Abstract
ATP-sensitive K+ (KATP) channels in pancreatic β-cells couple glucose metabolism to insulin secretion. Reduced KATP channel activity produces excessive insulin release and hyperinsulinism whereas increased KATP channel activity leads to lower insulin secretion and diabetes. Paradoxically, mice with genetic deletion of KATP channels, or loss-of-function mutations, are only transiently hypoglycaemic during the neonatal period and often display reduced glucose-stimulated insulin secretion subsequently. Mice with KATP channel gain-of-function mutations are hyperglycaemic and have impaired glucose-stimulated insulin secretion, a phenotype that accurately mimics human diabetes. This review discusses how mice expressing altered KATP channels have provided valuable insight into β-cell function.
Introduction
Blood glucose homeostasis is essential: too little glucose rapidly results in brain damage whereas elevation of blood glucose for an extended period leads to the complications of uncontrolled diabetes - retinopathy, nephropathy, and micro- and macro-vascular disease. Insulin is the only hormone capable of reducing blood glucose, which is why its impaired secretion can lead to diabetes (too little secretion) or hyperinsulinism (too much release). The mechanisms controlling insulin secretion from pancreatic β-cells are well understood and lessons from over 20 years of molecular, structural and whole animal studies have highlighted the essential role of the ATP-sensitive K+ (KATP) channel. In this review, we discuss the physiological and pathophysiological roles of KATP channels in controlling β-cell function in health and disease. Focusing specifically on animal models of KATP channels, we highlight how their study has advanced our understanding of β-cell dysfunction in metabolic disease.
Physiological role of KATP channels in β-cells
KATP channels link changes in circulating blood glucose to alterations in β-cell activity and insulin secretion. They are large macromolecular complexes composed of four Kir6.2 subunits, which form a central K+-selective pore, and four regulatory sulphonylurea (SUR) subunits, which modify and regulate the channel's properties. In β-cells, the KATP channel is composed of Kir6.2 and SUR1 isoforms [1,2]. Its activity is regulated by intracellular adenine nucleotides, being inhibited by binding of ATP to Kir6.2 and stimulated by MgATP/MgADP interaction with the nucleotide-binding domains of SUR1 [3,4]: the balance between these competing actions determines the level of KATP channel activity.
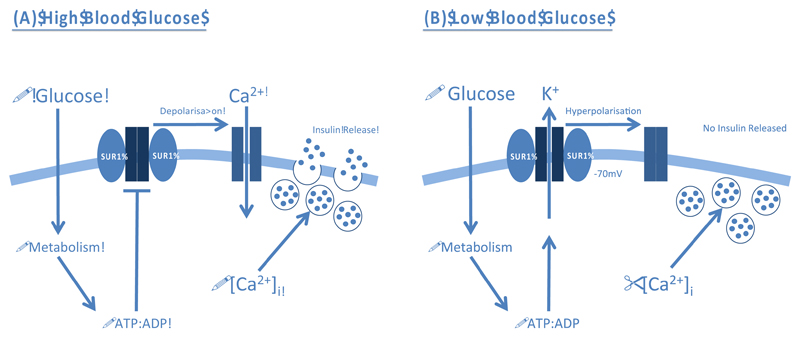
Electrophysiology studies in isolated pancreatic β-cells have demonstrated that increased glucose metabolism, elicited by a rise in blood glucose, promotes intracellular ATP formation and closure of KATP channels [5] (Figure 1A). The resulting membrane depolarisation opens voltage-gated Ca2+ channels, thereby permitting Ca2+ influx and exocytosis of insulin granules. Conversely, KATP channels open when metabolism falls in response to reduced blood glucose, which hyperpolarises the β-cell membrane potential and prevents electrical activity and insulin secretion (Figure 1B). Channel activity is also inhibited by sulphonylurea (SU) drugs, which bypass metabolic regulation and stimulate insulin secretion directly [6]: they are widely used to treat type 2 diabetes mellitus (T2DM).
Figure 1. Physiological role of ATP-sensitive K+ Channels (KATP) in controlling glucose-stimulated insulin secretion from pancreatic β-cells.
