Special Issue: stem cell therapy to rejuvenate mucosal immune responses
Keywords: aging, mucosa, secretory IgA, stem cells, vaccines
Abstract
Age-associated alterations in the mucosal immune system are generally termed mucosal immunosenescence. The major change seen in the aged mucosa is a failure to elicit an antigen-specific secretory IgA (SIgA) antibody response, which is a central player for host defense from various pathogens at mucosal surfaces. In this regard, it would be a first priority to compensate for mucosal dysregulation in the elderly in order to maintain their health in aging. We have successfully established antigen-specific SIgA antibody responses in aged (2 years old) mice, which provide protective immunity from Streptococcus pneumoniae and influenza virus infections, by using a new adjuvant system consisting of a plasmid encoding Flt3 ligand (pFL) and CpG ODN. In order to explore possible use of current mucosal vaccine strategies for the elderly, we have adoptively transferred adipose tissue-derived mesenchymal stem cells (AMSCs) to aged mice prior to mucosal vaccination. This immune therapy successfully resulted in protective antigen-specific antibody responses in the intestinal mucosa of aged mice that were comparable to those seen in young adult mice. In this regard, we postulate that adoptively transferred AMSCs could augment dendritic cell functions in aged mice. The potential cellular and molecular mechanisms whereby AMSCs restore mucosal immunity in immunosenescence are discussed in this short review. A stem cell transfer system could be an attractive and effective immunologic intervention strategy to reverse mucosal immunosenescence.
Introduction
The mucosae can be divided into organized tissues where initial induction of immunity occurs and more diffuse sites where actual effector immune responses take place (1, 2). Gut-associated lymphoid tissues (GALT) and nasopharyngeal-associated lymphoid tissues (NALT) appear to serve as major mucosal inductive sites. Both GALT and NALT are covered by a lymphoepithelium containing microfold (M) cells that continuously take up soluble and particulate antigens (1, 2). Furthermore, underlying the M cells are well organized regions that include the subepithelium with enriched antigen-presenting cells (APCs), a B-cell zone with germinal centers and adjacent T-cell areas including naive and memory T-cell phenotypes (1, 2). Upon antigen activation, memory B- and T-cell populations then emigrate from the mucosal inductive environment via lymphatic drainage, circulate through the bloodstream and home to mucosal effector sites such as the lamina propria of the gastrointestinal (GI), upper respiratory (UR) and reproductive tracts where abundant IgA-producing plasma cells are present (1, 2). Secretory IgA (SIgA) is the primary immunoglobulin involved in protecting mucosal surfaces and is locally produced in mucosal effector tissues (1, 2). In this regard, the majority of T and B cells in effector tissues are activated and express a memory phenotype (1, 2). One can appreciate this sophisticated mucosal immune system for the induction of antigen-specific mucosal immunity. However, age-associated changes are found in these immunocompromised hosts in whom there is an increased risk of infections, which mainly occur via mucosal surfaces. Mouse studies showed that the age-associated alterations, including a reduction in size of Peyer’s patches (PPs), intestinal antigen-specific SIgA antibody responses and lack of oral tolerance induction occur during the aging process (6–12-month-old mice) (3–6). In contrast, NALT functions remain intact during aging with some signs of immunosenescence seen only in fully aged mice (2 years old) (3, 4, 7). In this short review, we will discuss a novel mucosal vaccine strategy and a stem cell immune therapy to overcome impaired mucosal immune responses in the elderly.
