Direct effects of antibiotics in lung DC-mediated immune tolerance
Keywords: antibiotics, asthma, dendritic cell, germ-free, Th2 immunity
Abstract
Immune tolerance in the lung is important for preventing hypersensitivity, such as allergic asthma. Maintenance of tolerance in the lung is established by coordinated activities of poorly understood cellular and molecular mechanisms, including participation of dendritic cells (DCs). We have previously identified DC expression of the signaling molecule TRAF6 as a non-redundant requirement for the maintenance of immune tolerance in the small intestine of mice. Because mucosal tissues share similarities in how they interact with exogenous antigens, we examined the role of DC-expressed TRAF6 in the lung. As with the intestine, we found that the absence TRAF6 expression by DCs led to spontaneous generation of Th2-associated immune responses and increased susceptibility to model antigen-induced asthma. To examine the role of commensal microbiota, mice deficient in TRAF6 in DCs were treated with broad-spectrum antibiotics and/or re-derived on a germ-free (GF) background. Interestingly, we found that antibiotics-treated specific pathogen-free, but not GF, mice showed restored immune tolerance in the absence of DC-expressed TRAF6. We further found that antibiotics mediate microbiota-independent effects on lung T cells to promote immune tolerance in the lung. This work provides both a novel tool for studying immune tolerance in the lung and an advance in our conceptual understanding of potentially common molecular mechanisms of immune tolerance in both the intestine and the lung.
Introduction
Mucosal immune tolerance mechanisms are most prominently studied between the immune cells of the intestinal lining and commensal microbiota (1, 2). However, immune tolerance is also of great importance in other mucosal tissues, such as the lung, where the role of commensal microbiota is not well characterized (3, 4). Recently, it has become better appreciated that mechanisms of immune tolerance may be shared across various organs, especially between the mucosal tissues where similar resident cell types sample and surveil antigens at the external borders (5, 6). It is also possible that stimuli encountered in one mucosal location have effects on the maintenance of immune tolerance at another mucosal site because of trafficking of immune cells, soluble factors or even the stimuli themselves (7–9). Dendritic cells (DCs) are among the most important cellular regulators of immune tolerance in the mucosal tissues (10, 11). We have previously generated and described mice that lack TRAF6, an intracellular signaling mediator of various inflammatory pathways (12), specifically in the DC lineage (TRAF6ΔDC) (13). TRAF6ΔDC mice spontaneously develop Th2-associated pathology of the small intestine, with elevated tissue mRNA levels of pro-fibrogenic factors like Igf-1 and Acta2. TRAF6ΔDC mice exhibit increases in small intestine infiltration of eosinophils and IL-13+IL-4+ (Th2) CD4 T cells (concomitant with decreased Th1 cells) and have higher levels of serum IgE and IgG1. Additionally, significant deficiencies are found in TRAF6ΔDC Foxp3+ CD4 Treg numbers in the small intestine. Because Th2-linked hypersensitivity is of particular clinical importance to the lung, where dysregulation can result in the development and persistence of asthma (14, 15), we reasoned that TRAF6ΔDC mice could be a useful model to examine potential mechanistic links between gut and lung cross-talk with respect to immune tolerance. To this end, we examined the role of DC-expressed TRAF6 in the maintenance of immune tolerance of the lung. We report here that TRAF6-dependent signaling is essential to establish and/or maintain immune tolerance in the lung to prevent spontaneous Th2-associated hypersensitivity. Moreover, as we previously found in the gut, lung hypersensitivity also occurs in a microbiota-independent manner. These findings highlight the need to identify novel sources of inflammatory stimuli that interact with immune cells to break tolerance and drive pathologic hypersensitivity in mucosal tissues.
