Abstract
Introduction
Staple-line bleeding and leakage are the most common serious complications of laparoscopic sleeve gastrectomy. The relationship between multiple stapler firings and higher risk of postoperative complications is well defined in colorectal surgery but has not been addressed in bariatric procedures so far. Identification of new factors such as “the numbers of stapler firings used during laparoscopic sleeve gastrectomy (LSG)” as a predictor for complications can lead to optimization of the patient care at bariatric centers.
Aim
To determine the association between perioperative morbidity and the number of stapler firings during laparoscopic sleeve gastrectomy.
Material and methods
This observational study was based on retrospective analysis of prospectively collected data in patients operated on for morbid obesity in a teaching hospital/tertiary referral center for general surgery. The patients who underwent LSG were analyzed in terms of the number of stapler firings used as a new potential risk predictor for postoperative complications after surgery, adjusting for other patient- and treatment-related factors. The study included 333 patients (209 women, 124 men, mean age: 40 ±11).
Results
During the first 30 days after surgery, complications were observed in 18 (5.41%) patients. Multivariate analysis showed that prolonging operative time increased morbidity (every minute, OR = 1.01; 95% CI: 1.00–1.02) and the complication rate increased with the number of stapler firings (every firing, OR = 1.91; 95% CI: 1.09–3.33; p = 0.023).
Conclusions
Additional stapler firings above the usual number and a prolonged operation should alert a surgeon and the whole team about increased risk of postoperative complications.
Keywords: sleeve gastrectomy, postoperative complications, risk factors, stapler firings
Introduction
In recent years, the number of bariatric procedures has grown [1]. Laparoscopic sleeve gastrectomy (LSG) has become one of the most common bariatric operation worldwide [2]. Staple-line bleeding and leakage are the most common serious complications of LSG, with incidence between 0.5% and 20% [3–5]. In 90% of cases leaks occur in the angle of His, and probably they are related to technical error during stapler firing [6]. The relationship between multiple stapler firings and higher risk of postoperative complications is well defined in colorectal procedures but has not been addressed in bariatric surgery so far. Identification of new factors such as “the numbers of stapler firings used during LSG” as a predictor for complications can lead to optimization of patient care at bariatric centers.
Aim
The aim of this study was to determine the association between complication rate and number of stapler firings during laparoscopic sleeve gastrectomy.
Material and methods
This observational study design was based on retrospective analysis of prospectively collected data in patients operated on for morbid obesity at a tertiary referral for general surgery, academic, teaching hospital. Guidelines of the Metabolic and Bariatric Surgery Section of the Polish Surgical Society were used as criteria for surgical treatment, i.e., body mass index (BMI) ≥ 35 kg/m2 with obesity comorbidities, or BMI ≥ 40 kg/m2. Inclusion criteria: 18–65 years old, meeting criteria for morbid obesity surgical treatment. Exclusion criteria: lack of necessary data, previous operations for morbid obesity. Data obtained from patients’ history and 30-days follow-up included: factors associated with patient characteristics, e.g. age, sex, preoperative body weight, BMI, smoking, main comorbidities (cardiovascular diseases, hypertension, ischemic heart disease, chronic heart failure, respiratory diseases including obstructive sleep apnea syndrome, chronic obstructive pulmonary disease, diabetes mellitus type II, dyslipidemia, hepatic steatosis) and factors related to the surgical procedure, e.g. operative time, number of stapler firings used, intraoperative adverse effects and surgeon’s experience. Perioperative complications were defined as adverse events occurring within 30 days of the procedure and they were classified according