ABSTRACT
Vaccination represents the most effective intervention to prevent infection, hospitalization and mortality due to influenza. This meta-analysis quantifies data reporting influenza vaccine effectiveness (VE) on influenza visits and hospitalizations of case-control and cohort studies among high-risk groups.
A systematic literature review including original articles published between 2007 and 2016, using a protocol registered on Prospero with No. 42017054854, and a meta-analysis were conducted.
For 3 high-risk groups (subjects with underlying health conditions, pregnant women and health care workers) only a qualitative evaluation was performed. The VE quantitative analysis demonstrated a clear significant overall effect of 39% (95%CI: 32–46%) for visits and 57% (95%CI: 30–74%) for hospitalization among children. Considering the elderly influenza VE had a clear effect of 25% (95%CI: 6–40%) for visits and 14% (95%CI: 7–21%; p<0.001) for hospitalization.
This study showed the high VE of influenza vaccination among high-risk groups, representing a tool for public health decision-makers to develop evidence-based preventive interventions to avoid influenza outcomes.
KEYWORDS: children, chronic disease, effectiveness, elderly subjects, health care worker, hospitalization, influenza, pregnancy, vaccine, visit
Introduction
Influenza is a respiratory infectious disease responsible for thousands of infections, hospitalizations and deaths worldwide.1-3 Influenza viruses mainly affect lungs, higher and lower respiratory tract, representing one of the main causes of deaths and hospitalization especially during winter seasons.4,5 In particular, higher morbidity and mortality rates were observed among the elderly, individuals with underlying health conditions, children and pregnant, that are particularly at risk for developing influenza complications, such as bacterial pneumonia.6-11
At the same time, health care workers (HCWs) represent a group at higher risk of contracting influenza illness and transmitting the disease to their patients or to the general population.12-14 Reported estimates of influenza infection among HCWs each season are various (ranging from 20% to 47.5%) and many of them continue working while infected,13-15 favoring the spread of influenza virus.13 For these reasons, hospitalized patients could acquire influenza not only from other patients or visitors but also from hospital employees and only high influenza vaccination coverage of health care personnel could prevent nosocomial influenza transmission, reducing influenza-like illness (ILI) mortality among more frail patients.16,17
In general, influenza vaccination represents the most effective public health intervention to prevent seasonal influenza infection, hospitalization and mortality.18-21 All the preventive policies and international guidelines regarding influenza vaccination are primarily focused on protection of individuals at higher risk, by vaccinating themselves or those who could infect them.19-21
The principal challenge of this systematic literature review is to analyze studies that reported influenza vaccine effectiveness (VE) data on reducing laboratory confirmed cases, hospitalization, morbidity or mortality due to influenza and to quantify its impact among high-risk groups.
In particular the data were separately discussed among the following major high-risk groups identified in literature: children, subjects with underlying health conditions at any age, pregnant women, HCWs, and the elderly.
Results
Systematic literature review
As illustrated in the flowchart (Fig. 1), an initial number of 2,461 articles were retrieved through the selected databases. About one third of the manuscript (n = 775/2,461) was identified as duplicates and removed. Through the initial screening of titles and abstracts 1,496 articles were excluded and overall 190 full text articles were assessed for eligibility. A total of 38 studies met all the inclusion criteria of which 13 were included in the qualitative synthesis, whereas 25 took place in the meta-analysis (quantitative synthesis). For 3 major high-risk groups, namely subjects with underlying health conditions, pregnant women and HCWs, only a qualitative evaluation was conducted. Of note subjects with underlying health condition hadn't the same comorbidities so they weren't pooled together with meta-analysis. At the same time, both for 2 cohort studies about children/elderly and for case-control studies on pregnant women/HCWs (2 studies for each high-risk group), only a qualitative analysis was performed due to limited data available to conduct a quantitative evaluation. Out of the 25 remaining studies, 2 quantitative synthesis analyses were conducted for the high-risk groups of children and older people (12 manuscripts for children, 9 for the elderly, 4 conducted in both the high-risk groups). Table 1 describes the studies included both in qualitative or quantitative synthesis. In particular, 69% (n = 25/36) of them referred to hospitalized patients, while 47% (n = 17/36) were conducted in pediatric settings. Furthermore, 83% (n = 30/36) of selected studies confirmed influenza vaccination status by at least one objective source of information (registries, electronic data set, etc) and 78% (n = 28/36) were case control studies conducted by using the test-negative design.
Figure 1.
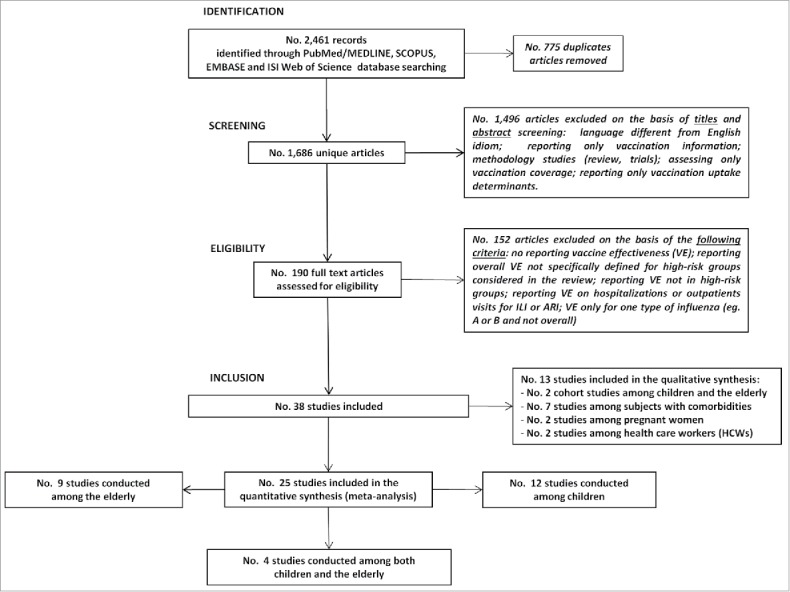
Flowchart of the systematic literature review process about influenza vaccine effectiveness among high risk groups.
Table 1.
Characteristics of included studies on anti-influenza vaccine effectiveness among at risk-group.
