Abstract
In a scenario involving a nuclear detonation during war or a terrorist attack, acute radiation exposure combined with thermal and blast effects results in severe skin injury. Although the cutaneous injury in such a scenario may not be lethal, it may lead to inflammation, delayed wound healing and loss of the skin barrier, resulting in an increased risk of infection. In this study, we tested the potential use of timolol, a beta-adrenergic receptor antagonist, to improve epidermal wound closure after combined burn and radiation injury using an ex vivo human skin culture model. Daily application of 10 µM timolol after combined injury (burn and 10 Gy ex vivo irradiation) increased wound epithelialization by 5–20%. In addition, exposure to 10 Gy significantly suppressed epidermal keratinocyte proliferation by 46% at 48 h postirradiation. Similar to what has been observed in a thermal burn injury, the enzyme phenylethanolamine N-methyltransferase (PNMT), which generates epinephrine, was elevated in the combined thermal burn and radiation wounds. This likely resulted in elevated tissue levels of this catecholamine, which has been shown to delay healing. Thus, with the addition of timolol to the wound to block the binding of locally generated epinephrine to the beta-adrenergic receptor, healing is improved. This work suggests that by antagonizing local epinephrine action within the wound, a beta-adrenergic receptor antagonist such as timolol may be a useful adjunctive treatment to improve healing in the combined burn and radiation injury.
INTRODUCTION
With the exception of radiotherapy, human acute exposure to moderate (2–10 Gy) and higher doses of ionizing radiation is usually accidental; however, it can also be intentional, as in the case of a nuclear detonation during terrorist attacks or war. In addition to radiation injuries, nuclear explosions produce thermal and blast injuries that often occur in combination. In such a scenario, a majority of the casualties (~70%) are likely to sustain a radiation combined skin-burn injury (RCI) (1). In RCI, the lethality of a radiation dose can be as low as 2.5 Gy (2). In humans, acute whole- or partial-body exposure to >2 Gy radiation causes acute radiation syndrome or sickness (ARS) (3, 4), leading to hematopoietic, gastrointestinal, cutaneous and cerebrovascular damages. Cutaneous radiation syndrome is not lethal per se, but contributes to multi-organ failure (5, 6) and delayed wound closure, thereby also increasing risk of infection (7, 8). Currently, no specific treatments are available for victims of radiation exposure alone or RCI (9), and supportive care in irradiated humans has been estimated to increase LD50 values from 3.5–4 to 6–7 Gy (10). Efforts to develop new medical countermeasures to treat ARS and RCI are the focus of the NIH Radiation and Nuclear Countermeasures Program, coordinated by the National Institute of Allergy and Infection Diseases (11). Preclinical investigations, several human case reports and a case series (60 patients) in which timolol is used to heal venous ulcers (12–16) suggest that cutaneous wound healing is improved by topically applied timolol maleate, a first-generation nonspecific beta 1, 2 adrenergic blocker, which is FDA approved for the treatment of glaucoma (17). Here, we examine the potential of timolol to improve healing in an ex vivo human skin model of combined radiation and burn injury.
MATERIALS AND METHODS
Human Skin Collection
Normal human skin was obtained from surgical procedures for cosmetic breast reduction or abdominoplasty. This study was approved by the Institutional Review Board (IRB) at the University of California, Davis and skin samples were obtained from nine female adult donors with the majority being Caucasian and an average age of 46 years. All patients had been cleared for this cosmetic surgery, and while protected health data regarding prescription drug use that could affect wound healing were not captured from patient records per IRB protocol, the assumption is that for clearance for elective plastic surgical procedures, these were limited.
