Abstract
Bowman-Birk inhibitor (BBI) is a soybean-derived protease inhibitor that has anti-inflammation and anti-HIV effect. Here, we further investigated the anti-HIV action of BBI in macrophages, focusing on its effect on viral entry. We found that BBI could significantly block HIV entry into macrophages. Investigation of the mechanism (s) of the BBI action on HIV inhibition showed that BBI down-regulated the expression of CD4 receptor (as much as 80%) and induced the production of the CC chemokines (up to 60 folds at protein level) in macrophages. This inhibitory effect of BBI on HIV entry could be blocked by the neutralization antibodies to CC chemokines. These findings indicate that BBI may have therapeutic potential as a viral entry inhibitor for the prevention and treatment of HIV infection.
Keywords: Bowman-Birk inhibitor, HIV, CD4, CCR5, CC chemokine
1. Introduction
In the absence of a protective vaccine or a cure, HIV infection persists as a major cause of morbidity in both developed and non-developed countries. An estimated 36.7 million people are infected with HIV worldwide. Therefore, prevention and access to antiretroviral treatments (ART) are the best options against HIV infection. Although significant advances in ART have been made since the introduction of zidovudine (AZT) in 1987, ART does not eradiate HIV and causes severe side effects and the development of drug resistant viruses. Thus, there is an urgent need to develop new and safe agents to prevent and treat HIV infection. Apparently, to design anti-HIV strategies that target the early stages of HIV infection is critical in the control of HIV spread.
It has been well known that HIV entry into target cells begins with binding of the viral envelope glycoprotein (gp120) to the primary receptor (CD4) and co-receptors (CCR5, CXCR4). There are three crucial steps in the HIV entry process, each of which could serve as a therapeutic target: binding of HIV gp120 with CD4 receptor, binding of the envelope-CD4 complex to CC chemokine receptors (CCR5 and CXCR4), and fusion of the viral and cell membranes (Chan and Kim, 1998; Wyatt and Sodroski, 1998; D’Souza et al., 2000). The CC chemokine receptor CCR5 is used for entry by most laboratory-adapted macrophage-tropic HIV strains. The CC chemokine ligands, CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES) are natural ligands for HIV co-receptor, CCR5. Thus these chemokines could inhibit the infection of CCR5-using HIV (R5) strains at the level of entry (Alkhatib et al., 1996; Ketas et al., 2003).
Serine proteases are known to be actively involved in pro-inflammatory actions (Safavi and Rostami, 2012), including the production of inflammatory cytokines (Breen et al., 1990; Lacroix et al., 1993; Contreras et al., 2003; Calabrese et al., 2004; Rizzi et al., 2006). BBI, as a serine proteases inhibitor, has anti-inflammatory effects (Ware et al., 1999; Dia et al., 2008; Dai et al., 2011; Li et al., 2011; Safavi and Rostami, 2012; Aboud et al., 2014). The importance of BBI in the inhibition of inflammation is highlighted by its ability to decrease LPS-induced inflammatory cytokines (TNF-α, IL-1β, IL-6) and increase anti-inflammatory cytokine (IL-10) in macrophages (Li et al., 2011). In vivo studies demonstrated that BBI exerts the immune-regulatory and anti-inflammatory effects in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (Safavi et al., 2013). Several studies (Ye et al., 2001; Ye and Ng, 2009; Prasad et al., 2010) also showed that BBI could inhibit HIV reverse transcriptase in cell-free systems. We showed that BBI inhibits HIV replication in macrophages through the induction of the intracellular antiviral factors (Ma et al., 2016). Here, we investigated whether BBI can block HIV entry into macrophages. We also examined the mechanisms involved in the BBI action on HIV.
2. Materials and methods
2.1. Reagents and antibodies
Bowman-Birk inhibitor (BBI) isolated from soybean (Glycine max) was purchased from Sigma-Aldrich (St. Louis, MO). BBI consists of 90% protein as assayed by Biuret, with the remainder of a phosphate buffer salt. The stock solution of BBI was prepared in sterile culture grade water at the concentration of 1 mg/ml. Polybrene was purchased from Sigma-Aldrich (St. Louis, MO). PE-cy7 anti-human CD4 (clone: SK3), FITC anti-human CD195 (CCR5), PE anti-human CXCR4 antibodies for flow cytometry were purchased from BD Bioscience (San Jose, CA), eBiosciences (San Diego, CA), BD Bioscience (San Jose, CA), respectively. The ELISA kits for RANTES, MIP-1α and MIP-1β were purchased from Biolegend Inc. (San Diego, CA), Raybiotech Inc. (Norcross, GA), and Raybiotech Inc. (Norcross, GA), respectively. The neutralization antibodies to RANTES, MIP-1α, MIP-1β were purchased from R & D Systems Inc. (Minneapolis, MN).