Mucosal immunosenescence
It has been shown through extensive immunologic analyses that a dysregulation occurs in GALT with an overall decline in mucosal immunity in the GI tract during aging (3). Although total IgA antibody levels in mucosal secretions in humans and experimental rodents were either increased or remained unchanged in aging, antigen-specific IgA antibody responses in the GI tract were significantly diminished in elderly humans (8, 9) and experimental animals (10–12) including non-human primates (13). It has been shown that the size of PPs in aged mice was significantly reduced (3, 4) and the actual numbers of naive CD4+ T cells are diminished (7). The reduced size of PPs could be due in part to thymic atrophy. However, B-cell follicles, which contribute significant parts to PPs, are also reduced in size. In this regard, this reduced size of PPs could be the outcome of reduced lymphotoxin β (LTβ) signals, which play key roles in tissue organogenesis of PPs and their maintenance (14, 15). Thus, it has been shown that blockage of LTβ signals by an LTβ receptor–immunoglobulin fusion protein induces reduced sized (flattened) PPs in adult mice that are similar to PPs seen in aged mice (14). In addition, recent studies showed that the numbers of mature M cells, which take up luminal antigens into the GALT from the gut lumen, are significantly decreased in aged mice (16). These findings suggest that altered PP functions contribute to impaired antigen-specific immunity in the GI tract. Of importance, our previous finding showed that age-associated alterations are already initiated in the mucosal immune system of the GI tract during a relatively early stage of their life span. Thus, when 1-year-old mice were orally immunized with ovalbumin (OVA) plus cholera toxin (CT), reduced levels of OVA- and B subunit of CT (CT-B)-specific mucosal and systemic immune responses were noted when compared with those exhibited in young adult mice, which resembled those seen in 2-year-old mice given the same oral vaccine (6). Further, reduced sizes of PPs and diminished numbers of naive CD4+ T cells were also noted in 1-year-old mice (3, 4, 7). Taken together, one could infer that an impaired GI tract immunity represents the first sign of mucosal senescence.
As mucosal inductive tissues, PPs and NALT share common features; however, a compartmentalization occurs between the GALT- and NALT-based mucosal immune systems for the induction of antigen-specific immune responses (17). In addition, the progression during organogenesis and lymphocyte trafficking are distinctly regulated (15, 18, 19). Furthermore, our previous studies provided evidence that the aging process in NALT is also distinctly regulated when compared with that of GALT (3, 7). For example, the overall numbers of naive CD4+, CD45RB+ T cells in NALT were comparable between aged and young adult mice. Thus, when 1-year-old mice were nasally immunized with OVA plus CT, elevated levels of antigen-specific mucosal SIgA and plasma IgG antibody responses were induced (7). These results clearly show that both mucosal and systemic immunity occurred in 1-year-old mice following nasal immunization. However, when 2-year-old mice were immunized nasally with OVA and CT as adjuvant, the mice failed to undergo induction of antigen-specific SIgA antibody responses (7, 20). Of interest, these aged mice showed intact plasma anti-OVA IgG antibody responses that were comparable to those induced in young adult mice (7, 20). In this regard, CD4+ T cells from the spleen of aged mice showed significant T-cell proliferation and a Th2 cytokine profile when stimulated with OVA (7). These results indicate that although mucosal immunosenescence takes place at 2 years of age in both GI and UR tracts of mice, the NALT-based mucosal immune system has advantages in that their aging process occurs later than seen with GALT-derived immunity.
A combined adjuvant for rejuvenation of mucosal immunity
It is generally agreed that pathogen-specific SIgA antibody responses are a necessary component for providing a first line of effective immunity against major respiratory pathogens at their entry site. However, antigen-specific mucosal SIgA antibody responses are diminished during the aging process despite slower development of immunosenescence in the NALT-based mucosal immune system. In order to establish effective protection at the mucosa of the elderly, we have begun to target mucosal tissues and immune cells for vaccine delivery. To this end, mucosal dendritic cell (DC)-targeting antigen-delivery systems have been shown to induce antigen-specific SIgA antibody responses (Fig. 1). In order to more broadly stimulate DCs and to avoid polarized Th1 (inflammatory)- or Th2 (allergic)-type immune responses in the elderly, a double adjuvant system has been developed using a combination of the plasmid encoding the Flt3 ligand cDNA (pFL) and CpG ODN. When aged mice were nasally immunized with OVA plus pFL and CpG ODN, significantly increased levels of OVA-specific, SIgA and plasma IgG antibody responses were induced (20) (Fig. 1). Importantly, this double adjuvant system elicited a balanced Th1- and Th2-type cytokine response with essentially no potential inflammatory IL-17 responses (20). In order to assess the functional properties of SIgA antibody responses in aged mice induced by a combined adjuvant system, aged mice were nasally immunized with bacterial- or viral-antigen plus pFL and CpG ODN. When aged mice were nasally immunized with pneumococcal surface protein A (PspA) plus a combination of pFL and CpG ODN, elevated levels of PspA-specific SIgA antibody responses in external secretions were induced that provided protective immunity against nasal Streptococcus pneumoniae colonization (21). In addition, we have shown that a nasal influenza vaccine containing our double adjuvant system pFL and CpG ODN enhanced influenza virus-specific immunity for the prevention of influenza virus infection in aged mice (22).
