Abstract
Introduction
Implementation of the laparoscopic approach in colorectal surgery has not happened as rapidly as in cholecystectomy, because of concerns about oncological safety. The results of controlled trials in multiple centers showed the method to be safe. Consequently, surgeons decided to try the approach with colorectal surgery. This process, in our clinic, began in earnest about four years ago.
Aim
To analyze and present the clinical outcomes of applying the laparoscopic approach to colorectal surgery in a single center.
Material and methods
We retrospectively identified patients from a hospital database who underwent colorectal surgery – laparoscopic and open – between 2013 and 2016. Our focus was on laparoscopic cases. Study points included operative time, duration of the hospital stay, postoperative mortality and rates of complications, conversion, reoperation and readmission.
Results
Of 534 cases considered, the results showed that the relation between open and laparoscopic procedures had reversed, in favor of the latter method (2013: open: 82% vs. laparoscopic: 18%; 2016: open: 22.4% vs. laparoscopic: 77.6%). The most commonly performed procedure was right hemicolectomy. The total complication rate was 22%. The total rate of conversion to open surgery was 9.3%. The postoperative mortality rate was 3%.
Conclusions
Use of the laparoscopic approach in colorectal surgery has increased in recent years world-wide – including in Poland – but the technique is still underused. Rapid implementation of the miniinvasive method in colorectal surgery, in centers with previous laparoscopic experience, is not only safe and feasible, but also highly recommended.
Keywords: laparoscopy, colorectal surgery, implementation, outcomes, miniinvasive surgery
Introduction
Laparoscopy is not applied as widely to colorectal surgery in Poland as might be expected, because of lingering concern about oncological safety, which are no longer valid.
Implementation and rapid adoption of the laparoscopic approach in cholecystectomy revolutionized this surgical procedure and had an influence on other areas of surgery. Clearly, laparoscopic cholecystectomy has been shown to have significant benefits, such as faster recovery, limited operative trauma, and reduced postoperative pain and length of hospital stay. These factors led to general acceptance of the laparoscopic method as an alternative to conventional open surgery in cholecystectomy [1, 2]. There has been nothing to suggest that colorectal surgery would not see the same benefits from the laparoscopic method.
With the implementation of the laparoscopic approach in colorectal surgery there is a chance that we will see an increase in the instances of some undesirable outcomes. Those increases would be short term, though. Long term, we would actually see decreases, where operative time and complication rate are concerned [3]. Work that has been done at our clinic substantiates these assumptions.
The first reports of laparoscopic colon resection in the world were published in 1991, with the procedures being performed independently by Jacobs and Fowler [4–8]. The first colorectal resection in our clinic – sigmoidectomy – was successfully performed by Prof. Stanowski in 1993 [6]. Around that time and before, due to a lack of adequate equipment and because the procedure itself was seen as very convoluted and technically difficult, surgeons eschewed colorectal resections. One of the major reasons for slow implementation of laparoscopy in colorectal surgery, especially in Poland, was higher costs compared to those of open approach [9]. Early case reports showing a high number of abdominal port-site recurrences did not help [8, 10].
Due to safety concerns the miniinvasive technique was more voluntarily used in benign colorectal diseases [11]. Over time, the results of randomized controlled trials performed at multiple centers showed the laparoscopic method to be at least comparable to the open one in terms of oncologic safety. Furthermore, laparoscopy might even have some additional advantages over the latter method [12–15].
Surgeons from our clinic, encouraged by these reports and having acquired greater facility with laparoscopy, decided to implement this method in colorectal surgery. This process began in earnest about four years ago.
Aim
In this study we aim to analyze and present the clinical outcomes of applying the laparoscopic approach in colorectal surgery.
Material and methods
Study design
We retrospectively identified patients from a hospital database, who underwent colorectal surgery – laparoscopic and open – between 1st January 2013 and 31st December 2016, in our department. Institutional review board approvals were obtained.
