Abstract
Objective
The comparative safety of immunosuppressive drugs, biologicals and glucocorticoids (GC) for patients with SLE remains controversial. We aimed to investigate the specific side effects of the available SLE drugs in this population of patients.
Methods
Electronic databases were systematically searched through September 2017 for randomised trials in patients with SLE. The primary outcomes were all-cause mortality and withdrawal related to adverse events (AEs). We performed a random-effects network meta-analysis to obtain estimates for primary and secondary outcomes and presented these estimates as ORs with 95% CIs.
Results
Forty-four studies comprising 9898 participants were included in the network meta-analysis. No drug regimen was considered to be safer for reducing all-cause mortality. However, compared with cyclophosphamide, azathioprine (OR 3.04, 95% CI (1.44 to 6.42)) and cyclosporine (OR 3.28, 95% CI (1.04 to 10.35)) were significantly less safety in AE-related withdrawals, and GC was ranked lowest and led to higher withdrawal rates. Tacrolimus (TAC) was ranked high and showed a benefit in many outcomes. Biologicals and chloroquine also showed good safety in all of the available outcomes, while the beneficial effects of other immunosuppressive drugs were not substantial in different types of serious adverse events.
Conclusions
TAC is the safest strategy for patients with SLE. Biologicals and chloroquine are also fairly safe for patients with SLE. The use of other immunosuppressive drugs and GC needs to be balanced against the potential harms of different types of AEs, and the practical safety of drug combinations still requires further trials to evaluate.
Keywords: systemic lupus erythematosus, dmards (biologic), treatment, safety, adverse events
Introduction
SLE is known as an autoimmune disease with complex pathogenic mechanisms that always lead to multisystem damage; the long duration of use of immunosuppressive drugs and glucocorticoids (GCs) increases the risk of premature death.1–3
During the treatment, nearly all patients report one or more adverse events (AEs), and these AEs shape doctors’ preferences, especially when two drugs are considered to be equivalent. Serious AEs (SAEs) refer to events that result in death, are life threatening, require inpatient hospitalisation or cause prolongation of existing hospitalisation, result in persistent or significant disability/incapacity, lead to a congenital anomaly/birth defect or that require intervention to prevent permanent impairment or damage; these effects can directly demonstrate the safety of available drugs in different aspects, eliminating the interference of more mild AEs. However, mainly because of an absence of head-to-head trials and SAE data, the comparative safety is largely unknown.
In a network meta-analysis published in 2017, researchers found that, in patients with proliferative lupus nephritis, mycophenolate mofetil (MMF) combined with calcineurin inhibitor therapy was less likely to cause ovarian failure, while the regimens generally had similar odds of major infection.4 Another network meta-analysis published in 2016 showed that tacrolimus (TAC) compared with other agents can reduce the risk of serious infection in lupus nephritis.5 Limited by the differing inclusion criteria, the divergence between these two studies cannot be ignored. To obtain an impartial comparison of the important AEs among the available drugs for all patients with SLE, the aim of our study was to assess the comparative effects of all available effective agents in patients with SLE using network meta-analysis.
Materials and methods
Search strategy
A systematic search of the scientific literature was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis. The searches included PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL) and Embase (from their inception to September 2017) using a combination of keywords and search strategies with Medical Subject Headings (online supplementary appendix 1). The search included only randomised controlled trials (RCTs) that reported the outcomes of interest. Additionally, reference lists from trials, review articles and reports were manually scanned to identify any other eligible studies.
lupus-2017-000253supp001.doc (3.7MB, doc)
Study selection
To make a comprehensive evaluation of the risk of AEs with agents used to treat SLE, our exposure of interest was treatment with immunosuppressants or biologicals (we only included rituximab (RTX) and belimumab due to their validated efficacy) or GC. Patients who met the 1987 American College of Rheumatology Classification criteria for SLE were included. Our primary outcomes were all-cause mortality and AE-related withdrawals. Secondary outcomes included AEs, SAEs, cardiovascular events (CVEs) (acute coronary syndrome, chronic ischaemic heart disease, coronary revascularisation, cardiovascular disease (CVD) death, cerebrovascular events or peripheral vascular events), serious infections (serious infection, major infection, severe infection, sepsis, cardiovascular infection or bacterial pneumonia), bone toxicity (avascular necrosis or fracture), malignant transformation, serious gastrointestinal events (leading to dose reduction or withdrawal), ovarian failure (sustained amenorrhea), menstrual disorder, new-onset hypertension, serious leucopenia (white cell count <2×109 L leading to dose reduction or withdrawal), leucopenia and hyperglycaemia (hyperglycaemia or new-onset diabetes).
Duplicate reports, studies that did not report on the outcomes of interest or in which all arms had 0 events, studies that lasted 24 weeks or less, studies that included children younger than 10 years old or women during pregnancy or lactation and studies that included fewer than 20 patients. All lupus nephritis diagnoses should have been confirmed by biopsy. We also excluded scientific reports that presented pooled trial data for which the individual trials could not be identified to prevent double counting.
Screening and data extraction
Standardised data forms and data extraction training exercises were developed to achieve a high level of consensus between reviewers. Two reviewers independently assessed the full text of the articles to confirm their eligibility. Any disagreement was resolved by consensus discussion.6 7 All studies included were listed in online supplementary appendix 2.
Assessment of methodology quality
Two authors evaluated the eligible studies from seven domains in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews (online supplementary appendix 3).
Statistical analysis
First, we conducted pairwise meta-analyses using the random-effects model.8 All results are expressed as ORs with 95% CIs. The heterogeneity of the data was assessed using the I2 test, with a p<0.05 indicating significant heterogeneity.