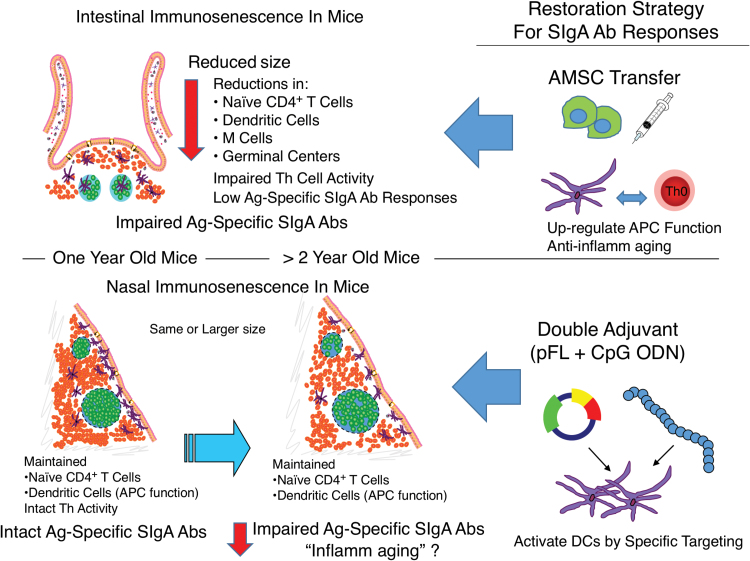
Fig. 1.
Strategies for restoration of mucosal immunosenescence. Reduced induction of antigen-specific intestinal SIgA antibody responses were noted in 1-year-old mice. Adoptive transfer of AMSCs prior to oral vaccination successfully induced protective antigen-specific SIgA antibody responses. In contrast, NALT functions remained intact during aging with notable signs of mucosal immunosenescence (loss of antigen-specific SIgA antibody responses) seen only in 2-year-old mice. To overcome impaired mucosal immunity, vaccines containing pFL and CpG ODN have been developed for protective antigen-specific SIgA antibody induction.
Adipose tissue-derived mesenchymal stem cell therapy restores protective antigen-specific SIgA antibody responses in the GI tract
Although the combined adjuvant strategy successfully elicited pathogen-specific SIgA antibody responses that provided protective immunity in aged mice, the intrinsic aged condition may not be changed. In this regard, we have assessed the potential of mesenchymal stem cells (MSCs), especially adipose tissue-derived MSCs (AMSCs) for the restoration of mucosal immunity in aged mice since various clinical trials have shown the regenerative capacity of MSCs (23–25). For example, a combination of AMSCs and fibrin glue therapy improved healing in complex perianal fistulas, which could potentially be relevant for the treatment of inflammatory bowel diseases (23). Others have shown that transplantation of monolayered MSCs is an effective new therapeutic strategy for cardiac tissue regeneration (24). In addition, it was shown that AMSCs could be used for treatment of Alzheimer’s disease (26) and periodontal disease (27).