| Reference article | At risk-group | Outcome | Publication year | Influenza season | Age range | Sample size | Country | Influenza vaccine type | Influenza virus diagnsosis among cases | Vaccine status | Study design | Qualitative/Quantitative analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Szilagyi PG22 | children | outpatient visit | 2008 | from 2003–2004 to 2004–2005 | from 6 months to 6 years | 10,906 | US | trivalent inactivated | A(H3N2) | Confirmed | Cohort | Qualitative |
| Ridenhour BJ23 | older | hospitalization/ deaths | 2013 | from 1993–1994 to 2007–2008 | ≥ 65 years | 21,180,919 | Canada | N.A. | N.A. | Confirmed | Cohort | Qualitative |
| Andrews N28 | comorbidity | outpatient visit | 2011 | 2009–2012 | <5 and ≥ 65 years | 2,153 | UK | adiuvated pH1N1 | A(H1N1) | Confirmed | Case-control | Qualitative |
| Emborg HD29 | comorbidity | outpatient visit / hospitalization | 2011 | 2009–2010 | <65 years | 388,069 | Denmark | adiuvated pH1N1 | A(H1N1) | Confirmed | Cohort | Qualitative |
| MacIntyre CR25 | comorbidity | hospitalization | 2013 | from 2008 to 2010 | ≥ 18 years | 599 | Australia | trivalent inactivated | A and B | Confirmed | Cohort | Qualitative |
| Perez-Romero P30 | comorbidity | hospitalization | 2012 | 2010–2011 | >16 years | 64 | Spain | trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Cohort | Qualitative |
| Steens A27 | comorbidity | hospitalization | 2011 | 2009–2011 | from 1 to 84 years | 10,968 | Netherlands | adiuvated pH1N1 | A(H1N1) | Confirmed | Case-control | Qualitative |
| Thompson MG31 | pregnant women | outpatient visit | 2013 | 2010–2011 and 2011–2012 | from 22 to 38 years | 492 | US | trivalent inactivated | A(H1N1) | Confirmed | Case-control | Qualitative |
| Regan AK32 | pregnant women | outpatient visit / hospitalization | 2016 | 2012–2013 | ≥ 18 years | 2,962,374 | Australia | trivalent inactivated | A(H1N1) | Confirmed | Cohort | Qualitative |
| Costa JT33 | health care workers | outpatient visit | 2012 | 2009–2010 | ≥ 18 years | 245 | Portugal | adiuvated pH1N1 | A(H1N1) | Confirmed | Case-control | Qualitative |
| Igari H34 | health care workers | hospitalization | 2011 | 2009–2013 | ≥ 20 years | 1,817 | Japan | adiuvated pH1N1 | A(H1N1) | Confirmed | Cohort | Qualitative |
| Blyth CC71 | children | outpatient visit | 2016 | 2008 and from 2010 to 2013 | from 6 months to 18 years | 2,205 | Australia | trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Sullivan SG48 | children and older | outpatient visit | 2014 | 2012 | < 18 and >65 years | 488 | Australia | trivalent inactivated | A(H1N1), A(H3N2) and B | Not confirmed | Case-control | Quantitative |
| Mc Lean HK72 | children and older | outpatient visit | 2014 | 2012–2013 | from 6 months to 17 y and ≥ 65 years | 3,145 | US | trivalent inactivated, adiuvated and live attenuated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Belongia EA73 | children | outpatient visit | 2011 | 2007–2008 | from 6 months to 6 years | 412 | US | trivalent inactivated | A(H3N2) and B Yamagata | Confirmed | Case-control | Quantitative |
| Joshi AY74 | children | outpatient visit | 2009 | from 1999–2000 to 2006–2007 | from 6 months to 6 years | 206 | US | trivalent inactivated | A(H1N1), A(H3N2) and B Victoria | Confirmed | Case-control | Quantitative |
| Eisenberg KW50 | children | outpatient visit | 2008 | from 2003–2004 to 2004–2005 | from 6 months to 6 years | 2,534 | US | trivalent inactivated | N.A. | Confirmed | Case-control | Quantitative |
| Shuler CM75 | children | outpatient visit | 2007 | 2003–2004 | from 6 months to 6 years | 870 | US | trivalent inactivated | N.A. | Confirmed | Case-control | Quantitative |
| Chiu SS76 | children | hospitalization | 2016 | from 2009–2010 to 2013–2014 | from 6 months to 17 years | 6,257 | Hong Kong | trivalent inactivated | B Yamagata and B Victoria | Not confirmed | Case-control | Quantitative |
| Blith CC54 | children | hospitalization | 2015 | 2009 and from 2010 to 2014 | from 6 months to 6 years | 712 | Australia | trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Grijalva CC77 | children and older | hospitalization | 2015 | from 2009–2010 to 2011–2012 | from 6 months to 17 y and ≥ 65 years | 1,806 | US | pandemic, trivalent inactivated and live attenuated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Cowling BJ78 | children | hospitalization | 2014 | from 2009–2010 to 2012–2013 | from 6 months to 17 years | 5,399 | Hong Kong | pandemic and trivalent inactivated | A(H1N1), A(H3N2) and B | Not confirmed | Case-control | Quantitative |
| Ferdinands JM79 | children | hospitalization | 2014 | from 2010–2011 to 2011–2012 | from 6 months to 17 years | 309 | US | N.A. | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Gilca R80 | children | hospitalization | 2011 | 2009–2010 | from 6 months to 9 years | 884 | Canada | adiuvated pH1N1 | pH1N1 | Confirmed | Case-control | Quantitative |
| Griffin MR81 | children | hospitalization | 2011 | 2009–2010 | from 6 months to 9 years | 2,168 | US | live attenuated and inactivated pH1N1 | pH1N1 | Confirmed | Case-control | Quantitative |
| Dixon GA53 | children | hospitalization | 2010 | 2008 | from 6 months to 6 years | 76 | Australia | trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Orellano PW59 | children and older | hospitalization | 2010 | 2009 | <5 y and >65 years | 1,115 | Argentina | trivalent inactivated | pH1N1 | Confirmed | Case-control | Quantitative |
| Chen Q82 | older | outpatient visit | 2014 | from 2006–2007 to 2008–2009, from 2010–2011 to 2011–2012 | ≥ 65 years | 927 | US | trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Havers F83 | older | hospitalization | 2016 | 2010–2011 | >50 years | 1,141 | US | trivalent inactivated | A(H1N1), A(H3N2) and B | Not confirmed | Case-control | Quantitative |
| Cheng AC24 | older and comorbidity | hospitalization | 2015 | 2014 | >65 y and ≥ 16 y for comorbidity | 3,217 | Australia | trivalent inactivated | A(H1N1), A(H3N2) and B | Not confirmed | Case-control | Quantitative |
| Gilca R60 | older | hospitalization | 2015 | 2014–2015 | ≥ 65 years | 314 | Canada | adiuvated trivalent inactivated | A(H3N2) | Not confirmed | Case-control | Quantitative |
| Puig-Barberà J84 | older | hospitalization | 2015 | 2014–2015 | ≥ 65 years | 1,108 | Spain | trivalent inactivated | A(H3N2) | Confirmed | Case-control | Quantitative |
| Castilla J85 | older | hospitalization | 2014 | 2013–2014 | >65 years | 239 | Spain | trivalent inactivated | A(H1N1) and A(H3N2) | Confirmed | Case-control | Quantitative |
| Kwong JC86 | older | hospitalization | 2013 | 2010–2011 | >65 years | 2,230 | Canada | trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Puig-Barberà J26 | older and comorbidity | hospitalization | 2012 | 2010–2011 | >60 y and ≥ 18 y for comorbidity | 379 | Spain | adiuvated trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
| Van Vuuren A87 | older | hospitalization | 2008 | 2004–2005 | ≥ 65 years | 6,410 | South Africa | trivalent inactivated | A(H1N1), A(H3N2) and B | Confirmed | Case-control | Quantitative |
Qualitative analysis
Cohort studies conducted among children and the elderly
Only 2 cohort studies examining effectiveness of influenza vaccine among children and the elderly were selected and included in the qualitative synthesis (Table 1). In particular, Szilagyi PG et al evaluated the effect of influenza vaccine on the number of outpatient visits and reported a VE range 7–52% among children aged 6 to 59 months, during 2 consecutive influenza seasons (2003–2004 and 2004–2005) in 3 different American counties.22 On the other hand, a retrospective cohort study conducted among Ontario residents aged ≥ 65 y from 1993–1994 through 2007–2008 seasons reported 22% VE for all influenza-associated deaths, 25% VE for deaths occurring within 30 d after and 19% VE for influenza-associated pneumonia/influenza hospitalization, respectively.23
Subjects with underlying health conditions
At the end of the revision process of studies that evaluated influenza VE in subjects with comorbidities, 5 case control and 2 cohort studies were selected and included in the qualitative analysis (Table 1). Cheng AC et al reported a 51.3% (95%CI: 40.7%–60.1%) reduction of hospitalization due to influenza disease in an Australian population (aged ≥ 18 years) with at least one chronic condition during 2014 season.24 In Sidney, a reduction of 83.6% (95%CI: 27.6%–96.3%) for acute myocardial infarction hospitalization was reported, after influenza vaccination, among 599 adults with previous cardiovascular event from 2008 to 2010 influenza seasons.25 Also, among a Spanish group of subjects aged 18 y or older with high-risk conditions, was reported an adjusted VE of 53% (95%CI: 4–77%) in reducing hospitalizations during the 2010–2011 influenza season.26 Furthermore, a reduction of 49% (95%CI: 16–69%) in hospitalization of a Dutch population 1–84 y old, with a diagnosis of laboratory confirmed A(H1N1)pdm09 influenza and affected by at least one underlying medical condition (pulmonary or cardiac disease, diabetes mellitus, chronic kidney failure, cancer and immunocompromised condition), was observed in 2009–2010 season due to the adjuvanted pandemic vaccine,27 as also documented by Andrews N et al in reducing outpatient visits in England (62%; 95%CI: 33–78%).28
On the other hand, with regard to cohort studies on influenza vaccination effectiveness, Emborg HD et al reported a reduction of 49% on general practitioners (GPs) consultation, as well as 44% in hospitalization of subjects <65 y old with underlying chronic diseases in Denmark.29 Moreover, a study conducted among 64 Spanish solid organ transplant (SOT) recipient, reported an influenza VE of 85% (95%CI: 40–97%) in reduction the hospitalizations during 2010–2011 season.30
Pregnant women
The qualitative analysis included 2 manuscripts on influenza VE among pregnant women (Table 1). A population based case control study conducted in California and Oregon evaluated prevention of Polymerase chain reaction confirmed influenza cases, in pregnancy, and reported, using influenza-negative controls, a VE of 57% during the 2010–2011 season and 27% during the 2011–2012 season, respectively.31
Furthermore, a retrospective cohort study conducted in Western Australia among 34,701 pregnant women reported a VE of 81% (95%CI: 31–95%) in decreasing emergency department visit for influenza and 65% reduction (95%CI: 3–87%) in hospital admission of pregnant women, during the 2012 and 2013 influenza seasons.32
Health care workers
After the revision process only 2 manuscripts concerning influenza VE among HCWs were included in the systematic review (Table 1). In detail, a case control study reported a VE of 90.5% (95%CI: 73.5%–97.3%) in reducing emergency department visit for influenza A(H1N1), among the employees of Sao João Hospital of Porto during 2009–2010 season.33 Another study showed a VE of 70.5% in reducing influenza A(H1N1) hospitalization, among a cohort of Japanese HCWs during 2009–2010 influenza season.34
Quantitative analysis
Children
Overall, 7 of the 16 studies included in the meta-analysis evaluated the VE against influenza visits, while 9 focused on influenza hospitalization among children aged 6 months to 18 y.
