Abstract
BACKGROUND
Angiotensin converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARB), beta-blockers and statins are recommended after acute myocardial infarction (AMI). Patients may adhere to some but not all therapies.
OBJECTIVE
We investigated the effect of trade-offs in adherence to ACEI/ARBs, beta-blockers, and statins on survival among older people after AMI.
METHODS
We identified 90,869 Medicare beneficiaries aged ≥65 who had prescription of ACEI/ARBs, beta-blockers and statins and survived ≥180 days after AMI hospitalization in 2008−2010. Adherence was measured by proportion of days covered (PDC) during 180 days following hospital discharge. Mortality follow-up extended up to 18 months after this period. We used Cox proportional hazards models to estimate hazard ratios of mortality for groups adherent to 2, 1 or none of the therapies versus group adherent to all 3 therapies.
RESULTS
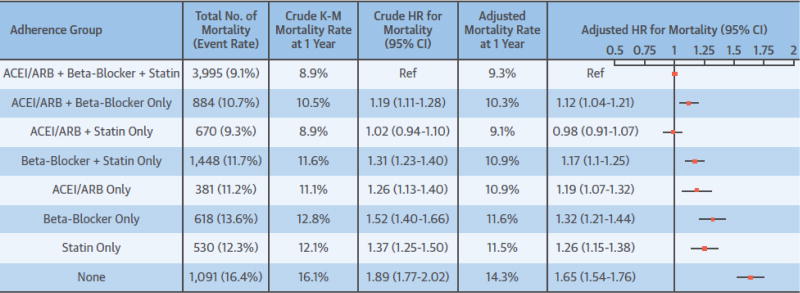
Only 49% of the patients adhered (PDC ≥80%) to all 3 therapies. Compared to being adherent to all 3 therapies, multivariable-adjusted hazard ratios (95% confidence intervals [95%CI]) for mortality were 1.12 (1.04 to 1.21) for being adherent to ACEI/ARBs and beta-blockers only, 0.98 (0.91 to 1.07) for ACEI/ARBs and statins only, 1.17 (1.10 to 1.25) beta-blockers and statins only, 1.19 (1.07 to 1.32) for ACEI/ARBs only, 1.32 (1.21 to 1.44) for beta-blockers only, 1.26 (1.15 to 1.38) statins only, and 1.65 (1.54 to 1.76) for being nonadherent (PDC <80%) to all 3 therapies.
CONCLUSIONS
Patients adherent to ACEI/ARBs and statins only had similar mortality as those adherent to all 3 therapies, suggesting limited additional benefit for beta-blockers in patients who were adherent to statins and ACE/ARBs. Nonadherence to ACEI/ARBs and/or statins was associated with higher mortality.
Keywords: medication adherence, myocardial infarction, secondary prevention, older adults
Introduction
Clinical guidelines recommend prescribing angiotensin converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARB), beta-blockers and statins after an acute myocardial infarction (AMI). The effectiveness of these guideline-recommended preventive therapies is dependent on patient adherence (1–4). However, a recent U.S. study reported that almost 40% of the patients who initiated use of ACEI/ARBs, beta-blockers or statins following hospitalization for AMI became nonadherent during the first treatment year (5). Many seem to do so already during the first 6 months (6). Studies from other countries also suggest sub-optimal adherence to preventive therapies for the secondary prevention of cardiovascular disease (CVD) (7–9).
Adhering to multiple therapies can present considerable challenges for older adults with multiple comorbidities and medications. The proportion of adults aged 65 and older who take 5 or more prescription medications tripled from13% to 39% between 1988 and 2010 (10). Patients with multiple comorbidities and polypharmacy have an increased risk of drug-drug interactions and adverse drug events (11). Furthermore, therapeutic and medication regimen complexity may decrease medication adherence (12,13). Patients may have trade-offs in adherence; in other words, they may choose to adhere to some post-AMI preventive therapies but not to others. Studies have shown notable variation in adherence across post-AMI preventive therapies (1,5,9,14,15). Clinicians who manage patients with complex treatment regimens are required to balance benefits and risks of preventive therapies, because evidence from randomized clinical trials mostly relates to the efficacy of a single preventive therapy on survival following AMI rather than combinations of therapies (16,17). Indeed, post-AMI beta-blocker trials were largely performed before statin use became widespread, and additive efficacy of beta-blockers in statintreated patients remains undetermined.
If a patient is not able to adhere to all post-AMI preventive therapies long term, which therapies should clinicians emphasize for patient adherence? Little is known about the clinical impact of the trade-offs in adherence made among the preventive therapies after AMI. Thus, the objective of this study was to investigate the effects of trade-offs in adherence to ACEI/ARBs, beta-blockers, and statins on all-cause mortality after AMI in a large cohort of Medicare beneficiaries.
Methods
Data Sources and Study Cohort
Data were sourced from the Center for Medicare & Medicaid Services Medicare Chronic Condition Data Warehouse 2007 to 2011 files that include enrollment summaries and inpatient, outpatient, skilled-nursing facility, physician office visits, and prescription claims. We first identified all Medicare beneficiaries meeting the following eligibility criteria: 1) aged 65 or older; 2) continuous enrollment for ≥365 days before and ≥180 days after the index AMI hospitalization in the Medicare fee-for-service and Part D prescription benefits; 3) index AMI hospitalization between January 1, 2008 and December 31, 2010; 4) discharge to home, and 5) survival for >180 days after the index hospitalization (Figure 1). Patients hospitalized for AMI were identified using an International Classification of Diseases, Ninth Revision, code of 410.x1 recorded either in the primary or secondary discharge diagnosis field in the inpatient files (5). The index AMI hospitalization was defined as each patient’s first hospitalization for AMI between 2008 and 2010. The final study population comprised patients who had all 3 preventive therapies (ACEI/ARBs, beta-blockers, and statins) within 30 days of the index hospital discharge (Figure 1). Having a preventive therapy was defined as either 1) having filled a prescription during the 30-day period, or 2) having enough medication supply from a prescription filled before the AMI hospitalization to cover the 30-day period after discharge.