Aged (>18 months old) mice were adoptively transferred with or without AMSCs (2 × 106, <10 passages in culture). AMSCs were isolated from adipose tissue of either young C57BL/6 mice or a young female human subject by a combination of enzymatic digestion and centrifugation (28). Two weeks later, both groups of mice were orally immunized with OVA plus CT three times at weekly intervals. Seven days after the final immunization, when fecal extract samples and plasma were subjected to OVA- and CT-B-specific ELISA, elevated levels of mucosal SIgA and plasma IgG antibody responses were noted in aged mouse recipients when compared with aged mice without AMSC transfer (Fig. 1) (28). The induction of antigen-specific SIgA antibody responses was supported by increased levels of IL-4 production in mucosal tissues of aged mice that was achieved by pre-treatment with AMSCs (28). Of importance, antigen-specific SIgA antibodies in aged mice restored by adoptive transfer of AMSCs were functional. Thus, fecal extracts containing CT-B-specific SIgA antibodies exhibited neutralization activity against CT intoxication (28). This is a unique and new feature of AMSCs that contrasts with previous studies generally showing that MSCs down-regulate various immunocompetent cells (29–31). More recently, MSCs were reported to inhibit both CD4+ and CD8+ T-cell proliferation following co-culture and polyclonal stimulation (32–34). Other in vitro studies showed reduced antibodies in mixed lymphocyte cultures (35) as well as diminished B-cell proliferation and antibody synthesis (36). Finally, co-culture of MSCs with splenic B cells induced IL-10-producing regulatory B cells that ameliorated autoimmunity and antibody synthesis (37). Our new system has taken a separate approach that allows AMSCs to restore an impaired mucosal immune system. The major difference with the studies of others is that we have assessed AMSC functions by adoptive transfer in vivo in a mouse model. Further, we employed serum-free medium to expand AMSCs. Thus, transferred AMSCs and their soluble products including exosomes may totally differ from others and up-regulate various immunocompetent cells. In this regard, it will be important to establish the minimum numbers of AMSCs that can reverse impaired mucosal immunity. Further, it would be of great interest to determine whether AMSCs from aged mice or humans could provide similar functions. These points are currently under investigation in our laboratory.
Potential mechanisms for overcoming mucosal immunosenescence
One of the features of immunosenescence is an increased baseline of inflammation known as ‘inflamm-aging’ (38). Thus, chronic inflammatory responses may hamper induction of antigen-specific immune responses when active immunization is initiated, since it is essential to induce a transient inflammatory innate immune response in order to elicit subsequent acquired immunity (39). It has been shown that MSCs also exhibited potential roles for anti-inflammatory functions (40). Thus, MSCs have been employed as therapeutic strategies for various immune disorders including graft-versus-host disease (41–43), organ transplantation (44–47), autoimmune diseases (48–52) and inflammatory bowel disease (53, 54). Indeed, MSCs interact with T cells to reduce their pro-inflammatory cytokine production (31, 55), while increasing their production of anti-inflammatory cytokines, including IL-4 and IL-10 (56, 57). In this regard, we considered the possibility that AMSC transfer into aged mice could reduce inflamm-aging and facilitate the subsequent restoration of antigen-specific immune responses when mice were orally immunized with OVA and CT (Fig. 2). Of importance, our studies noted increased numbers of IL-4-producing CD4+ T cells with increased levels of OVA-induced IL-4 production by CD4+ T cells in PPs. Since IL-4 is an essential Th2-type cytokine for adjuvant activity of CT (58, 59), these results clearly indicate that AMSCs enhanced IL-4 production in aged mice, which could also potentially down-regulate inflammatory responses and simultaneously allow CT to enhance OVA-specific antibody responses.
Fig. 2.
Possible scenario for adoptive transfer of AMSCs for anti-aging in the GI tract. The ‘inflamm-aging’, chronic inflammatory responses may hamper induction of antigen-specific immune responses when active immunization is initiated. It is possible that AMSCs could reduce inflamm-aging and facilitate the subsequent restoration of antigen-specific protective immunity. Further, AMSC adoptive transfer may increase the numbers of immature DCs which leads to up-regulation of APC functions. Finally, adoptive transfer of AMSCs could result in changes in the microbiota in order to correct intestinal dysbiosis in aged mice.