Our focus was on laparoscopic cases. Three senior surgeons performed 267 (94%) of these procedures. The remaining cases were seen as outliers, and therefore we excluded them from our study. Eight cases, due to unresectable malignancy, ended with ostomy being employed. Consequently, we also did not take these cases into consideration. There were no selection criteria for the laparoscopic approach. Application of the miniinvasive approach was based on the surgeon’s experience, as well as access to an operating theater and essential instruments.
A perioperative study was conducted in patients with tumor pathology, which included colonoscopies with biopsy, computed tomography (CT) scan of the abdomen and pelvis, X-ray of the chest and a blood test for tumor markers.
The operations were performed under general anesthesia with endotracheal intubation following preoperative antibiotic and antithrombotic prophylaxis and bladder catheterization. All the patients underwent mechanical bowel preparation.
Study variables
Study variables were analyzed in each case together with patients’ demographic variables. The following baseline variables were collected: gender, age, body mass index (BMI), American Society of Anesthesiologist score, diagnostic group and previous abdominal surgery.
Surgical variables include the following: type of resection (right hemicolectomy, left hemicolectomy, sigmoidectomy, rectal resection, colectomy and other), operative time, rate of conversion, type of pathology (malignant, benign, i.e. polyps, diverticulitis, inflammatory bowel disease and other), length of hospital stay, postoperative mortality and rates of complications (bleeding, anastomotic leak, abdominal access and others), conversion, reoperation and readmission within 30 days. The anatomopathological variables include TNM staging, G-staging.
Highly skilled surgical oncologists – each with at least 12 years of experience in surgery, post-residency, and 10 years of experience in laparoscopy – performed all of the analyzed procedures.
Results
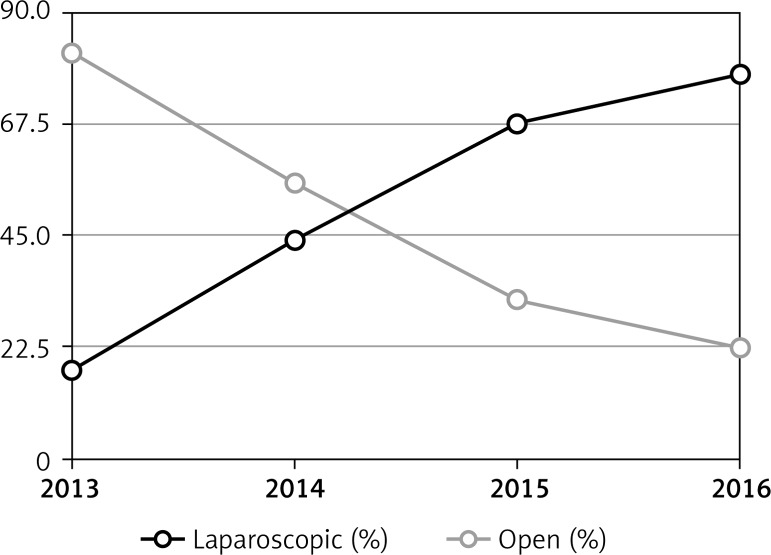
Five hundred and thirty-four patients were included in the study (284 via laparoscopic approach; 250 via open approach). Figure 1 illustrates the change in trend that occurred between laparoscopic and open colorectal resections between 2013 and 2016. When the first laparoscopic procedures were performed in 2013, open procedures were the majority (open: 82% vs. laparoscopic: 18%), but by 2016 those percentages had reversed (open: 22.4% vs. laparoscopic: 77.6%).
Figure 1.
Laparoscopic versus open colorectal resections 2013–2016
Two hundred fifty-nine patients receiving laparoscopic approach were analyzed. The base variables are shown in Table I. The vast majority of patients had comorbidities (85.9%), and the majority were over 65 years old. One hundred (38.6%) patients had previously undergone abdominal procedures, of which 16% were colorectal resections. The procedures were performed due to malignancy in 218 (84.2%) cases (1 case of sarcoma), benign lesion, i.e. diverticulitis and polyps in 35 (13.5%) cases, inflammatory bowel disease in 2 cases (0.8%) and other pathologies in 4 (1.5%) cases. Surgical variables are shown in Table II.