Figure 1. Flow chart of the study population.
ACEI/ARB = angiotensin converting enzyme inhibitors/angiotensin II receptor blockers; AMI = acute myocardial infarction.
Assessment Of Adherence and Adherence Trade-Off
A timeline for measurement of patient characteristics, adherence and outcomes is shown in Online Figure 1. We measured adherence for 180 days following hospital discharge. We calculated proportion of days covered (PDC) over the entire 180 days using Medicare Part D prescription claims files to measure patient adherence to a therapy (5). The PDC was calculated using dates and days of supply of the prescriptions filled. We classified patients as adherent (PDC ≥80%) or nonadherent (<80%) separately to each of the 3 preventive therapies. Previous research has shown that post-AMI patients benefit from use of preventive therapies at the adherence levels of ≥80% (2). Trade-offs in medication adherence were assessed by adherence categories to the 3 therapies. We had the following 8 categories: 1) adherent to all 3 therapies, 2) adherent to ACEI/ARBs and beta-blockers only, 3) adherent to ACEI/ARBs and statins only, 4) adherent to beta-blockers and statins only, 5) adherent to ACEI/ARBs only, 6) adherent to beta-blockers only, 7) adherent to statins only, and 8) adherent to none of the 3 therapies.
Assessment of Outcome
Mortality was measured using the verified date of death from the Medicare enrollment file. Patients were followed-up for death from the end of the 180-day adherence assessment period up to 18 months (Online Figure 1).
Patient Characteristics
All covariates were measured prior to the adherence assessment period (Online Figure 1). Clinical characteristics included the Charlson Comorbidity Index, diagnoses of any CVD and other risk factors for mortality in the 365-day baseline period prior to index AMI hospitalization. The baseline CVD diagnoses and risk factors included AMI, coronary artery bypass graft surgery (CABG), percutaneous coronary intervention (PCI), stroke/transient ischemic attack, unstable angina, angina pectoris, ischemic heart disease, heart failure, atrial fibrillation, peripheral vascular disease, hypertension, diabetes mellitus, hyperlipidemia, cancer, depression and dementia/Alzheimer’s disease; baseline potential intolerant conditions/ contraindications to the preventive therapies including chronic kidney disease, liver disease, chronic obstructive pulmonary disease, and asthma. In addition, dispensations of ACEI/ARBs, beta-blockers and statins within the 180 days prior to the index AMI hospitalization were included as were AMI type (ST-elevation versus non-ST-elevation myocardial infarction), revascularization procedures (angiography, CABG, PCI, cardiac catheterization, infusion of thrombolytic and/or platelet inhibitors), complications (heart failure, cardiogenic shock, acute renal failure, hypotension, cardiac dysrhythmias), total intensive care unit and inpatient days measured during the index hospitalization, and sociodemographic variables (sex, age, median household income of US Census block groups, state of residence, and insurance status) measured prior to the index AMI hospitalization.
We included an additional set of covariates to reduce potential confounding bias by frailty. Frailty is a strong risk factor for mortality in older people (18,19) and may affect adherence. This additional set included the following variables previously found to predict dependency in activities of daily living in Medicare population: use of ambulance transport, wheelchair, podiatric care, rehabilitation services, and screening tests, treatment for coagulation deficiency and lipid abnormality, as well as diagnoses for decubitus ulcer, falls/difficulty walking, obesity, bladder dysfunction, infection/sepsis, neurological disorder, osteoarthritis, paralysis, Parkinson’s Disease, pulmonary circulation disorder, vertigo, weakness, and weight loss (19).
Statistical Analyses
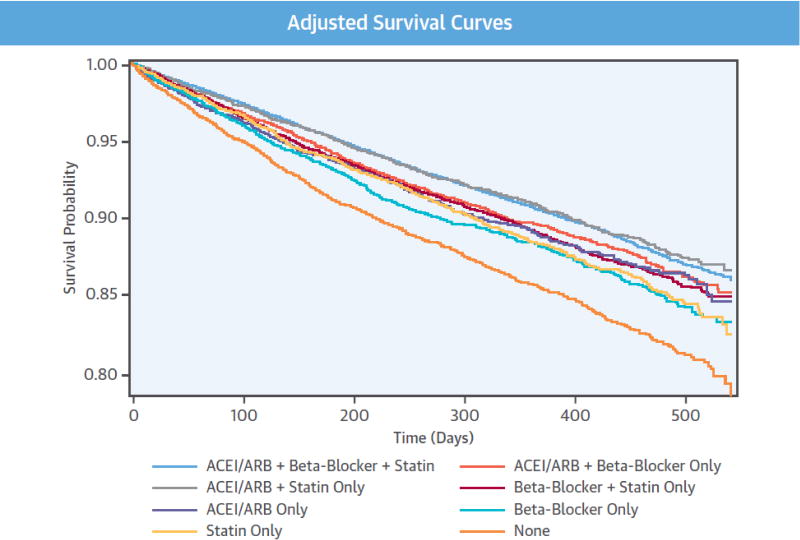
The distributions of patient characteristics, adherence and outcome events were assessed by the categories of adherence to the therapies. Categorical variables were expressed as numbers and percentages and continuous variables as means ± standard deviations. Kaplan-Meier estimators were applied to estimate the crude mortality rate at 1-year follow-up. We used Cox proportional hazards models to estimate hazard ratios (HR) and their 95% CIs for all-cause mortality associated with other adherence groups in comparison with group adherent to all 3 therapies. The model adjusted for all patient characteristics measured. The adjusted survival curves of the adherence groups were also plotted (20). The adjusted mortality rates at 1-year follow-up were estimated from the model. We assessed the proportional hazards assumption using 2 methods: Schoenfeld residuals test (Online Table 4) and graphical examination for cross-over of Kaplan-Meier curves (Central Illustration). The Schoenfeld residuals test only showed very weak correlation between the Schoenfeld residuals for the groups adherent to ACEIs/ARBs only and adherent to none of the therapies and time, and there was no cross-over between their curves and the curve of the reference group (adherent to all 3 therapies). The assessment suggests that although the HRs may not be constant over time for the 2 adherence groups inference on their HRs as average effects over time will still be valid.