We then performed network meta-analyses to obtain estimates for primary and secondary outcomes and presented these estimates as ORs with 95% CIs.9 Network meta-analyses assumes transitivity, which means one can learn about treatment A versus treatment B via treatment C (eg, learning about cyclophosphamide (CYC) vs TAC) via MMF). We implemented network meta-regression within the frequentist framework for outcomes to evaluate the assumptions in the studies and provide graphical representation of the results.
We investigated the extent of heterogeneity in every network by comparing the magnitude of τ for the network with an empirical distribution of heterogeneity variances specific to the types of outcomes and treatments being compared.10 Values lower than 0.1 were considered low, outcomes from 0.1 to 1.0 were considered moderate and outcomes higher than 1.0 represented high heterogeneity.
Disagreement between direct and indirect evidence can suggest that the transitivity assumption might not hold. We used a loop-specific approach to investigate the consistency within every closed triangular or quadratic loop in every network as the difference between direct and indirect estimates for a specific treatment comparison (RoR - ratio of odds ratio) in the loop.11 12 We identified inconsistent loops as those yielding a 95% CI excluding 1. This approach can be easily applied and indicate loops with large inconsistency but cannot infer consistency of the entire network or identify the particular comparison that is problematic. To check the assumption of consistency in the entire network, we then used the design-by-treatment interaction model that provides a single inference, and the χ² tests were adopted.13 For the generalisability of the findings, sensitivity analyses were then assessed by restricting analyses to studies with the following design characteristics: patients with lupus nephritis, follow-up longer than 24 months and without the use of biological agents.
To rank the treatments for an outcome, we calculated different ranks possibility of each agent, and then reported probabilities for all ranks and created a line graph showing cumulative ranks. In the line graph, each column represented a treatment, and different safety ranks were represented by different colours; the percentage of a colour was corresponding to the possibility of certain rank. The default was set to report only the probabilities of being the treatment with minimum frequency in the outcome, so the treatment that had the largest proportion of first rank colour in the graph indicated the safest treatment for the outcome. We also used forest plots to obtain an intuitive and full comparison of the safety of these agents.14 15
Results
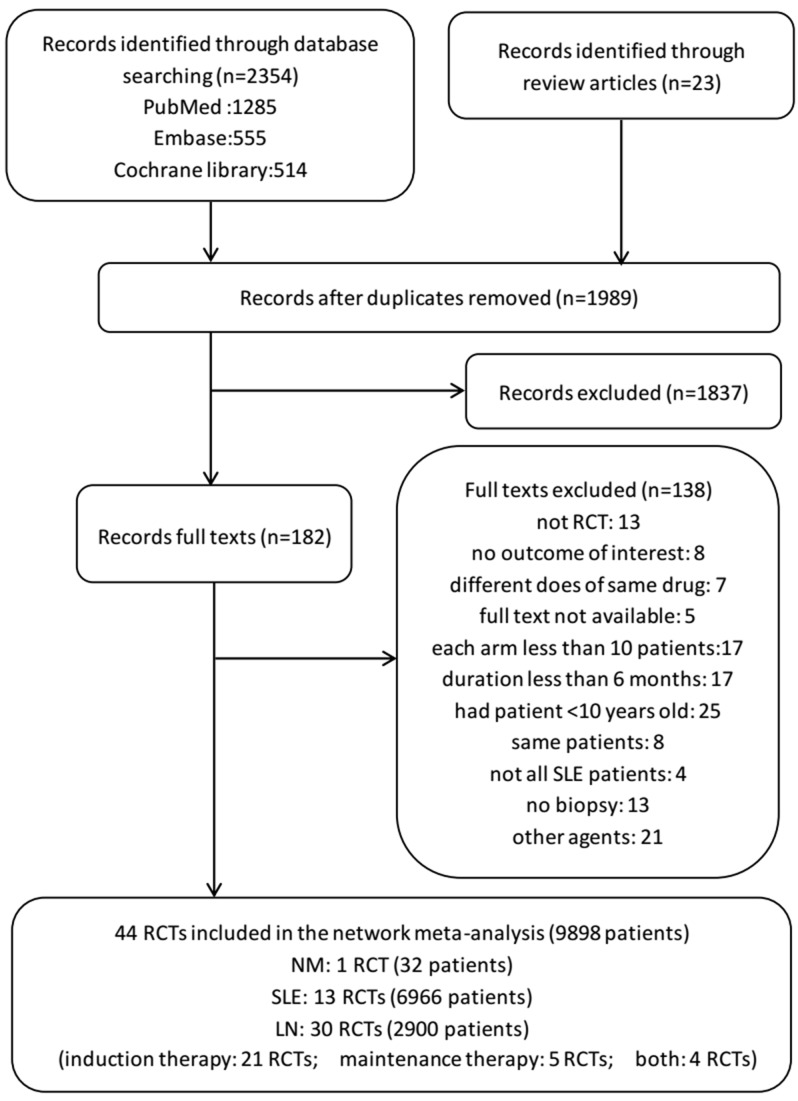
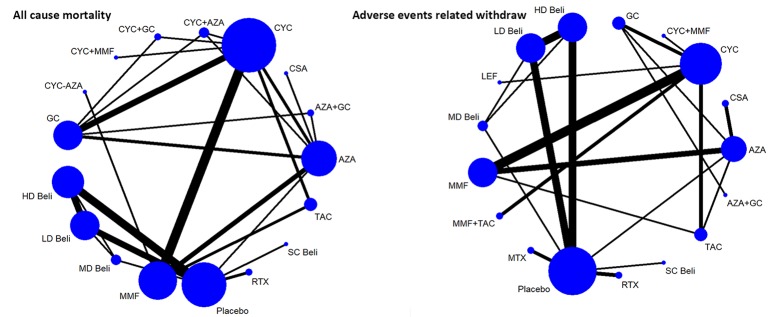
A total of 2377 relevant articles were searched, and 44 studies with 9898 patients were finally identified and included.16–59 Of the RCTs included in the systematic review, nine were three-arm trials, one was a four-arm trial and one was a five-arm trial. The selection process, reasons for exclusion and information for interventions are detailed in figure 1. Networks of eligible comparisons for the primary outcomes are presented in figure 2.