Methods
Animals
TRAF6ΔDC mice were described previously (9). Specific pathogen-free (SPF) C57BL/6 mice were purchased from Jackson Laboratory. Mice were maintained under SPF conditions in the animal care facility at POSTECH. As described previously (16), a colony of germ-free (GF) C57BL/6 mice was established at POSTECH from breeders obtained from Dr Andrew Macpherson (Bern University, Switzerland). Twelve SPF C57BL/6 mice and 26 GF C57BL/6 mice were used as recipients for bone marrow (BM) chimeras and antibiotic treatment experiments. All mice received care in compliance with the protocols approved by the Institutional Animal Care and Use Committees (IACUC) of the Pohang University of Science and Technology (POSTECH-2016-0019). GF mice were maintained in sterile flexible film isolators (Class Biological Clean Ltd, USA) and GF status was monitored monthly by anaerobic and aerobic culture of cecal contents. Normal chow diet (2018S, Harlan Laboratories) and purified water were supplied after sterilization by autoclave. All mice were humanely euthanized with CO2 before analysis or collecting cell populations.
Buffers and media
FACS buffer containing 2% fetal bovine serum (FBS) and 0.7mM EDTA was used to prepare, stain and wash isolated cells. Complete RPMI 1640 medium containing 10% FBS, 10 U ml−1 penicillin, 10 mg ml−1 streptomycin, 2 mM l-glutamine (Invitrogen) and 50 mM β-mercaptoethanol (Sigma) was used for cell culture and stimulation.
Cell isolation
Lung tissues were extracted from mice after transcardial perfusion to remove circulating blood cells. The lung tissues were minced and incubated in 3% FBS RPMI 1640 medium containing 2 mg ml−1 collagenase D (Roche), 100 μg ml−1 DNaseI (Sigma), 1 mM sodium pyruvate, 1 mM non-essential amino acid and 20 mM HEPES at 37°C for 40 min in a shaker. Mononuclear cells were collected at the interface between 40 and 70% Percoll (GE Healthcare) after centrifuging at 2500 rpm at room temperature for 20 min with no brake.
Flow cytometry
For immunophenotypic analyses, 106 cells were stained with fluorochrome-conjugated antibodies including those specific for CD4 (RM4-5), CD8 (53-6.7), CD90.2 (53-2.1), CD44 (IM-7), CD62L (Mel-14), CD11c (N418) and Siglec-F (E50-2440). For intracellular staining of Foxp3 transcription factor and proliferation marker (Ki-67), 106 cells were fixed and permeabilized with fixation/permeabilization reagent (eBioscience) after staining for surface markers including CD4, CD8 and CD90.2. Intracellular staining was performed by using antibodies specific for Foxp3 (FJK-16 s) and Ki-67 (SolA15). For detection of T cells producing cytokines, 106 cells were stimulated in round-bottom 96-well plates with complete RPMI 1640 medium containing 500 ng ml−1 ionomycin and 5 ng ml−1 PMA (Sigma) in the presence of Brefeldin A (BD) for the final 3 h. After surface staining, the cells were fixed and permeabilized with fixation/permeabilization reagent (BD) and stained with intracellular protein-specific antibodies specific for IL-13 (eBio13A) and IFN-γ (XMG1.2). All stained samples were analyzed on an LSR Fortessa flow cytometer (BD) and the raw data were calculated and visualized with FlowJo software (Tree Star). All of the antibodies were purchased from BD, eBioscience, Biolegend and Tonbo Biosciences.
Histological analysis
Lung specimens were fixed in 4% paraformaldehyde solution (Sigma) and paraffin-embedded sections were performed. Routine hematoxylin and eosin (H&E) staining was performed using hematoxylin for nucleus staining, and eosin for cytoplasm and muscle layer.
Quantitative PCR
Lung tissues were homogenized in TRIzol (Invitrogen) after freezing in liquid nitrogen. After, cDNA was synthesized using a QuantiTect Reverse Transcription Kit (QIAGEN), and quantitative PCR was performed using TaqMan gene probes with TaqMan Universal PCR Master Mix (Applied Biosystems) on a ViiA7 Real-time PCR System (Applied Biosystems): 2 min at 50°C, 10 min at 95°C, 50 cycles of 15 s 95°C, 1 min at 60°C, and signals were detected during the annealing step (60°C). Relative mRNA expression levels of all samples were normalized to 18S mRNA. The following TaqMan gene probes (Applied Biosystems) were used: Igf1 (Mm00439560_m1), Il12b (Mm99999067_m1), Il13 (Mm99999190_m1), Il5 (Mm00439646_m1) and 18S ribosomal RNA (Hs99999901_s1).