Central Illustration. Adherence trade-off to preventive therapies and survival.
Adjusted survival curves and hazard ratios (95% confidence intervals) of all-cause mortality by adherence categories to preventive therapies. ACEI/ARB = angiotensin converting enzyme inhibitors/angiotensin II receptor blockers; CI = confidence interval; HR = hazard ratio. Reference group: patients who were adherent to all 3 preventive therapies. Hazard ratios are adjusted for patient characteristics shown in Online Table 1.
Given the compelling indication of ACEI/ARBs for managing heart failure and diabetes, and polypharmacy burden for cognitively impaired patients, we additionally conducted subgroup analyses stratified by heart failure (either pre-admission or during admission), diabetes and dementia as well as age group (65 to 74, 75 to 84, 85+) and sex. We tested for statistical significance of the heterogeneity of the association between adherence group and mortality across the subgroups defined by presence of heart failure by including a product term “heart failure*adherence group” in the model, and similarly for presence of diabetes and dementia, and finally for age group and sex.
In sensitivity analysis, we additionally adjusted for polypharmacy (total number of unique medication classes with prescription filled in the 30 days after hospital discharge) and average daily dose of the last prescription for each of the three therapies filled in the 30 days after hospital discharge from Part D prescription files. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Results
Study Cohort and Adherence Groups
Overall 466,385 beneficiaries suffered an index AMI during the study period. Among these beneficiaries 192,746 patients met all the eligibility criteria. Of these patients 63,715 (33.1%) did not fill any prescriptions for ACEI/ARBs; 52,584 (27.3%) filled no prescriptions for beta-blockers, and 32,262 (16.7%) filled no prescriptions for statins within 30 days after the index discharge. The final study populations consisted of 90,869 patients who had all 3 therapies within the 30 days.
More than half of the patients (51.5%) were nonadherent to 1 or more of the 3 preventive therapies during the 180-day adherence assessment period. Overall 27,911 patients (30.7%) were nonadherent to ACEI/ARBs, 21,589 (23.8%) were nonadherent to beta-blockers, and 20,861 (23.0%) were nonadherent to statins. Table 1 shows selected baseline characteristics of the final study population according to the adherence category (see Online Table 1 for the distributions of all covariates).
Table 1.
Selected baseline characteristics of the study population stratified by adherence to the three classes of preventive therapies
| Adherent to | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Cohort | All three therapies |
ACEI/ARB + beta- blocker only |
ACEI/ARB + statin only |
Beta- blocker + statin only |
ACEI/ARB only |
Beta- blocker only |
Statin only |
None | |
|
| |||||||||
| N (%) | 90869 (100%) |
44051 (48.5%) |
8269 (9.1%) |
7242 (8.0%) |
12401 (13.6%) |
3396 (3.7%) |
4559 (5.0%) |
4304 (4.7%) |
6647(7.3%) |
| Sociodemographics, % | |||||||||
| Age, years | |||||||||
| 65–74 | 43.6 | 44.6 | 43.3 | 43.1 | 41.2 | 43.1 | 41.8 | 43.5 | 43.7 |
| 75–84 | 39.7 | 39.2 | 40.1 | 43.7 | 41.3 | 39.7 | 40.3 | 40.4 | 38.4 |
| 85+ | 16.7 | 16.1 | 17.6 | 17.2 | 17.6 | 17.2 | 17.9 | 16.2 | 17.9 |
| Gender (Male) | 45.2 | 45.4 | 40.9 | 45.5 | 45.9 | 41.4 | 43.0 | 51.5 | 47.3 |
| Race | |||||||||
| White | 85.0 | 86.1 | 84.5 | 84.8 | 87.1 | 80.0 | 85.1 | 86.2 | 76.6 |