Figure 1.
Summary of evidence search and selection. A total of 19 immunosuppressants or biologicals alone or in combination were involved in our analyses: intravenous CYC (0.5–1 g/m2 body surface area monthly) (21 trials), AZA (1–4 mg/kg/day) (12 trials), MMF (500–3000 mg/day) (12 trials), TAC (0.05–0.1 mg/kg/day) (four trials), oral CYC (1–4 mg/kg/day) (two trials), CSA (1–5 mg/kg/day) (five trials), MTX (7.5–20 mg/week) (three trials), RTX (1 g/day) (two trials), LD belimumab (1 mg/kg) (four trials), MD belimumab (4 mg/kg) (one trial), HD belimumab (10 mg/kg) (five trials), SC belimumab (200 mg/week) (one trial), LEF (1 mg/kg/day) (one trial), chloroquine (150 mg/day) (one trial), AZA+GC (one trial), MMF+TAC (two trials), intravenous CYC+MMF (one trial), CYC-AZA (two trials) and AZA+CYC (one trial). AZA, azathioprine; CSA, cyclosporine; CYC-AZA, CYC followed by AZA; CYC, cyclophosphamide; GC, glucocorticoid; HD, high dose; LD, low dose; LEF, leflunomide; MD, moderate dose; MMF, mycophenolate mofetil; MTX, methotrexate; RCT, randomised controlled trial; RTX, rituximab; SC, subcutaneous; TAC, tacrolimus, NM, neurological manifestations; LN, lupus nephritis
Figure 2.
Networks of treatment comparisons for primary outcomes of SLE agents in patients with SLE. The size of the nodes (blue circles) corresponds to the number of trials of the treatments. Comparisons are linked with a line, the thickness of which corresponds to the number of trials that assessed the comparison. +, combined with; AZA, azathioprine; Beli, belimumab; CSA, cyclosporine; CYC-AZA, CYC followed by AZA; CYC, cyclophosphamide; GC, glucocorticoid; HD, high dose; LD, low dose; LEF, leflunomide; MD, moderate dose; MMF, mycophenolate mofetil; MTX, methotrexate; RTX, rituximab; SC, subcutaneous; TAC, tacrolimus.
Risk of bias
The risk of bias in studies contributing to the primary outcomes was generally low, suggesting no evidence of small-study effects in the network. Moreover, in the risk of bias summary graph, the risk of bias was determined to be low for most criteria and unclear for some criteria (online supplementary appendix 3).
Heterogeneity and inconsistency
In pairwise comparisons of the primary outcomes, no evidence of statistical heterogeneity was seen in general (online supplementary appendix 5). In the network meta-analyses, statistical heterogeneity was low in most networks, moderate in networks for serious gastrointestinal events and ovarian failure and substantial in networks for AEs and new-onset hypertension. Treatment estimates from direct and indirect evidence in general did not show evidence of statistical inconsistency except for one loop of evidence for AEs (CYC–MMF–TAC). Global inconsistency was not noted within any network except for AEs (online supplementary appendix 6).
Outcomes
Data for direct comparisons and network estimates for statistically significant outcomes are shown in table 1, and complete outcomes are listed in online supplementary appendix 5 and 7.
Table 1.
Results from pairwise meta-analysis and network meta-analysis ORs (and 95% CI) for statistically significant outcomes
| Comparisons | Direct drug comparisons/participants (n/N) | Pairwise meta-analysis | Network meta-analysis | |
| Adverse events-related withdraw | ||||
| AZA versus | MMF | 3/572 | 2.13 (1.30 to 3.47) | 2.08 (1.29 to 3.36) |
| CYC | 3.04 (1.44 to 6.42) | |||
| TAC | 3.63 (1.05 to 12.61) | |||
| CSA versus | CYC | 3.28 (1.04 to 10.35) | ||
| Adverse events | ||||
| TAC versuss | CYC | 1/40 | 0.03 (0.00 to 0.56) | |
| AZA versus | GC | 1/28 | 0.06 (0.01 to 0.61) | |
| Chloroquine versus | MTX | 1/37 | 0.09 (0.02 to 0.44) | 0.09 (0.02 to 0.44) |
| Placebo | 0.16 (0.03 to 0.96) | |||
| RTX | 0.10 (0.02 to 0.68) | |||
| LD belimumab | 0.15 (0.02 to 0.92) | |||
| MD belimumab | 0.08 (0.01 to 0.62) | |||
| HD belimumab | 0.16 (0.03 to 0.98) | |||
| Serious adverse events | ||||
| SC belimumab versus | Placebo | 1/836 | 0.65 (0.43 to 0.99) | 0.65 (0.43 to 0.99) |
| LD belimumab | 0.57 (0.36 to 0.91) | |||
| Serious infection | ||||
| TAC versus | CYC | 2/113 | 0.23 (0.06 to 0.91) | 0.32 (0.12 to 0.83) |
| AZA | 0.34 (0.12 to 0.96) | |||
| MMF+TAC | 0.16 (0.04 to 0.62) | |||
| CYC-AZA | 0.22 (0.05 to 0.95) | |||
| Serious gastrointestinal events | ||||