(A) A rise in blood glucose increases β-cell metabolism. The resulting increase in intracellular ATP (and fall in MgADP) promotes closure of KATP channels and membrane depolarisation. This triggers opening of voltage-gated Ca2+ channels (VGCCs), Ca2+ influx and exocytosis of insulin granules. (B) A decrease in blood glucose reduces metabolism and the ATP:ADP ratio within the β-cell. This opens the KATP channel and hyperpolarises the membrane, preventing VGCC opening. Thus, no insulin is released.
Pathophysiological role of KATP channels in β-cells
Given the crucial role KATP channels play in controlling insulin secretion, it is not surprising that diseases characterised by excessively high, or low, circulating glucose are associated with altered KATP channel expression and/or function. Below, we highlight three diseases where KATP channel dysfunction is a common factor.
Congenital Hyperinsulinism
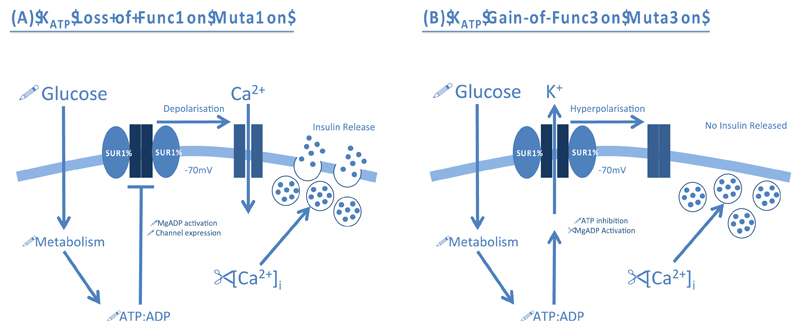
Loss of KATP channel activity leads to congenital hyperinsulinaemia (CHI), which is characterised by continuous and unregulated insulin secretion in response to low blood glucose levels [7–9]. It affects around 1 in 50,000 live births and patients present early in life with hypoglycaemia. If left untreated, it can cause brain damage. Over 100 loss-of-function mutations in Kir6.2 and SUR1 have been associated with CHI. Some affect the expression, maturation, assembly or trafficking of the KATP channel, resulting in a lower channel density. Others prevent the ability of MgADP/MgATP to stimulate channel activity. All mutations lead to a loss of KATP channel activity that causes permanent depolarisation of the β-cell membrane and excessive insulin secretion (Figure 2A).
Figure 2. Pathophysiological role of β-cell ATP-sensitive K+ Channels (KATP) in disease.
(A) KATP channel loss-of-function mutations impair MgADP activation or reduce channel expression. This results in permanent depolarisation of the β-cell membrane and opening of voltage-gated Ca2+ channels (VGCCs). The subsequent Ca2+ influx promotes exocytosis of insulin granules even when blood glucose and metabolism are low. (B) KATP channel gain-of-function mutations increase the sensitivity of the channel to ATP inhibition, or enhance activation by MgADP, rendering the channel inappropriately open. The resulting hyperpolarisation of the β-cell membrane prevents VGCC opening and Ca2+ influx. Therefore, insulin secretion is prevented even when blood glucose is elevated.
Patients with mutations that cause mild CHI can sometimes be treated by diet or oral administration of the KATP channel activator, diazoxide [10]; this implies KATP channels are present in their β-cells but that they fail to open when blood glucose levels fall. Patients with functionally more severe CHI mutations (that prevent the channel from reaching the plasma membrane), fail to respond to diazoxide therapy and are usually treated by sub-total pancreatectomy. In individuals with embryonic loss of heterozygosity, leading to focal homozygous expression of a paternally derived mutation, the lesion can often be revealed by 18-fluoro L-3,4-dihydroxyphenylalanine (18F(DOPA) positron emission tomography (PET) scanning and its surgical removal can result in a cure [11]. In diffuse CHI, where up to 80% of the pancreas may require removal, patients are at increased risk of subsequently developing diabetes. Alternative methods to treat these patients would be valuable. Although most diffuse CHI mutations are inherited recessively, a few are inherited in a dominant fashion. Some dominant mutations cause mild CHI that does not require pancreatectomy and may even predispose to type 2 diabetes in later life [12,13].