In addition to interacting with T and B cells, MSCs also play regulatory roles for innate immune cells including monocytes, macrophages and DCs (60–65). Notably, DCs co-cultured with MSCs showed aberrant maturation, cytokine production and down-regulation of activated T cells (60, 62, 63, 65). Of interest, when mature DCs were co-cultured with MSCs, these DCs lost high levels of CD11c, MHC class II and co-stimulatory molecules. Further, MSC-co-cultured DCs exhibited CD11b molecule expression and phagocytic activity, which characterize immature DCs (65). Although in vitro MSC-treated, mature DCs failed to induce T-cell proliferation, this DC population expressed CD11c and MHC class II when adoptively transferred into recipient mice (65). These results suggest that in vivo interactions of MSCs and mature DCs may induce an immature DC phenotype with APC function for activation of naive T cells and subsequent antigen-specific antibody responses. Even though in vitro MSC-treated, mature DCs express Jagged-2, which is essential for down-regulation of activated T cells (65), it has been shown that Jagged-2 expressed by DCs interacts with Notch-expressing, naive CD4+ T cells and thus plays a central role in the induction of effector Th2-type CD4+ T cells. Indeed, our current findings showed that adoptive transfer of AMSCs prior to oral immunization elicited IL-4-producing, antigen-specific CD4+ T cells in aged mice. Thus, it is possible that AMSCs interact with mature DCs to regenerate immature DCs for increased total numbers of APCs in order to restore impaired mucosal immune responses in aged mice (Fig. 2). To support this view, our previous studies (discussed above) showed that the DC-targeting mucosal adjuvant pFL and CpG ODN successfully induced antigen-specific SIgA antibody responses in aged mice (20–22). Studies to address these issues are currently under investigation.
It has been suggested that two major differences associated with aging are the decline in the gut immune system and changes in the distribution of the intestinal microbiota, termed dysbiosis (66). Indeed, it has been reported that significant alterations in the intestinal microflora occur in the elderly (>65 years old) (67, 68). In contrast, other human microbiome analyses showed that centenarians were associated with increased inflammatory cytokine responses, but not in the elderly group (average age 70 ± 3), which exhibited a change in their microbiota (69). On the basis of these findings, one could predict that adoptive transfer of AMSCs possibly re-shapes the dysbiosis of the intestinal microflora in aged mice to more resemble that seen in young adult mice (Fig. 2). To test this hypothesis, we have characterized the microbiota of aged mice given AMSCs via adoptive transfer by meta-genomic analyses. Our preliminary studies showed that fecal pellets of aged mice exhibited significant changes in bacterial species after AMSC transfer. We are currently confirming these findings and comparing the microflora of aged mice given AMSCs by adoptive transfer with those of young adult mice to determine if an actual re-shaping of the microbiota occurs.
Conclusion
In this review, we showed that a DC-targeting double adjuvant system and AMSC transfer immune therapy are potential strategies for restoration of mucosal immunity in aging (Fig. 1). Thus, a combination of pFL and CpG ODN effectively activates DCs in the mucosal inductive tissues and subsequently induces antigen-specific protective SIgA antibody responses in aged mice. AMSC transfer potentially down-regulates pre-existing inflammatory responses in aged mice by enhancing APC functions that regulate the induction of DC-mediated mucosal immunity (Fig. 2). Our present findings exploit a novel anti-aging role for AMSCs to restore functions in the GI tract of the mucosal immune system. Although it seems that the up-regulation of APC functions is the key element of both anti-aging strategies, we still need to address other cellular and molecular mechanisms for the reversal of immunosenescence. It is essential to further study the precise cellular and molecular mechanisms where a combined adjuvant and AMSCs restore the mucosal immune system in aging in order to expand this knowledge to potential clinical applications.
Funding
A portion of the work described in this review was supported by research funding from the BioMimetics Sympathy Inc. (Tokyo, Japan), the National Institutes of Health (NIH) grant AG 025873 (to K.F.), Japan Society for the Promotion of Science (JSPS) grant JP25461558, JP26670514 and JP16K10039 (to D.T.) as well as The Morinaga Foundation for Health & Nutrition (to D.T.).