Ex Vivo Skin Culture and Wounding
Full-thickness skin, including epidermis, dermis and subcutaneous fat, was collected at the operating room within 4 h of the surgical procedure. After collection, skin was immediately placed in cold sterile phosphate buffered saline (PBS; pH 7.2) containing antibiotics (Invitrogen™, Carlsbad, CA). After removal of the fat layer under sterile conditions, the skin was cut into ~15 × 20-cm pieces, and maintained in cold PBS until the experiments were initiated, within the next 4 h. The skin was subjected to one of three injuries: irradiation with burn; burn with irradiation; or burn only (control). Combined injuries were spaced by 15-min intervals, where the skin was left at room temperature and kept moist using Dulbecco’s modified Eagle medium (DMEM; Invitrogen). For radiation injury, 10 Gy of X rays were delivered using a clinical irradiator (Elekta Synergy®, Stockholm, Sweden) at a dose rate of 1 Gy/min at 15 MeV. For thermal burn, skin was dried with sterile gauze prior to 1-s application of a stainless-steel rod (1 mm in diameter) heated to 200°C on a heating plate. DMEM was applied to the skin immediately after burning. The skin tissue was cut into 1 × 1-cm squares after the wounds were created, submerged in culture media [DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotics] and cultured for 10 days before fixation. To observe the effect of timolol (Sigma-Aldrich® LLC, St. Louis, MO) on wound healing of injured skin pieces, timolol (10 µM) was added to the culture media (18). The culture media was refreshed daily. The number of tissue pieces for the nine donors across the six conditions varied but averaged out to three, and the number of skin sections per piece varied but also averaged out to three.
Hematoxylin and Eosin Staining
Skin samples were fixed in 10% buffered formalin at room temperature for 24 h, followed by another week or more at +4°C, prior to paraffin embedding. Skin sections, 5 µm in thickness, were stained with hematoxylin and eosin (H&E).
PNMT Staining
To perform the phenylethanolamine N-methyltransferase (PNMT) staining, the skin samples were fixed and embedded in paraffin and sectioned to 5 µm. The sections were deparaffinized, rehydrated and incubated with sodium citrate buffer (pH 6.0) at 95°C for 20 min to retrieve the antigen and blocked with 4% bovine serum albumin (BSA) for 1 h. Primary antibody against PNMT (rabbit antibody, cat. no. ab154282, 1:100; Abcam®, Cambridge, MA) was incubated with the samples overnight at 4°C, followed by three PBS washes. Secondary antibody, anti-rabbit-HRP (1:500; Cell Signaling Technology® Inc., Danvers, MA), was incubated with the sections for 2 h, followed by 10-min incubation of Vector® DAB (Vector Laboratories, Burlingame, CA) and counterstaining of the nuclei with hematoxylin. The slides were then mounted and imaged.
Image Analysis
Wound healing was scored by measuring the percentage of wound re-epithelialization. The skin sections were scanned at 10× by a BioRevo BZ-9000 microscope with the imaging software (Keyence Corp. of America, Itasca, IL). The wound edge was indicated by the detached epidermis. The wound length and re-epithelialization from both wound edges were digitally measured to determine the percentage of healing. The PNMT slides were scanned at 10× using a BioRevo BZ-9000 microscope. The epidermal area adjacent to the wounds was selected to measure the average PNMT staining intensity. The average PNMT intensity from three patients (two sections/sample) in each treatment group was compared to the control by t test.
Keratinocyte Proliferation
Isolated human keratinocytes between passage 3–6 were cultured in keratinocyte growth medium [EpiLife™ KGM™ with human keratinocyte growth supplements (HKGS) and ABAM], seeded at 3 × 105/ml on 60-mm culture plates on day 0, received 10 Gy of radiation and incubated for 24–48 h. Cells were collected at 24 and 48 h and counted to determine the proliferation rates. Three keratinocyte strains from donors were used and three independent experiments were repeated.
Statistical Analysis
Percentage healing (Fig. 1E) was modeled as a function of treatment (i.e., burn; burn with timolol; burn with irradiation; burn with irradiation and timolol; irradiation with burn; or irradiation with burn and timolol), using a Tobit model (20) to address the substantial number of sections with 0% healing. A random effect for donor was included in the model. The wound healing analyses were performed using R version 2.13.0 (21). Tobit modeling was performed using the R package AER (22). Skin samples from nine donors were used in three repeated experiments. For each skin donor, an average of three skin samples and an average of three sections per sample were scored in each treatment group.