Neonatal Diabetes
Neonatal diabetes (ND) is characterised by severe hyperglycaemia within the first six months of life. It can be caused by mutations in a number of genes, including those encoding Kir6.2 (KCNJ11) and SUR1 (ABCC8) [8,14,15]. Gain-of-function mutations render the KATP channel inappropriately active and its failure to close in response to glucose metabolism means that insulin secretion is prevented, even when blood glucose is elevated (Figure 2B). Prior to the discovery that ND can be caused by KATP channel mutations, patients required daily insulin injections to maintain normoglycaemia. Now ˜90% of ND patients diagnosed with KATP channel mutations have transferred to oral SU drugs, which block the KATP channel and thereby stimulate insulin secretion [16].
Some ND mutations, with severe functional effects, lead to diabetes that is accompanied by neurological problems such as developmental delay, epilepsy and muscle hypotonia [14,17]. This condition, termed DEND syndrome, affects ˜3% of patients; rather more (˜20%) show developmental delay but not epilepsy and are said to have intermediate DEND syndrome. Electrophysiology studies of ND mutant channels expressed in a heterologous system reveal a correlation between the magnitude of KATP current at physiologically relevant ATP concentrations and the clinical phenotype: the larger the current, the more severe the disease [18]. It is now clear that the neurological phenotype is not a secondary consequence of hyperglycaemia, but due to expression of the mutant KATP channel in neurones [19].
Type 2 Diabetes Mellitus (T2DM)
T2DM is also characterised by reduced β-cell function and reduced glucose-dependent insulin secretion [20]. It develops in later life and is extremely common, affecting 336 million people worldwide. T2DM has multiple aetiologies and is also influenced by environmental and lifestyle factors, such as age and obesity. Genetic polymorphisms that predispose to T2DM occur in a large number of genes. Of relevance here, however, is that two common variants in the KATP channel genes KCNJ11 (Kir6.2-E23K) and ABCC8 (SUR1-S1369A) which are in strong linkage disequilibrium (i.e. individuals carry both variants) predispose to T2DM [21,22]. In heterologous studies, the K23/A1369 variant causes a mild decrease in channel inhibition by ATP, but which variant is the more important remains controversial [23,24] and how these variants lead to diabetes in later life is unclear. Mouse models may help answer these questions.
T2DM is initially managed with diet and lifestyle modifications. As the disease progresses, pharmacological intervention is required and patients may switch first to oral SU therapy and subsequently to insulin. Why SU therapy eventually fails in T2DM but not ND, and why hypoglycaemia is less common in ND patients, is still unclear. Potentially, animal models may help resolve this puzzle.
Mouse models of β-cell KATP channel function
Why use mouse models to study metabolic disease?
The use of the mouse as a model system to study the molecular mechanisms of human disease is well established. Its many advantages include the fact that the mouse is mammalian, has a short life cycle, and the full genomic sequence of the C57BL/6J strain is available. Furthermore, such is the elegance of the molecular biology techniques used to generate genetically modified mice that a single nucleotide base pair can be changed and its effect studied in a specific cell type and/or at a defined time-point during the mouse’s life.
Mouse models have been employed for both in vivo and in vitro studies of metabolic disorders including diabetes. The latter is especially useful, as access to human islets, and diabetic islets in particular, is limited. However, like any animal model, extrapolation of findings in a diabetic mouse to human diabetes has to be made with caution. The difference in size and circadian rhythm between the two species can influence metabolism and hormone secretion, and differences in islet architecture and the ion channels that underlie action potential firing in β-cells are also evident [25,26]. Despite these potential caveats, however, mouse models have proven valuable for elucidating the molecular mechanisms underlying β-cell dysfunction in diabetes and, to a lesser extent, CHI.