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Fujihashi K. Boyaka P. N. and McGhee J. R. 2013. Host defenses at mucosal surfaces. In Rich R. T. Fleisher T. A. Shearer W. T. Schroeder H. W. Frew A. J. and Weyand C. M, eds., Clinical Immunology, 4th edn., p. 287 Mosby Elsevier, Philadelphia, PA. [Google Scholar]
- 2. Kiyono H. Kunisawa J. McGhee J. R. and Mestecky J. 2008. The mucosal immune system. In Paul W. E, ed., Fundamental Immunology, 5th edn., p. 983 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3. Fujihashi K. and Kiyono H. 2009. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 30:334. [DOI] [PubMed] [Google Scholar]
- 4. Fujihashi K. and McGhee J. R. 2004. Mucosal immunity and tolerance in the elderly. Mech. Ageing Dev. 125:889. [DOI] [PubMed] [Google Scholar]
- 5. Kato H., Fujihashi K., Kato R., et al. 2003. Lack of oral tolerance in aging is due to sequential loss of Peyer’s patch cell interactions. Int. Immunol. 15:145. [DOI] [PubMed] [Google Scholar]
- 6. Koga T. McGhee J. R. Kato H. Kato R. Kiyono H. and Fujihashi K. 2000. Evidence for early aging in the mucosal immune system. J. Immunol. 165:5352. [DOI] [PubMed] [Google Scholar]
- 7. Hagiwara Y., McGhee J. R., Fujihashi K., et al. 2003. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J. Immunol. 170:1754. [DOI] [PubMed] [Google Scholar]
- 8. Powers D. C. 1992. Immunological principles and emerging strategies of vaccination for the elderly. J. Am. Geriatr. Soc. 40:81. [DOI] [PubMed] [Google Scholar]
- 9. Schmucker D. L. Heyworth M. F. Owen R. L. and Daniels C. K. 1996. Impact of aging on gastrointestinal mucosal immunity. Dig. Dis. Sci. 41:1183. [DOI] [PubMed] [Google Scholar]
- 10. Enioutina E. Y. Visic V. D. and Daynes R. A. 2000. Enhancement of common mucosal immunity in aged mice following their supplementation with various antioxidants. Vaccine 18:2381. [DOI] [PubMed] [Google Scholar]
- 11. Schmucker D. L. Daniels C. K. Wang R. K. and Smith K. 1988. Mucosal immune response to cholera toxin in ageing rats. I. Antibody and antibody-containing cell response. Immunology 64:691. [PMC free article] [PubMed] [Google Scholar]
- 12. Thoreux K. Owen R. L. and Schmucker D. L. 2000. Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology 101:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor L. D. Daniels C. K. and Schmucker D. L. 1992. Ageing compromises gastrointestinal mucosal immune response in the rhesus monkey. Immunology 75:614. [PMC free article] [PubMed] [Google Scholar]
- 14. Dohi T., Rennert P. D., Fujihashi K., et al. 2001. Elimination of colonic patches with lymphotoxin beta receptor-Ig prevents Th2 cell-type colitis. J. Immunol. 167:2781. [DOI] [PubMed] [Google Scholar]
- 15. Kunisawa J. Nochi T. and Kiyono H. 2008. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 29:505. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi A., Donaldson D. S., Erridge C., et al. 2013. The functional maturation of M cells is dramatically reduced in the Peyer’s patches of aged mice. Mucosal Immunol. 6:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmgren J. and Czerkinsky C. 2005. Mucosal immunity and vaccines. Nat. Med. 11:S45. [DOI] [PubMed] [Google Scholar]
- 18. Macpherson A. J. McCoy K. D. Johansen F. E. and Brandtzaeg P. 2008. The immune geography of IgA induction and function. Mucosal Immunol. 1:11. [DOI] [PubMed] [Google Scholar]
- 19. Pascual D. W. Riccardi C. and Csencsits-Smith K. 2008. Distal IgA immunity can be sustained by αEβ7+ B cells in L-selectin-/- mice following oral immunization. Mucosal Immunol. 1:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukuiwa T., Sekine S., Kobayashi R., et al. 2008. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine 26:4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukuyama Y., King J. D., Kataoka K., et al. 2011. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J. Immunol. 186:2454. [DOI] [PubMed] [Google Scholar]