Considering outpatient or emergency department visits, VE demonstrated a clear significant overall effect of 39% (95%CI: 32–46%) of influenza vaccines among cases when compared with control children (Fig. 2). Since low heterogeneity was present between studies (I2 = 48.1%; p = 0.052), for this analysis a fixed-effect model instead of a random-effect model was used.
Figure 2.
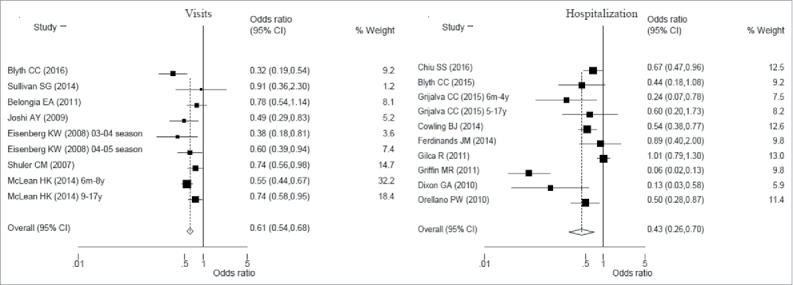
Forest plot of influenza visits and hospitalization vaccine effectiveness (1-Odds ratio) among children from 6 months to 18 year old.
On the other hand, studies evaluating the overall influenza hospitalization VE were analyzed using random effect model. Indeed, using inverse-variance weighting to calculate fixed and random effects summary estimate, there was an higher moment base estimate between studies variance (Chi2 = 0.40; p<0.001). The analysis on influenza hospitalization VE among children (Fig. 2) showed a clear overall effect of 57% (95%CI: 30–74%; p<0.001) even if with a higher between studies heterogeneity (I2 = 86.1%; p<0.001). To explain this phenomenon, a meta regression analysis was conducted including independent variables such as studies considering children (< 9 years) vaccinated for the first time with at least 2 doses and hemisphere where the study was conducted. Moreover, other 2 independent variables integrated the meta regression analysis: mismatch between influenza A or B viruses included in vaccine and influenza viruses A or B circulating among cases and control. As a result, the log odds ratio of influenza hospitalization VE was estimated to decrease of 0.91 (p = 0.043) among studies conducted in Northern hemisphere. The estimated between studies variance reduced from 0.40 to null.
Elderly subjects
There was a clear effect of 25% (95%CI: 6–40%; p = 0.012) using fixed effect model, when considering the 3 studies included in meta-analysis on VE for influenza visits among the elderly, although the heterogeneity between studies was very low (I2 = 0; p = 0.864) (Fig. 3).
Figure 3.
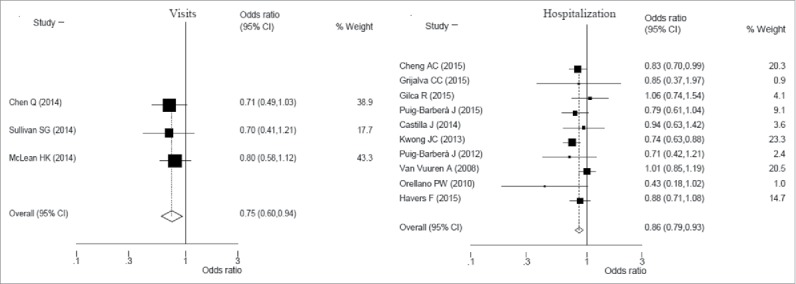
Forest plot of influenza visits and hospitalization vaccine effectiveness (1-Odds ratio) among elderly subjects.
Additionally, among 10 studies considered about elderly a clear effect of 14% VE (95%CI: 7–21%; p<0.001) was observed in reducing hospital admission due to influenza with low heterogeneity between studies (I2 = 19.2%; p = 0.286).
Risk of bias across studies
The symmetry of the funnel plots was examined to search for possible publication bias or even heterogeneity. Asymmetry was found for studies reporting influenza hospitalization VE among children (Table 2).
Table 2.
Analysis for funnel plot asymmetry of studies reporting vaccine effectiveness, estimated by Egger's regression test.
| No. studies | coefficient | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Vaccine effectiveness on influenza visits among children | 9 | −0.78 | −3.51 | 1.94 | 0.520 |
| Vaccine effectiveness on influenza hospitalization among children | 10 | −3.05 | −5.93 | −0.18 | 0.040 |
| Vaccine effectiveness on influenza visits among elderly subjects | 3 | −1.06 | −16.41 | 14.29 | 0.541 |
| Vaccine effectiveness on influenza hospitalization among elderly subjects | 10 | −0.52 | −2.35 | 1.31 | 0.531 |
Discussion
This study provide an up-to-date review of VE on reducing measurable outcomes in health care, such as outpatient visits and hospitalization, among 5 of the most important high-risk groups to which was strongly recommended influenza vaccination.35 Other reviews beforehand conducted, demonstrated that considerable variations could be observed in reported influenza VE estimates due to differences in circulating viral strains among countries, proportion of influenza strains within one region, type of vaccine used, age-specific vaccine coverage, type of population studied, season definition, case definition, ascertainment of vaccination status, differences in surveillance time-period, variables included or omitted in the statistical model, kind of model, and measured outcomes (admission, outpatient contact or infection).36-38 For these reasons, our study aimed to generate different model of systematic literature review (SLR) according to high-risk group considered, and to systematize the differences between other variables that make changing influenza VE.
