Abstract
Objective
The main goal of dopamine cell replacement therapy in Parkinson disease (PD) is to provide clinical benefit mediated by graft survival with nigrostriatal reinnervation. We report a dichotomy between graft structure and clinical function in a patient dying 16 years following fetal nigral grafting.
Methods
A 55-year-old levodopa-responsive woman with PD received bilateral putaminal fetal mesencephalic grafts as part of an NIH-sponsored double-blind sham-controlled trial. The patient never experienced clinical benefit, and her course was complicated by the development of graft-related dyskinesias. Fluorodopa positron emission tomography demonstrated significant increases postgrafting bilaterally. She experienced worsening of parkinsonism with severe dyskinesias, and underwent subthalamic nucleus deep brain stimulation 8 years after grafting. She died 16 years after transplantation.
Results
Postmortem analyses confirmed the diagnosis of PD and demonstrated >300,000 tyrosine hydroxylase (TH)-positive grafted cells per side with normalized striatal TH-immunoreactive fiber innervation and bidirectional synaptic connectivity. Twenty-seven percent and 17% of grafted neurons were serine 129-phosphorylated α-synuclein positive in the left and right putamen, respectively.
Interpretation
These findings represent the largest number of surviving dopamine neurons and the densest and most widespread graft-mediated striatal dopamine reinnervation following a transplant procedure reported to date. Despite this, clinical recovery was not observed. Furthermore, the grafts were associated with a form of dyskinesias that resembled diphasic dyskinesia and persisted in the off-medication state. We hypothesize that the grafted cells produced a low level of dopamine sufficient to cause a levodopa-independent continuous form of diphasic dyskinesias, but insufficient to provide an antiparkinsonian benefit.
It has been 40 years since the modern era of neural transplantation began with the seminal paper by Björklund and Stenevi demonstrating the viability and innervation of fetal monoaminergic allografts in lesioned rodents.1 Subsequently, numerous studies evaluated transplantation of embryonic dopaminergic neurons grafted into the striatum in rodent and nonhuman primate models of Parkinson disease (PD).2–7 These studies consistently demonstrated cell survival, site-specific reinnervation, synapse formation, increased dopamine production, and functional recovery.7
Laboratory studies were subsequently performed to determine optimal parameters for human studies such as the appropriate embryonic donor age, number of donors, site of implantation, and storage techniques.8 Subsequently, a series of open label clinical studies reported clinical benefit and fluorodopa positron emission tomography (FD-PET) recovery in PD patients. Definitive evidence of dopaminergic graft survival came from postmortem studies demonstrating robust survival of implanted dopamine neurons with evidence of extensive striatal reinnervation and synaptic connectivity.9–17 However, 2 double-blind and sham-surgery–controlled studies failed to meet their primary endpoints.9,10 Furthermore, as many as 50% of grafted patients experienced a novel form of dyskinesia that persisted even after discontinuation of L-dopa.18
Initial reports suggested that graft viability was associated with clinical benefit.11,13,19,20 However, these studies were open label and uncontrolled and are thus subject to placebo effect and bias.21 In this regard, it is noteworthy that postmortem cases from double-blinded studies showed good graft viability with prominent striatal innervation, although these studies failed to meet their primary endpoints, and the clinical benefit if any was marginal, challenging a clear structure–function association. 9,10 Furthermore, the mechanism responsible for the development of graft-induced dyskinesia that persisted despite withdrawal of L-dopa remains uncertain. We postulated, based on their clinical manifestation, that they represented a form of diphasic dyskinesia that resulted from graft survival with continuous release of low levels of dopamine sufficient to induce diphasic dyskinesias but insufficient to provide a full therapeutic response.18 In addition, a number of cases that survived at least 10 years postgrafting displayed Lewy bodies in grafted neurons. 14–17,22 suggesting that some implanted neurons may be dysfunctional and complicating our understanding of the relationship between graft survival and clinical response.
The present study documents the clinical–pathological findings in a patient who underwent fetal nigral grafting as part of our NIH double-blind study. She experienced remarkable graft survival and striatal innervation that was associated with graft-induced dyskinesias but not clinical benefit. This case provides unique insights into the potential of transplantation as a strategy for treating PD.
Subjects and Methods
The original clinical protocol was performed with the approval of the Mount Sinai School of Medicine, Rush University School of Medicine, University of South Florida School of Medicine, and University of British Columbia institutional review boards.