- 22. Asanuma H., Zamri N. B., Sekine S., et al. 2012. A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine 30:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia-Olmo D. Garcia-Arranz M. and Herreros D. 2008. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn’s disease. Expert Opin. Biol. Ther. 8:1417. [DOI] [PubMed] [Google Scholar]
- 24. Miyahara Y., Nagaya N., Kataoka M., et al. 2006. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 12:459. [DOI] [PubMed] [Google Scholar]
- 25. Psaltis P. J. Zannettino A. C. Worthley S. G. and Gronthos S. 2008. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26:2201. [DOI] [PubMed] [Google Scholar]
- 26. Katsuda T., Tsuchiya R., Kosaka N., et al. 2013. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 3:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tobita M. Orbay H. and Mizuno H. 2011. Adipose-derived stem cells: current findings and future perspectives. Discov. Med. 11:160. [PubMed] [Google Scholar]
- 28. Aso K., Tsuruhara A., Takagaki K., et al. 2016. Adipose-derived mesenchymal stem cells restore impaired mucosal immune responses in aged mice. PLoS ONE 11:e0148185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aggarwal S. and Pittenger M. F. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815. [DOI] [PubMed] [Google Scholar]
- 30. Glennie S. Soeiro I. Dyson P. J. Lam E. W. and Dazzi F. 2005. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105:2821. [DOI] [PubMed] [Google Scholar]
- 31. Krampera M. Glennie S. Dyson J. Scott D. Laylor R. Simpson E. and Dazzi F. 2003. Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood 101:3722. [DOI] [PubMed] [Google Scholar]
- 32. Cuerquis J. Romieu-Mourez R. Francois M. Routy J. P. Young Y. K. Zhao J. and Eliopoulos N. 2014. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-gamma and tumor necrosis factor-alpha stimulation. Cytotherapy 16:191. [DOI] [PubMed] [Google Scholar]
- 33. Dorronsoro A., Ferrin I., Salcedo J. M., et al. 2014. Human mesenchymal stromal cells modulate T-cell responses through TNF-α-mediated activation of NF-κB. Eur. J. Immunol. 44:480. [DOI] [PubMed] [Google Scholar]
- 34. Malcherek G., Jin N., Huckelhoven A. G., et al. 2014. Mesenchymal stromal cells inhibit proliferation of virus-specific CD8(+) T cells. Leukemia 28:2388. [DOI] [PubMed] [Google Scholar]
- 35. Comoli P., Ginevri F., Maccario R., et al. 2008. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol. Dial. Transplant. 23:1196. [DOI] [PubMed] [Google Scholar]
- 36. Corcione A., Benvenuto F., Ferretti E., et al. 2006. Human mesenchymal stem cells modulate B-cell functions. Blood 107:367. [DOI] [PubMed] [Google Scholar]
- 37. Park M. J. Kwok S. K. Lee S. H. Kim E. K. Park S. H. and Cho M. L. 2015. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 24:2367. [DOI] [PubMed] [Google Scholar]
- 38. Franceschi C., Capri M., Monti D., et al. 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128:92. [DOI] [PubMed] [Google Scholar]
- 39. Iwasaki A. and Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ho M. S. Mei S. H. and Stewart D. J. 2015. The immunomodulatory and therapeutic effects of mesenchymal stromal cells for acute lung injury and sepsis. J. Cell. Physiol. 230:2606. [DOI] [PubMed] [Google Scholar]
- 41. Le Blanc K., Frassoni F., Ball L., et al. ; Developmental Committee of the European Group for Blood and Marrow Transplantation 2008. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579. [DOI] [PubMed] [Google Scholar]
- 42. Perez-Simon J. A., Lopez-Villar O., Andreu E. J., et al. 2011. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica 96:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prasad V. K., Lucas K. G., Kleiner G. I., et al. 2011. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol. Blood Marrow Transplant. 17:534. [DOI] [PubMed] [Google Scholar]
- 44. Casiraghi F. Perico N. and Remuzzi G. 2013. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr. Opin. Organ Transplant. 18:51. [DOI] [PubMed] [Google Scholar]
- 45. Liang J., Zhang H., Hua B., et al. 2010. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann. Rheum. Dis. 69:1423. [DOI] [PubMed] [Google Scholar]