Qualitative analysis
Subjects with underlying health conditions
Subjects with underlying health conditions are recognized as a core group for influenza vaccination administration. Each co-morbidity represents a consistent increasing risk for influenza infection, complications and death. Furthermore, the association of several chronic conditions could enhance the risk for unvaccinated subjects during every influenza season.18,39 According to main public health authorities, all individuals >6 months old, with at least one chronic illness that represent a risk factor for influenza or complications, should be yearly and actively vaccinated against influenza.21
In particular, some case-control studies among subjects with comorbidities reported similar VE values, in the qualitative synthesis analysis, for hospitalization reduction (around 50%) despite different influenza seasons considered.24,26,27 Moreover, a reduction of 62% in outpatient visits and 84% in acute myocardial infarction hospitalization after influenza vaccination was demonstrated, as described by other authors.25,28,40 Also a cohort study conducted in Denmark reported a similar VE value (44%) in reducing hospitalization, while another cohort study among SOT found an higher value of VE (85%), evidencing the key role of influenza vaccination in preventing hospitalization in this particular high-risk group.29,30,41
Pregnant women
Both studies analyzed in the SLR conducted among pregnant women demonstrated a good VE in decreasing the total number of laboratory confirmed influenza cases,31 emergency department visits and hospitalizations in different influenza seasons.32 The consistent difference of VE among vaccinated pregnant women observed in US between the seasons 2010–2011 and 2011–2012 could be due to residual or unmeasured confounding, even if it was similar when stratified by season and influenza virus type.31 The magnitude effect of influenza vaccination during pregnancy was justified especially by 2 main factors: the rapid clinical deterioration observed in some patients in respect to the typical course of seasonal influenza, especially when infected with A(H1N1)pdm09 strains,9,42 and the higher prevalence of cleft lip–palate, neural-tube defects and cardiovascular malformations in newborns of mother with confirmed diagnosis of influenza during the second and/or third month of pregnancy.43
Heath care workers
Influenza vaccination of HCWs is the most effective public health strategies for preventing nosocomial influenza transmission and reducing ILI mortality among elderly and high-risk patients, as well as for minimizing absenteeism during annual epidemics.12,14,16,18
The 2 studies included in the SLR throughout the qualitative synthesis were both related to VE during the pandemic influenza season and the use of adjuvanted monovalent influenza vaccine against A(H1N1)pdm09.33,34 The very high level of VE in reducing emergency department visits and hospitalization for influenza A(H1N1)pdm09 confirmed the specific tropism of pandemic influenza strains for younger people but also the very high efficacy of the influenza vaccines quickly developed worldwide.44,45
Quantitative analysis
Children
During each seasonal outbreak, children sustain the highest burden of influenza. A systematic review of the global disease burden of influenza in children >5 y estimated that there were 90 million (95%CI: 49–162 millions) cases during the 2008 influenza season, 20 million (95%CI: 13–32 millions) cases of influenza-associated acute lower respiratory infections (ALRI), and 1–2 million cases of influenza associated severe ALRI, including 28,000 – 111,500 deaths.46 A review from 1982 to 2012, estimated that influenza resulted in approximately 374,000 (95%CI: 264,000 – 539,000) hospitalizations in children <1 y old, of which 228,000 (95%CI: 150,000 – 344,000) occurred among children <6 months, and 870,000 (95%CI: 610,000 – 1,237,000) in children <5 y of age, annually.47 According to data of this meta-analysis, influenza vaccination was protective against outpatient visits among children, especially considering studies with children <9 y old and in the US, with a confirmed vaccination status. The lower value of VE for outpatient influenza visits among children, were found by Sullivan SG et al.48 This latter could be due to unadjusted VE by distance of influenza visits and influenza vaccine administration. A combination of 2 possible mechanisms could explain this reduced VE. Firstly, seasonal variations of circulating viruses, due both to the appearance of another virus type or to the antigenic drift of circulating strains, could be responsible of a partial vaccine mismatch.49 Secondly, a waning immunity one month after administration of the influenza vaccine was described even among children.50 Furthermore, to assess vaccination status of enrolled children, this study used a not confirmed method, and this could further reduce the specificity of results on vaccination status. In particular, a study suggested that specificity of self-reported influenza vaccination status can be lowest for young children, whose parents may easily confuse influenza vaccine with other routine childhood vaccines.49
Better results about influenza visits VE were reported by Eisemberg KW et al,50 that estimated the influenza VE for children during the 2003–2004 and 2004–2005 seasons, although the matching between circulating influenza viruses and those included in the vaccine was considered suboptimal for both seasons.51,52
A better VE was found in reduction of influenza hospitalizations than outpatient influenza visits. Among studies focusing influenza hospitalization VE, the majority were conducted among children aged 6 months to 17 years, in Northern hemisphere, with diagnosis of influenza A or B infection and with a confirmation of vaccination status. Only studies conducted in Southern hemisphere were associated with an increase of influenza hospitalization VE, and this result can be explained because more frequently patients of studies conducted in Southern hemisphere were recruited from tertiary pediatric referral hospital as in Blyth CC et al and Dixon GA et al.53,54 These studies may have included more severe infections or complicated comorbidities, when compared with children admitted to more general pediatric wards. Furthermore, a recent global estimates of hospitalization for acute lower respiratory infections, among children <17 y old, including data from systematic review and surveillance platforms, showed that pooled percentages of positivity for influenza among hospitalized children with respiratory illness, varied among World Health Organization (WHO) regions with the highest values in Western Pacific and Southeast Asia (8.5% in both cases) and the lowest in the Americas and Europe (4.6% and 7.1%, respectively).47 These data confirm a different frequency of severe influenza illness between Southern and Northern hemispheres that could partially explain the VE variability. Even if differences in hospitalization practices, applications of case definitions and factors, such as time from symptom onset to specimen collection, could make detection of influenza viruses more or less likely, and therefore this could bias the outcome.
Elderly subjects
All of the 3 studies included in VE analysis and concerning the reduction of outpatient visits were conducted among confirmed influenza A and B individuals aged >65 y. More frequently were conducted in Northern hemisphere and the confirmation of influenza vaccine status collected through registries. The better influenza VE among elderly was found in Sullivan SG et al even with any limitations.48 In particular, these authors did not adjust for distance of influenza visit and influenza vaccine administration, and did not collect data on the presence of comorbidities predisposing to severe influenza, such as asthma, obesity and immunocompromising conditions.48 Failure to adjust for this important confounder may have accounted for the unexpected age effects. In these patients many mechanisms of failed response were related to frailty driven by chronic inflammation and age, even if one more established, but still controversial, explanation is the concept of original antigenic sin.55 This means that previous exposure to an antigen resulted in a sub-standard immune response, when exposure to a novel but closely related antigen occurs.56
In McLean HK et al was found a lower value of influenza visits VE among elderly, in particular for influenza A(H3N2).11 This estimated VE was consistent with laboratory findings from the US national virological surveillance during the same influenza season.57 Although virological surveillance indicated no antigenic drift between the circulating influenza A(H3N2) viruses and the cell grown reference vaccine virus, the egg-propagated A/Victoria/361/2011 reassortant virus used in vaccine production acquired 3 amino acid changes in the antigenic region of HA (at positions H156Q, G186V and S219Y), which significantly altered its antigenicity.57 Furthermore, this low VE against A(H3N2) suggests that other factors in addition to immunosenescence, may be important modifiers in this age group.55 In particular, additional studies are needed to understand the impact of previous infections, vaccinations, and antigenic variability on the risk of illness.58
In the elderly influenza VE was lower in hospitalization than outpatient visits. The studies reported in the meta-analysis of influenza hospitalization VE were more frequently among people >65 y old, conducted in Northern hemisphere and regarding trivalent inactivated influenza vaccines. The better influenza hospitalization VE was found by Orellano PW et al,59 even if socioeconomic status, place of residence, medical consultation, or past hospitalizations were not included in this study. This means that severe or mild influenza cases may be different in terms of background characteristics, and this might bias the estimated VE.55
On the other hand, lower influenza hospitalization VE was revealed by Gilca R et al.60 This can be consistent with mismatch during 2014–2015 influenza season, when the majority of A/H3N2 strains circulating in the Northern hemisphere were antigenically mismatched to the A/Texas/50/2012 H3N2 vaccine strain.61 Furthermore, hospitalization VE was evaluated considering a self-reported vaccination status and this may have resulted in exposure misclassification.49
Only 3 studies reporting VE among elderly who received adjuvanted vaccine did not calculate VE by vaccine type.26,60,72 The authors justified this due to small number of elderly vaccinated with adjuvanted vaccine compared with other trivalent inactivated vaccine. In future, would be beneficial that seasonal VE estimates will be reported by vaccine type to facilitate valid comparisons.