Table I.
Patients’ demographic characteristics
| Parameter | N (%) |
|---|---|
| Gender: | |
| Female | 101 (39) |
| Male | 158 (61) |
| Age group [years]: | |
| < 35 | 6 (2.3) |
| 35–50 | 29 (11.2) |
| 51–65 | 86 (33.2) |
| ≥ 65 | 138 (53.3) |
| BMI [kg/m2]: | |
| < 18.5 | 6 (2.3) |
| 18.5–25 | 92 (35.9) |
| 26–30 | 101 (39.5) |
| ≥ 30 | 57 (22.3) |
| ASA: | |
| I | 36 (14.1) |
| II | 157 (61.3) |
| III | 61 (23.8) |
| IV | 2 (0.8) |
| Diagnostic group: | |
| Benign | 35 (13.5) |
| Malignant | 218 (84.2) |
| IBD | 2 (0.8) |
| Other | 4 (1.5) |
| Previous surgery: | |
| Abdominal | 100 (38.6) |
| Colorectal | 16 (16) |
| Comorbidities | 220 (85.9) |
Table II.
Surgical variables
| Parameter | Value |
|---|---|
| Number of patients | 259 |
| Indication for surgery | |
| Malignant/benign pathology, n (%) | 218 (84.2)/41 (15.8) |
| Type of resection, n (%): | |
| Right hemicolectomy | 93 (35.9) |
| Left hemicolectomy | 25 (9.7) |
| Sigmoidectomy | 68 (26.3) |
| Rectal resection | 63 (24.3) |
| Colectomy | 6 (2.3) |
| Others | 4 (1.5) |
| Operative time, mean (range) [min] | 151 (30–150) |
| Rate of conversion, n (%) | 24 (9.3) |
| Rate of complication, n (%) | 57 (22) |
| Rate of reoperations, n (%) | 34 (13.1) |
| Morbidity, n (%) | 8 (3) |
Right-sided resections constituted the majority, with sigmoidectomy being the second most common procedure and rectal resection the third. In the four analyzed types of procedure, the mean operative time was 151 min (range: 30–350 min). Mean operative times, more specifically, were as follows: right hemicolectomy, 133 min (range: 30–350 min); left hemicolectomy, 170 min (range: 60–290 min); sigmoidectomy, 137 min (range: 50–295 min); rectal resection, 168 min (range: 70–335 min).
The total complication rate was 22%. Complications that occurred most frequently, n = 33 (12.7%) were what we described as ‘others’, which for the most part were instances of post-operative wound infection, ileus and bowel obstruction, dehiscence, and strangulation of inguinal hernia. Anastomotic leak was the second most common complication, n = 16 (6.2%), followed by bleeding, n = 6 (2.3%), and abdominal abscess, n = 2 (0.8%). Not all of the complications required reoperation, although reoperation was needed in 34 (13.1%) cases. The total rate of conversion to open surgery was 9.3%. Reasons for conversion included poor views, tumor infiltration into the surrounding tissue and adhesions. The number of readmissions within 30 days was 8 (3.1%), due to wound infection or ileus and small bowel obstruction. The mean duration of the hospital stay was 8 days (range: 2–103 days). The postoperative mortality rate was 3% (n = 8). Two patients died due to complications of freeing massive adhesions of the small bowel resulting in enterocutaneous fistula, 2 due to dehiscence of anastomosis, and the rest were associated with patients’ comorbidities following intraoperative bleeding (1 case) or bleeding from anastomosis (2 cases).
The anatomopathological variables in malignant disease according to the AJCC 7th edition TNM staging system are shown in Table III.
Table III.