Intranasal ovalbumin administration
Ten-week-old TRAF6ΔDC and control mice were gently anesthetized and delivered an intranasal administration of 100 μg ovalbumin (OVA; Sigma) in 20 μl PBS. The mice were administrated on 3 consecutive days and another set of three consecutive day OVA administrations was performed from 7 days after the first administration using the same protocol.
Antibiotic treatment
Broad-spectrum antibiotic treatment was supplied for 2 weeks in drinking water containing ampicillin (1 g l−1, Sigma), vancomycin (0.5 g l−1, Calbiochem), neomycin sulfate (1 g l−1, Calbiochem) and metronidazole (1 g l−1, Sigma). All antibiotic solutions were prepared by sterile by 0.2-μm membrane filtration. The antibiotic solution was replaced every 4 days.
Establishment of BM chimeras
The protocol for establishment of BM chimeras was previously described (16). Briefly, for irradiation of GF recipients, the mice were transferred to an autoclaved plastic cylinder sealed with mylar film. After irradiation, the cylinder was connected to the GF isolator and mice were transferred back to the isolator. BM stem cells were isolated from tibias and femurs of TRAF6ΔDC and control mice and T cells were removed by employing combined anti-CD4, anti-CD8 and anti-CD90.2 MACS depletion (Miltenyi Biotec) followed by transfer into autoclaved amber glass vials with silicone caps. The vials were imported into a GF isolator after sterilizing with 2% peracetic acid solution. Cells (5 × 106) were transferred via retro-orbital injection into irradiated GF or SPF C57BL/6 recipient mice. Chimeric mice were used for experiments upon hematopoietic reconstitution ~8 weeks after BM transplantation.
Statistics
Data were analyzed using Prism software (GraphPad) with an unpaired or paired Student’s t-test. P values <0.05 were considered significant.
Ethics statement
This research was approved by the IACUC of the Pohang University of Science and Technology (POSTECH-2016-0019). Mouse care and experimental procedures were performed in accordance with all institutional guidelines for the ethical use of non-human animals in research and protocols from IACUC of the Pohang University of Science and Technology.
Results
Spontaneous Th2 immunity in the lung of TRAF6ΔDC mice
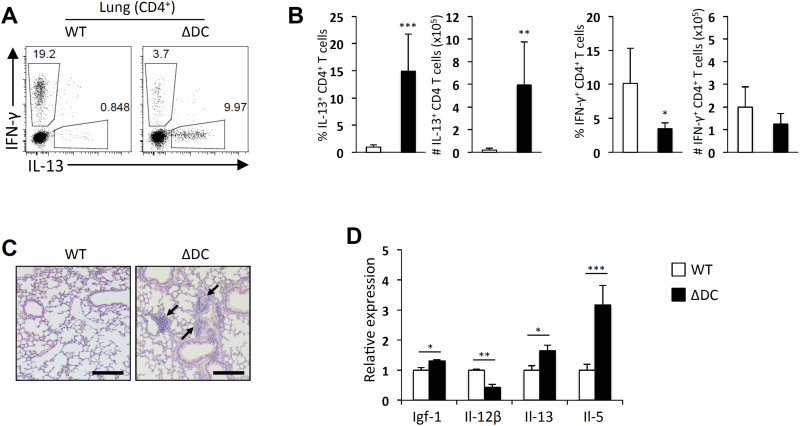
Because we previously observed spontaneous development of Th2-associated immunopathology in the small intestine of TRAF6∆DC mice, we investigated whether the lung is similarly affected in TRAF6∆DC mice. We first examined the cytokine production profiles of CD4 T cells harvested from lungs of 20-week-old control and TRAF6∆DC mice and found that TRAF6∆DC mice exhibited significant increases in both the frequency and numbers of IL-13-producing cells with slightly decreased frequency of IFN-γ-producing cells (Fig. 1A and B). Histological analysis showed increased cellular infiltration of the lungs of TRAF6∆DC mice compared to littermate control mice (Fig. 1C). Relative mRNA expression analysis of various factors in total lung tissue revealed that while the relative mRNA levels of the Th1-associated cytokine IL-12β was reduced in TRAF6∆DC lungs compared to control lungs, the mRNA levels of the pro-fibrogenic factor Igf-1 and the Th2-associated cytokines IL-13 and IL-5 were significantly increased in TRAF6∆DC lungs (Fig. 1D). These observations suggest that the spontaneous Th2-like phenotype observed in the gut of TRAF6∆DC extends to the lung.
