Abstract
Marijuana use is associated with psychosis, but its effects are understudied in individuals with preexisting risk for psychotic disorders. This preliminary study examined the acute psychological and physiological effects of smoked marijuana (0.0% or 5.5% Δ9-THC) in marijuana users at clinical high-risk (CHR; n = 6) to develop a psychotic disorder, and those not at risk (n = 6), under controlled laboratory conditions. CHR marijuana users exhibited temporary increases in psychotic-like states and decreases in neurocognitive performance during marijuana intoxication but control marijuana smokers did not. These findings, if replicated, may support a psychotogenic role for marijuana in CHR individuals.
Keywords: Cannabis, Prodromal psychosis, Ultra high-risk
1. Introduction
The association between marijuana smoking and the development of psychotic disorders (Gage et al., 2016) is concerning, but the cause-and-effect relationship between marijuana use and psychosis is not well-established (Auther et al., 2015; Haney and Evins, 2016). This impedes the development of clinical recommendations and interventions. Marijuana use and use disorder are common (Auther et al., 2015) in those at clinical high-risk (CHR) for a psychotic disorder (i.e., adolescents or young adults who exhibit attenuated psychotic symptoms and/or familial predisposition, with recent psychosocial decline or chronic low functioning; McGlashan et al., 2001). In this population, increases in reported marijuana use are associated with exacerbations of psychotic-like symptoms (Corcoran et al., 2008), both of which are predictive of psychosis onset (McHugh et al., 2016; Valmaggia et al., 2014).
In laboratory studies, administration of marijuana or its primary psychoactive component (Δ9-THC) may acutely produce psychotic-like and neurocognitive effects, with its most robust effects in participants with psychotic disorders (Sherif et al., 2016). However, to our knowledge no laboratory study has directly examined marijuana effects in CHR individuals, who have a distinct and appreciable risk to develop a psychotic disorder (Fusar-Poli et al., 2012). Further, existing laboratory studies in the psychosis spectrum may be limited in terms of ecological relevance and/or nonstandard measurement of marijuana effects (e.g., D'Souza et al., 2004). Therefore, in this preliminary study, we characterize the psychological and cardiovascular effects of active and placebo marijuana cigarettes (containing 5.5% and 0.0% Δ9-THC, respectively) in CHR and control marijuana users, under controlled laboratory conditions.
2. Methods1
This study was approved by the NYS Psychiatric Institute IRB and the NYS Office of Mental Health, and all participants provided written informed consent.
2.1. Participants
Participants were an ethnically-diverse sample (n = 12) of physically-healthy weekly marijuana users with minimal use of other illicit substances (verified by urine toxicology). They reported no prior serious adverse reactions to marijuana and denied seeking treatment for marijuana use. Half (n = 6; 5 M, 1 F) were a clinical sample that met operationalized criteria for a CHR syndrome (McGlashan et al., 2001), while the rest (n = 6; 4 M, 2 F) did not (controls). The groups were similar in demographic characteristics and marijuana/alcohol use patterns (p > 0.05), but not psychopathology (p < 0.05; see Supplemental [S] Table 1).
2.2. Procedures
During each laboratory session, participants completed a battery of subjective (e.g., visual analogue scale), neurocognitive (Keilp et al., 2014) and cardiovascular measures, smoked 50% of an active or placebo marijuana cigarette (provided by NIDA) according to a standardized procedure (e.g., Haney et al., 2016), and repeated the measures (Table S2). The strength of marijuana tested was randomized and double-blinded.
2.3. Statistical analyses
Due to the limited sample size, acute effects were examined for each group independently. Repeated measures ANOVA assessed the main effects of drug condition (active vs. placebo) and the drug condition × time (baseline vs. post-marijuana) interactions on dependent measures, with t-tests to probe significant interactions. Alpha was set at 0.05.