Limits
The studies included in the meta-analyses suffer from a limitation due to a potential overestimation of the vaccination status that could have occurred, since some examined studies used partially or totally referred vaccination status without validation technique. This could assess subjective measures of vaccine uptake that cause recall bias (e.g. past influenza vaccination uptake can be confused with the current one). Investigators who rely on self-reported influenza vaccination status, in particular for young children, should consider the possibility that up to 10% of individuals may be misclassified. So, whenever feasible, vaccination data should be validated by an external source to reduce misclassification.49
Also, a possible limit of the present study could be the different vaccine policies and strategies adopted in various countries, as well as the different type of influenza vaccines routinely available. All these factors could have influenced VE reported in different areas.
Regarding asymmetry resulted with influenza hospitalization VE among children, the analysis of funnel plot showed that missing studies were in a top right and bottom left area of significance, so publication bias was unlikely to be the underlying cause of asymmetry.
Conclusion
Influenza represents one of the leading causes of death worldwide. In particular, children, older people, subjects with underlying health conditions, pregnant women and health care workers are groups at higher risk of contracting influenza infection and its complication. Worldwide, vaccination constitutes the only recognized strategy to prevent the spread of influenza viruses as well as human-to-human transmission and infection, and the most important public health authorities strongly recommended vaccine administration among these high-risk groups.
Our SLR and meta-analysis demonstrated the high VE of influenza vaccination in all these high-risk groups, often regardless of season, circulating strain, type of vaccination. Furthermore, the reduction in hospitalization and outpatient visits represent not only a health benefit for individuals vaccinated but also an essential profit for National Health Systems.
Finally, may be suitable that this SLR and meta-analysis aim to provide a tool for public health decision makers to develop evidence based preventive interventions to contrast influenza infection, especially among high-risk groups.
Material and methods
Systematic literature review
A SLR was performed on influenza VE among high-risk groups. They, according to WHO position paper, were identified as people at increased risk of exposure to influenza virus as well as those at particular risk of developing severe disease (i.e. older people, children, people suffering from comorbidities and pregnant women).35 A written protocol was supplied to all investigators recruited, before starting SLR, and it was registered on Prospero with No. 42017054854 on 19 January 2017. Case-control and cohort studies on influenza health care outcomes, between vaccinated and unvaccinated risk groups, were selected through a SLR using key terms in combination and referred to vaccine/immunization, effectiveness, impact, at risk people and influenza/flu, with medical Subject Headings (MeSH) and MeSH Major Topics included in the syntax. The online databases PubMed/MEDLINE, SCOPUS, EMBASE, ISI Web of Science were considered, as well as the gray literature and a manual search from the references of the articles retrieved and it was performed in January 2017.
Original articles published between 1st of January 2007 and the 31st of December 2016 were retrieved, with restriction criteria applied: articles published in the English language and concerning influenza effectiveness in risk groups. Among all high-risk groups considered, elderly subjects (≥ 50 y old), children (≤ 18 y old), subjects with underlying health conditions at any age, pregnant women and HCW were included in the SLR. All influenza vaccines recommended by the WHO were considered to evaluate VE: trivalent inactivated vaccines and live attenuated influenza vaccines.35 For inclusion, studies were required to focus on at least one countable outcome related to influenza infection: GP or emergency department visits, hospital admission or death. Information were collected from patient consulting medical facilities or medical databases reporting health care outcomes. The following exclusion criteria were also applied during title and abstract screening: articles published in languages other than English, reporting only vaccination information, assessing only vaccination coverage, reporting only vaccination uptake determinants and review articles, trials and qualitative studies.
Other exclusion criteria used during full-text analysis were: no reporting VE, reporting overall VE not specifically defined for high-risk-groups considered in the review, reporting VE not in high-risk-groups and reporting VE on hospitalization or outpatients visit for ILI or acute respiratory infection. Only quantitative studies describing influenza VE among risk-groups were included in the review. Studies were then selected for the qualitative and quantitative analysis.
Variables extraction regarded: cases of influenza among high-risk-groups considered in the SLR, influenza VEs in selected group, laboratory diagnostic procedures for testing for influenza and strategies used to assess vaccination status of each participant. Four investigators independently conducted both a literature search and a systematic review considering the inclusion, eligibility criteria and quality. Incongruity between the investigators was resolved by further discussion, with involvement of an external investigator where necessary.
Meta-analysis
After studies have been selected, reporting number of vaccinated among cases and control and/or influenza incident cases among exposed and unexposed to influenza vaccine, a meta-analysis according to Cochrane guidelines,62 was conducted on the extracted measures to assess the overall effect. Crude ORs and RRs were considered where available. The logarithms were used for the meta-analysis, with exponentiated effect sizes and confidence intervals displayed in the forest plots. Vaccine effectiveness was calculated as VE = [(1-OR)x100] or VE = [(1-RR)x100] and crude ORs or RRs with relative 95% Confidence Interval (95%CI) were estimated for each risk-group.63
Pooled estimates were calculated using both fixed effects and DerSimonian and Laird random effects models, weighting individual study results by the inverse of their variances.64 Forest plots were used to visually assess the pooled estimates and corresponding 95%CI across studies. A test of heterogeneity was performed using a chi-square test at significance level of p<0.05 and reported with the I2 statistic together with a 25%, 50% or 75% cut-off, indicating low, moderate and high heterogeneity, respectively.65,66
When the test showed significant heterogeneity, the sources of heterogeneity were explored through pre-specified meta-regression and sensitivity analyses. The following variables were considered for a meta-regression analysis: vaccinated children (< 9 y old) who performed, for the first time, 2 doses of influenza vaccination (yes vs no), hemisphere where study was conducted (Northern vs Southern), year of study conduction before or after influenza pandemic season (before 2010 vs after 2010) and 2 variables that reported mismatch between influenza A or B viruses included in the seasonal vaccine and circulating viruses among cases and controls or exposed and unexposed (yes vs no), respectively. Sensitivity analyses were conducted to examine the contribution of each individual study by evaluating the impact of the outlier studies, eliminating each study from the meta-analysis and comparing the point estimates which included or excluded the study.