Fig. 1.
Spontaneous Th2 immunity in the TRAF6ΔDC lung. (A, B) Increases of IL-13-producing CD4 T cells were observed in the lungs of 20-week-old TRAF6ΔDC (ΔDC) mice. (A) The representative FACS plots gated on CD4 T cells of lungs show intracellular staining for IFN-γ and IL-13. (B) Percentage and number of IFN-γ- or IL-13-producing CD4 T cells are presented in histograms. (C) Histological analyses were performed by H&E staining of lungs from each mouse. Scale bars represent 200 μm. (D) Fibrosis marker (Igf-1), pro-inflammatory cytokine (Il-12β) and Th2 cell cytokines (Il-13 and Il-5) mRNA expression levels in the lung tissues. Histograms (mean ± SD) are representative of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
TRAF6ΔDC mouse susceptibility to model antigen-induced asthma
Th2-driven hypersensitivity of the lung is typically manifested in response to inhaled antigens (17). In order to determine whether Th2-associated pathology exhibited in the lung in TRAF6ΔDC mice is driven by inhaled antigen, we performed model antigen-induced asthma assays. Control and TRAF6ΔDC mice were exposed to 100 µg chicken OVA intranasally in PBS or PBS alone for 3 consecutive days and then re-challenged with intranasal OVA or PBS 1 week later for an additional 3 days. Analysis of the lungs of control and TRAF6ΔDC mice revealed that while eosinophil infiltration was higher in TRAF6ΔDC mice than in control mice given only PBS, eosinophil frequencies in the lungs of TRAF6ΔDC mice exposed to OVA were more dramatically increased compared to similarly treated control mice (Fig. 2A and B). Furthermore, histological analysis of lung tissue revealed significantly increased cellular infiltration in OVA-treated TRAF6∆DC mice compared to littermate control mice (Fig. 2C). These data further suggest that TRAF6ΔDC mice may be a suitable model for studying mechanisms of respiratory hypersensitivity driven by inhaled antigens.
Fig. 2.
TRAF6ΔDC susceptibility to model antigen-induced asthma. Intranasal OVA (100 μg in 20 μl PBS) administration was performed on TRAF6ΔDC (ΔDC) and control (WT) mice on 3 consecutive days (day 0–2) and another set of 3 consecutive day administrations was performed a week after the first administration (day 7–9). The mice were analyzed on day 10 from the first administration. (A) The representative FACS plots show the eosinophil population (Siglec-F+ CD11c−) in the lung tissues of each mouse and (B) the percentage of eosinophils is presented in the histogram (mean ± SD; *P < 0.05). (C) Histological analyses were performed by H&E staining of lungs from each condition. Scale bars represent 200 μm.
Effect of antibiotics on Th2 immunity in the TRAF6ΔDC lung
Commensal microbiota, found primarily in the gut, but also at lower numbers in the lung, is considered to have a significant role in influencing immune tolerance regulated via gut-lung cross-talk (18). We have previously shown that treatment of TRAF6ΔDC mice with broad-spectrum antibiotics abrogates the development of spontaneous Th2 responses in the small intestine. To determine the effects of antibiotic treatment on the phenotype of the TRAF6ΔDC lung, 20-week-old TRAF6ΔDC mice maintained under SPF conditions were provided broad-spectrum antibiotics containing 1 g l−1 ampicillin, 1 g l−1 neomycin, 0.5 g l−1 vancomycin and 1 g l−1 metronidazole ad libitum in drinking water for a period of 2 weeks. Cells were then isolated from lungs of both antibiotics-treated and untreated TRAF6ΔDC mice to reveal that antibiotics treatment significantly reduced the frequency and number of activated (CD44hiCD62Llo) lung CD4 T cells (Fig. 3A). Furthermore, in vitro stimulation of lung CD4 T cells with PMA/ionomycin showed that antibiotics treatment significantly reduced the frequency and number of IL-13-producing cells, while no significant effect on IFN-γ-producing cells was observed (Fig. 3B). These data show that antibiotics treatment ameliorates spontaneous Th2 responses in the TRAF6ΔDC lung and suggest that they may act in a manner similar to that observed in the small intestine of the same mice.