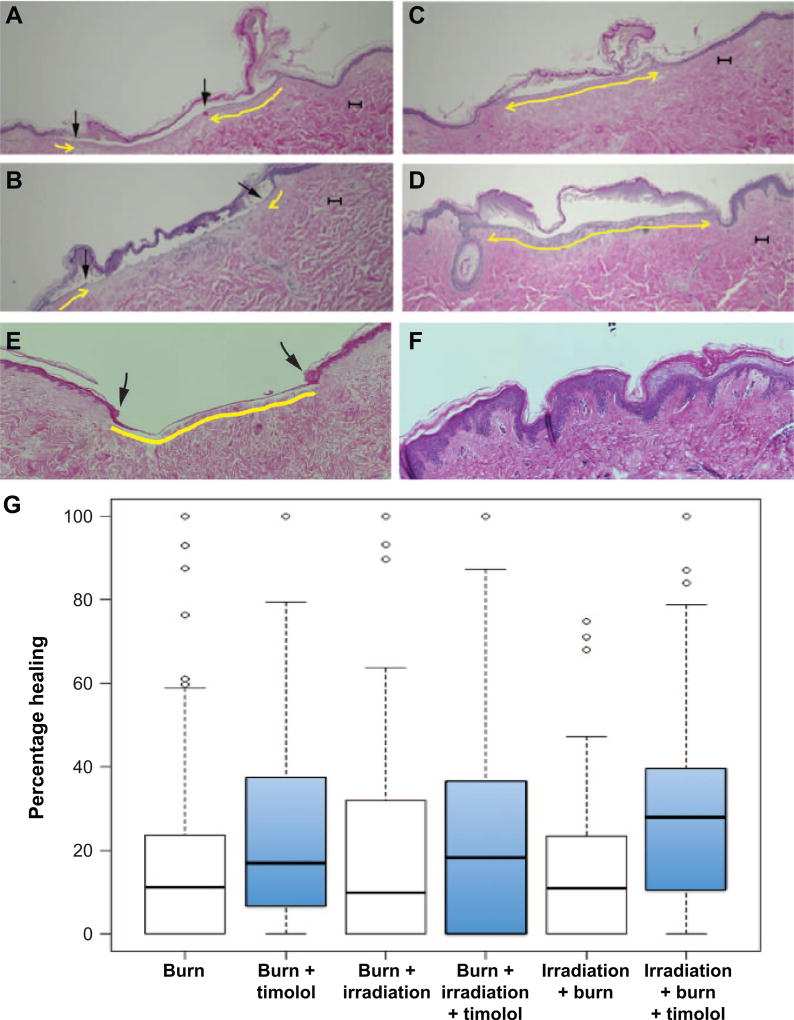
FIG. 1.
Timolol promotes re-epithelialization in the combined burn/radiation wounds. Sections of human skin that received combined burn and radiation injury were incubated for 10 days, fixed, then H&E stained for histological analysis. Scale bars = 100 µm. Panels A and B: Partially re-epithelialized wounds. Arrows indicate the re-epithelialization. Panels C and D: Fully re-epithelialized wounds. Panel E: Positive control of fully re-epithelialized incisional wound. Panel F: Untreated human skin to present the epidermis. Yellow line indicates newly re-epithelialized dermal surface, underlying the injured and necrotic separated stratum corneum. The epidermis layer does not regenerate to full thickness under the culture conditions. Panel G: Boxplots of percentage healing in six treatment groups: burn; burn + timolol; burn + irradiation; burn + irradiation + timolol; irradiation + burn; irradiation + burn + timolol. Timolol-treated groups are shown as blue boxes to the right of their untreated controls. The horizontal line in the middle of each box represents the median, the lower and upper edges of the box represent the first and third quartiles, respectively, and the whiskers represent the smallest and largest nonoutlying observations, where an outlying observation (indicated with a circle) is defined here as any observation lying more than 1.5 interquartile ranges from the box.