3. Results
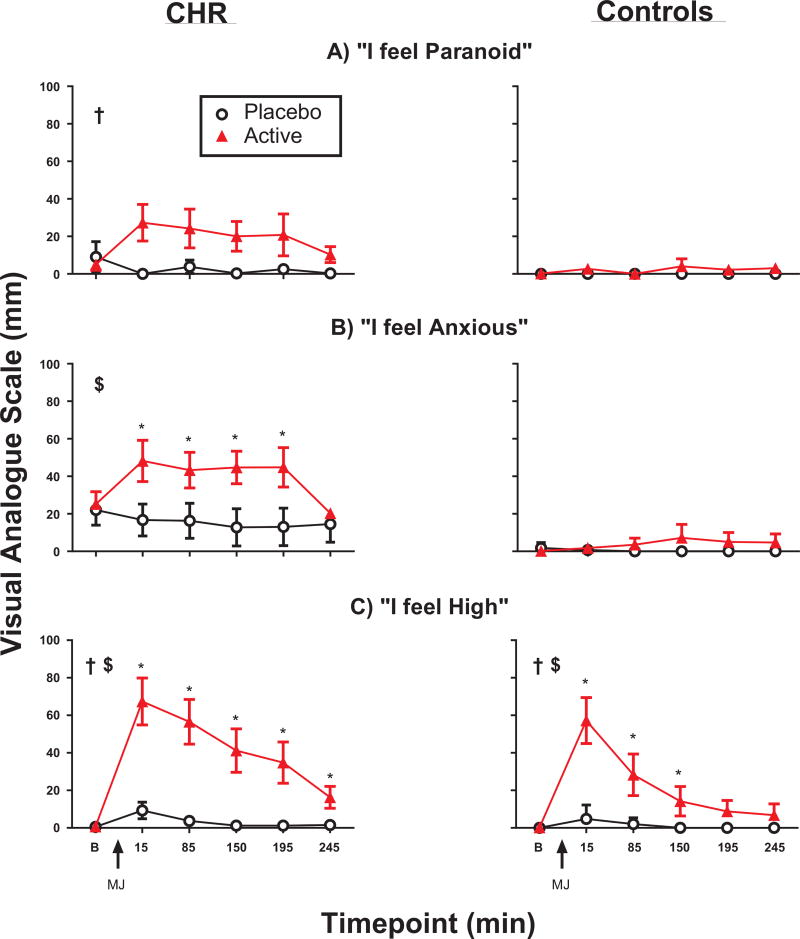
In the CHR group, active marijuana (relative to placebo) increased (p < 0.05) subjective ratings of paranoia (Fig. 1A), anxiety (1B), slowed time perception, visual illusions, feelings of strangeness and inattention (Table S3a), and decreased (p < 0.05) objective performance on tasks of working memory and response inhibition (Table S4a). These effects were not observed (p > 0.05) in the control group (Fig. 1A–B, Tables S3b–4b). In both groups, active marijuana increased (p < 0.05) measures of intoxication (“High”) and arousal (heart rate, “Stimulated”), relative to placebo (Fig. 1C and Table S3, respectively). Active marijuana did not significantly affect (p > 0.05) subjective ratings of auditory or visual hallucinations, extra-sensory perception, depression, loneliness, or quickened time perception (data not shown), or objective measures of sustained attention or complex reaction time (Table S4), in either group. The marijuana was relatively well tolerated, with only 1 temporary adverse reaction (e.g., dizziness, nausea) in each group.
Fig. 1.
Selected subjective ratings before and after marijuana administration as a function of group (Clinical High-Risk [CHR], left side; Controls, right side). On x-axis: B = baseline; MJ = marijuana administration. Error bars reflect SEM. †main effect of drug condition; $drug condition × time interaction; *active (5.5% THC) differs from placebo (0.0% THC); p < 0.05. Full ANOVA results in Table S2.