Fig. 3.
Effect of antibiotics on Th2 immunity in the TRAF6ΔDC lung. Cells were isolated from lung tissues of TRAF6ΔDC mice. Some of TRAF6ΔDC mice were provided with antibiotics (Abx) containing 1 g l−1 ampicillin, 1 g l−1 neomycin, 0.5 g l−1 vancomycin and 1 g l−1 metronidazole ad libitum in water for the final 2 weeks. (A) The representative FACS plots gated on lung CD4 T cells show naive (CD44lo CD62Lhi) and activated (CD44hi CD62Llo) populations, and histograms present percentage and number of activated CD4 T cells in the lung of each condition. (B) Intracellular staining was performed for IFN-γ and IL-13 cytokine production in the lung CD4 T cells. The FACS plots were gated on CD4 and histograms show proportion and cell number of IFN-γ- or IL-13-producing CD4 T cells. *P < 0.05; **P < 0.01.
GF conditions do not restore immune tolerance in the TRAF6ΔDC lung
The effects of antibiotics on the TRAF6ΔDC lung phenotype may imply a crucial role for microbiota in driving loss of immune tolerance in the lung. However, we have previously observed that antibiotics have effects on immune tolerance in the small intestine even in the absence of commensal microbiota (16). To determine whether the effects of antibiotics treatment on the TRAF6ΔDC lung are due to the elimination of commensal microbiota, we examined the phenotypes of lungs from BM chimera mice generated using control wild-type control (WT) or TRAF6ΔDC BM on either SPF or GF backgrounds. As expected, we observed that the lungs of SPF mice reconstituted TRAF6ΔDC BM cells exhibited increased frequency of IL-13-producing CD4 T cells compared to WT BM chimeras. Strikingly, the frequencies of IL-13-producing CD4 T cells in TRAF6ΔDC BM chimeras generated in GF hosts were increased compared to the frequencies observed in SPF hosts (Fig. 4A and B). At the same time, IFN-γ-producing cells were present in significantly lower frequencies in TRAF6ΔDC chimeras compared to WT chimeras under both SPF and GF conditions but exhibited no change in the frequency of IFN-γ-producing cells between SPF and GF conditions (Fig. 4B). Therefore, it is likely that the stimuli driving loss of immune tolerance in the TRAF6ΔDC lung is not derived from a microbial source.
Fig. 4.
GF conditions do not restore immune tolerance in the TRAF6ΔDC lung. BM chimeras were established in both SPF and GF conditions as described in Methods. The mice were analyzed 8 weeks after BM stem cell transfer. (A, B) Increase of IL-13-producing CD4 T cells in the lung tissues of TRAF6ΔDC (ΔDC) or control (WT) BM chimera in GF conditions compared to SPF conditions. (A) The representative FACS plots gated on CD4 T cells of lung tissues show intracellular staining for IFN-γ and IL-13. (B) Scatter plot graphs present percentage of IFN-γ- or IL-13-producing CD4 T cells in the lung. *P < 0.05; **P < 0.01.
Microbiota-independent effects of antibiotics in the TRAF6ΔDC lung
Because broad-spectrum antibiotics treatment of TRAF6ΔDC mice and re-derivation of TRAF6ΔDC BM chimera generated in GF mice yielded conflicting results with respect to development of spontaneous Th2-associated immune responses, it is possible that antibiotics are acting in a manner that is independent of the anti-microbial activity. To examine this possibility, we again generated TRAF6ΔDC BM chimeras in GF hosts, but this time treated these chimeras with or without broad-spectrum antibiotics for 2 weeks. After the treatment period, CD4 T cells from lungs of antibiotics-treated GF chimeras exhibited significantly lower frequencies and numbers of IL-13-producing cells compared to untreated GF chimeras (Fig. 5A and B). Histology performed on sections of lung tissue from TRAF6∆DC GF chimeras showed decreased cellular infiltration in antibiotics-treated versus untreated chimeras (Fig. 5C). Further, we examined relative total lung mRNA expression of Igf-1, IL-13 and IL-5 from TRAF6ΔDC GF chimeras and found that the levels of each were significantly decreased by antibiotics treatment compared to untreated controls (Fig. 5D). Therefore, these data suggest that the observed effects of broad-spectrum antibiotics treatment on the lung resulted not from removal of commensal microbiota, but rather from side effects of the antibiotics.