| RTX versus | AZA | 0.04 (0.00 to 0.49) | ||
| MMF | 0.09 (0.01 to 0.70) | |||
| CSA | 0.02 (0.00 to 0.26) | |||
| Placebo versus | AZA | 0.07 (0.01 to 0.60) | ||
| MMF | 2/127 | 0.10 (0.02 to 0.57) | 0.15 (0.03 to 0.80) | |
| CSA | 0.03 (0.00 to 0.33) | |||
| Serious leucopenia | ||||
| MMF versus | CYC | 2/87 | 0.14 (0.03 to 0.63) | 0.12 (0.03 to 0.49) |
| AZA | 2/226 | 0.19 (0.04 to 0.86) | 0.22 (0.05 to 0.91) | |
| TAC versus | CYC | 0.06 (0.01 to 0.29) | ||
| AZA | 1/70 | 0.11 (0.03 to 0.42) | 0.11 (0.03 to 0.39) | |
| Leucopenia | ||||
| AZA versus | MMF | 2/345 | 7.68 (1.94 to 30.40) | 5.81 (2.10 to 16.06) |
| TAC | 1/70 | 9.25 (2.39 to 35.80) | 7.74 (2.31 to 25.92) | |
| CYC | 3.20 (1.13 to 9.06) | |||
| GC | 12.30 (1.35 to 112.26) | |||
| CSA | 2/158 | 4.00 (1.85 to 8.33) | 4.55 (2.22 to 9.09) | |
| MMF+TAC | 15.51 (2.79 to 86.12) | |||
| CYC versus | MMF+TAC | 4.84 (1.24 to 18.93) | ||
| MMF | 1.81 (1.05 to 3.14) | |||
| Ovarian failure | ||||
| CYC versus | AZA | 1/39 | 15.00 (3.17 to 71.00) | |
| GC versus | CYC | 3/149 | 0.12 (0.03 to 0.46) | 0.13 (0.02 to 0.71) |
| CYC+GC | 1/55 | 0.11 (0.02 to 0.54) | 0.11 (0.01 to 0.95) | |
| CYC-AZA | 0.07 (0.01 to 0.92) | |||
| Menstrual disorder | ||||
| CYC versus | MMF+TAC | 2/402 | 3.94 (1.07 to 14.50) | 3.94 (1.07 to 14.49) |
| MMF | 2.15 (1.00 to 4.60) | |||
| New-onset hypertension | ||||
| AZA versus | GC | 1/28 | 0.10 (0.01 to 0.93) | |
| CSA versus | GC | 1/27 | 84.14 (3.90 to 1814.89) | |
| HD belimumab versus | Placebo | 1/577 | 0.53 (0.29 to 0.99) | |
+, combined with; AZA, azathioprine; CSA, cyclosporine; CYC, cyclophosphamide; CYC-AZA, CYC followed by AZA; GC, glucocorticoid; HD, high dose; IV, intravenous infusion; LD, low dose; MD, moderate dose; MMF, mycophenolate mofetil; MTX, methotrexate; RTX, rituximab; SC, subcutaneous; TAC, tacrolimus.
Primary outcomes
For primary outcomes, all-cause mortality was reported in 29 studies (8762 participants), but because data were scant for some treatments, the results of both the pairwise and network estimates were not significant. Compared with CYC alone (the most commonly used drug), the ORs ranged from 0.44 (95% CI 0.84 to 2.31) for the highest ranked treatment strategy (TAC) to 5.76 (95% CI 0.24 to 137.52) for the lowest ranked agent (CYC followed by azathioprine (AZA)). AE-related withdrawal was reported in 29 studies (8371 participants). AZA was significantly less safe compared with CYC, TAC and MMF (ORs 3.04, 95% CI (1.44 to 6.42), 3.63 (1.05 to 12.61) and 2.08 (1.29 to 3.36), respectively), and CSA was also significantly less secure compared with CYC (3.28 (1.04 to 10.35)). AZA combined with GC, CYC combined with MMF and moderate dose (MD) belimumab were also ranked highly, indicating high safety, while GC with the lowest rank was considered to lead to a higher withdrawal rate (online supplementary appendix 7).
Secondary outcomes
Adverse events
Chloroquine ranked highest in all treatments for the risk of AEs and was significantly safer than methotrexate (MTX), placebo, RTX, low dose (LD) belimumab, MD belimumab and high dose belimumab (OR 0.09, 95% CI (0.02 to 0.44), 0.16 (0.03 to 0.96), 0.10 (0.02 to 0.68), 0.15 (0.02 to 0.92), 0.08 (0.01 to 0.62), 0.16 (0.03 to 0.98), respectively). TAC was safer compared with CYC (0.03 (0.00 to 0.56)), and AZA was safer compared with GC (0.06 (0.01 to 0.61)). CYC followed by AZA ranked the lowest and increased the risk of AEs (online supplementary appendix 7).
SAEs
For SAEs, subcutaneous (SC) belimumab was significantly better than placebo and LD belimumab (0.65 (0.43 to 0.99) and 0.57 (0.36 to 0.91), respectively). MD belimumab ranked the lowest and increased the risk of SAEs (online supplementary appendix 7).
Serious infection
TAC was superior to CYC, AZA, MMF combined with TAC and CYC followed by AZA for prevention of serious infection (0.32 (0.12 to 0.83), 0.34 (0.12 to 0.96), 0.16 (0.04 to 0.62), and 0.22 (0.05 to 0.95), respectively), whereas the effects of other drugs were not significant or were very imprecise. Additionally, CYC combined with AZA, CYC combined with MMF, and TAC alone ranked highest and indicated a lower possibility of suffering serious infection, while MMF combined with TAC ranked lowest (online supplementary appendix 7).