| Black | 8.0 | 7.1 | 8.7 | 7.9 | 6.6 | 11.4 | 8.7 | 7.0 | 14.0 |
| Hispanic | 2.9 | 2.7 | 3.2 | 3.2 | 2.4 | 4.3 | 2.9 | 2.5 | 4.4 |
| Asian | 2.2 | 2.2 | 1.7 | 2.1 | 2.3 | 2.4 | 1.5 | 2.4 | 2.4 |
| Other | 1.9 | 1.9 | 1.9 | 2.0 | 1.6 | 1.9 | 1.8 | 1.9 | 2.7 |
| Income proxy* | |||||||||
| ≤$30000 | 47.2 | 46.9 | 47.7 | 46.4 | 45.6 | 51.4 | 46.0 | 44.5 | 52.1 |
| $30001–$60000 | 41.4 | 41.7 | 41.2 | 42.3 | 42.4 | 37.9 | 42. | 42.5 | 37.5 |
| $60001–$100000 | 9.2 | 9.18 | 9.1 | 9.1 | 9.7 | 8.3 | 9.9 | 10.4 | 8.3 |
| $100001–$150000 | 1.7 | 1.7 | 1.6 | 1.7 | 1.6 | 1.9 | 1.5 | 2.0 | 1.7 |
| ≥$150001 | 0.5 | 0.5 | 0.4 | 0.6 | 0.6 | 0.5 | 0.4 | 0.7 | 0.4 |
| Having Part D prescription drug benefit gap (“doughnut hole”) | 12.7 | 13.5 | 12.0 | 13.1 | 13.4 | 11.6 | 11.3 | 12.0 | 9.3 |
| Medicare & Medicaid dual eligibility | 23.5 | 24.4 | 21.5 | 22.6 | 21.2 | 25.7 | 19.8 | 19.3 | 29.6 |
|
| |||||||||
| Clinical characteristics (within 12 months prior to index admission), % | |||||||||
| AMI | 2.8 | 2.7 | 3.0 | 2.6 | 2.8 | 2.5 | 3.6 | 3.0 | 3.1 |
| CABG | 0.7 | 0.7 | 0.6 | 0.8 | 0.9 | 0.6 | 0.8 | 0.9 | 0.6 |
| PCI | 4.8 | 4.8 | 4.8 | 4.8 | 4.7 | 4.7 | 5.7 | 4.6 | 4.6 |
| Stroke/TIA | 6.0 | 5.6 | 5.9 | 6.4 | 6.1 | 6.2 | 7.3 | 6.5 | 7.2 |
| Unstable Angina | 3.8 | 3.7 | 3.8 | 3.9 | 4.2 | 4.5 | 4.2 | 3.5 | 3.5 |
| Angina Pectoris | 6.2 | 6.2 | 6.1 | 6.5 | 6.5 | 6.3 | 7.2 | 5.9 | 5.4 |
| IHD | 44.0 | 43.0 | 45.2 | 44.8 | 45.4 | 43.5 | 47.9 | 43.8 | 43.0 |
| CHF | 20.7 | 19.8 | 21.7 | 19.6 | 22.5 | 21.4 | 22.8 | 19.5 | 22.8 |
| Atrial Fibrillation | 9.7 | 9.3 | 10.4 | 9.9 | 10.9 | 9.3 | 10.9 | 9.94 | 8.1 |
| Hypertension | 75.6 | 75.2 | 78.2 | 76.7 | 76.6 | 77.9 | 78.7 | 71.4 | 71.1 |
| PVD | 17.3 | 16.6 | 17.6 | 17.3 | 18.6 | 16.8 | 19.5 | 17.3 | 17.8 |
| Hyperlipidemia | 59.3 | 59.5 | 60.0 | 60.2 | 61.5 | 56.4 | 60.7 | 60.5 | 51.2 |
| Diabetes | 40.0 | 40.0 | 40.7 | 39.8 | 40.3 | 41.0 | 42.0 | 38.0 | 38.3 |
| CKD | 11.4 | 10.2 | 10.5 | 10.4 | 15.1 | 10.6 | 14.8 | 12.5 | 11.9 |
| COPD | 21.3 | 19.8 | 21.7 | 21.8 | 22.5 | 23.5 | 21.2 | 23.9 | 24.7 |
| Asthma | 5.1 | 4.6 | 5.2 | 5.4 | 5.5 | 6.4 | 5.0 | 5.7 | 6.2 |
| Liver Disease | 1.5 | 1.5 | 1.8 | 1.1 | 1.4 | 1.9 | 1.6 | 1.5 | 1.8 |
| Cancer | 10.4 | 9.9 | 10.8 | 10.4 | 11.2 | 9.8 | 12.1 | 11.6 | 9.76 |
| Depression | 13.0 | 12.1 | 13.1 | 13.3 | 13.7 | 14.2 | 14.6 | 14.5 | 15.3 |
| Dementia/Alzheimer ’s Disease | 9.5 | 7.2 | 7.0 | 8.4 | 7.4 | 9.8 | 8.7 | 8.7 | 11.8 |
| Charlson Comorbidity | |||||||||
| Index | |||||||||
| 0 | 30.7 | 32.2 | 29.7 | 30.4 | 28.8 | 29.0 | 27.0 | 30.4 | 30.0 |
| 1–2 | 39.8 | 40.1 | 40.3 | 40.8 | 38.5 | 41.4 | 39.2 | 39.0 | 39.1 |
| 3–5 | 23.4 | 22.2 | 24.3 | 23.3 | 25.3 | 24.1 | 25.5 | 23.9 | 24.3 |
| 6–8 | 5.0 | 4.6 | 4.7 | 4.5 | 6.3 | 4.4 | 6.7 | 5.4 | 5.4 |
| 9+ | 1.1 | 0.9 | 1.0 | 1.0 | 1.2 | 1.2 | 1.6 | 1.4 | 1.2 |
|
| |||||||||
| Pre-admission medication use (within 180 days prior to index admission), % | |||||||||
| ACEI/ARB | 69.5 | 70.8 | 73.5 | 73.0 | 69.1 | 73.9 | 66.7 | 60.5 | 58.9 |
| Beta-blocker | 56.6 | 58.8 | 61.6 | 51.1 | 58.2 | 48.3 | 62.9 | 48.6 | 44.3 |
| Statin | 61.2 | 63.7 | 55.5 | 67.0 | 64.5 | 52.0 | 54.3 | 63.9 | 47.7 |
|
| |||||||||
| Characteristics of index admission, % | |||||||||
| NSTEMI | 71.9 | 70.7 | 72.7 | 73.8 | 72.4 | 74.3 | 73.8 | 71.4 | 73.7 |
| CHF | 35.3 | 34.7 | 35.1 | 31.8 | 38.4 | 34.5 | 37.6 | 35.0 | 37.0 |
| Cardiogenic Shock | 2.7 | 2.7 | 2.4 | 2.0 | 3.6 | 2.2 | 2.8 | 3.4 | 2.3 |
| Cardiac Arrest | 1.2 | 1.2 | 1.1 | 0.9 | 1.2 | 0.8 | 1.5 | 1.6 | 0.9 |
| Acute Renal Failure | 12.2 | 10.3 | 11.0 | 10.7 | 18.0 | 10.4 | 15.5 | 14.6 | 13.3 |