to the Clavien-Dindo classification [7]. Rhabdomyolysis was defined as at least elevated biochemical test results of creatine phosphokinase (CPK > 1000 IU/l) with a concomitant increase in myoglobin level. Next, univariate and multivariate analyses were performed to identify potential risk factor for postoperative complications with particular attention paid to the number of stapler firings. In order to minimize bias, patients’ care was standardized in accordance with the principles of the multimodal ERAS pathway and the surgical technique for LSG was standardized in all patients. The patients’ data were collected prospectively by the authors directly involved in bariatric patients’ treatment and follow-up. Patients were treated in accordance with the principles of the multimodal ERAS pathway, including preoperative, intraoperative and postoperative interventions [8, 9]. Preoperative interventions included extensive perioperative counseling, shortened fluid fasts, preoperative high protein and carbohydrate drink, and optimized operating scheduling times. Intraoperatively, the optimized bariatric anesthetic protocol was introduced using multimodal analgesia. There was no routine use of nasogastric tubes or intra-abdominal drains. Postoperative interventions included early mobilization, analgesia without opioids, administration of an IPP (40 mg of pantoprazole) antagonist, early enteral feeding and discharge planning. Anti-thrombotic prophylaxis was administered up to 14 postoperative days using enoxaparin. Patients were scheduled for postoperative appointment 2 weeks after discharge, then 1 month and 3 months after discharge. The surgical technique for LSG was standardized and procedures were performed by a team consisting of 5 surgeons. A Veress needle was used for pneumoperitoneum (15 mm Hg) and five ports were routinely inserted. A Nathanson liver retractor was also in routine use. A sealer/divider or ultrasonic shears were used for dissection and coagulation (LigaSure Atlas, Covidien or SonoSurg, Olympus). The calibration of the gastric sleeve was done with a 34-French gastric bougie inserted into the stomach along the lesser curvature. The transection of the stomach was begun 5–6 cm from the pylorus with continuously applied linear staplers, starting with 2 firings of 60 mm, Ethicon Echelon EndoFlex with gold cartridges (3.8 mm open stapler height, 1.8 mm closed stapler height), then continued with blue cartridges (3.6 mm open stapler height, 1.5 mm closed stapler height) to the angle of His. The entire staple line was oversewn by a continuous seromuscular invaginating suture using 3-0 PDS, starting from the angle of His. The resected stomach was removed through the left flank trocar site. At the end of the procedure, an intraoperative leak test was conducted with methylene blue solution through a nasogastric tube after the pylorus was occluded with a soft clamp. No drains were left after operations. On the first postoperative day, upper gastrointestinal study with Gastrografin was performed. The information about the number of stapler firings was taken from the surgery protocol, where each surgery was recorded. The endpoint was determining the influence of the number of stapler firings on perioperative morbidity (in the 30-day postoperative period), by performing multivariate logistic regression analysis adjusting for other patient- and surgery-related factors.
All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent for surgical treatment was obtained from all patients before surgery. The study was approved by the local Ethics Review Committee (KBET/62/B/2011). Informed consent was obtained from all individual participants included in the study.
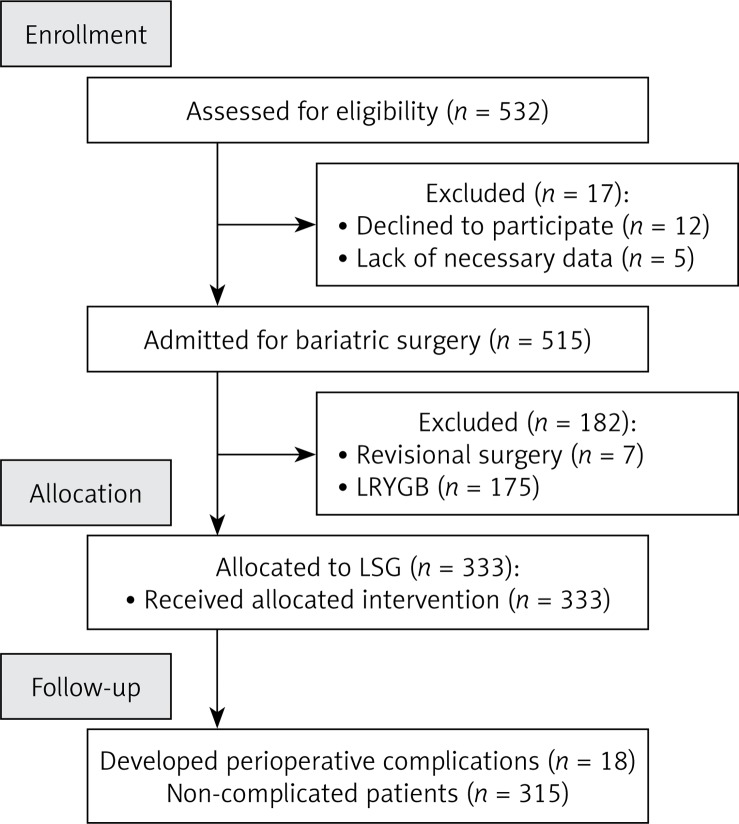
From April 2013 to September 2015, 532 patients were treated for morbid obesity (336 women, 196 men, mean age: 43 ±11 years). The study included 333 patients who underwent LSG (209 women, 124 men, mean age: 40 ±11). Medians of maximal perioperative weight and BMI were 132 kg and 45.91 kg/m2. Patients’ baseline characteristics are presented in Table I. A flowchart of patients in the study is presented in Figure 1.