Serious gastrointestinal events
RTX and placebo ranked high in all treatments and both had significant reductions in serious gastrointestinal events compared with AZA (0.04 (0.00 to 0.49) and 0.07 (0.01 to 0.60), respectively), MMF (0.09 (0.01 to 0.70) and 0.15 (0.03 to 0.80), respectively), and CSA (0.02 (0.00 to 0.26) and 0.03 (0.00 to 0.33), respectively). TAC alone also showed great benefit of serious gastrointestinal events. Compared with CSA alone, the combination with AZA could reduce the risk of serious gastrointestinal events (online supplementary appendix 7).
Serious leucopenia
TAC and MMF showed significantly rates of serious leucopenia compared with CYC (0.12 (0.03 to 0.49) and 0.06 (0.01 to 0.29), respectively) and AZA (0.22 (0.05 to 0.91) and 0.11 (0.03 to 0.39), respectively). Belimumab also had beneficial effects, while CYC alone, CYC followed by AZA and AZA alone demonstrated a higher possibility of suffering serious leucopenia (online supplementary appendix 7).
Leucopenia
Similar to the result of serious leucopenia, CYC and AZA increased the risk of suffering leucopenia compared with MMF, TAC, GC, CSA and MMF combined with TAC (table 1), and AZA was considered the worst (compared with CYC: 3.20 (1.13 to 9.06)). The combination of AZA and CYC also ranked low, as did LEF. GC and MMF combined with TAC, on the contrary, played an important role in reducing leucopenia events (online supplementary appendix 7).
Ovarian failure
TAC, AZA and GC ranked high in ovarian failure, and GC showed significant reductions compared with CYC, CYC combined with GC and CYC followed by AZA (0.13 (0.02 to 0.71), 0.11 (0.01 to 0.95) and 0.07 (0.01 to 0.92), respectively). CYC alone and in combination increased the risk of ovarian failure (online supplementary appendix 7).
Menstrual disorder
Similar to the result of ovarian failure, TAC and AZA ranked high and reduced the risk of menstrual disorders. CYC ranked the lowest, and CSA also showed an increased tendency to cause menstrual disorders (online supplementary appendix 7).
New-onset hypertension
Different from pairwise analyses, the network results of new-onset hypertension were not significant. However, CSA was ranked lowest, which was also confirmed by direct comparison (compared with GC 84.14 (3.90 to 1814.89), while AZA compared with GC 0.10 (0.01 to 0.93)) indicated an increased risk of hypertension (online supplementary appendix 7).
Outcomes with no statistically significant
There was no evidence that any of these drugs had significantly different odds of CVEs, bone toxicity, malignant transformation and hyperglycaemia related to the limited data and follow-up duration. The rank results required cautious interpretation for the conflicting sort of these drugs alone or in combination (online supplementary appendix 7).
Sensitivity analyses
Results for serious infection were generally robust in sensitivity analyses restricted to lupus nephritis patients only and excluding biological agents, but it was imprecise when restricted to trials with follow-up of longer than 24 months (online supplementary appendix 8).
Discussion
Our network meta-analysis provides unified hierarchies of evidence for all available effective agents in patients who have SLE, overcoming the absence of comparative data in head-to head trials. Overall, no significant difference was observed in all cause mortality. However, GC ranked the lowest as the least effective agent for prevention of AE-related withdrawal, and AZA and CSA, which also ranked low, were significantly less secure compared with CYC. As for the result of all AEs, chloroquine ranked highest in all treatments and was significantly less safe than MTX and most biologicals; TAC compared with CYC and AZA compared with GC also had better safety. For SAEs, SC belimumab was significantly better than placebo and LD belimumab. These results provide us a comprehensive understanding of the agents’ safety and, compared with CYC, TAC and chloroquine, may have better safety, while GC is believed to be less safe.
Compared with the general population, patients with SLE have more than a sixfold higher risk of developing atherosclerotic lesions and much a higher risk of cardiovascular morbidity and mortality,60 61 and the multiple SLE therapies play important roles in the disease progression.62 63 Subsequent studies have confirmed it and have indicated that corticosteroids were linked to increased CVE risk, whereas antimalarial medications were protective.64 65 Moreover, a similar study found that corticosteroids and non-steroidal anti-inflammatory drugs were associated with an increased risk of CVEs in rheumatoid arthritis.66 However, limited to the simple size, we did not find significant results, and to exclude the effects of other factors, long-term follow-up is also required.
Serious infection is always a key concern for patients with SLE, since immunosuppressive drugs and GCs both suppress the immune system.67 68 Similar to Singh et al’s study,5 TAC was found to have a large advantage compared with several other agents for the prevention of serious infection. It also appears that the benefit of TAC was evident in lupus nephritis patients and in patients with SLE.
Bone toxicity, especially avascular necrosis, is a serious comorbidity in SLE patients, and strategies to minimise GC use are necessary to prevent this serious complication.69 70 Recent research has found that the use of immunosuppressive agents is also a significant risk factor, while antimalarial treatments played a protective role.71 However, since the incidence of bone toxicity in patients with SLE was affected by disease activity, a higher disease activity score is significantly associated with an accelerated incidence of bone toxicity; the influence of immunosuppressive agents should be cautiously estimated, since compared with the patients who do not use immunosuppressive agents, user conditions can be much worse and they are likely to have higher disease activity scores.
In the previous study, researchers found that compared with CYC, MMF incurred lower risks of nausea and vomiting, but it was more likely to cause diarrhoea.4 Although gastrointestinal events are quite common in treatments for SLE, few studies focus on it. Our results indicated that RTX, TAC and placebo, as well as CSA combined with AZA could reduce the risk of serious gastrointestinal events, while CSA was associated with a higher risk of suffering serious gastrointestinal events. The combination of these agents may indicate lower levels of gastrointestinal toxicity, an effect similar to those shown in the previous outcomes. The combination of two low-rank agents became safer, and whether it is just a coincidence or a feasible process for toxicity reduction, more studies will be needed to draw a conclusion.