| Cardiac Dysrhythmias | 30.2 | 29.9 | 31.6 | 31.0 | 31.2 | 29.3 | 31.0 | 30.7 | 27.3 |
| Hypotension | 5.0 | 4.8 | 4.5 | 5.1 | 5.6 | 4.7 | 5.0 | 6.1 | 4.6 |
| Angiocardiography | 67.4 | 69.1 | 66.3 | 67.5 | 66.3 | 65.6 | 64.2 | 67.9 | 62.0 |
| CABG | 7.72 | 7.04 | 6.71 | 7.24 | 11.0 | 6.48 | 8.62 | 10.0 | 6.39 |
| PCI | 48.6 | 51.6 | 47.0 | 48.4 | 46.0 | 46.4 | 42.9 | 46.8 | 41.6 |
| Cardiac Catheterization | 67.7 | 69.3 | 66.7 | 67.9 | 66.7 | 66.3 | 65.2 | 67.7 | 62.5 |
| Thrombolytic use for AMI | 0.7 | 0.7 | 0.8 | 0.6 | 0.5 | 0.8 | 0.6 | 0.7 | 0.7 |
| Antiplatelet use for AMI | 5.4 | 5.6 | 5.3 | 5.6 | 5.4 | 4.8 | 4.9 | 5.3 | 4.9 |
|
| |||||||||
| Adherence (PDC), mean (during 180 days after the index discharge) | |||||||||
| ACEI/ARB | 0.81 | 0.97 | 0.96 | 0.96 | 0.45 | 0.94 | 0.46 | 0.46 | 0.45 |
| Beta-blocker | 0.86 | 0.97 | 0.96 | 0.53 | 0.97 | 0.53 | 0.95 | 0.52 | 0.48 |
| Statin | 0.85 | 0.97 | 0.52 | 0.95 | 0.96 | 0.51 | 0.51 | 0.94 | 0.46 |
|
| |||||||||
| Follow-up days, mean | 347 | 351 | 347 | 352 | 344 | 345 | 344 | 347 | 335 |
Values are percentages if not otherwise stated.
ACEI/ARB = angiotensin converting enzyme inhibitors/angiotensin II receptor blocker; AMI = acute myocardial infarction; CABG = coronary artery bypass surgery; CHF = congestive heart failure; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; IHD = ischemic heart disease; NSTEMI = non-ST-elevation myocardial infarction; PDC = proportion of days covered; PCI = percutaneous coronary interventions; PVD = peripheral vascular disease; TIA = transient ischemic attack; PDC = proportion of days covered.
Average household income at Census block groups of residence among residents who were 65 years and older
Mortality in the Whole Cohort
Of the final study population, 9617 (10.6%) died during the mean follow-up of 347 days. Crude and adjusted mortality rates among patients who were adherent to all 3 therapies were 8.9% and 9.3% at 1-year follow-up, respectively (Figure 2.). The 1-year crude and adjusted mortality rates for patients who were nonadherent to all 3 therapies were 16.1% and 14.3%, respectively. Figure 2 shows that those who were adherent to ACEI/ARBs and statins only had similar mortality as those adhering to all 3 therapies (adjusted HR 0.98, 95% CI: 0.91 to 1.07). Those who were nonadherent to all 3 therapies had highest mortality (HR 1.65, 95% CI: 1.54 to 1.76), followed by those who were adherent to beta-blockers only (HR 1.32, 95% CI: 1.21 to 1.44), to statins only (HR 1.26, 95% CI: 1.15 to 1.38), to ACEI/ARBs only (HR 1.19, 95% CI: 1.07 to 1.32), to beta-blockers and statins only (HR: 1.17, 95% CI: 1.10 to 1.25), and to ACEI/ARBs and beta-blockers only (HR 1.12, 95% CI: 1.04 to 1.21). The adjusted survival curves are presented in the Central Illustration. Adjustment for additional variables suggestive of pre-admission frailty did not appreciably change the HRs already adjusted for conventional sociodemographic and clinical variables (Online Table 2). The sensitivity analysis by additionally adjusting for polypharmacy and dose of the 3 therapies yielded very similar and consistent findings (Online Table 3).
Figure 2. Crude and adjusted rates and hazard ratios (95% confidence intervals) of all-cause mortality by adherence categories to preventive therapies in the whole study cohort.
ACEI/ARB = angiotensin converting enzyme inhibitors/angiotensin II receptor blockers; K-M, Kaplan-Meier. Reference group: patients who were adherent to all 3 preventive therapies. Hazard ratios are adjusted for patient characteristics shown in Online Table 1.