Table I.
Patient’s characteristics
| Parameter | Result |
|---|---|
| Patients’ numbers, n (%) | 333 (100) |
| Gender, n (%): | |
| Female | 209 (63) |
| Male | 124 (37) |
| Age, mean ± SD [years] | 40 ±11 |
| Maximal preoperative weight, median (IQR) [kg] | 132 (120; 147) |
| Maximal preoperative body mass index (BMI), median (IQR) [kg/m2] | 45.91 (42.48; 50.19) |
| Weight at admission for operation, median (IQR) [kg] | 130 (117; 143) |
| BMI at admission for operation, median (IQR) [kg/m2] | 44.82 (41.33; 48.55) |
| Preoperative weight loss, median (IQR) [kg] | 2 (0; 6) |
| Operative time, median (IQR) [min] | 110 (85; 140) |
| Additional procedure during bariatric op., n (%) | 3 (1) |
| Intraoperative adverse effects*, n (%) | 4 (1.2) |
| Surgical experience of operator**, n (%): | |
| Exp. | 78 (23) |
| Learning | 255 (77) |
Intraoperative adverse effects: 2 bleeding from the stapler line, 1 liver injury after liver retractor, 1 stapler misfire.
We considered an experienced operator to be a surgeon who had independently performed at least 100 bariatric procedures.
Figure 1.
Study flow chart. More stapler firings increase risk of perioperative morbidity after laparoscopic sleeve gastrectomy
Statistical analysis
All data were analyzed with Statistica version 12.0 PL (StatSoft Inc., Tulsa, Oklahoma, USA). The results are presented as mean ± standard deviation (SD), median and interquartile range (IQR) and odds ratio (OR) with 95% confidence intervals (CI) when appropriate. Univariate and multivariate logistic regression analyses for complications were performed to assess the influence of selected parameters on outcomes. Results were considered statistically significant when the p-value was found to be less than 0.05. Pairwise deletion for missing data was used.
Results
During the first 30 days after surgery, complications were observed in 18 (5.41%) patients. The most common complications included biochemical rhabdomyolysis (6 cases; 1.8%), defined as elevation of myoglobin and/or CPK level on day 1 after surgery, delayed gastric emptying (4 cases; 1.2%), surgical site infection (1 case; 0.3%), staple-line leakage (5 cases; 1.5%), intra-abdominal bleeding from the staple line (1 case; 0.3%) and cardiorespiratory failure (1 case; 0.3%). There was no mortality (Table II).
Table II.