Fig. 5.
Microbiota-independent effects of antibiotics in the TRAF6ΔDC lung. A group of TRAF6ΔDC BM chimeras maintained under GF conditions was provided with antibiotics (Abx) containing 1 g l−1 ampicillin, 1 g l−1 neomycin, 0.5 g l−1 vancomycin and 1 g l−1 metronidazole for the final 2 weeks and the mice were analyzed 8 weeks in total after BM reconstitution. (A, B) Reduced IL-13-producing cells were observed in the lung tissue. (A) Intracellular staining for IFN-γ and IL-13 was performed on CD4 T cells isolated from the lung. The representative FACS plots were gated on CD4 T cells. (B) Histograms present percentage and number of IFN-γ- and IL-13-producing CD4 T cells in the lung tissues. (C) Histological analyses were performed by H&E staining of lung from each condition. Scale bars represent 200 μm. (D) Histograms show relative gene expression level of fibrosis marker (Igf-1) and Th2 cell cytokines (Il-13 and Il-5). Histograms (mean ± SD) are representative of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Antibiotics reduce TRAF6ΔDC lung T-cell proliferation
Our previous work suggests that antibiotics treatment may exert direct effects on the Treg population in the small intestine, and that this enhanced Treg activity may be sufficient to overcome any loss of immune tolerance cause by lack of DC-expressed TRAF6 (16). Even though we have found that the frequencies of Tregs in the lungs of GF-WT and GF-TRAF6∆DC mice are comparable, unlike the frequency of Tregs in the small intestine lamina propria, which is consistently reduced in GF-TRAF6∆DC (Supplementary Figure 1, available at International Immunology Online), we expected enhanced Treg numbers induced by antibiotics treatment might mitigate lung pathology observed in GF-TRAF6∆DC mice. To examine whether a similar mechanism may be operating in the lung, we examined the lung Foxp3+ Treg populations of antibiotics-treated and untreated TRAF6ΔDC GF BM chimeras and found that unlike the previously observed finding in the small intestine, the frequency of lung Tregs was unaltered by antibiotics treatment (Fig. 6A and B). We therefore shifted our focus to non-Treg CD4+ T-cell proliferation by Ki-67 expression. Interestingly, we found that while Foxp3+ Tregs in both antibiotics-treated and untreated TRAF6ΔDC GF chimeras exhibited modestly decreased frequencies of Ki-67+ cells, the decrease in Ki-67+ cells in the Foxp3– CD4+ T-cell population was much more substantial (Fig. 6C–E). Therefore, antibiotics may act directly on immune cells in the TRAF6ΔDC lung in a pro-tolerogenic manner that is either independent of Tregs, or possibly may act more efficiently on non-Tregs than on Tregs.
Fig. 6.