KATP Channel Loss-of-function Mouse Models: ß-cell Hyperexcitability
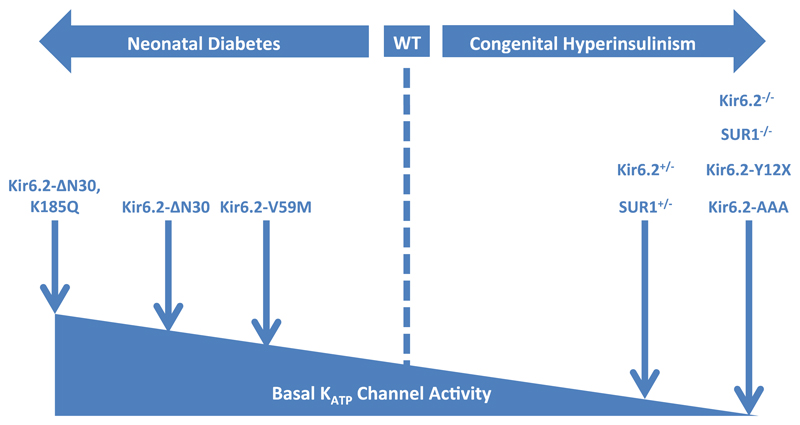
Mouse models of CHI have been generated by genetic deletion of Kir6.2 (Kir6.2-/-) and SUR1 (SUR1-/-) or loss-of-function mutations in these genes (Figure 3, Table 1). Unexpectedly, however, these models do not fully recapitulate the human phenotype.
Figure 3. Mouse models of β-cell KATP channel insulin secretory disorders.
An increase in basal KATP channel activity reduces insulin secretion and leads to neonatal diabetes. Mouse models of increased KATP channel activity display hyperglycaemia and hypoinsulinaemia: they include those with Kir6.2-V59M, Kir6.2-ΔN30, and Kir6.2-ΔN30,K185Q mutations. Reduced basal KATP channel activity results in congenital hyperinsulinism. Mouse models with partial deletion (Kir6.2+/-, SUR+/-) or ablated channel expression / function (Kir6.2-/-, SUR-/-, Kir6.2-Y12X, Kir6.2-AAA) have been generated but do not fully recapitulate the human disease.
Table 1. Mouse models of β-cell KATP channel insulin secretory disorders: Phenotype description.
| KATP Channel Loss-of-Function | ||||
|---|---|---|---|---|
| Mutation | KATP Effect | Phenotype | Reference | |
| SUR1-/- | Global SUR1 deletion from birth | β-cells |
|
[32–37] |
| Isolated islets |
|
|||
| Islet histology |
|
|||
| In vivo |
|
|||
| Kir6.2-/- | Global Kir6.2 deletion from birth | β-cells |
|
[27–28, 30–31] |
| Isolated islets |
|
|||
| Islet histology |
|
|||
| In vivo |
|
|||
Kir6.2-/- mice showed transient neonatal hypoglycaemia but subsequently became normoglycaemic, normoinsulinaemic and displayed mild glucose intolerance (Table 1) [27,28]. A similar phenotype was observed in mice expressing the human CHI homozygous mutation Kir6.2-Y12X (Table 1) [29]. In Kir6.2-/- mice there was a marked reduction in insulin secretion both in vivo and in vitro following glucose, arginine or GIP stimulation, despite enhanced insulin sensitivity [27,28,30,31]. Although KATP currents were absent, leading to spontaneous electrical activity at low glucose, there was only a modest increase in basal intracellular [Ca2+]i and insulin secretion from freshly isolated islets [31]. However, when cultured overnight, islets exhibited increased basal [Ca2+]i [27,31], and restored glucose-stimulated insulin secretion [31].