Mortality in Patient Subgroups
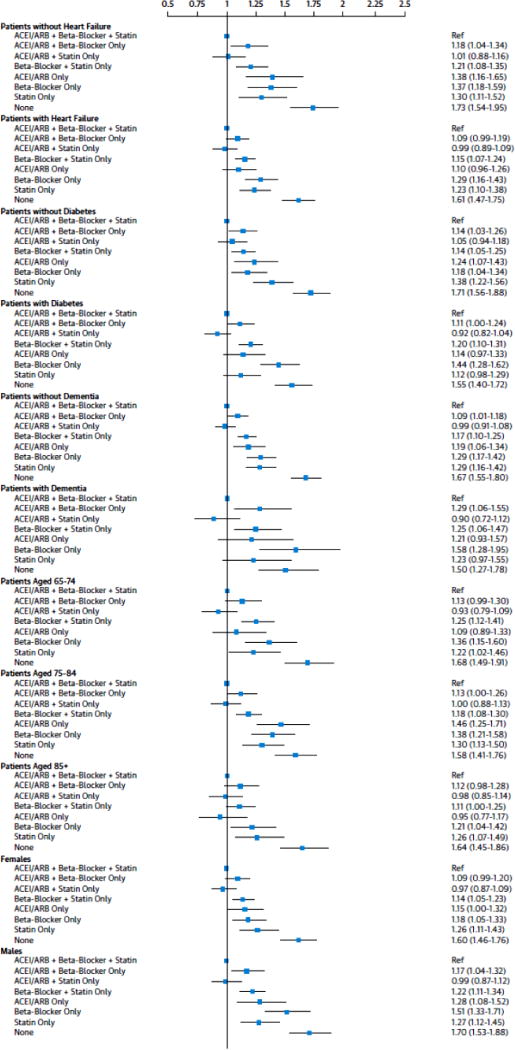
We observed some variation in the associations across subgroups of patients with and without heart failure, diabetes, and dementia (P values for interactions heart failure *adherence group 0.176, diabetes *adherence group 0.002, and dementia *adherence group 0.032). The results of subgroup analyses comparing other adherence groups to the group adherent to all 3 therapies are presented in Figure 3. Overall, directions of all associations between adherence groups and mortality in patients with heart failure and diabetes were similar to those in the whole study population, with patients who were nonadherent to all 3 therapies having the highest mortality. Mortality in patients who were adherent to ACEI/ARBs and statins only was not significantly different to mortality in those who were adherent to all 3 therapies in any of the subgroups. Nonetheless, the HR of mortality for patients who were adherent to ACEI/ARBs only versus all 3 therapies was 1.38 (95% CI: 1.16 to 1.65) among patients without heart failure and 1.10 (95% CI: 0.96 to 1.26) with heart failure. The HR of mortality for patients who were adherent to ACEI/ARB only versus all 3 therapies was 1.14 (95% CI: 0.97 to 1.33) among patients without diabetes and 1.24 (95% CI: 1.07 to 1.43) with diabetes. The HR of mortality for patients who were adherent to beta-blockers only versus all 3 therapies was 1.18 (95% CI: 1.04 to 1.34) among patients without diabetes and 1.44 (95% CI: 1.28 to 1.62) with diabetes. The HR of mortality for patients who were adherent to statins only versus all 3 therapies was 1.38 (95% CI: 1.07 to 1.43) among patients without diabetes and 1.12 (95% CI: 0.98 to 1.29) with diabetes. Compared to patients without dementia, patients with dementia had higher mortality when adherent to ACEI/ARB and beta-blockers only (HRs 1.09, 95% CI: 1.01 to 1.18 and 1.29, 95% CI: 1.06 to 1.55) and to beta-blockers only (HRs 1.29, 95% CI: 1.17 o t1.42 and 1.58, 95% CI: 1.28 to 1.95) versus 3 therapies. The effects of adherence trade-offs on mortality tended to be stronger in men than women (P value for sex*adherence group 0.031) and in younger age groups than the oldest one (P value for age group*adherence group 0.097).
Figure 3. Hazard ratios (95% confidence intervals) of all-cause mortality by adherence to various combinations of preventive therapies in subgroups stratified by presence heart failure, diabetes, and dementia and age and sex.
Reference group: patients who were adherent to all 3 preventive therapies. Hazard ratios are adjusted for patient characteristics shown in Online Table 1 and total intensive care unit and inpatient days. ACEI/ARB = angiotensin converting enzyme inhibitors/angiotensin II receptor blockers.
Discussion
In our cohort of older Medicare beneficiaries, 30% were nonadherent to ACEI/ARBs and almost 25% were nonadherent to beta-blockers and statins, respectively, at 6 months after discharge. These nonadherence rates are comparable to those reported recently among older post-AMI survivors in the USA (5). Among those who received ACEI/ARBs, beta-blockers and statins within a month after AMI hospitalization, all-cause mortality rates among patients who were adherent to ACEI/ARBs and statins only did not differ from rates among those who were adherent to all 3 therapies. Nonadherence to ACEI/ARBs or statins in any combination and nonadherence to all 3 therapies in particular was associated with notably higher mortality. These associations were broadly similar in patient subgroups defined by sex, age, presence of heart failure, diabetes and dementia.
Our findings are intriguing for long-term medical management of older patients after AMI. Clinical guidelines recommend all 3 therapies, ACEI/ARBs, statins and beta-blockers, for long-term use as secondary prevention after AMI; however, their benefits were demonstrated in randomized controlled trials for a single therapy rather than combinations (16,17). Clinical uncertainties exist as to the clinical impact of adherence to some therapies versus all 3 in the long-term. In clinical practice, this is a particularly challenging issue for older adults with multiple morbidities and polypharmacy. The high prevalence of comorbidities and polypharmacy may markedly increase the risk of adverse drug events and drug–drug interactions, which is further complicated by more prevalent cognitive impairment in older people. Occurrence of adverse drug events accompanied with the physical and cognitive burdens of taking many medications may render long-term adherence to all 3 AMI preventive therapies unrealistic though desired.
We found that, among patients who had all 3 therapies after AMI hospitalization, being adherent to ACEI/ARBs and statins only was associated with equal survival as being adherent to all 3 therapies. Nonadherence to ACEI/ARBs or statins in any combination in particular was associated with notably higher mortality. Thus, our findings suggest that long-term adherence to ACEI/ARBs and statins may be more important than adherence to beta-blockers after AMI. Prior, smaller studies from the United States (1,21) and other countries (3,9,22) also showed larger reductions in all-cause mortality for adherence to statins and ACEI/ARBs than adherence to beta-blockers. Accordingly, in a large observational study (>44,000 patients), beta-blockers were not associated with mortality benefit in patients with prior MI or those without a history of AMI (23). Our observations support the argument that in the current clinical practice incremental survival benefits associated with use of beta-blockers may be smaller than benefits associated with use of the other 2 evidence-based preventive therapies (23–25).