Index Case
This index case was diagnosed in 1991 with tremor predominant PD at age 47 years and had a good response to carbidopa/L-dopa. When first seen at Rush University Medical Center, in 1993, she was graded as Hoehn and Yahr (HY) stage 3 in the OFF state and 2 in the ON state, and was treated with 300mg carbidopa/L-dopa per day. From 1994 to 1995, her Unified Parkinson Disease Rating Scale (UPDRS) Motor Examination scores in the ON state ranged from 33 to 41 and were complicated by peak dose dyskinesia (HY stage=2–3). In the OFF state, she suffered gait impairment with falls (HY stage=4). During this time, she was treated with pergolide 0.5mg/day, bromocriptine up to 13.5mg/day, and L-dopa/carbidopa in doses ranging from 300 to 450mg/day with supplemental nighttime carbidopa/L-dopa Controlled Release. In 1999, due to her continued worsening, she was enrolled in the NIH-sponsored double-blind fetal transplant program, and randomized to active treatment. Details of the procedure are provided in the primary publication.10 Grafts derived from 4 embryonic ventral mesencephalon per side were implanted into the postcommissural putamen bilaterally. Postoperatively, her preoperative medical regimen was reinstituted. Cyclosporine was prescribed preoperatively and for 6 months after transplantation. FD-PET was performed at baseline, and at 1 and 2 years postgrafting. UPDRS Motor Examination scores were performed in the practically defined OFF and best ON states at 1, 3, 6, 9, 12, 15, 18, and 24 months. Because the blind was maintained until all patients completed the study, she received additional blinded evaluations through 31 months postgrafting. This patient was subsequently followed in an open label manner at Rush University Medical Center. Eight years after the grafting procedure, she received bilateral subthalamic deep brain stimulation because of worsening parkinsonism and the development of graft-induced dyskinesias. Thereafter, she received medical care from a local practitioner and eventually died 16 years after the transplant procedure.
Histology and Neuroanatomical Assessments
Autopsy was performed at Rush University Medical Center, where the brain was removed with a 6-hour postmortem interval, and processed as described previously.22 Several antibodies were employed to visualize expressions of dopaminergic, nondopaminergic, and pathological markers (Table). Stereological estimates23–25 of tyrosine hydroxylase–immunoreactive (TH-ir), serine 129 phosphorylated α-synuclein–immunoreactive (S129-αSyn-ir), and neuromelanin (NM)-containing neurons in each transplant deposit were performed bilaterally. To evaluate dopaminergic innervation of putamen, quantification of the relative optical density of TH-ir neurons was performed using Scion Image 1.63 (NIH).12
TABLE.
Antibodies
| Name | Catalogue #/Company | Host | Western Blot | Concentration |
|---|---|---|---|---|
| TH | 22941/ImmunoStar, Hudson, WI | Mouse | 60kDa | 1:10,000 |
| Alpha-synuclein (p-S129) | ab51253/Abcam, Cambridge, MA | Rabbit | 14~18kDa | 1:1,000 |
| Alpha-synuclein (LB509) | 180215/Life Technologies, Carlsbad, CA | Mouse | ~18kDa | 1:500 |
| Beta-amyloid (6E10) | SIG-39300/Covance, Princeton, NJ | Mouse | N/A | 1:2,000 |
| GAD | AB1511/Millipore, Billerica, MA | Rabbit | 65/67kDa | 1:500 |
| Iba1 | 019-19741/Wako, Richmond, VA | Rabbit | N/A | 1:2,000 |
| GFAP | ab48050/Abcam | Rabbit | 50kDa | 1:1,000 |
GAD=glutamate decarboxylase; GFAP=glial fibrillary acidic protein; N/A=not available; TH=tyrosine hydroxylase.
Ultrastructure
To determine whether grafted cells provided or received putative synaptic contacts with host neurons, sections were first stained for TH as described above. A subset of brain sections that were used to visualize TH-ir neurons at the light level were selected for analyses at the electron microscopic level (2 immunostained and 2 primary delete), as described previously.12
Controls
In addition to the index case, we studied an age-matched normal control and a nongrafted PD case stained simultaneously with the index case to assess the degree of reinnervation achieved by the grafts.