The methodological quality of studies included in the meta-analysis was assessed using revised versions of previously validated checklists for quantitative retrospective and prospective studies, as recommended by the Cochrane Collaboration.62,67
To assess a potential publication bias, a graphical plot of the logarithm effect estimates versus its standard error, for each study, was used, and the Egger test was performed.68,69
All data were analyzed using the statistical package STATA/MP 14.2 (StataCorp LP, College Station, TX, USA), with the “metan” command used for meta-analysis, “metafunnel,” “metabias” and “confunnel” for publication bias assessment.70
Abbreviations
- ALRI
influenza-associated acute lower respiratory infections
- GP
general practitioner
- HCW
Health Care Worker
- ILI
influenza-like illness
- OR
Odds ratio
- RR
Relative risk
- SOT
solid organ transplant
- SLR
systematic literature review
- VE
vaccine effectiveness
- WHO
World Health Organization
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
References
- [1].Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25(27):5086-96; PMID:17544181; https://doi.org/ 10.1016/j.vaccine.2007.03.046 [DOI] [PubMed] [Google Scholar]
- [2].Bonmarin I, Belchior E, Lévy-Bruhl D. Impact of influenza vaccination on mortality in the French elderly population during the 2000–2009 period. Vaccine 2015; 33(9):1099-1101; PMID:25604800; https://doi.org/ 10.1016/j.vaccine.2015.01.023 [DOI] [PubMed] [Google Scholar]
- [3].Molbak K, Espenhain L, Nielsen J, Tersago K, Bossuyt N, Denissov G, Baburin A, Virtanen M, Fouillet A, Sideroglou T, et al.. Excess mortality among the elderly in European countries, December 2014 to February 2015. Euro Surveill 2015; 20(11);pii:2106; https://doi.org/ 10.2807/1560-7917.ES2015.20.11.21065 [DOI] [PubMed] [Google Scholar]
- [4].Mazick A, Gergonne B, Nielsen J, Wuillaume F, Virtanen MJ, Fouillet A, Uphoff H, Sideroglou T, Paldy A, Oza A, et al.. Excess mortality among the elderly in 12 European countries, February and March 2012. Euro Surveill 2012; 17(14);pii:20138 [PubMed] [Google Scholar]
- [5].Michelozzi P, De' Donato F, Scortichini M, De Sario M, Asta F, Agabiti N, Guerra R, De Martino A, Davoli M. On the increase in mortality in Italy in 2015: analysis of seasonal mortality in the 32 municipalities included in the Surveillance system of daily mortality. Epidemiol Prev 2016; 40(1):22-8; PMID:26951698 [DOI] [PubMed] [Google Scholar]
- [6].Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997–2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses 2014; 8(5):507-15; PMID:24975705; https://doi.org/ 10.1111/irv.12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fischer WA, Gongz M, Bhagwanjeex S, Sevransky J. Global Burden of Influenza as a Cause of Cardiopulmonary Morbidity and Mortality. Global hearth 2014; 3:325-36; https://doi.org/ 10.1016/j.gheart.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beck CR, McKenzie BC, Hashim AB. Influenza Vaccination for Immunocompromised Patients: Systematic Review and Meta-analysis by Etiology. J Infect Dis 2012; 206:1250-9; PMID:22904335; https://doi.org/ 10.1093/infdis/jis487 [DOI] [PubMed] [Google Scholar]
- [9].Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N Engl J Med 2010; 362:27-35; PMID:20032319; https://doi.org/ 10.1056/NEJMoa0910444 [DOI] [PubMed] [Google Scholar]
- [10].Principi N, Esposito S, Marchisio P, Gasparini R, Crovari P. Socioeconomic impact of influenza on healthy children and their families. Pediatr Infect Dis J 2003; 22:S207-10; PMID:14551476; https://doi.org/ 10.1097/01.inf.0000092188.48726.e4 [DOI] [PubMed] [Google Scholar]
- [11].McLean HQ, Peterson SH, King JP, Meece JK, Belongia EA. School absenteeism among school-aged children with medically attended acute viral respiratory illness during three influenza seasons, 2012–2013 through 2014–2015. Influenza Other Respir Viruses 2017; 11(3):220-229; https://doi.org/ 10.1111/irv.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Amodio E, Restivo V, Firenze A, Mammina C, Tramuto F, Vitale F. Can influenza vaccination coverage among healthcare workers influence the risk of nosocomial influenza-like illness in hospitalized patients? J Hosp Infect 2014; 86(3):182-7; PMID:24581755; https://doi.org/ 10.1016/j.jhin.2014.01.005 [DOI] [PubMed] [Google Scholar]
- [13].Elder AG, O'Donnell B, McCruden EAB, Symington IS, Carman WF. Incidence and recall of influenza in a cohort of Glasgow healthcare workers during the 1993–4 epidemic: results of serum testing and questionnaire. BMJ 1996; 313:1241-2; https://doi.org/ 10.1136/bmj.313.7067.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Restivo V, Costantino C, Mammina C, Vitale F. Influenza like Illness among medical residents anticipates influenza diffusion in general population: data from a national survey among Italian medical residents. PLoS One 2016; 11(12):e0168546; PMID:27997602; https://doi.org/ 10.1371/journal.pone.0168546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hagel S, Ludewig K, Moeser A, Baier M, Löffler B, Schleenvoigt B, Forstner C, Pletz MW. Characteristics and management of patients with influenza in a German hospital during the 2014/2015 influenza season. Infection 2016; 44(5):667-72; PMID:27380386; https://doi.org/ 10.1007/s15010-016-0920-0 [DOI] [PubMed] [Google Scholar]
- [16].Dolan GP, Harris RC, Clarkson M, Sokal R, Morgan G, Mukaigawara M, Horiuchi H, Hale R, Stormont L, Béchard-Evans L, et al.. Vaccination of health care workers to protect patients at increased risk for acute respiratory disease. Emerg Infect Dis 2012; 18(8):1225-34; PMID:22840895; https://doi.org/ 10.3201/eid1808.111355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Costantino C, Vitale F. Influenza vaccination in high-risk groups: a revision of existing guidelines and rationale for an evidence-based preventive strategy. J Prev Med Hyg 2016; 57(1):E13-8; PMID:27346934 [PMC free article] [PubMed] [Google Scholar]
- [18].Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, Bresee JS. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016; 65(5):1-54; https://doi.org/ 10.15585/mmwr.rr6505a1 [DOI] [PubMed] [Google Scholar]
- [19].Armstrong C. ACIP Updates Influenza Vaccination Recommendations for 2016–2017. Am Fam Physician 2016; 94(8):668-70; PMID:2792922327929223 [Google Scholar]
- [20].Doherty M, Schmidt-Ott R, Santos JI, Stanberry LR, Hofstetter AM, Rosenthal SL, Cunningham AL. Vaccination of special populations: Protecting the vulnerable. Vaccine 2016; 34(52):6681-90; PMID:27876197; https://doi.org/ 10.1016/j.vaccine.2016.11.015 [DOI] [PubMed] [Google Scholar]
- [21].European Centre for Disease Prevention and Control Seasonal influenza vaccines. Influenza vaccination. [Accessed 2017Februaty 22] http://ecdc.europa.eu/en/healthtopics/seasonal_influenza/vaccines/Pages/influenza_vaccination.aspx#vaccinationstrategies [Google Scholar]
- [22].Szilagyi PG, Fairbrother G, Griffin MR, Hornung RW, Donauer S, Morrow A, Altaye M, Zhu Y, Ambrose S, Edwards KM, et al.. Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch Pediatr Adolesc Med 2008; 162(10):943-51; PMID:18838647; https://doi.org/ 10.1001/archpedi.162.10.943 [DOI] [PubMed] [Google Scholar]
- [23].Ridenhour BJ, Campitelli MA, Kwong JC, Rosella LC, Armstrong BG, Mangtani P, Calzavara AJ, Shay DK. Effectiveness of inactivated influenza vaccines in preventing influenza-associated deaths and hospitalizations among Ontario residents aged ≥ 65 years: estimates with generalized linear models accounting for healthy vaccinee effects. PLoS One 2013; 8(10):e76318; PMID:24146855; https://doi.org/ 10.1371/journal.pone.0076318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng AC, Kotsimbos T, Kelly PM, FluCAN Investigators . Influenza vaccine effectiveness against hospitalisation with influenza in adults in Australia in 2014. Vaccine 2015; 33(51):7352-6; PMID:26529066; https://doi.org/ 10.1016/j.vaccine.2015.10.016 [DOI] [PubMed] [Google Scholar]