Antibiotics reduce TRAF6ΔDC lung T-cell proliferation. Broad-spectrum antibiotics were given to GF TRAF6ΔDC BM chimeras for the final 2 weeks and the mice were analyzed 8 weeks in total after reconstitution. (A) FACS plots gated on CD4+ T cells show intracellular staining for Foxp3 in lung tissues of GF TRAF6ΔDC BM chimera. (B) Percentage of Foxp3+ CD4 T cells is shown from the lungs of GF TRAF6ΔDC BM chimera (ΔDC-BMC) in each indicated condition. (C) FACS histograms gated on Foxp3+ or Foxp3− CD4 T cells show intracellular staining for Ki-67, a marker to measure proliferation status. (D) Scatter plot graphs present percentage of Ki-67 positive CD4 T cells in each condition according to Foxp3 expression. (E) Ki-67+ ratio represents reduction efficiency of antibiotics treatment. The value of Ki-67+ ratio was calculated by dividing Ki-67+ percentage of antibiotics-treated mice with water only mice in each group of Foxp3+ and Foxp3− CD4 T cells. N.S., not significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
We have presented findings here that DC-expressed TRAF6 is important for immune tolerance in the lung, which, given the established role of DC-expressed TRAF6 in immune tolerance of the small intestine, lends support to the increasingly discussed concept of gut-lung cross-talk in the context of immune tolerance (8, 18). We further showed that while loss of tolerance caused by TRAF6 deficiency may be overcome by antibiotics treatment, the stimuli that signal through DC TRAF6 to maintain tolerance are likely not derived from commensal microbiota. These findings are consistent with previous results that suggest potential direct biotic effects of antibiotics (19), but it has not previously been demonstrated that such effects may impact immune tolerance in the lung. It will be important going forward to determine the source(s) and types of stimuli that regulate TRAF6-dependent immune tolerance of the lung. It also remains unclear, however, exactly how TRAF6 is functionally maintaining tolerance within the lung and the gut, and more specifically, whether the same or related DC subsets are involved. It is possible that TRAF6 is a crucial molecular intermediary that activates the same pro-tolerogenic downstream pathways, but in response to different external stimuli and/or signaling receptors, as might be expected given the different anatomic locations. Additional work will need to be performed to determine both the receptors or receptor families involved in this type of immune regulation, and on which DC subsets their expression is required.
Evidence exists that microbiota-derived stimuli are critical mediators of immune tolerance in the lung, and that these stimuli participate in gut-lung signaling cross-talk (18). Because TRAF6 is a signaling mediator of the TLR/IL-1R superfamily, it would make sense that immune tolerance regulated by a TRAF6-dependent mechanism might involve TLR stimulation through microbiota-derived factors. However, we have observed here that TRAF6ΔDC mice exhibit loss of immune tolerance in the lung regardless of the presence of commensal microbiota. Therefore, future efforts to determine the mechanistic role of TRAF6-mediated signaling responsible for maintaining tolerance in lung DCs may focus instead on cytokine receptor signaling pathways (e.g. TGFβR and/or TNFR superfamilies) that also employ TRAF6 for signal transduction and are involved in immune tolerance-related functions (20–22).
It is also important to determine the source of antigen relevant to TRAF6-dependent immune tolerance in the lung (as well as in the small intestine). We showed that the lungs of TRAF6ΔDC mice exhibit enhanced sensitivity to inhaled model antigen in the form of eosinophil infiltration. However, these data do not necessarily exclude the possibility that a different source of antigen drives spontaneous Th2-associated immunity in the lung. For instance, it is possible that antigen experienced through the small intestine contributes to responses observed in anatomically distant locations like the lung, and further work will need to be performed to better characterize this aspect of gut-lung cross-talk. It will be important, for instance, to investigate the role of oral antigens in regulating immune tolerance of the small intestine of TRAF6ΔDC mice, and further, whether the antigen experienced through this route contributes to immune responses in the lung. Similarly, it will be interesting to determine whether inhaled antigens impact gut immune tolerance in the absence of DC-expressed TRAF6.
As we have speculated previously with respect to antibiotic treatment of TRAF6ΔDC mice (16), it may be that increased Treg frequency observed in the small intestine is due, indirectly, to reduction of effector T cells by antibiotic treatment since metronidazole and vancomycin have previously been reported to directly inhibit proliferation of immune cells (23, 24). The small intestine (and mesenteric lymph nodes) would be the primary sites affected by oral antibiotics treatment, while lung tissue might more likely be indirectly affected via blood circulation. In addition, interestingly, Ki-67 frequency of lung Tregs appears much higher than other organs (Supplementary Figure 2, available at International Immunology Online) and the level of Ki-67 is sustained after antibiotics treatment (Fig. 6C and D). These phenomena imply that proliferative (Ki-67 positive) Treg cells prefer to migrate into lung tissue. Such accumulation of proliferative Tregs may contribute to establish immune tolerance in the lung.