GC, MMF, TAC and their combinations showed benefits in the reduction of all leucopenia events. AZA and CYC, on the contrary, increased the risk of all leucopenia events. The result from both serious leucopenia and leucopenia were consistent, similar to the results for ovarian failure and menstrual disorders. Previous studies have already confirmed that patients who used CYC had a higher occurrence of transient amenorrhea and premature menopause.72–74 Apart from these findings, our results found that TAC, GC and AZA had low ovarian toxicity and may be good alternative therapies for women of childbearing age, while the risk of ovarian toxicity with CSA use should be considered.
Our study has potential limitations. First, because of scant primary data, the effects of agents on CVEs, bone toxicity, malignant transformation, new-onset hypertension and hyperglycaemia were very uncertain, leading to a pivotal weakness in our understanding of these drugs. The present debate about optimum treatments in SLE would be assisted greatly by the collection of robust data for these outcomes in future trials. Second, data for the outcome of SAEs were only reported in some of the studies, and most of them used biologicals; thus, we cannot directly determine the incidence rate of SAEs for all these agents. Third, haemorrhagic cystitis was poorly defined, and scant evidence relating to this outcome does not allow us to make proper estimates of the risk benefit ratio of agents in SLE. Fourth, we did not restrict patients to only adults, as if we had done so, the studies included would have been insufficient to run the analyses. Fifth, due to lack of original data, we are not sure whether the influence of other factors, including the organs involved, underlying disease and demographics, affect the outcomes.
In summary, our analysis showed that TAC is the safest strategy and has benefits for nearly all the SAEs, while the benefits of other agents are not very substantial in different types of SAEs. Therefore, we must consider the potential harms of these treatments in individual patients and even in one patient in different conditions. So that, the status of specific AEs can be evaluated, and appropriate treatments with improved safety should be adopted. Surveillance for treatment-related AEs is important, as is better recording these different types of SAEs and an improved understanding of their outcomes, particularly in the context of future trials.
Footnotes
Contributors: JT contributed to design of the study, performed the systematic review and meta-analysis and drafted the manuscript. YL contributed to screen relevant studies, extract the data and drafted the manuscript. HW, HL and MZ contributed to screen relevant studies and extract the data. QL contributed to interpretation of the results and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81220108017, No. 81430074 and No. 81522038), the National Key Research and Development Program of China(2016YFC0903900) and the National Key Clinical Speciality Construction Project of National Health and Family Planning Commission of the People’s Republic of China.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet 2013;382:809–18. doi:10.1016/S0140-6736(13)60889-2 [DOI] [PubMed] [Google Scholar]
- 2.Gordon C, Amissah-Arthur M-B, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology 2017;358 doi:10.1093/rheumatology/kex286 [DOI] [PubMed] [Google Scholar]
- 3.Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet 2014;384:1878–88. doi:10.1016/S0140-6736(14)60128-8 [DOI] [PubMed] [Google Scholar]
- 4.Palmer SC, Tunnicliffe DJ, Singh-Grewal D, et al. Induction and maintenance immunosuppression treatment of proliferative lupus nephritis: a network meta-analysis of randomized trials. Am J Kidney Dis 2017;70:324–36. doi:10.1053/j.ajkd.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Hossain A, Kotb A, et al. Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis. BMC Med 2016;14137 doi:10.1186/s12916-016-0673-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. doi:10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W–94. doi:10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. doi:10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 9.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012;3:80–97. doi:10.1002/jrsm.1037 [DOI] [PubMed] [Google Scholar]
- 10.Turner RM, Davey J, Clarke MJ, et al. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818–27. doi:10.1093/ije/dys041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salanti G, Marinho V, Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol 2009;62:857–64. doi:10.1016/j.jclinepi.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 12.Veroniki AA, Vasiliadis HS, Higgins JP, et al. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013;42:332–45. doi:10.1093/ije/dys222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98–110. doi:10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111–25. doi:10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White IR. Multivariate random-effects meta-regression: updates to mvmeta. 2011.