2.2. Macrophages and HIV strain
Purified monocytes were obtained from Human Immunology Core at the University of Pennsylvania (Philadelphia, PA). The Core has the Institutional Review Board approval for blood collection from healthy donors. Freshly isolated monocytes were cultured in the 48-well plate (2.5×105 cells/well) in DMEM containing 10% FBS. Macrophages used in this study refer to 7-day-cultured monocytes at 37 °C, 5% CO2. The HIV R5 strains (Jago and the pseudotyped HIVADA) were obtained from the AIDS Research and Reference Program (National Institutes of Health, Bethesda, MD). HIV pseudotyped with the envelope glycoprotein of the R5 isolate HIVADA has a replication-defective viral genome that encoded a luciferase reporter gene, which allows a quantitative measure of the levels of single-round infection (Geijtenbeek et al., 2000). VSV-G pseudotyped HIV was packaged in 293 T cells with psPAX2, pMD2. G and pTRIPZ.
2.3. MTS
The effect of BBI on the viability of macrophages was analyzed by 3- (4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay. Macrophages cultured in a 96-well plate were treated with different concentrations of BBI (50, 100 μg/ml) for 6 days. For MTS assay, 20 μl of CellTiter 96® AQueous One Solution Reagent containing MTS and phenazine ethosulfate was added to each well of the 96-well plate. Absorbance at 490 nm was measured at 4 h after addition of the reagent.
2.4. BBI treatment and HIV infection
Macrophages were treated with different concentrations of BBI (25, 50, and 100 μg/ml) for 24 h prior to HIV (Jago) infection. After washing away unattached virus, macrophages were cultured in medium without BBI. Cells and supernatant were collected on day 5 post-infection. For pseudotyped virus infection, macrophages were treated with/without BBI (25, 50 and 100 μg/ml) for 24 h prior to pseudotyped HIVADA infection in the presence of polybrene. After washing away unattached virus, fresh medium without BBI was added. Macrophages were cultured for additional 48 h prior to cell lysis. Luciferase activities (relative light units, RLU) were measured by Spectra Max M5 (Molecular Devices, CA).
For VSVG pseudotyped HIV infection, Macrophages were treated with/without BBI (100 μg/ml) for 24 h prior to VSV-G pseudotyped HIV infection. The RFP protein expression was observed under a fluorescent microscope.
2.5. Reverse transcription and quantitative real-time PCR
Total DNA/RNA from macrophages or RNA from cell-free supernatant was extracted using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Reverse transcription was performed using the random primer, dNTP, AMV transcriptase and RNase inhibitor (Promega Co., Madison, WI) according to the manufacturer’s instruction. Quantitative real-time PCR (qRT-PCR) was performed with Brilliant SYBR Green Master Mix (Bio-Rad Laboratories, Hercules, CA). The primers used for the qRT-PCR amplifications are listed in Table 1. All values for RNA quantitative from macrophages were calculated using the delta delta Ct method (Schmittgen and Livak, 2008) and expressed as the changes relative to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Values for RNA (HIV Gag) quantitative from cell-free supernatant were determined by HIV standard (Kumar et al., 2002).
Table 1.
Primers for real-time PCR.
| Primer | Accession no | Orientation | Sequences |
|---|---|---|---|
| GAPDH | NM_002046 | Sense | 5′-GGTGGTCTCCTCTGACTTCAACA-3′ |
| Antisense | 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ | ||
| MIP1-α | NM_002983 | Sense | 5′-GCTGACTACTTTGAGACGAGC-3′ |
| Antisense | 5′-CCAGTCCATAGAAGAGGTAGC-3′ | ||
| MIP1-β | NM_002984 | Sense | 5′-CCAAACCAAAAGAAGCAAGC-3′ |
| Antisense | 5′-AGAAACAGTGACAGTGGACC-3′ | ||
| RANTES | NM_002985 | Sense | 5′-CTGCATCTGCCTCCCCATA-5′ |
| Antisense | 5′-GCGGGCAATGTAGGCAAA-3′ | ||
| CD4 | NM_000616 | Sense | 5′-GCACGACTCTGCAGAAGGAA-3′ |
| Antisense | 5′-CCTAAAAGGGACTCCCCGGT-3′ | ||
| CCR5 | NM_000579 | Sense | 5′-TCCAGTGAGAAAAGCCCGTAAA-3′ |
| Antisense | 5′-GGGAACGGATGTCTCAGCTC-3′ | ||
| CXCR4 | NM_003467 | Sense | 5′-ATCCCTGCCCTCCTGCTGACTATTC-3′ |
| Antisense | 5′-GAGGGCCTTGCGCTTCTGGTG-3′ | ||
| HIV Gag | AJ_437058 | Sense | 5′-ATAATCCACCTATCCCAGTAGGAGAAA-3′ |
| Antisense | 5′-TTTGGTCCTTGTCTTATGTCCAGAATGC-3′ | ||
| HIV LTR | HQ_846904 | Sense | 5′-TGGAGGACCCGGAGAAAGAA-3′ |
| Antisense | 5′-GCTCGATGTCAGCAGTCCTT-3′ |
2.6. Reverse transcriptase (RT) assay
HIV RT activity was determined based on the technique of Willey et al. (Ronald et al., 1988) with the modifications (Zhou et al., 2010). In brief, 10 μl of culture supernatant was added to 50 μl cocktail containing poly A, oligo-dT and (32P) dTTP and incubated overnight at 37 °C. The cocktail (30 μl) was then spotted onto pre-marked DE81 paper, dried for 15 min, and washed 4 times with 2× saline-sodium citrate buffer and once with 95% ethanol. The filter paper was then air-dried, cut into small pieces and putted into bottles which contain 1 ml scintillation liquid. Radioactivity was counted in a liquid scintillation counter (PerkinEImer, Inc. Shelton, CT).