Results
Clinical Response following Grafting
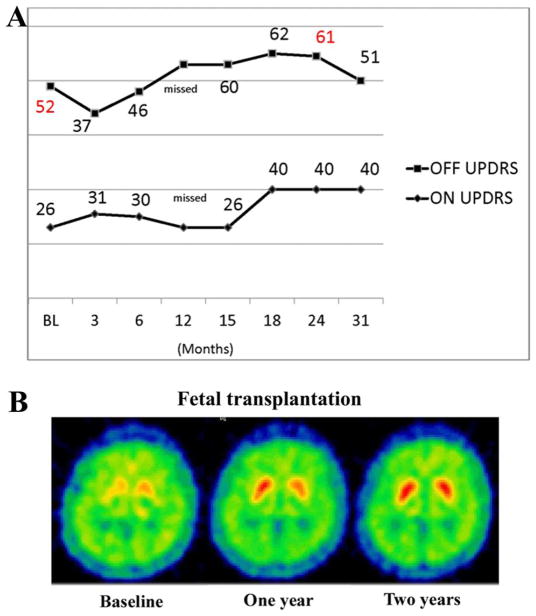
At study enrollment, the patient’s baseline UPDRS Motor Examination score in the OFF state was 52 with significant freezing (HY stage=4), and in the ON state it was 26 with marked peak dose dyskinesias (HY stage=2). Medications prior to surgery were liquid carbidopa/L-dopa 470mg/day plus bromocriptine 13.75mg/day. There was a transient and modest reduction in the UPDRS Motor Examination OFF score to 37 at 3 months, but the score rose to 46 at 6 months, with scores continuing to remain stable or increasing over the 31 months of blinded assessment (Fig 1). The ON UPDRS Motor Examination scores did not improve compared to baseline and worsened after 15 months (see Fig 1). Postoperative videotape assessments in practically defined OFF state, as per study protocol, revealed new dyskinesias primarily affecting the lower extremities. This type of off-medication dyskinesia was not seen in videotape recordings during the ON state and was typical of what we have described as off-medication or graft-induced dyskinesias.18 These dyskinesias were observed in the practically defined OFF state when the patient had been off medications for approximately 12 hours. This type of dyskinesia was different from the head, neck, and upper extremity dyskinesia seen at baseline and in postoperative ON recordings and was typical of the graft-induced off-medication dyskinesia that we have previously described.18 As per protocol, medications were not changed following grafting until the breaking of the blind. After the blind was broken in 2002, she was periodically seen at Rush for open label assessments. During these visits, ON UPDRS scores ranged from 34 to 36 with severe peak dose (HY stage=2–3). OFF periods were complicated by freezing and frequent falls, progressive worsening in UPDRS scores, and OFF dyskinesias described above. Because of progressive worsening in parkinsonism and dyskinesia, she received bilateral subthalamic nucleus deep brain stimulation in 2007 at her local hospital. She was not seen at Rush after this time, but medical records indicated significant improvement in motor function following deep brain stimulation (DBS; no UPDRS scores obtained), particularly with respect to peak dose dyskinesia. Initially after surgery, she walked with a walker, but persistent freezing and gait impairment led to the decision to keep her wheelchair bound except for transfers. She needed help with eating and dressing, and developed cognitive impairment, severe hypophonia, and swallowing difficulties. Visual hallucinations were prominent in the last 6 months of her life, with paranoia and anxiety that compromised family care. The patient died at home with the patient receiving palliative care from her family at the patient’s request.
FIGURE 1.
(A) Blinded clinical assessment of motor function using Unified Parkinson Disease Rating Scale (UPDRS) part III in OFF and ON state at baseline and over 31 months postgrafting. (B) Positron emission tomography images at baseline, and 1 and 2 years postgrafting showing enhanced fluorodopa uptake and binding following dopaminergic transplantation.
PET Findings
At baseline, there was a severe bilateral and mildly asymmetric loss of FD-PET uptake in the putamen (0.007 on the left, 0.005 on the right; see Fig 1B). These values increased following transplant (0.017 and 0.016 at 1 year; 0.018 and 0.016 at 2 years; Fig 1B, D). There was mild asymmetric reduction of fluorodopa uptake in the head of the caudate nucleus (left=0.0104, right=0.007), with a modest increase following the transplant procedure (0.015 and 0.015 at 1 year; 0.013 and 0.013 at 2 years; see Fig 1B, right). Normal values are approximately 0.02. All PET measures are striatal-occipital ratios of tracer uptake.
Neuropathology
Pathology was consistent with a diagnosis of PD based upon the loss of TH-ir nigral neurons especially in the ventrolateral tier of the substantia nigra, free NM within the nigra, numerous S129-αSyn-ir Lewy bodies and Lewy neurites, markedly reduced TH staining in the dorsal striatum, and the absence of S129-αSyn immunoreactivity in oligodendroglia. There was also substantial β-amyloid deposition in cortical but not subcortical sections (stage A1), with limited phospho-tau–immunopositive hippocampal and cortical neurons. There was a significant loss of cholinergic neurons in the nucleus basalis of Meynert, serotonergic neurons in the raphe, and noradrenergic neurons in the locus coeruleus as well.
Dopamine Graft Viability and Innervation
Qualitative observations revealed robust graft viability bilaterally (see Fig 2A–D) at all 8 deposit sites. The cytoarchitecture of the graft sites were for the most part classic, with TH-ir–positive grafted neurons preferentially located at the borders of the graft, but occasionally sites appeared completely filled with grafted cells. The majority of the cells appeared healthy morphologically (see Fig 2B, D), with seamless integration between the graft and host with numerous fibers crossing the interface.
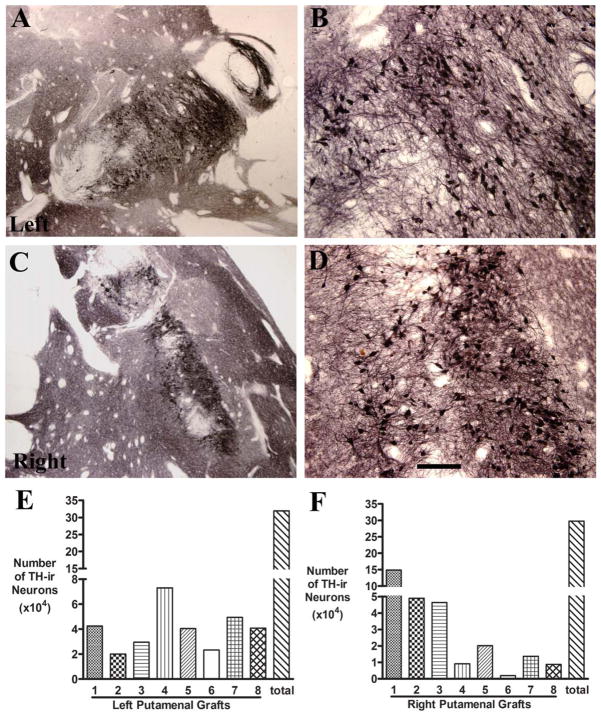
FIGURE 2.