4. Discussion
The results of this preliminary study are the first demonstration, to our knowledge, of marijuana producing some psychotic-like and neurocognitive effects under controlled conditions selectively in CHR individuals. These effects are consistent with the results of laboratory and naturalistic studies in samples of other populations within the broad psychosis spectrum (Mason et al., 2009; Sherif et al., 2016; Verdoux et al., 2003), and extend the laboratory results to those who are at heightened and imminent risk for psychotic disorders. The clinical relevance of the psychotic-like effects is demonstrated by a larger study (McHugh et al., 2016) that found that those CHR patients who endorsed experiencing marijuana-induced psychotic-like effects in the natural environment were almost 5 times more likely to develop a subsequent psychotic disorder than those who did not. Convergently, in the current study we observed apparently stronger marijuana effects (including psychotic-like) in the two CHR individuals who eventually developed a psychotic disorder than those who did not (see Fig. S1). Further, the neurocognitive functions affected by marijuana in this study were relatively higher-order functions that are considered uniquely relevant to psychosis development (Lewis et al., 2004; Vadhan et al., 2009).
The lack of psychotic-like and neurocognitive effects in the controls contrasts with other results (e.g., Bhattacharyya et al., 2012), but these earlier studies employed nonsmoking routes of drug administration and/or participants with minimal or varied prior experience with marijuana, which may impact its acute effects (D'Souza et al., 2008; Ramaekers et al., 2009). These factors were accounted for in the current study by employing smoked marijuana administration in experienced users. The seemingly aversive effects of marijuana in the CHR participants raise questions about their motivation for regular marijuana use. Marijuana's robust intoxication and arousing effects and its potential interaction with altered dopamine function (Kuepper et al., 2013; Mizrahi et al., 2014) in this population, suggest that coping with anhedonia should be examined as a possible motivation (Cressman et al., 2015; Gill et al., 2015).
These conclusions are limited by the sample size, which was constrained by the difficulty in recruiting eligible CHR marijuana smokers (i.e., regular users that were nontreatment-seeking and physically healthy), and the employment of multiple parametric comparisons. Although these methodological characteristics are consistent with other investigations involving acute administration of Δ9-THC to participants on the psychosis spectrum (e.g., D'Souza et al., 2005; Kuepper et al., 2013), the findings still should be viewed as preliminary. In sum, these results indicate the feasibility of marijuana administration research in the CHR population, and the possibility that the psychotic-like effects of marijuana may relate to an individual's preexisting level of risk for psychotic disorders. Replication research on this topic may be warranted.
Supplementary Material
Acknowledgments
Funding
All data were collected at the New York State Psychiatric Institute and an earlier version of this manuscript was written while the primary author was at Stony Brook University. We gratefully acknowledge the assistance of Elisa Payne, PhD, Olivia Wu, MA, Kristy Nguyen, MA, Michael Harakas, BA, Sarah Badach, BA, Ashley Danies, MA, Olivia Derella, MA, Bennett Wechsler, BS, Richard Foltin, PhD, Ragy Girgis, MD, Gary Brucato, PhD, Kelly Gill, MA, Shelly Ben-David, MA, Leigh Arndt, MA, Rachel Blair, MA, and Danusha Selva Kumar, BA for their study contributions. The authors thank Drs. Salil Vadhan, Christine DeLorenzo and Joseph Schwartz, and Rajapillai Pillai, for their comments on the earlier version of this manuscript.
Funding for this study was provided by a Brain and Behavior Research Foundation Young Investigator Award, NIH grants DA19239, DA09236, DA034877, MH086125, MH093398 and CUMC Irving Scholar Awards.
Footnotes
Full details on methodology, results and safety can be found in the online supplement.
All authors declare that they have no conflicts of interest.
Portions of this research were presented at the 2014 annual meeting of the American Psychiatric Association and the 2016 NIH Marijuana and Cannabinoids Neuroscience Research Summit.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.psychres.2017.07.070.