2.7. ELISA
Cell-free supernatant from macrophage cultures treated with/without different concentrations of BBI (25, 50, 100 μg/ml) for 24 h was collected for the analysis of protein levels of RANTES, MIP-1α and MIP-1β with ELISA kits. ELISA was performed according to the manufacturer’s instructions.
2.8. Flow cytometric analysis
The expression of CD4, CCR5 and CXCR4 were examined by flow cytometer. Macrophages in culture plates were detached with Versene buffer (8 g Nacl, 0.2 g Kcl, 1.15 g Na2HPO4, 0.2 g EDTA, 0.1 g Phenol Red in 1 L). After washing with phosphate-buffered saline containing 1% fetal bovine serum, macrophages were incubated with PE-cy7 anti-human CD4 (clone: SK3), FITC anti-human CD195 (CCR5), PE anti-human CXCR4 antibodies at room temperature for 30 min. For gp120 and CD4 binding assay, macrophages were pretreated with BBI (100 μg/ml) for 24 h at 37 °C. After washing, macrophages were incubated with recombinant HIV envelope gp120 conjugated to FITC (15 μg/ml) (USBiological, Salem, MA) for 2 h at room temperature. Unstained or isotype-matched mouse immunoglobulin G-stained cells were included as a negative control. Stained cells were acquired by fluorescence activated cell sorting (FACSCanto II; BD Bioscience, San Jose, CA) and analyzed using Flow-Jo software (Tree Star InC, Ashland, OR).
2.9. Neutralization of CC chemokines
Macrophages were treated with/without BBI (100 μg/ml) for 4 h, after washing with PBS, macrophages were cultured with fresh medium for additional 20 h. Supernatant was collected as BBI-conditioned medium. To neutralize the effects of CC chemokines on HIV, BBI-conditioned medium was incubated with the neutralization antibodies to RANTES, MIP-1α, MP-1β (20 μg/ml, respectively) for 1 h. The mixture was then added to the macrophage cultures prior to HIV (Jago) infection. After washing away unbound virus, BBI-conditioned medium and neutralization antibodies to CC chemokines were added to the cultures. Cell-free supernatant was collected on day 5 and determined by real time RT PCR for HIV Gag gene.
2.10. Statistical analysis
Where appropriate, data were expressed as mean±standard deviation (SD) of triplicate cultures. Statistical significance was assessed by Student’s t-test and differences were considered to be statistically significant when p<0.05. Statistical analyses were performed with GraphPad prism Statistical Software.
3. Results
3.1. BBI inhibits HIV infection of macrophages at the entry level
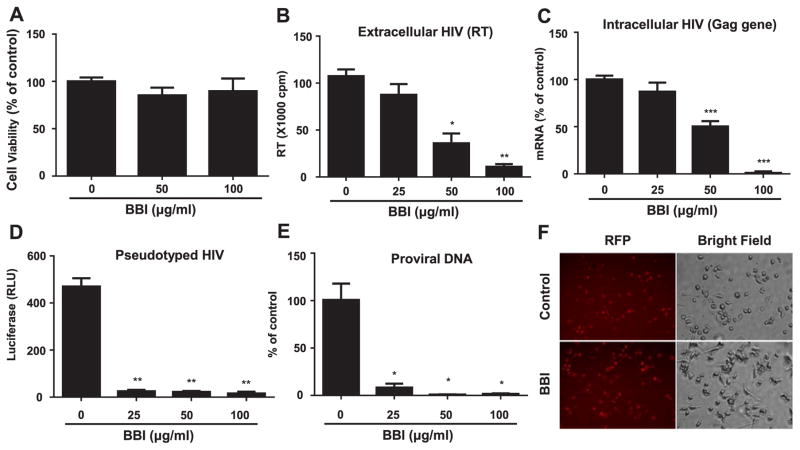
We firstly determined whether BBI has cytotoxicity on peripheral blood monocyte-derived macrophages. As showed in Fig. 1A, BBI at the dose of 50 or 100 μg/ml did not affect cell viability. To determine the anti-HIV effect of BBI, 7-day-cultured macrophages were pretreated with different concentrations of BBI (25, 50, and 100 μg/ml) prior to HIV (Jago strain) infection. Both extracellular HIV (RT activity) (Fig. 1B) and intracellular HIV (Gag gene expression) (Fig. 1C) were significantly inhibited by BBI in a dose-dependent manner without cytotoxicity (Fig. 1A). To determine whether BBI could inhibit HIV infection at the entry level, macrophages were first treated with BBI and then infected with pseudotyped HIV derived from ADA strain, which has a replication-defective HIV genome that encoded a luciferase reporter gene and allows a quantitative measure of the levels of single-round infection (Geijtenbeek et al., 2000). As shown in Fig. 1D, BBI blocked the pseudotyped HIV infection of macrophages. To confirm BBI could inhibit HIV entry into macrophages, cells were treated with BBI prior to HIV infection. As shown in Fig. 1E, HIV proviral DNA was significantly inhibited in BBI-treated macrophages. To further investigate whether BBI can inhibit the viral fusion to the cell membranes, BBI-pretreated macrophages were infected with VSV-G pseudotyped HIV. As shown in Fig. 1F, BBI had little effect on VSV-G pseudotyped HIV infection of macrophages.