- 46. Tan J., Wu W., Xu X., et al. 2012. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 307:1169. [DOI] [PubMed] [Google Scholar]
- 47. Yamout B., Hourani R., Salti H., et al. 2010. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J. Neuroimmunol. 227:185. [DOI] [PubMed] [Google Scholar]
- 48. Connick P., Kolappan M., Crawley C., et al. 2012. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 11:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Figueroa F. E. Carrión F. Villanueva S. and Khoury M. 2012. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol. Res. 45:269. [DOI] [PubMed] [Google Scholar]
- 50. Lee H. J., Lee J. K., Lee H., et al. 2012. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol. Aging 33:588. [DOI] [PubMed] [Google Scholar]
- 51. Lee P. H. Kim J. W. Bang O. Y. Ahn Y. H. Joo I. S. and Huh K. 2008. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin. Pharmacol. Ther. 83:723. [DOI] [PubMed] [Google Scholar]
- 52. Wang D., Zhang H., Liang J., et al. 2013. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 22:2267. [DOI] [PubMed] [Google Scholar]
- 53. Forbes G. M., Sturm M. J., Leong R. W., et al. 2014. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin. Gastroenterol. Hepatol. 12:64. [DOI] [PubMed] [Google Scholar]
- 54. Knyazev O. V. Parfenov A. I. Shcherbakov P. L. Ruchkina I. N. and Konoplyannikov A. G. 2013. Cell therapy of refractory Crohn’s disease. Bull. Exp. Biol. Med. 156:139. [DOI] [PubMed] [Google Scholar]
- 55. Di Nicola M. Carlo-Stella C. Magni M. Milanesi M. Longoni P. D. Matteucci P. Grisanti S. and Gianni A. M. 2002. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838. [DOI] [PubMed] [Google Scholar]
- 56. Kong Q. F., Sun B., Bai S. S., et al. 2009. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J. Neuroimmunol. 207:83. [DOI] [PubMed] [Google Scholar]
- 57. Prevosto C. Zancolli M. Canevali P. Zocchi M. R. and Poggi A. 2007. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 92:881. [DOI] [PubMed] [Google Scholar]
- 58. Okahashi N., Yamamoto M., VanCott J. L., et al. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vajdy M. Kosco-Vilbois M. H. Kopf M. Köhler G. and Lycke N. 1995. Impaired mucosal immune responses in interleukin 4-targeted mice. J. Exp. Med. 181:41. [DOI] [PubMed] [Google Scholar]
- 60. Beyth S., Borovsky Z., Mevorach D., et al. 2005. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T cell unresponsiveness. Blood 105:2214. [DOI] [PubMed] [Google Scholar]
- 61. Cho D. I., Kim M. R., Jeong H. Y., et al. 2014. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 46:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Djouad F., Charbonnier L. M., Bouffi C., et al. 2007. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25:2025. [DOI] [PubMed] [Google Scholar]
- 63. English K. Barry F. P. and Mahon B. P. 2008. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol. Lett. 115:50. [DOI] [PubMed] [Google Scholar]
- 64. Kim M. G., Kim S. H., Noh H., et al. 2013. CD11c(+) cells partially mediate the renoprotective effect induced by bone marrow-derived mesenchymal stem cells. PLoS ONE 8:e72544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang B., Liu R., Shi D., et al. 2009. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood 113:46. [DOI] [PubMed] [Google Scholar]
- 66. Sato S. Kiyono H. and Fujihashi K. 2015. Mucosal immunosenescence in the gastrointestinal tract: a mini-review. Gerontology 61:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Claesson M. J., Cusack S., O’Sullivan O., et al. 2011. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. (USA) 108(Suppl. 1):4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Woodmansey E. J. 2007. Intestinal bacteria and ageing. J. Appl. Microbiol. 102:1178. [DOI] [PubMed] [Google Scholar]
- 69. Biagi E., Nylund L., Candela M., et al. 2010. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 5:e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]