References
- Auther AM, Cadenhead KS, Carrión RE, Addington J, Bearden CE, Cannon TD, et al. Alcohol confounds relationship between cannabis misuse and psychosis conversion in a high-risk sample. Acta Psychiatr. Scand. 2015;132(1):60–68. doi: 10.1111/acps.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa J, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, et al. Induction of psychosis by Δ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch. Gen. Psychiatry. 2012;69(1):27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Kimhy D, Stanford A, Khan S, Walsh J, Thompson J, et al. Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophr. Res. 2008;20(2–3):286–293. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Schobel SA, Steinfeld S, Ben-David S, Thompson JL, Small SA, et al. Anhedonia in the psychosis risk syndrome: associations with social impairment and basal orbitofrontal cortical activity. Npj Schizophr. 2015;1:15020. doi: 10.1038/npjschz.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol. Psychiatry. 2005;57(6):594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33(10):2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol. Psychiatry. 2016;79(7):549–556. doi: 10.1016/j.biopsych.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Gill KE, Poe L, Azimov N, Ben-David S, Vadhan NP, Girgis R, et al. Reasons for cannabis use among youths at ultra high risk for psychosis. Early Interv. Psychiatry. 2015;9(3):207–210. doi: 10.1111/eip.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393–401. doi: 10.1038/npp.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41(8):1974–1982. doi: 10.1038/npp.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Beers SR, Burke AK, Melhem NM, Oquendo MA, Brent DA, et al. Neuropsychological deficits in past suicide attempters with varying levels of depression severity. Psychol. Med. 2014;44(14):2965–2974. doi: 10.1017/S0033291714000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuepper R, Ceccarini J, Lataster J, van Os J, van Kroonenburgh M, van Gerven JMA, et al. Delta-9-tetrahydrocannabinol-induced dopamine release as a function of psychosis risk: 18F-fallypride positron emission tomography study. PLoS One. 2013;8(7):e70378. doi: 10.1371/journal.pone.0070378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia.[see comment] Ann. N.Y. Acad. Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Mason O, Morgan CJA, Dhiman SK, Patel A, Parti N, Curran HV. Acute cannabis use causes increased psychotomimetic experiences in individuals prone to psychosis. Psychol. Med. 2009;39(06):951–956. doi: 10.1017/S0033291708004741. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L. Instrument for the assessment of prodromal symptoms and states. In: Miller T, Mednick S, Chauncey DL, Libiger T, Johanssen J, editors. Early Intervention in Psychotic Disorders. Kluwer Academic Publishers; Amsterdam: 2001. pp. 135–149. [Google Scholar]
- McHugh MJ, McGorry PD, Yung AR, Lin A, Wood SJ, Hartmann JA, et al. Cannabis-induced attenuated psychotic symptoms: implications for prognosis in young people at ultra-high risk for psychosis. Psychol. Med. 2016:1–11. doi: 10.1017/S0033291716002671. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology. 2014;39(6):1479–1489. doi: 10.1038/npp.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J. Psychopharmacol. 2009;23(3):266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Sherif M, Radhakrishnan R, D’Souza DC, Ranganathan M. Human laboratory studies on cannabinoids and psychosis. Biol. Psychiatry. 2016;79(7):526–538. doi: 10.1016/j.biopsych.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Serper MR, Haney M. Acute effects of smoked marijuana on working memory: implications for schizophrenia? Prim. Psychiatry. 2009;16(4):51–59. [PMC free article] [PubMed] [Google Scholar]
- Valmaggia LR, Day FL, Jones C, Bissoli S, Pugh C, Hall D, et al. Cannabis use and transition to psychosis in people at ultra-high risk. Psychol. Med. 2014;44(12):2503–2512. doi: 10.1017/S0033291714000117. [DOI] [PubMed] [Google Scholar]
- Verdoux H, Gindre C, Sorbara F, Tournier M, Swendsen JD. Effects of cannabis and psychosis vulnerability in daily life: an experience sampling test study.[see comment] Psychol. Med. 2003;33(1):23–32. doi: 10.1017/s0033291702006384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.