Perioperative (≤ 30 days) complications regarding Clavien-Dindo scale
| C-D Grade | Complications | N (% of patients) | N (% of complications) |
|---|---|---|---|
| 4b | Cardiorespiratory failure (ICU stay) | 1 (0.30) | 1 (5.56) |
| 3b | Staple-line leak* | 5 (1.50) | 5 (27.78) |
| Bleeding from suture line** (relaparoscopy) | 1 (0.30) | 1 (5.56) | |
| 1 | Delayed gastric emptying*** | 4 (1.20) | 4 (22.22) |
| Prolonged drainage | 1 (0.30) | 1 (5.56) | |
| Rhabdomyolysis | 6 (1.80) | 6 (33.33) | |
| Total | 18 (5.41) | 18 (100.00) |
Diagnosed clinically, confirmed with upper gastrointestinal study with water-soluble contrast, then if necessary computed tomography was performed
diagnosed clinically, confirmed with ultrasonography
diagnosed clinically, confirmed with upper gastrointestinal study with water-soluble contrast.
The median number of stapler firings in our group was 4 (minimum 3, maximum 8). The univariate logistic regression analysis showed that an increase in the number of stapler firings used during the operation significantly increased the rate of perioperative complications. Maximum preoperative body weight and BMI, as well as body weight and BMI on admission day, were also associated with occurrence of perioperative complications in univariate logistic regression. We found a correlation between longer operative time and higher complication rate (Table III).
Table III.
Univariate logistic regression analysis of parameters affecting occurrence of complications
| Parameter | Univariate logistic regression | ||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Gender (M vs. F) | 0.55 | 0.19–1.59 | 0.271 |
| Age | 1.02 | 0.97–1.06 | 0.431 |
| Maximal preoperative weight | 1.02 | 1.00–1.04 | 0.019 |
| Maximal preoperative BMI | 1.09 | 1.01–1.17 | 0.021 |
| Weight at admission for operation | 1.02 | 1.00–1.05 | 0.017 |
| BMI at admission for operation | 1.10 | 1.01–1.18 | 0.021 |
| Preoperative weight loss | 1.02 | 0.97–1.08 | 0.473 |
| Tobacco smoking (Yes vs. No) | 0.94 | 0.04–19.92 | 0.966 |
| Cardiovascular diseases (Yes vs. No) | 0.57 | 0.16–2.07 | 0.388 |
| Arterial hypertension (Yes vs. No) | 0.33 | 0.04–2.78 | 0.309 |
| Chronic heart disease (Yes vs. No) | 0.96 | 0.11–8.56 | 0.974 |
| Congestive heart failure (Yes vs. No) | 0.96 | 0.09–10.73 | 0.976 |
| Respiratory failure (Yes vs. No) | 0.77 | 0.24–2.54 | 0.672 |
| Obstructive sleep apnea (Yes vs. No) | 0.46 | 0.08–2.51 | 0.365 |
| Chronic obstructive pulmonary disease (Yes vs. No) | 0.96 | 0.12–7.82 | 0.973 |
| Diabetes mellitus (Yes vs. No) | 0.63 | 0.16–2.38 | 0.489 |
| Dyslipidemia (Yes vs. No) | 0.64 | 0.23–1.80 | 0.398 |
| Fatty liver disease (Yes vs. No) | 1.13 | 0.41–3.09 | 0.818 |
| Depression (Yes vs. No) | 0.96 | 0.09–10.73 | 0.976 |
| Varicose veins (Yes vs. No) | 1.09 | 0.33–3.57 | 0.881 |
| Operative time | 1.02 | 1.01–1.03 | < 0.001 |
| Additional procedure during bariatric op. (Yes vs. No) | 0.96 | 0.10–9.48 | 0.975 |
| Intraoperative adverse effects (Yes vs. No) | 0.96 | 0.11–8.39 | 0.973 |
| Numbers of stapler firings used | 2.40 | 1.44–3.99 | < 0.001 |
| Surgical experience of operator (learning vs. experienced) | 2.27 | 0.50–10.20 | 0.284 |
All significant factors from the univariate regression analysis were then used in the multivariate analysis model. The multivariate analysis showed that operative time increased the odds for complications (every minute, OR = 1.01; 95% CI: 1.00–1.02; p = 0.029) and the number of stapler firings used significantly contributed to the increase of complications (every firing, OR = 1.91; 95% CI: 1.09–3.33; p = 0.023) (Table IV).
Table IV.