Anatomopathological variables in malignant disease according to AJCC 7th edition TNM staging system (number of patients = 217)
| Variable | N (%) |
|---|---|
| pT: | |
| pTx | 1 (0.5) |
| pTis | 0 |
| pT0 | 0 |
| pT1 | 10 (4.6) |
| pT2 | 33 (15.2) |
| pT3 | 154 (71) |
| pT4a | 12 (5.5) |
| pT4b | 7 (3.2) |
| pN: | |
| pNx | 3 (1.4) |
| pN0 | 120 (55.3) |
| pN1a | 23 (10.6) |
| pN1b | 20 (9.2) |
| pN1c | 14 (6.5) |
| pN2a | 20 (9.2) |
| pN2b | 17 (7.8) |
| pM: | |
| pMx | |
| pM0 | 177 (81.6) |
| pM1a | 30 (13.8) |
| pM1b | 10 (4.6) |
| G: | |
| Gx | 0 |
| G1 | 12 (5.5) |
| G2 | 193 (88.9) |
| G3 | 11 (5.1) |
| G4 | 1 (0.5) |
Discussion
The impact of laparoscopy on long-term oncological treatment results was a subject of controversy for many years due to port-site metastases and concerns regarding the lower number of lymph nodes retrieved [16]. The high number of metastases in the abdominal wall, especially in trocar wounds – described in some of the first publications concerning laparoscopic colorectal surgery – caused widespread concerns regarding the safety of this approach. Some reports suggested a 10–20% risk of port-site metastases and peritoneal dissemination [17–19]. To further investigate the cause of these complications, factors related to laparoscopy, the patient and the tumor were analyzed in experimental studies [20, 21]. The so-called ‘chimney effect’, the leakage of CO2 alongside trocars through the trocar wound and aerosolization of tumor cells – these were among the main factors that contributed to the occurrence of unfavorable postoperative complications [22].
Based on these data, some changes in the laparoscopic technique were proposed to avoid, or at least reduce, the risk of port-site metastases [23, 24]. These recommendations included: emptying the CO2 through trocars, use of the ‘no touch technique’, i.e. avoiding touching or manipulating the tumor, protecting the wall incision with a special device, and closing the main vessels running to the tumor.
However, despite these initial concerns about oncological safety, well-designed, prospective, randomized, multi-centered trials, that compared mini-invasive and open approaches, have demonstrated no differences in the incidence of metastases in the surgical wound or in the oncological outcomes between these two types of procedures [10, 25–27].
Use of laparoscopy, in management of colorectal malignancies, is currently accepted worldwide [28]. Although use of the mini-invasive approach in colorectal surgery has been increasing in recent years, the percentage of patients who undergo laparoscopic surgery is still limited and there are also significant differences among centers [29, 30].
In our study we analyzed colorectal resections performed in our center between January 2013 and December 2016. Out of 534 patients who underwent major colorectal resection, 18% and 77.6% underwent mini-invasive surgery in 2013 and 2016, respectively, with a conversion rate of 9.3%.
In recent years, there have been several reports on implementation of laparoscopy in colorectal surgery. The Norwegian Colorectal Cancer Registry evaluated the use of laparoscopy for all colon cancer resections performed in 2007–2010. Out of 8707 patients with colon cancer who underwent major resections, 16% and 36% received laparoscopic treatment in 2007 and 2010, respectively. The conversion rate of laparoscopic procedures was 14.5% [31].
The Surgical Care and Outcomes Assessment Program analyzed the use of laparoscopy for elective colorectal resection at 48 hospitals in the United States from 2005 to 2010. The use of laparoscopic procedures increased from 23.3% in 2005 to 41.6% in 2010 [32].
In another recent study, using the University Health System Consortium administrative database – which included more than 300 academic hospitals and consisted of 85,712 patients who underwent colon resections between October 2008 and December 2011 – the mini-invasive approach was attempted in 36,228 (42.2%) patients, with 5751 (15.8%) patients requiring conversion to an open procedure. There was a trend toward increasing utilization of the mini-invasive approach from 37.5% in 2008 to 44.1% in 2011 [33].