RESULTS AND DISCUSSION
We examined wound healing in an ex vivo human skin model of combined burn and radiation injury. Wound closure was scored by histomorphometric analysis of re-epithelialization in H&E stained skin sections on day 10 after injury (Fig. 1). Representative images of partially healed wounds (Fig. 1A and B, epithelialization indicated by arrows) and fully healed wounds (Fig. 1C and D) are shown. Timolol demonstrates beneficial re-epithelialization effects in these wounds. Adding 10 µM timolol after injury improved wound epithelialization by 5–20% (see Fig. 1G, treatment groups: burn with timolol; burn with irradiation and timolol; irradiation with burn and timolol). Statistical analysis demonstrates that the timolol treatment increased healing among the three treatment groups (likelihood ratio test P < 0.001). The order in which injuries occurred (burn injury followed by radiation injury or vice versa) did not affect the healing rates (likelihood ratio test P = 0.330). The overall benefit of timolol across wound types is significant, and no difference in the response to timolol by wound type was observed (likelihood ratio test P = 0.414).
Radiation changes the proliferative capacity of epidermal cells, as well as the communication network of keratinocytes, fibroblasts and immune cells (6). Keratinocyte proliferation is required for wound re-epithelialization, and 10 Gy exposure significantly inhibited keratinocyte proliferation by 46% at 48 h postirradiation (Fig. 2A), which likely contributed to the decrease in re-epithelialization observed in post-burn tissues (Fig. 1; 10–25% re-epithelialization after 10 days of incubation).
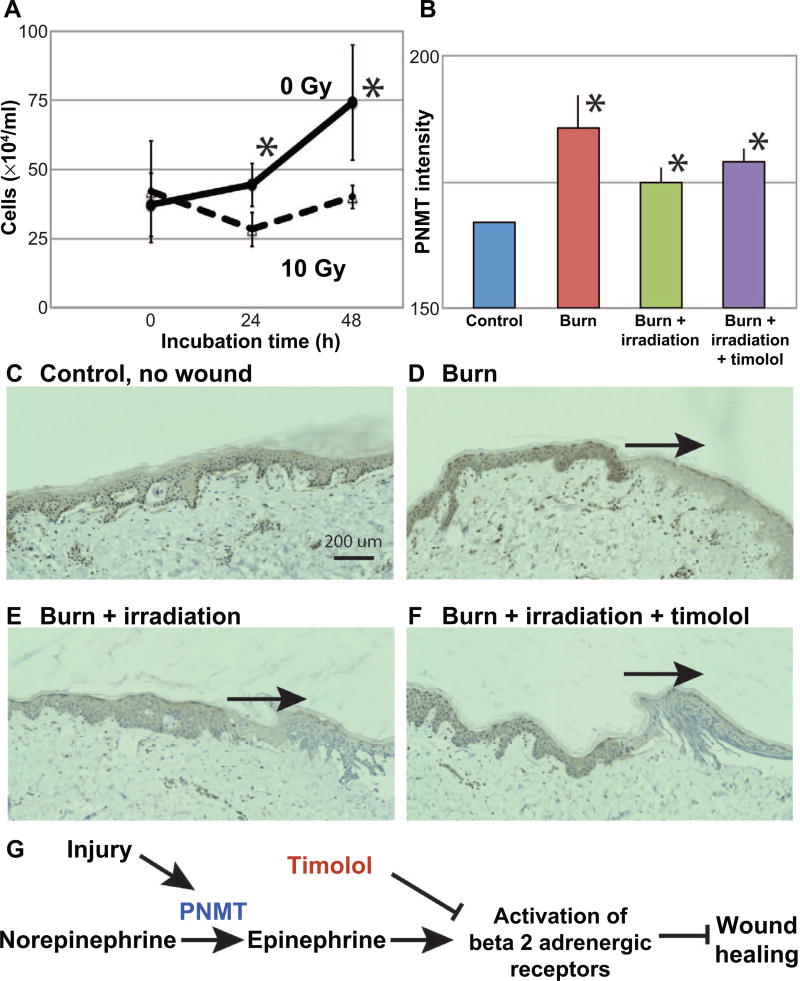
FIG. 2.