While the associations between adherence groups and mortality were generally similar among patients in subgroups as in the whole cohort, several variations are notable. The increase in mortality risk associated with being adherent to ACEI/ARBs only was considerably smaller among patients with heart failure than among patients without heart failure. Among patients with heart failure, there was no statistically significant difference in mortality risk between patients who were adherent to ACEI/ARBs only and those who were adherent to all 3 therapies. The findings are in line with the landmark clinical trials that have demonstrated ACEI/ARBs as cornerstone therapy in reducing mortality among patients with heart failure including those after AMI (26,27). In our heart failure subgroup, mortality in patients nonadherent to statins and those adherent patients did not differ. This is also consistent with 2 clinical trials that found no overall CVD risk reduction in Class II to IV heart failure patients treated with statins (28,29). The pivotal trials such as MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure), COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival) and CIBIS-II (Cardiac Insufficiency Bisoprolol Study II), showed that beta-blockers are associated with lower all-cause mortality in heart failure (30–32). Our study suggests large relative benefit of beta-blocker adherence in reducing mortality in comparison to nonadherence to all 3 therapies among patients with heart failure. However, there was no benefit of beta-blocker adherence in reducing mortality in comparison to patients with heart failure and already adherent to ACEI/ARBs. This finding was of surprise to us. One possible explanation has to do with age. In the MERIT-HF, COPERNICUS and CIBIS-II trials the mean age of the patients were 61 to 64 years, whereas in our study the patients were 77 years on average. In line with us, in the SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure) study in older heart failure patients (> 70 years) nebivolol, a beta-blocker, failed to reduce mortality (33). Further research on this is warranted.
In contrast, adherence to ACEI/ARBs and statins conferred a greater benefit in patients with diabetes than among patients without diabetes. These findings suggest that it may be most important to be adherent to ACEI/ARBs among patients with heart failure and to ACEI/ARBs and statins among patients with diabetes post-AMI. Meta-analyses of clinical trials have shown that ACEI/ARBs and statins reduce all-cause mortality among patients diabetes (34,35). Current U.S. national guidelines recommend ACEIs and statins as first-line therapy for patients with diabetes, hypertension, and CVD (36–38).
We found markedly higher mortality risk for being adherent to beta-blockers only among patients with diabetes or dementia than among patients without these conditions. This suggests that being adherent to beta-blockers in the long-term may not be as beneficial for patients with diabetes or dementia as among patients free of these conditions. Recently, a study conducted among ~16 000 US nursing home residents found that among AMI survivors with moderate or severe cognitive impairment, the use of beta-blockers was associated with a 30% increased risk of experiencing functional decline over a 3-month period post-AMI (39). No such association was observed among survivors without cognitive impairment. Concerns have also been raised regarding the negative effects of beta-blockers on glycemic control, insulin sensitivity, masking of hypoglycemia, and dyslipidemia. Meta-analyses of clinical trials have shown that use of beta-blockers was associated with higher risk of new-onset diabetes than non-diuretic antihypertensive medications (40,41). The clinical implications of our findings need to be investigated in further studies.
Our study has several limitations. First, we restricted our study population to the patients who filled prescriptions for each of the 3 therapies shortly after hospital discharge. This feature of our study most likely enhances comparability of adherence groups; however, it precludes generalization of our results to situations where decisions are made to stop or not initiate preventive therapies among patients who are not eligible to all the 3 therapies at discharge. Second, due to our reliance on prescription refill data, we could not differentiate the trade-off in adherence to multiple therapies as physicians’ decision to discontinue medication or patient’s/care taker’s decision not to refill prescriptions. Third, although we adjusted our outcome models for a comprehensive list of baseline risk factors for potential adverse effects or intolerant conditions for therapies and mortality, including variables suggestive of pre-admission frailty, residual confounding by unmeasured factors such as use of aspirin may exist. However, the residual confounding may be limited in the comparisons between patients who were adherent to at least 1 therapy and those adherent to all 3 therapies. In contrast, the estimates comparing patients who were adherent to none of the 3 therapies to those adherent to all therapies are likely to be affected by significant unmeasured confounding. Finally, due to relatively small numbers of patients in non-white subgroups of our study population, we did not conduct race-specific analyses. This important question should be addressed in future studies.
Conclusions
Those patients who were adherent to ACEI/ARBs and statins but nonadherent to beta-blockers had similar mortality risk as those adherent to all 3 therapies, suggesting the role of post-MI beta-blockers in the statin and ACE/ARB era deserved further investigation. Nonadherence to ACEI/ARB or statins in any combination and nonadherence to all 3 therapies in particular was associated with higher mortality.
Supplementary Material
Clinical Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS
Nonadherence to statin and angiotensin inhibitor therapy among survivors of myocardial infarction (MI) may reduce survival, while adherence to β-blockers may not be beneficial to patients with dementia or diabetes.
TRANSLATIONAL OUTLOOK
More research is needed to provide information on the risks and benefits of with various combinations of guideline-recommended post-MI preventive therapies in patients with specific comorbidities, such as heart failure, diabetes or dementia.
Acknowledgments
Funding: This study is supported in part by National Institute of Aging (NIA) grant 1R01AG046267-01A1 (PI: Fang) and 1R21AG043668-01A1 PI: Fang). The findings and views in this manuscript are the authors, and do not represent the official opinions and views of the NIA. MJK was supported by the Australian National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Frailty and Healthy Ageing.