- [25].MacIntyre CR, Heywood AE, Kovoor P, Ridda I, Seale H, Tan T, Gao Z, Katelaris AL, Siu HW, Lo V, et al.. I schaemic heart disease, influenza and influenza vaccination: a prospective case control study. Heart 2013; 99(24):1843-8; PMID:23966030; https://doi.org/ 10.1136/heartjnl-2013-304320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Puig-Barberà J, Díez-Domingo J, Arnedo-Pena A, Ruiz-García M, Pérez-Vilar S, Micó-Esparza JL, Belenguer-Varea A, Carratalá-Munuera C, Gil-Guillén V, Schwarz-Chavarri H. Effectiveness of the 2010–2011 seasonal influenza vaccine in preventing confirmed influenza hospitalizations in adults: a case-case comparison, case-control study. Vaccine 2012; 30(39):5714-20; PMID:22819720; https://doi.org/ 10.1016/j.vaccine.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Steens A, Wijnans EG, Dieleman JP, Sturkenboom MC, van der Sande MA, van der Hoek W. Effectiveness of a MF-59™-adjuvanted pandemic influenza vaccine to prevent 2009 A/H1N1 influenza-related hospitalisation; a matched case-control study. BMC Infect Dis 2011; 11:196; PMID:21767348; https://doi.org/ 10.1186/1471-2334-11-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Andrews N, Waight P, Yung CF, Miller E. Age-specific effectiveness of an oil-in-water adjuvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England. J Infect Dis 2011; 203(1):32-9; PMID:21148494; https://doi.org/ 10.1093/infdis/jiq014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Emborg HD, Krause TG, Hviid A, Simonsen J, Mølbak K. Effectiveness of vaccine against pandemic influenza A/H1N1 among people with underlying chronic diseases: cohort study, Denmark, 2009–10. BMJ 2011; 344:d7901; PMID:22277542; https://doi.org/ 10.1136/bmj.d7901 [DOI] [PubMed] [Google Scholar]
- [30].Perez-Romero P, Aydillo TA, Perez-Ordoñez A, Muñoz P, Moreno A, López-Medrano F, Bodro M, Montejo M, Gavaldà J, Fariñas MC, et al.. Reduced incidence of pneumonia in influenza-vaccinated solid organ transplant recipients with influenza disease. Clin Microbiol Infect 2012; 18(12):E533-40; PMID:23078072; https://doi.org/ 10.1111/1469-0691.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thompson MG, Li DK, Shifflett P, Sokolow LZ, Ferber JR, Kurosky S, Bozeman S, Reynolds SB, Odouli R, Henninger ML, et al.. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis 2014; 58(4):449-57; PMID:24280090; https://doi.org/ 10.1093/cid/cit750 [DOI] [PubMed] [Google Scholar]
- [32].Regan AK, Nd Klerk, Moore HC, Omer SB, Shellam G, Effler PV. Effectiveness of seasonal trivalent influenza vaccination against hospital-attended acute respiratory infections in pregnant women: A retrospective cohort study. Vaccine 2016; 34(32):3649-56; PMID:27216758; https://doi.org/ 10.1016/j.vaccine.2016.05.032 [DOI] [PubMed] [Google Scholar]
- [33].Costa JT, Silva R, Tavares M, Nienhaus A. High effectiveness of pandemic influenza A (H1N1) vaccination in healthcare workers from a Portuguese hospital. Int Arch Occup Environ Health 2012; 85(7):747-52; PMID:22045387; https://doi.org/ 10.1007/s00420-011-0714-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Igari H, Watanabe A, Chiba H, Shoji K, Segawa S, Nakamura Y, Watanabe M, Suzuki K, Sato T. Effectiveness and safety of pandemic influenza A (H1N1) 2009 vaccine in healthcare workers at a university hospital in Japan. Jpn J Infect Dis 2011; 64(3):177-82; PMID:21617299 [PubMed] [Google Scholar]
- [35].World Health Organization Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec 2012; 87(47):461-76; PMID:23210147 [PubMed] [Google Scholar]
- [36].Remschmidt C, Wichmann O, Harder T. Influenza vaccination in patients with end-stage renal disease: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness, and safety. BMC Med 2014; 12:244; PMID:25523432; https://doi.org/ 10.1186/s12916-014-0244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2012; 15(8):CD004879; PMID:22895945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine 2002; 20(13–14):1831-6; PMID:11906772; https://doi.org/ 10.1016/S0264-410X(02)00041-5 [DOI] [PubMed] [Google Scholar]
- [39].European Centre for Disease Prevention and Control Seasonal influenza vaccination in Europe: Overview of vaccination recommendations and coverage rates in the EU Member States for the 2012–13 influenza season. [Accessed 2017February22] http://ecdc.europa.eu/en/publications/Publications/Seasonal-influenza-vaccination-Europe-2012-13.pdf [Google Scholar]
- [40].Sung LC, Chen CI, Fang YA, Lai CH, Hsu YP, Cheng TH, Miser JS, Liu JC. Influenza vaccination reduces hospitalization for acute coronary syndrome in elderly patients with chronic obstructive pulmonary disease: a population-based cohort study. Vaccine 2014; 32(30):3843-9; PMID:24837769; https://doi.org/ 10.1016/j.vaccine.2014.04.064 [DOI] [PubMed] [Google Scholar]
- [41].Restivo V, Vizzini G, Mularoni A, Di Benedetto C, Gioè SM, Vitale F. Determinants of influenza vaccination among solid organ transplant recipients attending Sicilian reference center. Hum Vaccin Immunother 2017; 13(2):346-50; PMID:27929758; https://doi.org/ 10.1080/21645515.2017.1264792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rasmussen SA, Jamieson DJ, Bresee S. Pandemic influenza and pregnant women. Emerg Infect Dis 2008; 14:95-100; PMID:18258087; https://doi.org/ 10.3201/eid1401.070667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nandor ACS, Banhidy F, Puho E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Def Res 2005; 73:989-96; https://doi.org/ 10.1002/bdra.20195 [DOI] [PubMed] [Google Scholar]
- [44].Radigan KA, Mutlu GM. Markers of prognosis specific to influenza infection: are we there yet? Am J Respir Crit Care Med 2014; 189(10):1159-60; PMID:24832741; https://doi.org/ 10.1164/rccm.201403-0587ED [DOI] [PubMed] [Google Scholar]
- [45].Kidd M. Influenza viruses: update on epidemiology, clinical features, treatment and vaccination. Curr Opin Pulm Med 2014; 20:242-6; PMID:24637227; https://doi.org/ 10.1097/MCP.0000000000000049 [DOI] [PubMed] [Google Scholar]
- [46].Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, et al.. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378(9807):191; https://doi.org/ 10.1016/S0140-6736(11)61051-9 [DOI] [PubMed] [Google Scholar]
- [47].Lafond KE, Nair H, Rasooly MH, Valente F, Booy R, Rahman M, Kitsutani P, Yu H, Guzman G, Coulibaly D, et al.. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med 2016; 13(3):e1001977; PMID:27011229; https://doi.org/ 10.1371/journal.pmed.1001977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sullivan SG, Chilver MB, Higgins G, Cheng AC, Stocks NP. Influenza vaccine effectiveness in Australia: results from the Australian Sentinel Practices Research Network. Med J Aust 2014; 201(2):109-11; PMID:25045991 [DOI] [PubMed] [Google Scholar]
- [49].Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine 2009; 27(47):6546-9; PMID:19729083 [DOI] [PubMed] [Google Scholar]
- [50].Eisenberg KW, Szilagyi PG, Fairbrother G, Griffin MR, Staat M, Shone LP, Weinberg GA, Hall CB, Poehling KA, Edwards KM, et al.. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics 2008; 122(5):911-9; PMID:18977968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Centers for Disease Control and Prevention Update: influenza activity: United States and worldwide, 2003–04 season, and composition of the 2004–05 influenza vaccine. MMWR Morb Mortal Wkly Rep 2004; 53(25):547-52; PMID:15229411 [PubMed] [Google Scholar]
- [52].Centers for Disease Control and Prevention Update: influenza activity: United States and worldwide, 2004–05 season. MMWR Morb Mortal Wkly Rep 2005; 54(25):631-4; PMID:15988408 [PubMed] [Google Scholar]
- [53].Dixon GA, Moore HC, Kelly H, Jacoby P, Carcione D, Williams S, Smith D, Keil AD, Van Buynder P, Richmond PC, et al.. Lessons from the first year of the WAIVE study investigating the protective effect of influenza vaccine against laboratory-confirmed influenza in hospitalised children aged 6–59 months. Influenza Other Respir Viruses 2010; 4(4):231-4; PMID:20629773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Blyth CC, Cheng AC, Finucane C, Jacoby P, Effler PV, Smith DW, Kelly H, Macartney KK, Richmond PC. The effectiveness of influenza vaccination in preventing hospitalisation in children in Western Australia. Vaccine 2015; 33(51):7239-44; PMID:26549359 [DOI] [PubMed] [Google Scholar]