Fig. 1. BBI inhibits HIV infection of macrophages at the entry level.
(A) Seven-day-cultured macrophages were treated with/without BBI at indicated concentrations for 6 days. The cell viability was assessed by MTS assay. The data are expressed as the absorbance (490 nm) relative to untreated control, which is defined as 100%. Data are shown as mean±SD for three independent experiments. Macrophages derived from monocytes of the healthy donors were treated with/without indicated concentrations of BBI for 24 h prior to HIV (Jago) infection. After washing away unbound virus, fresh medium without BBI was added to the cultures. Cells and cell-free supernatant were collected on day 5 post-infection for HIV reverse transcriptase (RT) assay (B). Data are shown as mean±SD for three independent experiments. (C) Cellular RNA was subjected to real time RT PCR for HIV Gag and GAPDH RNA. The data are expressed as HIV RNA levels relative (%) to untreated control, which is defined as 100%. Data are shown as mean±SD for three independent experiments. (D) Macrophages were treated with/without the indicated concentrations of BBI for 24 h prior to pseudotyped HIVADA infection. After washing away unattached virus, fresh medium without BBI was added. Macrophages were cultured for additional 48 h and then lysed with cell lysis buffer. Luciferase activities (relative light units, RLU) were measured. The data are expressed as the RLU change vs untreated control. Data are shown as mean±SD for three independent experiments. (E) Macrophages were treated with/without the indicated concentrations of BBI for 24 h prior to HIV (Jago) infection. Macrophages were cultured for additional 24 h and then DNA was extracted from the cells. The proviral DNA was quantified with the real time PCR. Data are shown as mean±SD for three independent experiments. (*P<0.05, **P<0.01, ***P<0.001 when performing Student’s t-test). (F) Macrophages were treated with/without BBI (100 μg/ml) for 24 h prior to VSV-G pseudotyped HIV infection for additional 24 h. The RFP protein expression was observed under a fluorescent microscope.
3.2. BBI decreases the expression of CD4 and CCR5
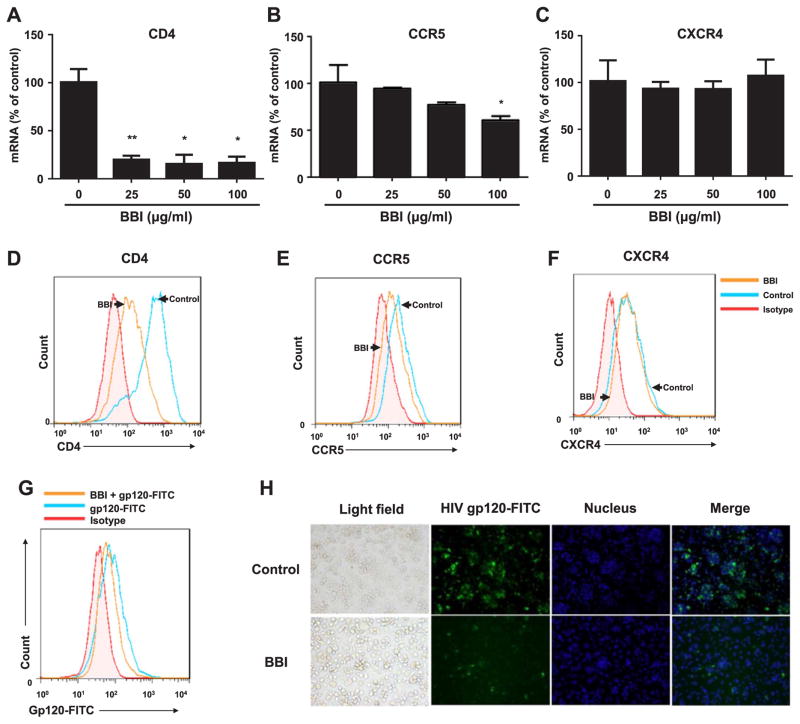
To examine the mechanism of BBI-mediated inhibition of HIV entry, we examined the impact of BBI on the expression of CD4 receptor, CCR5 and CXCR4 co-receptors in macrophages. As shown in Fig. 2, A and D, macrophages treated with BBI expressed significantly lower levels of CD4 at both mRNA and protein levels than untreated cells. In addition, BBI also inhibited the expression of CCR5 at both mRNA and protein levels in macrophages (Fig. 2, B and E). BBI treatment of macrophages, however, had little effect on the expression of CXCR4 in macrophages (Fig. 2, C and F). We also determined whether BBI could inhibit HIV gp120 binding to CD4 receptor. As shown in Fig. 2G–H, BBI treatment of macrophages inhibited gp120 binding to CD4.
Fig. 2. BBI decreases CD4 and CCR5.