JGR has received research grants to institution from Amarin, Amgen, Astra-Zeneca, Eli Lilly, Esai, Esperion, Glaxo-Smith Kline, Merck, Pfizer, Regeneron/Sanofi, Takeda. JGR is a consultant for Akcea/Ionis, Amgen, Dr Reddy, Eli Lilly, Esperion, Merck, Pfizer, Regeneron/Sanofi. JH has received research grants to institution from Biosense Webster, Medtronic, St Jude Medical, Boehringer Ingelheim, MSD, Amgen and AstraZeneca and is a member of advisory board for Biosense Webster, Medtronic, St Jude Medical, Boehringer Ingelheim, MSD, Amgen and AstraZeneca.
Abbreviation list
- ACEI
angiotensin converting enzyme inhibitor
- AMI
acute myocardial infarction
- ARB
angiotensin II receptor blocker
- CABG
coronary artery bypass graft
- CVD
cardiovascular disease
- HR
hazard ratio
- PCI
percutaneous coronary intervention
- PDC
proportion of days covered
- 95%CI
95% confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: MJK, IEA, RPH, JSB and GF disclose no conflict of interest.
References
- 1.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 2.Choudhry NK, Glynn RJ, Avorn J, et al. Untangling the relationship between medication adherence and post-myocardial infarction outcomes: medication adherence and clinical outcomes. Am Heart J. 2014;167:51–8. e5. doi: 10.1016/j.ahj.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Hamood H, Hamood R, Green MS, Almog R. Effect of adherence to evidence-based therapy after acute myocardial infarction on all-cause mortality. Pharmacoepidemiology Drug Saf. 2015;24:1093–104. doi: 10.1002/pds.3840. [DOI] [PubMed] [Google Scholar]
- 4.Bansilal S, Castellano JM, Garrido E, et al. Assessing the impact of medication adherence on long-term cardiovascular outcomes. J Am Coll Cardiol. 2016;68:789–801. doi: 10.1016/j.jacc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Lauffenburger JC, Robinson JG, Oramasionwu C, Fang G. Racial/Ethnic and gender gaps in the use of and adherence to evidence-based preventive therapies among elderly Medicare Part D beneficiaries after acute myocardial infarction. Circulation. 2014;129:754–63. doi: 10.1161/CIRCULATIONAHA.113.002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathews R, Wang TY, Honeycutt E, et al. Persistence with secondary prevention medications after acute myocardial infarction: Insights from the TRANSLATE-ACS study. Am Heart J. 2015;170:62–9. doi: 10.1016/j.ahj.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–8. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 8.Halava H, Korhonen MJ, Huupponen R, et al. Lifestyle factors as predictors of nonadherence to statin therapy among patients with and without cardiovascular comorbidities. CMAJ. 2014;186:E449–56. doi: 10.1503/cmaj.131807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenzi J, Rucci P, Castaldini I, et al. Does age modify the relationship between adherence to secondary prevention medications and mortality after acute myocardial infarction? A nested case-control study. Eur J Clin Pharmacol. 2015;71:243–50. doi: 10.1007/s00228-014-1793-8. [DOI] [PubMed] [Google Scholar]
- 10.Charlesworth CJ, Smit E, Lee DS, Alramadhan F, Odden MC. Polypharmacy among adults aged 65 years and older in the United States: 1988–2010. J Gerontol A Biol Sci Med Sci. 2015;70:989–95. doi: 10.1093/gerona/glv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried TR, O'Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62:2261–72. doi: 10.1111/jgs.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171:814–22. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 13.Wimmer BC, Cross AJ, Jokanovic N, et al. Clinical outcomes associated with medication regimen complexity in older people: a systematic review. J Am Geriatr Soc. 2017;65:747–53. doi: 10.1111/jgs.14682. [DOI] [PubMed] [Google Scholar]
- 14.Choudhry NK, Setoguchi S, Levin R, Winkelmayer WC, Shrank WH. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2008;17:1189–96. doi: 10.1002/pds.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gislason GH, Rasmussen JN, Abildstrøm SZ, et al. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153–8. doi: 10.1093/eurheartj/ehi705. [DOI] [PubMed] [Google Scholar]
- 16.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2013;61(4):485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e179–347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Chrischilles EA, Schneider KM, Schroeder MC, et al. Association between preadmission functional status and use and effectiveness of secondary prevention medications in elderly survivors of acute myocardial infarction. J Am Geriatr Soc. 2016;64:526–35. doi: 10.1111/jgs.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24:59–66. doi: 10.1002/pds.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Ho P, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–7. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 22.Tuppin P, Neumann A, Danchin N, et al. Evidence-based pharmacotherapy after myocardial infarction in France: adherence-associated factors and relationship with 30-month mortality and rehospitalization. Arch Cardiovasc Dis. 2010;103:363–75. doi: 10.1016/j.acvd.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Bangalore S, Steg G, Deedwania P, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–9. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 24.Mickley H, Eiskjaer H, Botker HE. Is an additional post-myocardial infarction beta-blocker trial required in the era of early revascularization? Eur Heart J. 2004;25:96–7. doi: 10.1016/j.ehj.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Roolvink V, Ibáñez B, Ottervanger JP, et al. Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol. 2016;67:2705–15. doi: 10.1016/j.jacc.2016.03.522. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Pitt B, Davis CE, Hood WB, Jr, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 27.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 28.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–61. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 29.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–9. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 30.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 31.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. New Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 32.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 33.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 34.Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014;174:773–85. doi: 10.1001/jamainternmed.2014.348. [DOI] [PubMed] [Google Scholar]
- 35.Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 38.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–26. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 39.Steinman MA, Zullo AR, Lee Y, et al. Association of β-blockers with functional outcomes, death, and rehospitalization in older nursing home residents after acute myocardial infarction. JAMA Intern Med. 2017;177:254–62. doi: 10.1001/jamainternmed.2016.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol. 2007;100:1254–62. doi: 10.1016/j.amjcard.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 41.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–7. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.