(A–D) Low-power (A, C) and high-power (B, D) photomicrographs of putamen showing the robust survival of tyrosine hydroxylase–immunoreactive (TH-ir) grafted neurons in the right and left hemisphere. Bar in D=0.5mm in A–D. (E, F) Histograms showing the number of TH-ir neurons in individual grafts as well as the total number.
A total of 332,022 TH-ir cells were estimated in the left putamen (see Fig 2E), with between 20,022 and 73,047 in each deposit. There were a total of 43,321 NM-positive cells that did not display TH. On the right side, a total of 327,585 TH-ir cells were estimated, with between 9,087 and 48,979 positive cells at each graft site (see Fig 2F). An additional 34,249 NM-containing cells did not express TH.
Qualitatively, the TH innervation of the putamen appears indistinguishable from the normal case (Fig 3A vs Fig 3C), with virtually complete reinnervation in a patch matrix fashion being achieved bilaterally in the putamen, including the precommissural regions that did not receive any graft deposits. The nongrafted PD case displayed a comprehensive absence of TH-ir fibers in the putamen (see Fig 3C, D) as previously reported.26
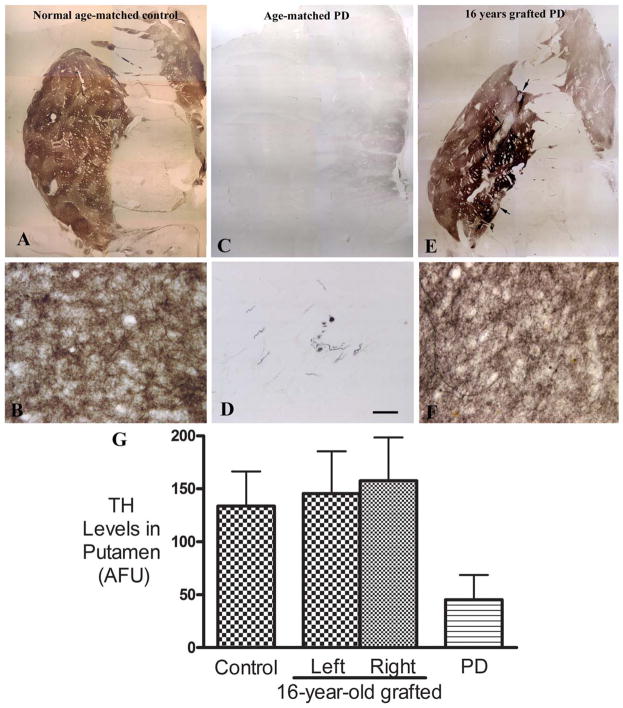
FIGURE 3.
(E–F) Comparison of tyrosine hydroxylase (TH) immunoreactivity in putamen from a normal age-matched control without Parkinson disease (PD; A, B), an age- and Hoehn and Yahr score (HY)-matched PD patient (C, D; from the Rush PD Brain Repository), and our PD patient, 16 years after fetal cell grafting (E, F). The grafted putamen displayed extensive and intense TH immunoreactivity similar in intensity, volume, and pattern to the age-matched control. At higher magnification, dense fine TH-immunoreactive (TH-ir) fibers consisted of the fine network in gray matter of putamen (F). In the nongrafted age- and HY-matched PD brain, TH-ir fibers were not visible throughout much of the putamen. Bar in D=24 μm in B, D, and F, 2.85mm in A, C, and E. (G) Histogram showing the relative levels (mean+standard error of the mean [SEM]) of TH-ir fibers in putamen from the 3 brains shown in Figure 3A–F: an age-matched non-PD control, an age- and HY-matched PD case, and our PD patient 16 years after fetal cell grafting. SEM represents variability from different sections. AFU=arbitrary fluorescence units.
Quantitative optical density measurements revealed that the grafted putamen displayed levels of TH-ir innervation throughout the putamen similar to the non-PD age-matched control bilaterally (see Fig 3G). In contrast, the nongrafted PD control displayed optical density measurements far less than the grafted PD case or the healthy control (see Fig 3G).