Multivariate logistic regression analysis of parameters affecting occurrence of procedural complications
| Parameter | Multivariate logistic regression | ||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Maximal preoperative weight | 1.01 | 0.99–1.04 | 0.171 |
| Maximal preoperative body mass index | 1.05 | 0.97–1.14 | 0.207 |
| Weight at admission for operation | 1.02 | 0.99–1.04 | 0.137 |
| Body mass index at admission for operation | 0.91 | 0.83–1.00 | 0.060 |
| Operative time | 1.01 | 1.00–1.02 | 0.029 |
| Number of stapler firings used | 1.91 | 1.09–3.33 | 0.023 |
Discussion
Laparoscopic sleeve gastrectomy is currently one of the most popular bariatric procedures [10]. The main reasons for that are the short learning curve, satisfactory weight loss effect and control of comorbidities, as well as wide potential for revisional surgery [11]. Identification of new factors as a predictor for complications can lead to optimization of the patient care at bariatric centers [12]. We aimed to determine the association between complication rate and number of stapler firings during laparoscopic sleeve gastrectomy. Our analysis revealed that the number of stapler firings during LSG could be a possible predictor for perioperative complications after LSG. We did not find this observation in the available literature. A similar relationship was found in colorectal surgery. During low rectal resection, multiple stapler firings increase the risk of anastomotic leak [13, 14]. We can also find some analogies in other fields of surgery. For example, in the case of laparoscopic cholecystectomy the number of clips used during surgery is also an important risk factor [15]. The use of more than four clips was shown to be associated with a higher rate of postoperative complications [16, 17].
In our observations, postoperative complications during the first 30 days after surgery occurred in 18 (5.41%) patients, and this number was similar to other reports [18]. The types of complications were comparable to the results presented in the literature [19, 20]. One of the most severe complications after LSG is bleeding from the staple line and leak. In our study, 1 (0.3%) patient had bleeding from the staple line and needed emergency reoperation and 5 (1.5%) patients suffered from staple-line leak, which were successfully treated with endoscopic stent and reoperation with peritoneal drainage. The rate of those complications reported in the literature ranges from 1% to 2.7% according to a previous study [18, 21]. Numerous potential risk factors for perioperative complications were identified, including age, gender, BMI, comorbidities, smoking, calibrating bougie size, and distance between pylorus and gastric transection line [22].
Quality of the staple line and stapler firing technique during LSG are, in our opinion, the most important aspects of the procedure. Some technical practices, such as reinforcement of the staple line (oversewing or buttressing), are considered to reduce the complication rate. However, recent studies showed that they do not have a clinically significant effect on the leak rate [22–24]. In 2015, Gagner and Buchwald reported that the leak rate in LSG was significantly lower when absorbable polymer membrane (APM) staple-line reinforcement was used compared to oversewing. However, they did not focus on the number of stapler firings, which, in our opinion, is essential [25]. The multivariate logistic regression analysis showed that use of a greater number of stapler cartridges caused a statistically significant increase in the risk of perioperative complications after LSG (OR = 1.91; 95% CI: 1.09–3.33; p = 0.023). In an additional analysis, we found that increased number of stapler firings was associated with longer operative time. A logical explanation of this observation leads to the conclusion that use of a greater number of stapler firings was more time-consuming and prolonged the operative time. In our opinion, additional stapler firings were primarily related to some technical problems during the operation which resulted in a higher postoperative complication rate.
The limitations of the present study are the non-randomized design, relatively small sample of patients, operations performed at a tertiary referral academic institution, and short follow-up.