- [55].Sanei F, Wilkinson T. Influenza vaccination for patients with chronic obstructive pulmonary disease: understanding immunogenicity, efficacy and effectiveness. Ther Adv Respir Dis 2016; 10(4):349-67; PMID:27193567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol 2009; 183(5):3294-301; PMID:19648276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Centers for Disease Control and Prevention Update: influenza activity–United States and worldwide, May 19-September 28, 2013. MMWR Morb Mortal Wkly Rep 2013; 62:838-42; PMID:24153315 [PMC free article] [PubMed] [Google Scholar]
- [58].Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001-6; PMID:10570188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Orellano PW, Reynoso JI, Carlino O, Uez O. Protection of trivalent inactivated influenza vaccine against hospitalizations among pandemic influenza A (H1N1) cases in Argentina. Vaccine 2010; 28(32):5288-91 [DOI] [PubMed] [Google Scholar]
- [60].Gilca R, Skowronski DM, Douville-Fradet M, Amini R, Boulianne N, Rouleau I, Martineau C, Charest H, De Serres G. Mid-Season estimates of influenza vaccine effectiveness against influenza A(H3N2) hospitalization in the elderly in Quebec, Canada, January 2015. PLoS One 2015; 10(7):e0132195; PMID:26200655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of hemagglutinin residues responsible for H3N2 antigenic drift during the 2014–2015 influenza season. Cell Rep 2015; 12(1):1-6; PMID:26119736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org [Google Scholar]
- [63].Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31(17):2165-8; PMID:23499601 [DOI] [PubMed] [Google Scholar]
- [64].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177-88; PMID:3802833; https://doi.org/ 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- [65].Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993; 2:121-45; PMID:8261254; https://doi.org/ 10.1177/096228029300200202 [DOI] [PubMed] [Google Scholar]
- [66].Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J 2003; 327:557-60; https://doi.org/ 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2012. [Accessed 2017February22]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- [68].Sterne JA, Egger M. Funnel plots for detecting bias in metaanalysis: Guidelines on choice of axis. J Clin Epidemiol 2001; 54:1046-55; PMID:11576817; https://doi.org/ 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- [69].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629-34; PMID:9310563; https://doi.org/ 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Palmer TM, Sterne JA, editors. Meta-Analysis in Stata: An Updated Collection from the Stata Journal. 2nd ed. Stata Press; 2015, 534 p [Google Scholar]
- [71].Blyth CC, Jacoby P, Effler PV, Kelly H, Smith DW, Borland ML, Willis GA, Levy A, Keil AD, Richmond PC, et al.. Influenza vaccine effectiveness and uptake in children at risk of severe disease. Pediatr Infect Dis J 2016; 35(3):309-15; PMID:26646548; https://doi.org/ 10.1097/INF.0000000000000999 [DOI] [PubMed] [Google Scholar]
- [72].McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, Piedra PA, Zimmerman RK, Nowalk MP, Raviotta JM, et al.. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015; 211(10):1529-40; PMID:25406334; https://doi.org/ 10.1093/infdis/jiu647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Belongia EA, Kieke BA, Donahue JG, Coleman LA, Irving SA, Meece JK, Vandermause M, Lindstrom S, Gargiullo P, Shay DK. Influenza vaccine effectiveness in Wisconsin during the 2007–08 season: comparison of interim and final results. Vaccine 2011; 29(38):655; https://doi.org/ 10.1016/j.vaccine.2011.07.002 [DOI] [PubMed] [Google Scholar]
- [74].Joshi AY, Iyer VN, St Sauver JL, Jacobson RM, Boyce TG. Effectiveness of inactivated influenza vaccine in children less than 5 years of age over multiple influenza seasons: a case-control study. Vaccine 2009; 27(33):4457-61; PMID:19490957; https://doi.org/ 10.1016/j.vaccine.2009.05.038 [DOI] [PubMed] [Google Scholar]
- [75].Shuler CM, Iwamoto M, Bridges CB, Marin M, Neeman R, Gargiullo P, Yoder TA, Keyserling HL, Terebuh PD. Vaccine effectiveness against medically attended, laboratory-confirmed influenza among children aged 6 to 59 months, 2003–2004. Pediatrics 2007; 119(3):e587-95; PMID:17332179; https://doi.org/ 10.1542/peds.2006-1878 [DOI] [PubMed] [Google Scholar]
- [76].Chiu SS, Feng S, Chan KH, Lo JY, Chan EL, So LY, Cowling BJ, Peiris JS. Hospital-based vaccine effectiveness against influenza B lineages, Hong Kong, 2009–14. Vaccine 2016; 34(19):2164-9; PMID:27013437; https://doi.org/ 10.1016/j.vaccine.2016.03.032 [DOI] [PubMed] [Google Scholar]
- [77].Grijalva CG, Zhu Y, Williams DJ, Self WH, Ampofo K, Pavia AT, Stockmann CR, McCullers J, Arnold SR, Wunderink RG, et al.. Association Between Hospitalization With Community-Acquired Laboratory-Confirmed Influenza Pneumonia and Prior Receipt of Influenza Vaccination. JAMA 2015; 314(14):1488-97; PMID:26436611; https://doi.org/ 10.1001/jama.2015.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cowling BJ, Chan KH, Feng S, Chan EL, Lo JY, Peiris JS, Chiu SS. The effectiveness of influenza vaccination in preventing hospitalizations in children in Hong Kong, 2009–2013. Vaccine 2014; 32(41):5278-84; PMID:25092636; https://doi.org/ 10.1016/j.vaccine.2014.07.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ferdinands JM, Olsho LE, Agan AA, Bhat N, Sullivan RM, Hall M, Mourani PM, Thompson M, Randolph AG, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US children, 2010–2012. J Infect Dis 2014; 210(5):674-83; PMID:24676207; https://doi.org/ 10.1093/infdis/jiu185 [DOI] [PubMed] [Google Scholar]
- [80].Gilca R, Deceuninck G, De Serres G, Boulianne N, Sauvageau C, Quach C, Boucher FD, Skowronski DM. Effectiveness of pandemic H1N1 vaccine against influenza-related hospitalization in children. Pediatrics 2011; 128(5):e1084-91; PMID:21987710; https://doi.org/ 10.1542/peds.2010-3492 [DOI] [PubMed] [Google Scholar]
- [81].Griffin MR, Monto AS, Belongia EA, Treanor JJ, Chen Q, Chen J, Talbot HK, Ohmit SE, Coleman LA, Lofthus G, et al.. Effectiveness of non-adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 U.S. communities. PLoS One 2011; 6(8):e23085; PMID:21857999; https://doi.org/ 10.1371/journal.pone.0023085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen Q, Griffin MR, Nian H, Zhu Y, Williams JV, Edwards KM, Talbot HK. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015; 211(7):1045-50; PMID:25336724; https://doi.org/ 10.1093/infdis/jiu578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Havers F, Sokolow L, Shay DK, Farley MM, Monroe M, Meek J, Daily Kirley P, Bennett NM, Morin C, Aragon D, et al.. Case-Control Study of Vaccine Effectiveness in Preventing Laboratory-Confirmed Influenza Hospitalizations in Older Adults, United States, 2010–2011. Clin Infect Dis 2016; 63(10):1304-11; PMID:27486114; https://doi.org/ 10.1093/cid/ciw512 [DOI] [PubMed] [Google Scholar]
- [84].Puig-Barbera J, Mira-Iglesias A, Tortajada-Girbes M, Lopez-Labrador FX, Belenguer-Varea A, Carballido-Fernandez M, Carbonell-Franco E, Carratala-Munuera C, Limon-Ramirez R, Mollar-Maseres J, et al.. Effectiveness of influenza vaccination programme in preventing hospital admissions, Valencia, 2014/15 early results. Euro Surveill 2015; 20(8);pii:21044; https://doi.org/ 10.2807/1560-7917.ES2015.20.8.21044 [DOI] [PubMed] [Google Scholar]
- [85].Castilla J, Martínez-Baz I, Navascués A, Fernandez-Alonso M, Reina G, Guevara M, Chamorro J, Ortega MT, Albéniz E, Pozo F, et al.. Vaccine effectiveness in preventing laboratory-confirmed influenza in Navarre, Spain: 2013/14 mid-season analysis. Euro Surveill 2014; 19(6);pii:20700; https://doi.org/ 10.2807/1560-7917.ES2014.19.6.20700 [DOI] [PubMed] [Google Scholar]
- [86].Kwong JC, Campitelli MA, Gubbay JB, Peci A, Winter AL, Olsha R, Turner R, Rosella LC, Crowcroft NS. Vaccine effectiveness against laboratory-confirmed influenza hospitalizations among elderly adults during the 2010–2011 season. Clin Infect Dis 2013; 57(6); PMID:23788243; https://doi.org/ 10.1093/cid/cit404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Van Vuuren A, Rheeder P, Hak E. Effectiveness of influenza vaccination in the elderly in South Africa. Epidemiol Infect 2009; 137(7):994-1002; PMID:18925986; https://doi.org/ 10.1017/S0950268808001386 [DOI] [PubMed] [Google Scholar]