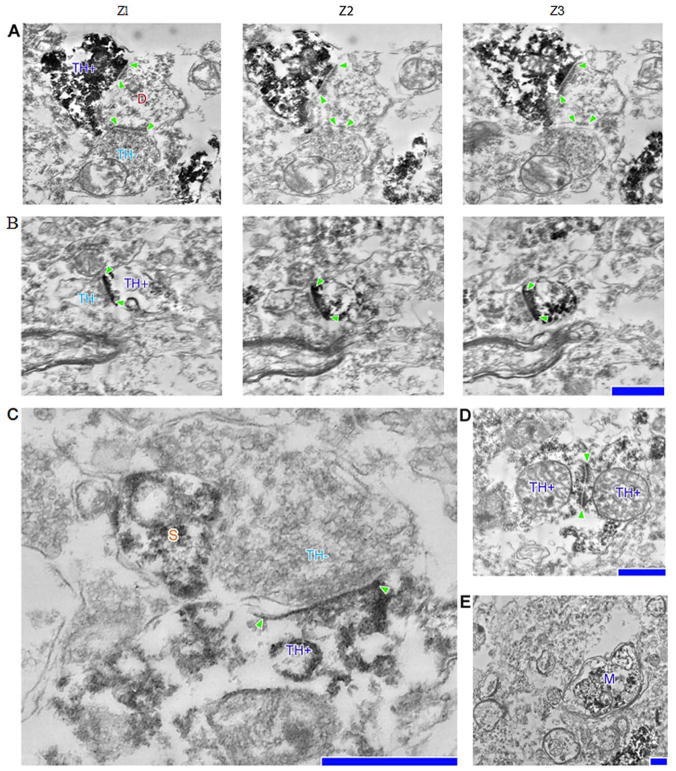
Ultrastructural analyses demonstrated unlabeled axoaxonic and axospiny synaptic contacts upon TH-ir cell bodies within the graft consistent with connectivity between graft and host neurons (Fig 4). Within the host, putative graft-derived TH-ir axons were also seen making synaptic contacts upon unlabeled host neurons. Dendrodendritic contacts between grafted neurons were observed (see Fig 4D). Myelinated putative graft-derived axons were also seen coursing through the host (see Fig 4E).
FIGURE 4.
(A, B) (2) Serial section electron micrographs depicting (1) a host, tyrosine hydroxylase–negative (TH−) dendrite postsynaptic to a graft, TH-positive (TH+), bouton as well as (3) a host, TH−, bouton (A); TH+, spine synapsing with a host, TH+, bouton (B). (C) Host bouton synapsing onto a grafted dendritic shaft, positioned next to a dendritic spine (S). (D) Two TH+ grafted neurons synapsing onto each other. (E) TH+ myelinated axon (M) in close proximity to several host myelinated axons. Postsynaptic densities are denoted with arrowheads. Scale bars=0.5 μm for A, B, D, and E; 0.2 μm for C.
Lewy Bodies and S129-αSyn
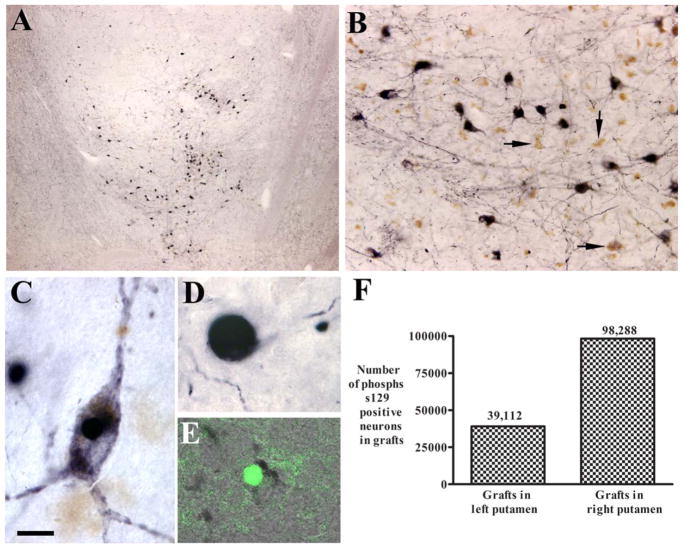
To assess for the presence of Lewy pathology within grafted neurons, we stained sections with S129-αSyn and thioflavin S (Fig 5). Stereological estimates of S129-αSyn revealed 98,288 positive grafted cells on the right side and 39,112 positive grafted cells on the left side. Virtually all of these cells were NM positive and reflected 27% and 10.7% of surviving grafted NM-positive cells in the left and right putamen, respectively. A small proportion of these cells were also thioflavin S positive, indicating beta-pleated sheet formation. In addition to S129-αSyn–positive cell bodies, there were numerous α-synuclein–positive aggregates within grafted neurites (Lewy neurites). It is important to note that there was also a significant amount of Lewy pathology within the host putamen.
FIGURE 5.