Radiation inhibits keratinocyte growth, and PNMT is elevated in the combined burn/radiation wounds. Panel A: Exposure (10 Gy) inhibited the growth of cultured keratinocytes at 48 h postirradiation compared to control. Immunohistochemistry was used to detect PNMT in the injured skin, and levels of intensity quantified in skin samples at day 10 (panel B). PNMT levels were increased in the burn, combined burn/ radiation and timolol-treated burn/radiation wounds compared to controls (*P < 0.05). Panels C–F: PNMT staining was observed in the epidermis (control, burn, burn/radiation wound and timolol-treated burn/radiation wound, respectively); wound sites are indicated by the arrows. The nonspecific staining of stratum corneum was not included in the quantification. Scale bar = 200 µm. Panel G: Schematic showing the regulation of beta 2 adrenergic receptors and wound healing.
Local generation of epinephrine, an endogenous ligand for the beta-adrenergic receptors, within a thermal burn wound has previously been demonstrated to impair healing (18), and here we demonstrate its role in the combined injury (schematic pathway, Fig. 2G). The burned epidermal tissue contributes to epinephrine generation by upregulation of the catecholamine biosynthetic enzyme PNMT (18). Similar to the thermal burn injury, epidermal PNMT levels were elevated in the combined thermal burn and irradiation wounds, even with the timolol treatment at day 10 (Fig. 2B, histological images in Fig. 2D–F).
Sivamani et al. (19) showed that the PNMT level may be regulated by the beta-adrenergic receptors by examining the very early, acute responses (30 min to 24 h) of PNMT levels in the keratinocyte scratch wounds. Here, we examined the delay in healing; thus, we probed tissue at day 10 after injury. This might explain why in the current study, the timolol treatment did not change the PNMT level, yet by blocking generated catecholamine activity, improved wound healing. We speculate that the beta-adrenergic receptor antagonist timolol inhibits the activity of the locally elevated epinephrine after wounding, resulting in improved keratinocyte migration and wound epithelialization in combined burn and radiation injury. Interestingly, a nonhealing, radiation-induced wound has been reported to heal when treated with timolol, providing support for the potential clinical translation of this therapeutic approach. Indeed, a federally-funded randomized controlled clinical trial in the U.S. to examine the efficacy of timolol for healing diabetic wounds has just recently been initiated (ClinTrials NCT03282981) and a non-U.S. trial for timolol treatment of venous ulcers is reported to be underway (ClinTrials NCT02422017). Further experiments to examine the levels of epinephrine and beta-adrenergic receptors in wound tissue will be helpful to more fully elucidate the mechanism of timolol in improving wound healing. Taken together, this work suggests that a beta-adrenergic receptor antagonist such as timolol may also be a useful adjunctive treatment in early combined radiation injury to the skin, improving healing and restoring the epidermal barrier earlier, thereby decreasing the likelihood of skin wound infection.
Acknowledgments
We thank the gracious personnel at the Plastic Surgery Clinic at UC Davis for assistance with obtaining informed consents from the skin donors, the radiation therapists at the UC Davis Medical Center for their patience and help with irradiation of ex vivo skin and Jessel Yee at Department of Dermatology, UC Davis, for technical support with immunostaining. This work was supported by the National Institute of Allergy and Infectious Diseases (grant no. R21/R33 AI080604 to DMR and RRI) and a NIH grant to UC Davis Clinical and Translational Science Center (NCATS UL1 TR001860).