Macrophages were treated with/without BBI at indicated concentrations for 6 h (mRNA) or BBI (100 μg/ml) for 24 h (protein). Cellular RNA was collected and subjected to real time PCR for the genes indicated and GAPDH RNA (A–C). The data are expressed as RNA levels percentage (%) for CD4, CCR5, CXCR4 to untreated control, which is defined as 100%. Data are shown as mean±SD for three independent experiments. (D–F) CD4 in the surface of human macrophages stained with PE-cy7 anti-human CD4 (clone: SK3) antibody, CCR5 stained with FITC anti-human CD195 (CCR5) antibody, CXCR4 stained with PE anti-human CXCR4 antibody and all of them were detected by flow cytometer (FACSCantoII), the arrows indicated macrophages stimulated with/without BBI for 24 h. Representative data from three independent experiments are shown. (*P<0.05, **P<0.01, when performing Student’s t-test). Macrophages were stimulated with BBI (100 μg/ml) for 24 h. After washing 3 times with PBS, Macrophages treated with/without BBI were incubated with gp120-FITC (15 μg/ml) for additional 2 h, the binding of gp120-FITC to CD4 was detected by flow cytometer (G) and fluorescence microscope (magnification, 200) (H). The data from flow cytometer were analyzed with Flow-Jo software. Representative data from three independent experiments are shown.
3.3. BBI increases CC chemokines
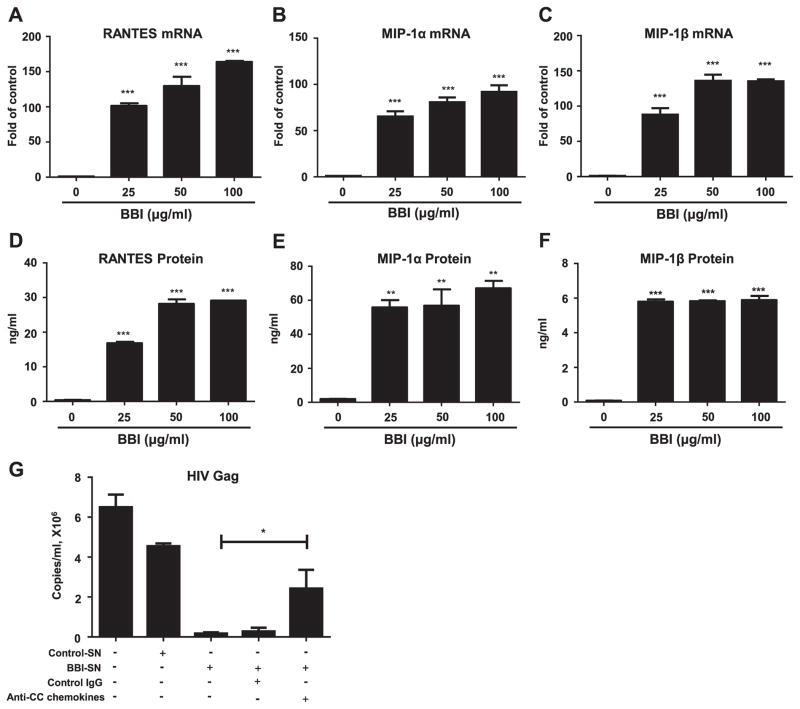
As the ligands of HIV co-receptor CCR5, CC chemokines could block HIV entry by competing with the virus for the CCR5 receptor (Alkhatib et al., 1996). We thus explored the effect of BBI on the expression of CC chemokines (RANTES, MIP-1α and MIP-1β) in macrophages. As shown in Fig. 3, BBI treatment of macrophages induced the production of RANTES, MIP-1α and MIP-1β at both mRNA (Fig. 3, A–C) and protein levels (Fig. 3, D–F). To confirm the role of the CC chemokines in BBI-mediated HIV inhibition at the entry level, we performed the neutralization assay with the antibodies to CC chemokines. As shown in Fig. 3G, the neutralization antibodies to CC chemokines could block the inhibitory effect of BBI on HIV infection of macrophages.
Fig. 3. BBI increases CC chemokines (ligands of HIV entry co-receptor CCR5).
Macrophages were treated with/without BBI at indicated concentrations for 6 h (mRNA) or 24 h (protein). Cellular RNA was collected and subjected to real time RT PCR for the genes indicated and GAPDH RNA (A–C). The data are expressed as RNA relative (fold) for RANTES, MIP-1α and MIP-1β to untreated control, which is defined as 1.0. Data are shown as mean±SD for three independent experiments. (D–F) RANTES, MIP-1α and MIP-1β proteins were analyzed by ELISA with the specific kits according to the manufacturer’s instructions. Data are shown as mean±SD for three independent experiments. (G) Macrophages were treated with/without BBI (100 μg/ml) for 4 h, and then washed with PBS. The cells were cultured with fresh medium for additional 20 h. The supernatant was collected as BBI-conditioned medium. BBI-conditioned medium was incubated with/without neutralization antibodies to RANTES, MIP-1α, MP-1β (CC chemokines) (20 μg/ml, respectively) for 1 h prior to HIV-1 (Jago) infection of macrophaegs. IgG antibody was used as the control. Cell-free supernatant was collected on day 5 for HIV Gag gene expression by the real time PCR. Data are shown as mean±SD for three independent experiments. (*P<0.05, **P<0.01, ***P<0.001 when performing Student’s t-test).