Conclusions
The risk of postoperative complications following laparoscopic sleeve gastrectomy increased with prolonged operative time and increase in the number of stapler firings used during operations. Additional stapler firings above the usual number and a prolonged operation should alert a surgeon and the whole team about increased risk of postoperative complications.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benaiges D, Mas-Lorenzo A, Goday A, et al. Laparoscopic sleeve gastrectomy: more than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21:11804–14. doi: 10.3748/wjg.v21.i41.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal RJ, Diaz AA, Arvidsson D, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis. 2012;8:8–19. doi: 10.1016/j.soard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015;29:3608–17. doi: 10.1007/s00464-015-4117-x. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S, Sharma AP, Ramaswamy N. Outcome of laparoscopic sleeve gastrectomy with and without staple line oversewing in morbidly obese patients: a randomized study. J Laparoendosc Adv Surg Tech A. 2013;23:895–9. doi: 10.1089/lap.2013.0137. [DOI] [PubMed] [Google Scholar]
- 6.Csendes A, Braghetto I, Leon P, Burgos AM. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg. 2010;14:1343–8. doi: 10.1007/s11605-010-1249-0. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matłok M, Pędziwiatr M, Major P, et al. One hundred seventy-nine consecutive bariatric operations after introduction of protocol inspired by the principles of enhanced recovery after surgery (ERAS®) in bariatric surgery. Med Sci Monit. 2015;21:791–7. doi: 10.12659/MSM.893297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Małczak P, Pisarska M, Piotr M, et al. Enhanced recovery after bariatric surgery: systematic review and meta-analysis. Obes Surg. 2017;27:226–35. doi: 10.1007/s11695-016-2438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janik MR, Stanowski E, Paśnik K. Present status of bariatric surgery in Poland. Videosurgery Miniinv. 2016;11:22–5. doi: 10.5114/wiitm.2016.58742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 12.Major P, Wysocki M, Pędziwiatr M, et al. Can the Obesity Surgery Mortality Risk Score predict postoperative complications other than mortality? Videosurgery Miniinv. 2016;11:247–52. doi: 10.5114/wiitm.2016.64448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawada K, Hasegawa S, Hida K, et al. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc. 2014;28:2988–95. doi: 10.1007/s00464-014-3564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Sugito M, Kobayashi A, et al. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis. 2008;23:703–7. doi: 10.1007/s00384-008-0470-8. [DOI] [PubMed] [Google Scholar]
- 15.Ghavidel A. Migration of clips after laparoscopic cholecystectomy; a case report and literature review. Middle East J Dig Dis. 2015;7:45–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Brutvan FM, Kampschroer BH, Parker HW. Vessel clip as a nidus for formation of common bile duct stone. Gastrointest Endosc. 1982;28:222–3. doi: 10.1016/s0016-5107(82)73080-9. [DOI] [PubMed] [Google Scholar]
- 17.Cetta F, Baldi C, Lombardo F, et al. Migration of metallic clips used during laparoscopic cholecystectomy and formation of gallstones around them: surgical implications from a prospective study. J Laparoendosc Adv Surg Tech A. 1997;7:37–46. doi: 10.1089/lap.1997.7.37. [DOI] [PubMed] [Google Scholar]
- 18.Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258:690–4. doi: 10.1097/SLA.0b013e3182a67426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanni A, Perez S, Medbery R, et al. Postoperative complications in bariatric surgery using age and BMI stratification: a study using ACS-NSQIP data. Surg Endosc. 2014;28:3302–9. doi: 10.1007/s00464-014-3606-7. [DOI] [PubMed] [Google Scholar]
- 20.Luppi CR, Balague C, Targarona EM, et al. Laparoscopic sleeve gastrectomy in patients over 60 years: impact of age on weight loss and co-morbidity improvement. Surg Obes Relat Dis. 2015;11:296–301. doi: 10.1016/j.soard.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5:469–75. doi: 10.1016/j.soard.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509–15. doi: 10.1007/s00464-011-2085-3. [DOI] [PubMed] [Google Scholar]
- 23.Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240–5. doi: 10.1007/s00464-012-2426-x. [DOI] [PubMed] [Google Scholar]
- 24.Kasalicky M, Dolezel R, Vernerova E, Haluzik M. Laparoscopic sleeve gastrectomy without over-sewing of the staple line is effective and safe. Videosurgery Miniinv. 2014;9:46–52. doi: 10.5114/wiitm.2014.40387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014;10:713–23. doi: 10.1016/j.soard.2014.01.016. [DOI] [PubMed] [Google Scholar]