References
- 1.Pellmar TC, Ledney GD. NATO RTG-099 2005. Bethesda, MD: Armed Forces Radiobiology Research Institute; 2005. Combined injury: Radiation in combination with trauma, infectious disease, or chemical exposure; pp. 1–9. [Google Scholar]
- 2.Planning guidance for response to a nuclear detonation. Washington, DC: Environmental Protection Agency; 2017. ( https://fas.org/irp/threat/detonation.pdf) [Google Scholar]
- 3.Dorr H, Meineke V. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Med. 2011;9:126–31. doi: 10.1186/1741-7015-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5:S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meineke V. The role of damage to the cutaneous system in radiation-induced multi-organ failure. BJR Suppl. 2005;27:85–99. [Google Scholar]
- 6.Peter RU. Cutaneous radiation syndrome in multi-organ failure. BJR Suppl. 2005;27:180–4. [Google Scholar]
- 7.Ran X, Cheng T, Shi C, Xu H, Qu J, Yan G, et al. The effects of total-body irradiation on the survival and skin wound healing of rats with combined radiation-wound injury. J Trauma. 2004;57:1087–93. doi: 10.1097/01.ta.0000141885.72033.c7. [DOI] [PubMed] [Google Scholar]
- 8.Ledney GD, Elliott TB. Combined injury: factors with potential to impact radiation dose assessments. Health Phys. 2010;98:145–52. doi: 10.1097/01.HP.0000348466.09978.77. [DOI] [PubMed] [Google Scholar]
- 9.Hafer N, Maidment BW, Hatchett RJ. The NIAID Radiation Countermeasures Program business model. Biosecur Bioterror. 2010;8:357–63. doi: 10.1089/bsp.2010.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003:473–96. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 11.DiCarlo AL, Ramakrishnan N, Hatchett RJ. Radiation combined injury: overview of NIAID research. Health Phys. 2010;98:863–7. doi: 10.1097/HP.0b013e3181a6ee32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen L, Tchanque-Fossuo CN, Gorouhi F, Boudreault D, Nguyen C, Fuentes JJ, et al. Combination therapy of autologous adipose mesenchymal stem cell-enriched, high-density lipoaspirate and topical timolol for healing chronic wounds. J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2390. [DOI] [PubMed] [Google Scholar]
- 13.Beroukhim K, Rotunda AM. Topical 0.5% timolol heals a recalcitrant irradiated surgical scalp wound. Dermatol Surg. 2014;40:924–6. doi: 10.1111/DSU.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 14.Braun LR, Lamel SA, Richmond NA, Kirsner RS. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400–2. doi: 10.1001/jamadermatol.2013.7135. [DOI] [PubMed] [Google Scholar]
- 15.Lev-Tov H, Dahle S, Moss J, Isseroff RR. Successful treatment of a chronic venous leg ulcer using a topical beta-blocker. J Am Acad Dermatol. 2013;69:e204–5. doi: 10.1016/j.jaad.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Thomas B, Kurien JS, Jose T, Ulahannan SE, Varghese SA. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg Venous Lymphat Disord. 2017;5:844–50. doi: 10.1016/j.jvsv.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Highlights of prescribing information. Report No. NDA 21-516/S-005. Silver Spring, MD: Food and Drug Administration; 2013. ( http://bit.ly/2BBjt1x) [Google Scholar]
- 18.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivamani RK, Shi B, Griffiths E, Vu SM, Lev-Tov HA, Dahle S, et al. Acute wounding alters the beta2-adrenergic signaling and catecholamine synthetic pathways in keratinocytes. J Investig Dermatol. 2014;134:2258–66. doi: 10.1038/jid.2014.137. [DOI] [PubMed] [Google Scholar]
- 20.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 21.R Development Core Team. R: A language and environment for statistical computing. 1. Vienna, Austria: The R Foundation for Statistical Computing; 2011. [Google Scholar]
- 22.Kleiber C, Zeileis A. Applied econometrics with R. 1. New York: Springer-Verlag; 2008. [Google Scholar]