4. Discussion
HIV entry is an essential step for initiation, spread, and replication of the virus, which represents an interesting target for the antiviral therapy. In the present study, we identified that BBI, a natural product in soybean, is a potent entry inhibitor of HIV infection of macrophages. BBI has been shown to have several therapeutic activities, including anti-inflammation (Dai et al., 2012; Safavi and Rostami, 2012) and anti-cancer (Souza Lda et al., 2014). Several studies (Ye et al., 2001; Ye and Ng, 2009; Prasad et al., 2010) showed that BBI could inhibit HIV reverse transcriptase in cell-free systems, with the IC50 of 49 μM (equal to 387 μg/ml) (Ye et al., 2001). We recently reported that BBI inhibits HIV replication in macrophages through the induction of the multiple intracellular antiviral factors (Ma et al., 2016). Here, we showed for the first time that BBI was able to block HIV entry into macrophages (Fig. 1). As a natural product from soybean, BBI may have safety advantage in use for HIV prevention and therapy. It was reported that BBI concentrate (BBIC), a soybean extract enriched in BBI (Kennedy et al., 1993), had little toxicity effect in the phase I randomized double-blind placebo-controlled trials (Lin et al., 2014). Our in vitro experiments showed that BBI at the concentration of 100 μg/ml had little cytotoxicity effect on macrophages (Fig. 1A). The cytotoxicity effect of BBI could also be ruled out by the observation that BBI induced the expression of the CC chemokines in macrophages (Fig. 3A–F).
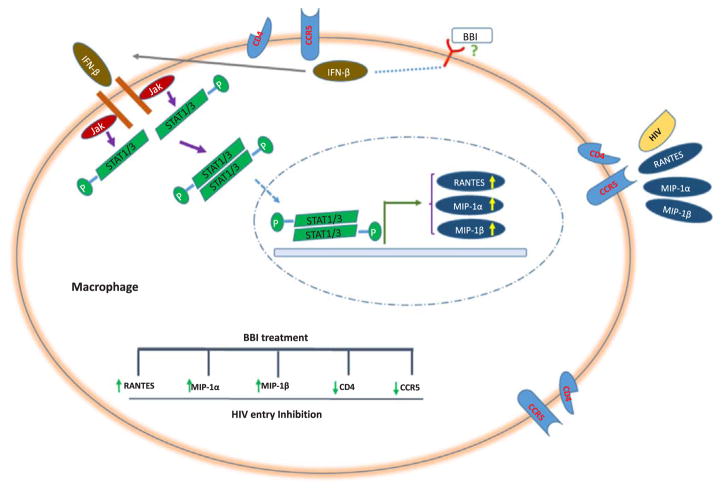
Because the initial binding of HIV envelope gp120 to the CD4 receptors on the target cells is a key step for the viral entry, there has been a great interest in finding molecules that block the binding of HIV to its entry receptors as an effective way to inhibit HIV infection. Several compounds have been developed to inhibit HIV entry through various mechanisms. Soluble CD4 (sCD4) are a logical choice as the inhibitors of HIV attachment to CD4 receptor on target cells (Lagenaur et al., 2010). As the microbicides that block HIV initial attachment on the target cells, PRO2000 (Abdool Karim et al., 2011) and Cyanovirin-N (Alexandre et al., 2010) could prevent HIV infection. Some microbicides could inhibit HIV entry by binding to HIV gp120, such as HIV-binding peptides and lectin (Mahalingam et al., 2011). In addition, the agents that mimic the ligands of CCR5 can block HIV entry. For example, Maraviroc and Vicriviroc, the selective CCR5 antagonists, specifically bind to CCR5 and block cell migration that depends on CCL3, CCL4, CCL5, and CCR5-mediated intracellular signaling (Dorr et al., 2005; Caseiro et al., 2012). As compare with these inhibitors of HIV entry, BBI may have some advantages, as it has low toxicity, minor side-effects and is cost-effective. Importantly, BBI not only downregulated the expression of CD4 and CCR5 (Fig. 2A–B and D–E), but also upregulated the production of the CC chemokines (Fig. 3A–F). The role of CC chemokines in the BBI action on HIV entry was confirmed by the finding that antibodies to CC chemokines could compromise the inhibitory effect of BBI on HIV infection (Fig. 3G). Our previous study (Ma et al., 2016) showed that BBI inhibits HIV replication in macrophages through the induction of the multiple intracellular antiviral factors, including IFNs. It was reported (Cremer et al., 2000) that IFNbeta could up-regulate the expression of CC chemokines and downregulate the expression of CCR5. Therefore, it is likely that BBI-induced IFN-beta may contribute to the induction of CC chemokines (see diagram in Fig. 4). To combine our early observations with the findings in this study, we conclude that at least two distinct elements are involved in BBI-mediated anti-HIV activities: the induction of extracellular factors, CC chemokines that block HIV entry into macrophages, and the activation of intracellular HIV restriction factors that inhibit HIV at different steps of the viral replication cycle. These anti-HIV mechanisms by BBI are important, as they make HIV difficult to develop resistance.
