et al. Extensive shared chemosensitivity between malaria and babesiosis blood-stage parasites. Antimicrob Agents Chemother 2016; 60: 5059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.