SUR1-/- mice were hypoglycaemic on the first day of life, but subsequently became normoglycaemic and showed impaired glucose tolerance [32,33]. Like Kir6.2-/- mice, freshly isolated islets showed impaired insulin secretion [32,34], but following overnight culture, resting [Ca2+]i and basal insulin secretion were elevated, and glucose-stimulated insulin release was greater than in control islets [34,35]. Unlike Kir6.2-/- mice [30], SUR1-/- mice showed impaired GLP-1-mediated insulin secretion, despite normal cAMP levels [36]. This may reflect the fact that SUR1 interacts with Epac2, which mediates the PKA-independent effect of cAMP on exocytosis [37]: perhaps SUR1 serves as a scaffold protein to anchor Epac2 in the correct location. The reduced GLP-1 response may account for the impaired oral glucose tolerance [33] and lack of post-prandial hypoglycaemia [32]. In contrast to Kir6.2-/- mice, insulin sensitivity was normal [32]: this is expected because skeletal muscle KATP channels are composed of Kir6.2/SUR2 subunits rather than Kir6.2/SUR1 subunits [18].
Why Kir6.2-/- or SUR1-/- mice fail to show a hypoglycaemic/hyperinsulinaemic phenotype is unclear. No evidence for β-cell loss has been reported for these mice [27,28,30,32]. It has been proposed that hyperexcitability drives the β-cell into secretory failure [38]. Others have suggested that an inhibitory signal constrains release in vivo [35], or a reduced incretin response may also contribute [30,33,36].
Interestingly, heterozygous knockout of KATP channels in β-cells (Kir6.2+/-, SUR1+/-; 60-70% reduction in functional channels) resulted in hyperinsulinaemia, enhanced glucose tolerance and increased glucose-stimulated insulin secretion, despite no change in islet size or composition (Table 1) [28]. A similar result was observed for mice expressing a dominant negative transgene (Kir6.2-AAA residues 156-158), selectively in ~70% of β-cells [28,39,40]. However, Kir6.2+/-, SUR1+/- and Kir6.2-AAA mice do not perfectly mimic human CHI as they are normoglycaemic (Table 1).
KATP Channel Gain-of-function Mouse Models: ß-cell Hypoexcitability
A mouse model of enhanced KATP channel activity was first described by Nichols and colleagues [39] (Figure 3, Table 1). Expression of Kir6.2 with an N-terminal truncation of 30 amino acids (Kir6.2-ΔN30), resulted in a 7-fold reduction in ATP inhibition. Transgenic mice with this mutation were severely hyperglycaemic and hypoinsulinaemic, and died within 5 days of birth. No abnormalities in islet size and distribution were observed but there was a decrease in insulin-positive β-cells and an increase in glucagon-positive α-cells, as seen in islets from patients with T2DM [41]. Addition of a point mutation at residue 185 (Kir6.2-ΔN30,K185Q) and targeted expression of the Kir6.2-ΔN30,K185Q transgene to β-cells in adult life using Cre-lox technology, also resulted in hyperglycaemia and undetectable levels of circulating insulin, due to impaired glucose-stimulated insulin secretion [42]. This was attributed to a failure of glucose to elevate intracellular calcium, as a result of the KATP channel hyperactivity [43].
A second mouse model of ND has also been generated that expresses a human Kir6.2 mutation (V59M) under the control of the endogenous ROSA26 promoter: this drives relatively weak expression and mimics the heterozygous state observed in ND patients [44] (Figure 3, Table 1). Kir6.2-V59M mice were hyperglycaemic, hyperinsulinaemic and hyperglucagonaemic by 5 weeks of age. Insulin content and β-cell area were reduced and islet architecture affected [44], as also seen in Kir6.2-ΔN30,K185Q mice [42], and β-cell proliferation decreased [45].
In islets isolated from Kir6.2-V59M mice, addition of the sulphonylurea tolbutamide to block KATP channels restored glucose-stimulated insulin secretion and normal Ca2+ dynamics [44]. This suggests that the mutant β-cells retain their capacity to produce and secrete insulin confirming the defect lies in KATP closure and explaining why ND patients respond to SU therapy. Further evidence for this idea comes from the fact that transplantation of normal islets under the kidney capsule, or administration of SUs in the form of a subcutaneous pellet prior to gene induction, prevented the development of hyperglycaemia, hypoinsulinaemia and loss of islet insulin content and β-cell architecture in Kir6.2-ΔN30,K185Q mice [42]. This has important implications for ND patients as it highlights the need for rapid transfer to SUs following diagnosis. No mice expressing KATP channels with a gain-of-function SUR1 mutation have yet been reported.