Fig. 4. Schematic diagram of mechanisms involved in BBI-mediated HIV entry inhibition in macrophage.
IFN-β released from BBI-treated macrophages binds to type I IFN receptors on the cell membrane, and activates intracellular JAK/STAT pathway, leading to the induction of CC chemokines and reduction of CCR5, which contributes to the BBI-mediated inhibition of HIV entry into macrophages.
Taken together, while the precise mechanism(s) of the BBI actions on CD4/CCR5 and CC chemokines are complexed and remain to be determined, our findings are clinically significant and relevant to HIV prevention and treatment. Given the fact that there is limited access to conventional antiretroviral therapy (ART) in developing countries and emergence of resistant mutants of HIV, BBI and related natural products may provide an additional and excellent source for developing new and affordable anti-HIV drugs. Therefore, there is a necessity of further in vivo studies on the anti-HIV effect of BBI in order to develop BBI-based supplementary therapy for prevention and/or treatment of HIV infection, particularly for those in resource poor settings.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant number: 81271334, 81571962, 81201261, 81301428) and the National Institute on Drug Abuse (grant numbers:DA042373, DA41302, DA40329, MH109385, DA022177 and DA027550).
Footnotes
Conflicts-of-interest disclosure
The authors declare no competing financial interests.
Authorship contributions
Contribution: T-C. M., W-Z. H., X. W. and W. H. conceived of and designed the experiments; T-C. M., R-H. Z. and L. G. performed the experiments; T-C. M., Y. Z. and J-B. L. analyzed the data; T-C. M. made the figures; W-Z. H. and J-L. L. contributed reagents/materials/analysis tools; T-C. M. and W-Z. H. wrote the paper. All authors reviewed the manuscript.
References
- Abdool Karim SS, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25(7):957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud L, Ball TB, Tjernlund A, Burgener A. The role of serpin and cystatin antiproteases in mucosal innate immunity and their defense against HIV. Am J Reprod Immunol. 2014;71(1):12–23. doi: 10.1111/aji.12166. [DOI] [PubMed] [Google Scholar]
- Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, et al. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology. 2010;402(1):187–196. doi: 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144(2):480–484. [PubMed] [Google Scholar]
- Calabrese LH, Zein N, Vassilopoulos D. Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis. 2004;63(Suppl 2):ii18–ii24. doi: 10.1136/ard.2004.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseiro MM, Nelson M, Diaz RS, Gathe J, de Andrade Neto JL, Slim J, et al. Vicriviroc plus optimized background therapy for treatment-experienced subjects with CCR5 HIV-1 infection: final results of two randomized phase III trials. J Infect. 2012;65(4):326–335. doi: 10.1016/j.jinf.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93(5):681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- Contreras X, Bennasser Y, Chazal N, Bahraoui E. HIV-1 Tat induces TNF-alpha production by human monocytes: involvement of calcium and PKC pathways. J Soc Biol. 2003;197(3):267–275. [PubMed] [Google Scholar]
- Cremer I, Vieillard V, De Maeyer E. Retrovirally mediated IFN-beta transduction of macrophages induces resistance to HIV, correlated with up-regulation of RANTES production and down-regulation of C-C chemokine receptor-5 expression. J Immunol. 2000;164(3):1582–1587. doi: 10.4049/jimmunol.164.3.1582. [DOI] [PubMed] [Google Scholar]
- D’Souza MP, Cairns JS, Plaeger SF. Current evidence and future directions for targeting HIV entry: therapeutic and prophylactic strategies. JAMA. 2000;284(2):215–222. doi: 10.1001/jama.284.2.215. [DOI] [PubMed] [Google Scholar]
- Dai H, Ciric B, Zhang GX, Rostami A. Bowman-Birk Inhibitor attenuates experimental autoimmune encephalomyelitis by delaying infiltration of inflammatory cells into the CNS. Immunol Res. 2011;51(2–3):145–152. doi: 10.1007/s12026-011-8254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Ciric B, Zhang GX, Rostami A. Interleukin-10 plays a crucial role in suppression of experimental autoimmune encephalomyelitis by Bowman-Birk inhibitor. J Neuroimmunol. 2012;245(1–2):1–7. doi: 10.1016/j.jneuroim.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dia VP, Berhow MA, Gonzalez De Mejia E. Bowman-Birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J Agric Food Chem. 2008;56(24):11707–11717. doi: 10.1021/jf802475z. [DOI] [PubMed] [Google Scholar]
- Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AR, Szuhaj BF, Newberne PM, Billings PC. Preparation and production of a cancer chemopreventive agent, Bowman-Birk inhibitor concentrate. Nutr Cancer. 1993;19(3):281–302. doi: 10.1080/01635589309514259. [DOI] [PubMed] [Google Scholar]