The low rate of conversion in our data could be biased by the high percentage of right-sided lesions and proportionally lower percentage of rectal resection, which are the most demanding cases. Where achievement of nearly 80% employment of the laparoscopic approach in colorectal surgery is concerned, the determining factor may have been the 3 surgeons who performed the operations. Their work greatly shortened the learning curve and improved results.
Conclusions
Although use of the laparoscopic approach in colorectal surgery has increased in recent years worldwide – including in Poland – several studies have shown that mini-invasive techniques are still underused and there are also great differences among centers. In our opinion, fast implementation of laparoscopy in colorectal surgery, in centers with previous laparoscopic experience, is safe and feasible. A high volume of cases per surgeon is an important factor for shortening the learning curve and improving the outcomes.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kwon S, Billingham R, Farrokhi E, et al. Adoption of laparoscopy for elective colorectal resection: a report from the Surgical Care and Outcomes Assessment Program. J Am Coll Surg. 2012;214:909–18. doi: 10.1016/j.jamcollsurg.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascual M, Salvans S, Pera M. Laparoscopic colorectal surgery: current status and implementation of the latest technological innovations. World J Gastroenterol. 2016;22:704–17. doi: 10.3748/wjg.v22.i2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piątkowski J, Jackowski M. Laparoscopic colon resections – own experience report. Videosurgery Miniinv. 2009;4:135–7. [Google Scholar]
- 4.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection laparoscopic colectomy. Surg Laparosc Endosc. 1991;1:144–50. [PubMed] [Google Scholar]
- 5.Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Stanek A. Rys historyczny chirurgii laparoskopowej w Polsce. In: Kostewicz W, editor. Chirurgia laparoskopowa. Vol. 1. Warsaw: Wydawnictwo Lekarskie PZWL; 2002. pp. 33–42. [Google Scholar]
- 7.Leszczyszyn J. Chirurgia laparoskopowa jelita grubego. In: Kostewicz W, editor. Chirurgia laparoskopowa. Vol. 1. Warsaw: Wydawnictwo Lekarskie PZWL; 2002. pp. 355–67. [Google Scholar]
- 8.Blackmore AE, Wong MT, Tang CL. Evolution of laparoscopy in colorectal surgery: an evidence-based review. World J Gastroenterol. 2014;20:4926–33. doi: 10.3748/wjg.v20.i17.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pędziwiatr M, Wierdak M, Nowakowski M, et al. Cost minimization analysis of laparoscopic surgery for colorectal cancer within the enhanced recovery after surgery (ERAS) protocol: a single-center, case-matched study. Videosurgery Miniinv. 2016;11:14–21. doi: 10.5114/wiitm.2016.58617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo J. Laparoscopic colorectal surgery. Perm J. 2008;12:27–31. doi: 10.7812/tpp/07-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulskas A, Samalavicius NE, Gupta RK, Zabulis V. Laparoscopic colorectal surgery for colorectal polyps: single institution experience. Videosurgery Miniinv. 2015;10:73–8. doi: 10.5114/wiitm.2015.49752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopic-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 13.Clinical Outcomes of Surgical Therapy Study Group A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 14.Bonjer HJ, Hop WC, Nelson H, et al. Laparoscopically assisted vs. open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007;142:298–303. doi: 10.1001/archsurg.142.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Skrovina M, Czudek S, Bartos J, et al. Colorectal cancer complications of laparoscopic resection. Videosurgery Miniinv. 2006;1:142–9. [Google Scholar]
- 16.Martinez J, Targarona EM, Balagué C, et al. Port site metastasis. An unresolved problem in laparoscopic surgery. A review. Int Surg. 1995;80:315–21. [PubMed] [Google Scholar]