The Future: KATP Channels, β-cells and Disease
Collectively, these mouse models highlight the importance of KATP channel activity for regulating β-cell function and insulin secretion. They have also been helpful in understanding the molecular mechanisms that underlie altered insulin secretion in ND caused by gain-of-function KATP channel mutations. Furthermore, they serve as a valuable tool for exploring how SU treatment may prevent or reverse disease progression in ND patients. Potentially, mice expressing gain-of-function KATP channel mutations selectively in pancreatic β-cells may also be used to explore the effects of hypoinsulinaemia/hyperglycaemia on both pancreatic function and extra-pancreatic tissues. Their advantages over other models of T2DM are that they exhibit a known genetic defect that is confined to the β-cell, and that can be reversed by SU or insulin therapy. Models of CHI have been less successful as they fail to recapitulate human CHI and illustrate the point that it is wise to confirm all data obtained from mouse models in humans, wherever possible. Nevertheless, they too have provided valuable insight into β-cell function.
Despite significant progress gained from studies of mouse models in recent years, much remains to be done. It will be interesting, for example, to determine the effects of β-cell specific knockout models and SUR1 gain-of-function mutations. No doubt, they will provide further insight into β-cell function as well as human disease.
Acknowledgements
We thank the Wellcome Trust and the European Union for supporting the work in our laboratory. MFB holds an OXION Wellcome Trust Training Fellowship.
Footnotes
Conflict of interest
The author(s) have no conflict of interest to declare.
References
- 1.Inagaki N, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270(5239):1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 2.Sakura H, et al. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377(3):338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 3.Nichols CG, et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272(5269):1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 4.Tucker SJ, et al. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387(6629):179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 5.Ashcroft FM, et al. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 6.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46(7):875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 7.Dunne MJ, et al. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev. 2004;84(1):239–275. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan SE, et al. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30(2):170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- 9.Ashcroft FM. The Walter B. Cannon Physiology in Perspective Lecture, 2007. ATP-sensitive K+ channels and disease: from molecule to malady. Am J Physiol Endocrinol Metab. 2007;293(4):E880–889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 10.Dekel B, et al. Compound heterozygosity for the common sulfonylurea receptor mutations can cause mild diazoxide-sensitive hyperinsulinism. Clin Pediatr (Phila) 2002;41(3):183–186. doi: 10.1177/000992280204100310. [DOI] [PubMed] [Google Scholar]
- 11.Hardy OT, et al. Accuracy of [18F]fluorodopa positron emission tomography for diagnosing and localizing focal congenital hyperinsulinism. J Clin Endocrinol Metab. 2007;92(12):4706–4711. doi: 10.1210/jc.2007-1637. [DOI] [PubMed] [Google Scholar]
- 12.Huopio H, et al. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet. 2003;361(9354):301–307. doi: 10.1016/S0140-6736(03)12325-2. [DOI] [PubMed] [Google Scholar]
- 13.Ocal G, et al. Clinical characteristics of recessive and dominant congenital hyperinsulinism due to mutation(s) in the ABCC8/KCNJ11 genes encoding the ATP-sensitive potasium channel in the pancreatic beta cell. J Pediatr Endocrinol Metab. 2011;24(11–12):1019–1023. doi: 10.1515/jpem.2011.347. [DOI] [PubMed] [Google Scholar]
- •14.Gloyn AL, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 15.Babenko AP, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355(5):456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 16.Pearson ER, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355(5):467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 17.Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54(9):2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 18.McTaggart JS, et al. The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J Physiol. 2010;588(Pt 17):3201–3209. doi: 10.1113/jphysiol.2010.191767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •19.Clark RH, et al. Muscle dysfunction caused by a KATP channel mutation in neonatal diabetes is neuronal in origin. Science. 2010;329(5990):458–461. doi: 10.1126/science.1186146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashcroft FM, Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell. 2012;148(6):1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloyn AL, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52(2):568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 22.Florez JC, et al. Haplotype structure and genotype-phenotype correlations of the sulfonylurea receptor and the islet ATP-sensitive potassium channel gene region. Diabetes. 2004;53(5):1360–1368. doi: 10.2337/diabetes.53.5.1360. [DOI] [PubMed] [Google Scholar]