- Ketas TJ, Klasse PJ, Spenlehauer C, Nesin M, Frank I, Pope M, et al. Entry inhibitors SCH-C, RANTES, and T-20 block HIV type 1 replication in multiple cell types. AIDS Res Hum Retrovir. 2003;19(3):177–186. doi: 10.1089/088922203763315678. [DOI] [PubMed] [Google Scholar]
- Kumar R, Vandegraaff N, Mundy L, Burrell CJ, Li P. Evaluation of PCR-based methods for the quantitation of integrated HIV-1 DNA. J Virol Methods. 2002;105(2):233–246. doi: 10.1016/s0166-0934(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Lacroix F, Zhao DY, Izaguirre CA, Filion LG. Suppression by HIV of IL-1 and IL-6 secretion in accessory cells: ac function defect partially corrected with exogenous IL-1 and IL-6. Clin Immunol Immunopathol. 1993;67(2):109–116. doi: 10.1006/clin.1993.1052. [DOI] [PubMed] [Google Scholar]
- Lagenaur LA, Villarroel VA, Bundoc V, Dey B, Berger EA. sCD4-17b bifunctional protein: extremely broad and potent neutralization of HIV-1 Env pseudotyped viruses from genetically diverse primary isolates. Retrovirology. 2010;7:11. doi: 10.1186/1742-4690-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ye L, Cook DR, Wang X, Liu J, Kolson DL, et al. Soybean-derived Bowman-Birk inhibitor inhibits neurotoxicity of LPS-activated macrophages. J Neuroinflamm. 2011;8:15. doi: 10.1186/1742-2094-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LL, Mick R, Ware J, Metz J, Lustig R, Vapiwala N, et al. Phase I randomized double-blind placebo-controlled single-dose safety studies of Bowman-Birk inhibitor concentrate. Oncol Lett. 2014;7(4):1151–1158. doi: 10.3892/ol.2014.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Zhou RH, Wang X, Li JL, Sang M, Zhou L, et al. Soybean-derived Bowman-Birk Inhibitor (BBI) Inhibits HIV Replication in Macrophages. Sci Rep. 2016;6:34752. doi: 10.1038/srep34752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam A, Geonnotti AR, Balzarini J, Kiser PF. Activity and safety of synthetic lectins based on benzoboroxole-functionalized polymers for inhibition of HIV entry. Mol Pharm. 2011;8(6):2465–2475. doi: 10.1021/mp2002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad ER, Dutta-Gupta A, Padmasree K. Purification and characterization of a Bowman-Birk proteinase inhibitor from the seeds of black gram (Vigna mungo) Phytochemistry. 2010;71(4):363–372. doi: 10.1016/j.phytochem.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Rizzi C, Crippa MP, Jeeninga RE, Berkhout B, Blasi F, Poli G, et al. Pertussis toxin B-oligomer suppresses IL-6 induced HIV-1 and chemokine expression in chronically infected U1 cells via inhibition of activator protein 1. J Immunol. 2006;176(2):999–1006. doi: 10.4049/jimmunol.176.2.999. [DOI] [PubMed] [Google Scholar]
- Ronald L, willey DHS, Lasky Laurence A, Theodore Theodore S, Earl Patricia L, Moss Bernard, Capon Daniel J, Martin Malcolm A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus That Is critical for infectivity. J Virol. 1988;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi F, Rasouli J, Mari E, Zhang GX, Rostami AM. Bowman-Birk protease inhibitor (BBI) induces IL10 production in human T cells and suppresses effector phase of experimental autoimmune encephalomyelitis (EAE) by Tr1 induction. Mult Scler J. 2013;19(11):233–233. [Google Scholar]
- Safavi F, Rostami A. Role of serine proteases in inflammation: bowman-birk protease inhibitor (BBI) as a potential therapy for autoimmune diseases. Exp Mol Pathol. 2012;93(3):428–433. doi: 10.1016/j.yexmp.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- da Souza LC, Camargo R, Demasi M, Santana JM, de Sa CM, de Freitas SM. Effects of an anticarcinogenic Bowman-Birk protease inhibitor on purified 20S proteasome and MCF-7 breast cancer cells. PLoS One. 2014;9(1):e86600. doi: 10.1371/journal.pone.0086600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek, Teunis BH, SKD, Ruurd Torensma SJvV, van Duijnhoven JM, Gerard CF, Cornelissen HSLMN, Ine LMH, KewalRamani DRL, Vineet N, Figdor aYvK, Carl G. DC-sign, a DendriticCell–specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Ware JH, Wan XS, Newberne P, Kennedy AR. Bowman-Birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis. Dig Dis Sci. 1999;44(5):986–990. doi: 10.1023/a:1026616832119. [DOI] [PubMed] [Google Scholar]
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280(5371):1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- Ye X, Ng TB. A trypsin-chymotrypsin inhibitor with antiproliferative activity from small glossy black soybeans. Planta Med. 2009;75(5):550–556. doi: 10.1055/s-0029-1185312. [DOI] [PubMed] [Google Scholar]
- Ye XY, Ng TB, Rao PF. A Bowman-Birk-type trypsin-chymotrypsin inhibitor from broad beans. Biochem Biophys Res Commun. 2001;289(1):91–96. doi: 10.1006/bbrc.2001.5965. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang X, Liu M, Hu Q, Song L, Ye L, et al. A critical function of toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology. 2010;131(1):40–49. doi: 10.1111/j.1365-2567.2010.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]