- 16.Stohl W, Schwarting A, Okada M, et al. Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: A Fifty-Two-Week Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheumatol 2017;69:1016–27. doi:10.1002/art.40049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis 2017;76:1575–82. doi:10.1136/annrheumdis-2016-210882 [DOI] [PubMed] [Google Scholar]
- 18.Zhang F, Bae SC, Bass D, et al. Arthritis and rheumatology Conference: american college of rheumatology/association of rheumatology health professionals annual scientific meeting, ACR/ARHP A pivotal phase III, randomized, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan, and South Korea. The Netherlands: John Wiley and Sons Inc, 2016:68. [Google Scholar]
- 19.Rathi M, Goyal A, Jaryal A, et al. Comparison of low-dose intravenous cyclophosphamide with oral mycophenolate mofetil in the treatment of lupus nephritis. Kidney Int 2016;89:235–42. doi:10.1038/ki.2015.318 [DOI] [PubMed] [Google Scholar]
- 20.Mok CC, Ying KY, Yim CW, et al. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: a randomised controlled trial and long-term follow-up. Ann Rheum Dis 2016;75:30–6. doi:10.1136/annrheumdis-2014-206456 [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Zhang H, Ji Y, et al. Efficacy and safety of cyclophosphamide combined with mycophenolate mofetil for induction treatment of class IV lupus nephritis. Int J Clin Exp Med 2015;8:21572–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Zhang H, Liu Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med 2015;162:18–26. doi:10.7326/M14-1030 [DOI] [PubMed] [Google Scholar]
- 23.Wallace DJ, Navarra S, Petri MA, et al. Safety profile of belimumab: pooled data from placebo-controlled phase 2 and 3 studies in patients with systemic lupus erythematosus. Lupus 2013;22:144–54. doi:10.1177/0961203312469259 [DOI] [PubMed] [Google Scholar]
- 24.van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012;71:1343–9. doi:10.1136/annrheumdis-2011-200937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012;64:1215–26. doi:10.1002/art.34359 [DOI] [PubMed] [Google Scholar]
- 26.Li X, Ren H, Zhang Q, et al. Mycophenolate mofetil or tacrolimus compared with intravenous cyclophosphamide in the induction treatment for active lupus nephritis. Nephrology Dialysis Transplantation 2012;27:1467–72. doi:10.1093/ndt/gfr484 [DOI] [PubMed] [Google Scholar]
- 27.Islam MN, Hossain M, Haq SA, et al. Efficacy and safety of methotrexate in articular and cutaneous manifestations of systemic lupus erythematosus. Int J Rheum Dis 2012;15:62–8. doi:10.1111/j.1756-185X.2011.01665.x [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Liu Q, Chen W, et al. Outcomes of maintenance therapy with tacrolimus versus azathioprine for active lupus nephritis: a multicenter randomized clinical trial. Lupus 2012;21:944–52. doi:10.1177/0961203312442259 [DOI] [PubMed] [Google Scholar]
- 29.Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. doi:10.1016/S0140-6736(10)61354-2 [DOI] [PubMed] [Google Scholar]
- 30.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. doi:10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dooley MA, Jayne D, Ginzler EM, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011;365:1886–95. doi:10.1056/NEJMoa1014460 [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Tang X, Liu Q, et al. Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: A multicenter randomized clinical trial. Am J Kidney Dis 2011;57:235–44. doi:10.1053/j.ajkd.2010.08.036 [DOI] [PubMed] [Google Scholar]
- 33.Zavada J, Pesickova S, Rysava R, et al. Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: the Cyclofa-Lune study. Lupus 2010;19:1281–9. doi:10.1177/0961203310371155 [DOI] [PubMed] [Google Scholar]
- 34.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62:222–33. doi:10.1002/art.27233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houssiau FA, D’Cruz D, Sangle S, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis 2010;69:2083–9. doi:10.1136/ard.2010.131995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffiths B, Emery P, Ryan V, et al. The BILAG multi-centre open randomized controlled trial comparing ciclosporin vs azathioprine in patients with severe SLE. Rheumatology 2010;49:723–32. doi:10.1093/rheumatology/kep396 [DOI] [PubMed] [Google Scholar]
- 37.El-Shafey EM, Abdou SH, Shareef MM. Is mycophenolate mofetil superior to pulse intravenous cyclophosphamide for induction therapy of proliferative lupus nephritis in Egyptian patients? Clin Exp Nephrol 2010;14:214–21. doi:10.1007/s10157-010-0270-7 [DOI] [PubMed] [Google Scholar]
- 38.Austin HA, Illei GG, Braun MJ, et al. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 2009;20:901–11. doi:10.1681/ASN.2008060665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009;20:1103–12. doi:10.1681/ASN.2008101028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HY, Cui TG, Hou FF, et al. Induction treatment of proliferative lupus nephritis with leflunomide combined with prednisone: a prospective multi-centre observational study. Lupus 2008;17:638–44. doi:10.1177/0961203308089408 [DOI] [PubMed] [Google Scholar]
- 41.Fortin PR, Abrahamowicz M, Ferland D, et al. Steroid-sparing effects of methotrexate in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2008;59:1796–804. doi:10.1002/art.24068 [DOI] [PubMed] [Google Scholar]
- 42.Bao H, Liu ZH, Xie HL, et al. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008;19:2001–10. doi:10.1681/ASN.2007121272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moroni G, Doria A, Mosca M, et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol 2006;1:925–32. doi:10.2215/CJN.02271205 [DOI] [PubMed] [Google Scholar]
- 44.Grootscholten C, Ligtenberg G, Hagen EC, et al. Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int 2006;70:732–42. doi:10.1038/sj.ki.5001630 [DOI] [PubMed] [Google Scholar]
- 45.El-Sehemy MS, Al-Saaran AM, Baddour NM, et al. Comparative clinical prospective therapeutic study between cyclophosphamide, cyclosporine and azathioprine in the treatment of lupus nephritis. Egypt J Immunol 2006;13:39–52. [PubMed] [Google Scholar]