- 23.Schwanstecher C, et al. K(IR)6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic beta-cell ATP-sensitive K(+) channels. Diabetes. 2002;51(3):875–879. doi: 10.2337/diabetes.51.3.875. [DOI] [PubMed] [Google Scholar]
- 24.Hamming KS, et al. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K(+) channel. Diabetes. 2009;58(10):2419–2424. doi: 10.2337/db09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levetan CS, Pierce SM. Distinctions Between the Islets of Mice and Men: Implications for New Therapies for Type 1 and 2 Diabetes. Endocr Pract. 2012:1–36. doi: 10.4158/EP12138.RA. [DOI] [PubMed] [Google Scholar]
- 26.Rorsman P, Braun M. Regulation of Insulin Secretion in Human Pancreatic Islets. Annu Rev Physiol. 2012 doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- ••27.Miki T, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95(18):10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••28.Remedi MS, et al. Hyperinsulinism in mice with heterozygous loss of K(ATP) channels. Diabetologia. 2006;49(10):2368–2378. doi: 10.1007/s00125-006-0367-4. [DOI] [PubMed] [Google Scholar]
- 29.Hugill A, et al. A mutation in KCNJ11 causing human hyperinsulinism (Y12X) results in a glucose-intolerant phenotype in the mouse. Diabetologia. 2010;53(11):2352–2356. doi: 10.1007/s00125-010-1866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miki T, et al. Distinct effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 on insulin secretion and gut motility. Diabetes. 2005;54(4):1056–1063. doi: 10.2337/diabetes.54.4.1056. [DOI] [PubMed] [Google Scholar]
- 31.Ravier MA, et al. Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology. 2009;150(1):33–45. doi: 10.1210/en.2008-0617. [DOI] [PubMed] [Google Scholar]
- ••32.Seghers V, et al. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275(13):9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 33.Shiota C, et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277(40):37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- •34.Szollosi A, et al. Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. J Biol Chem. 2007;282(20):14768–14776. doi: 10.1074/jbc.M701382200. [DOI] [PubMed] [Google Scholar]
- 35.Nenquin M, et al. Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic beta-cells. J Biol Chem. 2004;279(31):32316–32324. doi: 10.1074/jbc.M402076200. [DOI] [PubMed] [Google Scholar]
- 36.Nakazaki M, et al. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes. 2002;51(12):3440–3449. doi: 10.2337/diabetes.51.12.3440. [DOI] [PubMed] [Google Scholar]
- 37.Shibasaki T, et al. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004;279(9):7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 38.Nichols CG, Remedi MS. The diabetic beta-cell: hyperstimulated vs. hyperexcited. Diabetes Obes Metab. 2012;14(Suppl 3):129–135. doi: 10.1111/j.1463-1326.2012.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •39.Koster JC, et al. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100(6):645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- ••40.Koster JC, et al. Hyperinsulinism induced by targeted suppression of beta cell KATP channels. Proc Natl Acad Sci U S A. 2002;99(26):16992–16997. doi: 10.1073/pnas.012479199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahier J, et al. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24(5):366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- ••42.Remedi MS, et al. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of K(ATP)-induced neonatal diabetes mellitus. Cell Metab. 2009;9(2):140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benninger RK, et al. Defects in beta cell Ca(2)+ signalling, glucose metabolism and insulin secretion in a murine model of K(ATP) channel-induced neonatal diabetes mellitus. Diabetologia. 2011;54(5):1087–1097. doi: 10.1007/s00125-010-2039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •44.Girard CA, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119(1):80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porat S, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13(4):440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]