(A–E) Low-power (A) and high-power (B–E) microphotographs illustrating serine 129 phosphorylated α-synuclein–immunoreactive (S129-αSyn-ir; A–D) and thioflavin staining (E) pattern in the graft. Grafted neurons displayed S129-αSyn immunoreactivity (A, B). Some neurons exhibited dark soma and processes (B, C), and others were round (B, D). Many grafted neurons displayed neuromelanin without S129-αSyn immunoreactivity (B, arrows). Some grafted neurons displayed thioflavin aggregation (E). (F) In the grafted neurons, 27.11 % (right) and 10.45% (left) were S129-αSyn-ir. Bar in C=12 μm in C and E, 260 μm in A, 60μm in B, and 6 μm in D.
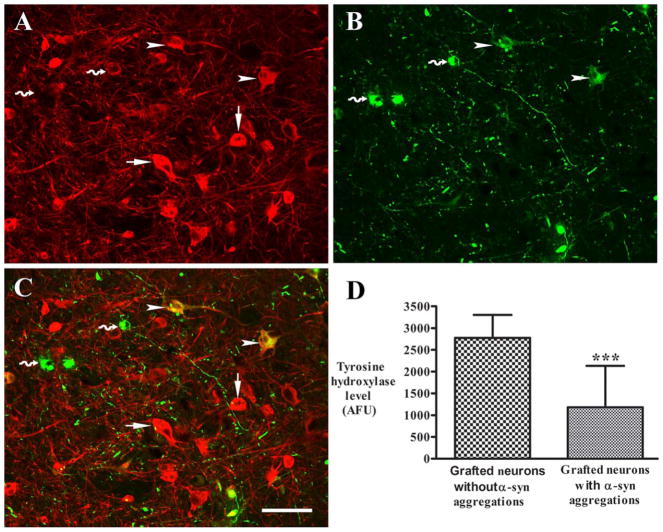
We have previously demonstrated that host nigral neurons in PD with alpha synuclein aggregates display diminished TH.27 Therefore, we performed quantitative optical density measurements on TH-stained grafted neurons with and without S129-αSyn expression to assess the effect of Lewy pathology on their dopaminergic phenotype. As illustrated in Figure 6, we found that grafted midbrain dopamine neurons that coexpressed S129-αSyn displayed diminished TH immunofluorescence relative to neighboring neurons that did not express Lewy pathology.
FIGURE 6.
(A–C) Confocal microscopic images of a graft showing immunostaining pattern of tyrosine hydroxylase (TH; A, red) and serine 129 phosphorylated α-synuclein (S129-αSyn; B, green), and colocalization of TH and S129-αSyn (C, merged). Neurons with S129-αSyn inclusions had undetectable TH (curved arrows), and neurons with nonaggregated monomeric S129-αSyn (arrowheads) had diminished TH intensity compared with neurons without S129-αSyn immunoreactivity (arrows). (D) Quantification of relative fluorescent intensities revealed that optical densities of TH were significantly reduced in neurons with S129-αSyn inclusions relative to neurons with absent of S129-αSyn immunoreactivity. *** denotes p < .001. Bar in C=110 μm in A–C. AFU=arbitrary fluorescence units.
Nondopaminergic Findings
Few serotonergic or γ-aminobutyric acidergic neurons were seen within the graft, but they were seen in the raphe and hippocampus of the host, which served as a positive immunohistochemical control. The density of glial fibrillary acidic protein–positive astrocytes and ionized calcium-binding adaptor molecule 1–positive microglia was similar between the graft and host. The latter is particularly interesting, as we have previously demonstrated robust microglial infiltration in grafts surviving for shorter time periods.10
Discussion
The present study reports clinical and postmortem findings of a PD patient who received bilateral fetal dopamine transplants 16 years prior to death. This patient displayed the most robust graft survival (>300,000 dopamine cells per putamen). This level of survival represents approximately 60% of the dopaminergic nigral population in the normal adult midbrain.28 Striatal innervation occurred in a patch-matrix manner and the breadth, and intensity of the innervation was indistinguishable from a healthy age-matched control. Bidirectional synapses between host and graft were seen ultrastructurally. These findings were observed throughout the putamen, and to a lesser degree the caudate nucleus, although transplants were only performed in the postcommissural putamen. These changes were associated with significant increases in striatal FD-PET bilaterally.
Despite these findings, this patient never displayed meaningful clinical benefit on both blinded, and later open label, assessments. In comparison, clinical benefit has been reported with cell counts ranging from 80,000 to 200,000 grafted dopamine cells per putamen in open label studies.11–13 Recently reported longstanding bilateral clinical benefits in an open label assessment were sufficient to withdraw L-dopa following a unilateral transplant, with only 42,000 residual grafted dopamine neurons.22
The reasons for these discrepancies are not clear. Open label assessments may have overemphasized any benefit that may have occurred, as 2 double-blind trials have failed to replicate the results of open label studies following surgical procedures in PD.21 Improvement in motor UPDRS of approximately 30% has been reported for >12 months with double-blind sham surgical procedures. 29 Furthermore, progression of disability may be due to degeneration of non-nigrostriatal systems and thus would not be altered by fetal nigral transplants.