- 17.Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet. 1994;344:58. doi: 10.1016/s0140-6736(94)91079-0. [DOI] [PubMed] [Google Scholar]
- 18.Lacy AM, Delgado S, García-Valdecasas JC, et al. Port site metastases and recurrence after laparoscopic colectomy. A randomized trial. Surg Endosc. 1998;12:1039–42. doi: 10.1007/s004649900776. [DOI] [PubMed] [Google Scholar]
- 19.Vukasin P, Ortega AE, Greene FL, et al. Wound recurrence following laparoscopic colon cancer resection. Results of the American Society of Colon and Rectal Surgeons Laparoscopic Registry. Dis Colon Rectum. 1996;39:S20–3. doi: 10.1007/BF02053801. [DOI] [PubMed] [Google Scholar]
- 20.Bouvy ND, Marquet RL, Jeekel H, Bonjer HJ. Impact of gas(less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg. 1996;224:694–700. doi: 10.1097/00000658-199612000-00005. discussion 700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson DI, Mathew G, Ellis T, et al. Gasless laparoscopy may reduce the risk of port-site metastases following laparoscopic tumor surgery. Arch Surg. 1997;132:166–8. doi: 10.1001/archsurg.1997.01430260064014. discussion 169. [DOI] [PubMed] [Google Scholar]
- 22.Whelan RL, Lee SW. Review of investigations regarding the etiology of port site tumor recurrence. J Laparoendosc Adv Surg Tech A. 1999;9:1–16. doi: 10.1089/lap.1999.9.1. [DOI] [PubMed] [Google Scholar]
- 23.Franklin ME, Rosenthal D, Abrego-Medina D, et al. Prospective comparison of open vs. laparoscopic colon surgery for carcinoma. Five-year results. Dis Colon Rectum. 1996;39:S35–46. doi: 10.1007/BF02053804. [DOI] [PubMed] [Google Scholar]
- 24.Lacy AM, García-Valdecasas JC, Piqué JM, et al. Short-term outcome analysis of a randomized study comparing laparoscopic vs open colectomy for colon cancer. Surg Endosc. 1995;9:1101–5. doi: 10.1007/BF00188996. [DOI] [PubMed] [Google Scholar]
- 25.Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 26.Hazebroek EJ; Color Study Group COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc. 2002;16:949–53. doi: 10.1007/s00464-001-8165-z. [DOI] [PubMed] [Google Scholar]
- 27.Jacob BP, Salky B. Laparoscopic colectomy for colon adenocarcinoma: an 11-year retrospective review with 5-year survival rates. Surg Endosc. 2005;19:643–9. doi: 10.1007/s00464-004-8921-y. [DOI] [PubMed] [Google Scholar]
- 28.Pascual M, Salvans S, Pera M. Laparoscopic colorectal surgery: current status and implementation of the latest technological innovations. World J Gastroenterol. 2016;22:704–17. doi: 10.3748/wjg.v22.i2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reames BN, Sheetz KH, Waits SA, Dimick JB, Regenbogen SE. Geographic variation in use of laparoscopic colectomy for colon cancer. J Clin Oncol. 2014;32:3667–72. doi: 10.1200/JCO.2014.57.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo H, Niland J, Milne D, et al. Incidence of minimally invasive colorectal cancer surgery at National Comprehensive Cancer Network centers. J Natl Cancer Inst. 2015;107:362. doi: 10.1093/jnci/dju362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stormark K, Søreide K, Søreide JA, et al. Nationwide implementation of laparoscopic surgery for colon cancer: short-term outcomes and long-term survival in a population-based cohort. Surg Endosc. 2016;301:4853–64. doi: 10.1007/s00464-016-4819-8. [DOI] [PubMed] [Google Scholar]
- 32.Surgical Care and Outcomes Assessment Program (SCOAP) Collaborative. Kwon S, Billingham R, Farrokhi E, et al. Adoption of laparoscopy for elective colorectal resection: a report from the Surgical Care and Outcomes Assessment Program. J Am Coll Surg. 2012;214:909–18.e1. doi: 10.1016/j.jamcollsurg.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simorov A, Shaligram A, Shostrom V, et al. Laparoscopic colon resection trends in utilization and rate of conversion to open procedure: a national database review of academic medical centers. Ann Surg. 2012;256:462–8. doi: 10.1097/SLA.0b013e3182657ec5. [DOI] [PubMed] [Google Scholar]