- 46.Ong LM, Hooi LS, Lim TO, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology 2005;10:504–10. doi:10.1111/j.1440-1797.2005.00444.x [DOI] [PubMed] [Google Scholar]
- 47.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate Mofetil or Intravenous Cyclophosphamide for Lupus Nephritis. N Engl J Med Overseas Ed 2005;353:2219–28. doi:10.1056/NEJMoa043731 [DOI] [PubMed] [Google Scholar]
- 48.Barile-Fabris L, Ariza-Andraca R, Olguín-Ortega L, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis 2005;64:620–5. doi:10.1136/ard.2004.025528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med Overseas Ed 2004;350:971–80. doi:10.1056/NEJMoa031855 [DOI] [PubMed] [Google Scholar]
- 50.Mok CC, Ho CT, Siu YP, et al. Treatment of diffuse proliferative lupus glomerulonephritis: a comparison of two cyclophosphamide-containing regimens. Am J Kidney Dis 2001;38:256–64. doi:10.1053/ajkd.2001.26084 [DOI] [PubMed] [Google Scholar]
- 51.Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med 2000;343:1156 62. doi:10.1056/NEJM200010193431604 [DOI] [PubMed] [Google Scholar]
- 52.Carneiro JR, Sato EI. Double blind, randomized, placebo controlled clinical trial of methotrexate in systemic lupus erythematosus. J Rheumatol 1999;26:1275–9. [PubMed] [Google Scholar]
- 53.Gourley MF, Austin HA, Scott D, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med 1996;125:549 doi:10.7326/0003-4819-125-7-199610010-00003 [DOI] [PubMed] [Google Scholar]
- 54.Sesso R, Monteiro M, Sato E, et al. A controlled trial of pulse cyclophosphamide versus pulse methylprednisolone in severe lupus nephritis. Lupus 1994;3:107–12. doi:10.1177/096120339400300209 [DOI] [PubMed] [Google Scholar]
- 55.Boumpas DT, Austin HA, Vaughn EM, et al. Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 1992;340:741–5. doi:10.1016/0140-6736(92)92292-N [DOI] [PubMed] [Google Scholar]
- 56.Steinberg AD, Steinberg SC. Long-term preservation of renal function in patients with lupus nephritis receiving treatment that includes cyclophosphamide versus those treated with prednisone only. Arthritis Rheum 1991;34:945–50. doi:10.1002/art.1780340803 [DOI] [PubMed] [Google Scholar]
- 57.Donadio JV, Holley KE, Ferguson RH, et al. Treatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamide. N Engl J Med 1978;299:1151–5. doi:10.1056/NEJM197811232992102 [DOI] [PubMed] [Google Scholar]
- 58.Hahn BH, Kantor OS, Osterland CK. Azathioprine plus prednisone compared with prednisone alone in the treatment of systemic lupus erythematosus. Report of a prospective controlled trial in 24 patients. Ann Intern Med 1975;83:597–605. [DOI] [PubMed] [Google Scholar]
- 59.Cade R, Spooner G, Schlein E, et al. Comparison of azathioprine, prednisone, and heparin alone or combined in treating lupus nephritis. Nephron 1973;10:37–56. doi:10.1159/000180176 [DOI] [PubMed] [Google Scholar]
- 60.Bartels CM, Buhr KA, Goldberg JW, et al. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J Rheumatol 2014;41:680–7. doi:10.3899/jrheum.130874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Björnådal L, Yin L, Granath F, et al. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964-95. J Rheumatol 2004;31:713–9. [PubMed] [Google Scholar]
- 62.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE--mechanisms and management. Nat Rev Rheumatol 2012;8:214–23. doi:10.1038/nrrheum.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmad Y, Shelmerdine J, Bodill H, et al. Subclinical atherosclerosis in systemic lupus erythematosus (SLE): the relative contribution of classic risk factors and the lupus phenotype. Rheumatology 2007;46:983–8. doi:10.1093/rheumatology/kem002 [DOI] [PubMed] [Google Scholar]
- 64.Tselios K, Sheane BJ, Gladman DD, et al. Optimal monitoring for coronary heart disease risk in patients with systemic lupus erythematosus: a systematic review. J Rheumatol 2016;43:54–65. doi:10.3899/jrheum.150460 [DOI] [PubMed] [Google Scholar]
- 65.Wu GC, Liu HR, Leng RX, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev 2016;15:22–37. doi:10.1016/j.autrev.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 66.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9. doi:10.1136/annrheumdis-2014-206624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia Popa-Lisseanu MG, Greisinger A, Richardson M, et al. Determinants of treatment adherence in ethnically diverse, economically disadvantaged patients with rheumatic disease. J Rheumatol 2005;32:913–9. [PubMed] [Google Scholar]
- 68.Chambers SA, Raine R, Rahman A, et al. Why do patients with systemic lupus erythematosus take or fail to take their prescribed medications? A qualitative study in a UK cohort. Rheumatology 2009;48:266–71. doi:10.1093/rheumatology/ken479 [DOI] [PubMed] [Google Scholar]
- 69.Becker A, Fischer R, Scherbaum WA, et al. Osteoporosis screening in systemic lupus erythematosus: impact of disease duration and organ damage. Lupus 2001;10:809–14. doi:10.1177/096120330101001108 [DOI] [PubMed] [Google Scholar]
- 70.Dubois EL, COZEN L, Avascular CL. Avascular (aseptic) bone necrosis associated with systemic lupus erythematosus. JAMA 1960;174:966–71. doi:10.1001/jama.1960.03030080028005 [DOI] [PubMed] [Google Scholar]
- 71.Zhang K, Zheng Y, Jia J, et al. Systemic lupus erythematosus patients with high disease activity are associated with accelerated incidence of osteonecrosis: a systematic review and meta-analysis. Clin Rheumatol 2018;37 doi:10.1007/s10067-017-3820-5 [DOI] [PubMed] [Google Scholar]
- 72.Singh G, Misra R, Aggarwal A. Ovarian insufficiency is major short-term toxicity in systemic lupus erythematosus patients treated with cyclophosphamide. J Assoc Physicians India 2016;64:28–31. [PubMed] [Google Scholar]
- 73.Mayorga J, Alpízar-Rodríguez D, Prieto-Padilla J, et al. Prevalence of premature ovarian failure in patients with systemic lupus erythematosus. Lupus 2016;25:675–83. doi:10.1177/0961203315622824 [DOI] [PubMed] [Google Scholar]
- 74.Akawatcharangura P, Taechakraichana N, Osiri M. Prevalence of premature ovarian failure in systemic lupus erythematosus patients treated with immunosuppressive agents in Thailand. Lupus 2016;25:436–44. doi:10.1177/0961203315617539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2017-000253supp001.doc (3.7MB, doc)