A second possibility for the clinical failure following surgery could be a slow-growing transplant that ultimately filled the putamen by the time of her death, but was not vital during the time of blinded clinical assessments. However, this too would not seem to be the case, because FD-PET scans revealed increases in fluorodopa binding by 1 year postsurgery with no improvement clinically on blinded assessments.
Third, it is possible that despite robust expression of TH and the present electron microscopic findings, synaptic dopamine is not being synthesized or released properly. Ultrastructural analyses revealed putative dopaminergic synapses upon host striatal neurons, and the comprehensive loss of putaminal TH-ir fibers in advanced PD makes it likely that these synapses are graft-derived.30 However, it is impossible to state conclusively that the innervation and synapses are functional.
Another consideration is that implanted neurons were dysfunctional based upon the presence of Lewy pathology and reductions in TH-ir expression in individual grafted neurons. Many of the>300,000 TH/NM-positive cells in the graft contained α-synuclein aggregates, had reduced TH staining, and were thioflavin positive, indicating abnormal protein misfolding. We14–17,22 have postulated that these inclusions are likely transferred to healthy embryonic dopamine neurons from PD-affected host neurons and are consistent with a prion-like process.31–35 This could impair cell function, as noted by reports of reduced dopamine transporter–immunoreactive and TH-ir staining in surviving neurons.16–19 Although these pathological aggregates could negatively impact the function of the graft, they appear to develop in a time-dependent manner and have not yet been seen until at least 10 years postgrafting, 14–17,22 whereas our patient did not benefit from the graft at any time. The failure to improve clinically in this case stands in stark contrast to the findings of the Lund group, which reported dramatic benefits in a single patient sufficient to stop L-dopa in open label assessment with only 40,000 surviving cells, 27% of which displayed S129-αSyn-ir–positive inclusions.22 This patient gradually lost motor benefits after approximately 10 years. An autopsy was performed 24 years after the transplant, and it is possible that benefits at an earlier time point were observed with a different histologic picture.22
It is also possible that patient-specific factors determine whether there is clinical benefit. In the 2 double-blind NIH trials, post hoc analyses suggested that patients younger than 60 years,9 or with milder disease (UPDRS Motor Examination OFF state score<45),10 benefited from transplant procedure.10 The patient in the present study was 55 years of age at the time of surgery, but her UPDRS Motor Examination OFF state score was 52. She also had cortical β-amyloid deposition at autopsy, although when this occurred is not known.
Therefore, it is possible that the patient’s disease was too severe at the time of grafting for benefit to occur. However, this patient had a good response to L-dopa and experienced motor complications, making such an explanation unlikely.
One of the most fascinating aspects of this case is the development of graft-induced dyskinesias. These dyskinesias differ from peak dose dyskinesias, which are typically choreiform in nature and occur in association with the peak L-dopa plasma concentrations and the best antiparkinsonian response. By contrast, graft-induced dyskinesias are phenomenologically more similar to diphasic dyskinesia.36 Diphasic dyskinesias are characterized by asymmetric, stereotypic involuntary movements that tend to primarily affect the lower extremities. They are typically very short in duration (seconds to minutes) and are associated with relatively low levels of plasma L-dopa, disappearing as the L-dopa dose rises or falls. Although the dyskinesias seen following grafting were similar in appearance, they are long-lasting and can persist in the absence of L-dopa. Perhaps the most parsimonious explanation for the findings in the present case is that the grafts are producing dopamine sufficient to cause persistent diphasic dyskinesias but insufficient to provide an antiparkinsonian benefit.
Our study raises several important issues with the regard to the future of cell replacement strategies, particularly with relevance to embryonic stem cells or induced pluripotent stem cells. We show here that despite the most robust graft survival reported to date, clinical benefit following fetal transplantation was not observed.
However, if our hypothesis is correct that despite robust graft viability and innervation, the grafts are producing functional dopamine at a level too low to provide clinical benefit, this implies that to achieve clinical benefit, even greater numbers of surviving grafted dopamine neurons may be required, or that more efficient dopamine release or physiologic connectivity is required. The production of greater levels of dopamine and/or functional dopamine release obviates the development of graft-induced dyskinesias. This goal might be more readily achieved with stem cells, as they can be produced in greater numbers. Nonetheless, even if successful, it remains to be determined how transplant strategies will provide benefit in comparison to available surgical therapies such as DBS and Duodopa, and how they could address nondopaminergic features of PD such as falling and dementia, which are currently the most disabling aspects of the disease.
Acknowledgments
Supported by a grant from the NIHNINDS (NS32842; C.W.O., C.G.G., T.B.F., J.H.K., A.J.S.) and a Center Grant from the Parkinson’s Disease Foundation (C.G.G., J.H.K.). G.M.H. is supported by a National Health and Medical Research Council Senior Principal Research Fellowship (1079679).
We thank the family of the patient presented in this report for their diligence in optimizing the brain donation and for their time in providing medical records and information about the deceased.
Footnotes
Author Contributions
J.H.K., C.G.G., A.J.S., T.B.F., and C.W.O. contributed to the conception and design of the study. A.J.S., V.S., Y.C., G.M.H., D.A.N., T.M., and D.J.M. participated in the acquisition and analysis of data. J.H.K., C.G.G., Y.C., G.M.H., D.A.N., A.J.S., and C.W.O. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
Nothing to report.
References
- 1.Stenevi U, Björklund A, Svendgaard NA. Transplantation of central and peripheral monoamine neurons to the adult rat brain: techniques and conditions for survival. Brain Res. 1976;114:1–20. doi: 10.1016/0006-8993(76)91003-9. [DOI] [PubMed] [Google Scholar]
- 2.Perlow MJ, Freed WJ, Hoffer BJ, et al. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 3.Björklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177:555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- 4.Freed WJ, Perlow MJ, Karoum F, et al. Restoration of dopaminergic function by grafting of fetal rat substantia nigra to the caudate nucleus: long-term behavioral, biochemical, and histochemical studies. Ann Neurol. 1980;8:510–519. doi: 10.1002/ana.410080508. [DOI] [PubMed] [Google Scholar]
- 5.Bakay RA, Barrow DL, Fiandaca MS, et al. Biochemical and behavioral correction of MPTP Parkinson-like syndrome by fetal cell transplantation. Ann N Y Acad Sci. 1987;495:623–640. doi: 10.1111/j.1749-6632.1987.tb23705.x. [DOI] [PubMed] [Google Scholar]
- 6.Redmond DE, Sladek JR, Jr, Roth RH, et al. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet. 1986;1:1125–1127. doi: 10.1016/s0140-6736(86)91839-8. [DOI] [PubMed] [Google Scholar]
- 7.Bjorklund A, Kordower JH. Cell therapy for Parkinson’s disease: what next? Mov Disord. 2013;28:110–115. doi: 10.1002/mds.25343. [DOI] [PubMed] [Google Scholar]
- 8.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson’s disease. Trends Neurosci. 1996;19:102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 9.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 10.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 11.Kordower JH, Freeman TB, Snow BJ, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 12.Kordower JH, Rosenstein JM, Collier TJ, et al. Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J Comp Neurol. 1996;370:203–230. doi: 10.1002/(SICI)1096-9861(19960624)370:2<203::AID-CNE6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kordower JH, Chu Y, Hauser RA, et al. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 15.Kordower JH, Chu Y, Hauser RA, et al. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 16.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 17.Li JY, Englund E, Widner H, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25:1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- 18.Olanow CW, Gracies J-M, Goetz CC, et al. Clinical pattern and risk factors for dyskinesias following fetal nigral transplantation in Parkinson’s disease: a double-blind video-based analysis. Mov Disord. 2009;24:336–343. doi: 10.1002/mds.22208. [DOI] [PubMed] [Google Scholar]
- 19.Lindvall O, Sawle G, Widner H, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann Neurol. 1994;35:172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- 20.Freeman TB, Olanow CW, Hauser RA, et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson’s disease. Ann Neurol. 1995;38:379–388. doi: 10.1002/ana.410380307. [DOI] [PubMed] [Google Scholar]
- 21.Alterman RL, Tagliati M, Olanow W. Observer bias in biological/surgical trials of novel Parkinson’s disease therapies. Mov Disord. 2009;24:254. [Google Scholar]
- 22.Li W, Englund E, Widner H, et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating Parkinsonian brain. Proc Natl Acad Sci U S A. 2016;113:6544–6549. doi: 10.1073/pnas.1605245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 25.Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Review Ann N Y Acad Sci. 2010;1184:55–67. doi: 10.1111/j.1749-6632.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- 26.Chu Y, Goldman J, Kelly L, et al. Abnormal alpha-synuclein reduces nigral voltage-dependent anion channel 1 in sporadic and experimental PD. Neurobiol Dis. 2014;69:1–14. doi: 10.1016/j.nbd.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Chu Y, Kordower JH. Age-associated increases of a-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Pakkenberg B, Møller A, Gundersen HJ, et al. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedorow H, Halliday GM, Rickert CH, et al. Evidence for specific phases in the development of human neuromelanin. Neurobiol Aging. 2006;27:506–512. doi: 10.1016/j.neurobiolaging.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kordower JH, Brundin P. Propagation of host disease to grafted neurons: accumulating evidence. Exp Neurol. 2009;220:224–225. doi: 10.1016/j.expneurol.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Brundin P, Kordower JH. Neuropathology in transplants in Parkinson’s disease: implications for disease pathogenesis and the future of cell therapy. Prog Brain Res. 2012;200:221–224. doi: 10.1016/B978-0-444-59575-1.00010-7. [DOI] [PubMed] [Google Scholar]
- 33.Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28:31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 34.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci U S A. 2009;106:12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kordower JH, Olanow CW. Fetal grafts for Parkinson’s disease: decades in the making. Proc Natl Acad Sci U S A. 2016;113:6332–6334. doi: 10.1073/pnas.1606342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu Y, Morfini G, Langhammer L, et al. Axonal transport defects in sporadic and experimental PD. Brain. 2012;135:2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]