Overall, we have presented data here using TRAF6ΔDC mice that bolsters the notion that immune tolerance in mucosal tissues is regulated across anatomically distant locations like the gut and lung by a form of regulatory cross-talk, and further, that TRAF6ΔDC mice may be employed as a useful tool for better characterizing the mechanisms controlling immune tolerance in the lung.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
This work was supported by the Institute for Basic Science (IBS-R005-D1 to D.H., K.S.K., S-W.H., J.L., J.Y., C.D.S.), Ministry of Education, Science and Technology (MEST) and in part by the National Institutes of Health (AI064909, AI123771 to Y.C.).
Supplementary Material
Acknowledgements
We thank T. Kim and J. Seo for maintenance of the germ-free mouse facility and H. Jung for assistance with FACS analysis.
Conflict of Interest statement: The authors declared no conflicts of interest.
References
- 1. Chistiakov D. A., Bobryshev Y. V., Kozarov E., et al. 2015. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Front. Microbiol. 5:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hasegawa M. and Inohara N. 2014. Regulation of the gut microbiota by the mucosal immune system in mice. Int. Immunol. 26:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous 2015. The lungs at the frontlines of immunity. Nat Immunol. 16:17. [DOI] [PubMed] [Google Scholar]
- 4. Lambrecht B. N. and Hammad H. 2015. The immunology of asthma. Nat. Immunol. 16:45. [DOI] [PubMed] [Google Scholar]
- 5. Keely S. Talley N. J. and Hansbro P. M. 2012. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J. Li F. Wei H. Lian Z. X. Sun R. and Tian Z. 2014. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 211:2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akbari O. Stock P. DeKruyff R. H. and Umetsu D. T. 2003. Mucosal tolerance and immunity: regulating the development of allergic disease and asthma. Int. Arch. Allergy Immunol. 130:108. [DOI] [PubMed] [Google Scholar]
- 8. Tulic M. K. Piche T. and Verhasselt V. 2016. Lung-gut cross-talk: evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin. Exp. Allergy 46:519. [DOI] [PubMed] [Google Scholar]
- 9. Hauptmann M. and Schaible U. E. 2016. Linking microbiota and respiratory disease. FEBS Lett. 590:3721. [DOI] [PubMed] [Google Scholar]
- 10. Coombes J. L. and Powrie F. 2008. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 8:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merad M. Sathe P. Helft J. Miller J. and Mortha A. 2013. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh M. C. Lee J. and Choi Y. 2015. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 266:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han D., Walsh M. C., Cejas P. J., et al. 2013. Dendritic cell expression of the signaling molecule TRAF6 is critical for gut microbiota-dependent immune tolerance. Immunity 38:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuipers H. and Lambrecht B. N. 2004. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr. Opin. Immunol. 16:702. [DOI] [PubMed] [Google Scholar]
- 15. Willart M. and Hammad H. 2011. Lung dendritic cell-epithelial cell crosstalk in Th2 responses to allergens. Curr. Opin. Immunol. 23:772. [DOI] [PubMed] [Google Scholar]
- 16. Han D., Walsh M. C., Kim K. S., et al. 2015. Microbiota-independent ameliorative effects of antibiotics on spontaneous Th2-associated pathology of the small intestine. PLoS ONE 10:e0118795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen K. and Kolls J. K. 2013. T cell-mediated host immune defenses in the lung. Annu. Rev. Immunol. 31:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Budden K. F., Gellatly S. L., Wood D. L., et al. 2017. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 15:55. [DOI] [PubMed] [Google Scholar]
- 19. Aminov R. I. 2013. Biotic acts of antibiotics. Front. Microbiol. 4:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cejas P. J., Walsh M. C., Pearce E. L., et al. 2010. TRAF6 inhibits Th17 differentiation and TGF-beta-mediated suppression of IL-2. Blood 115:4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walsh M. C. Kim G. K. Maurizio P. L. Molnar E. E. and Choi Y. 2008. TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL. PLoS ONE 3:e4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao X., Balasubramanian S., Liu W., et al. 2012. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 13:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Vlem B. Vanholder R. De Paepe P. Vogelaers D. and Ringoir S. 1996. Immunomodulating effects of antibiotics: literature review. Infection 24:275. [DOI] [PubMed] [Google Scholar]
- 24. Olver S. D. Price P. and Karthigasu K. T. 1991. Potentiation of murine cytomegalovirus pneumonitis by antibiotics in clinical use. J. Antimicrob. Chemother. 27:81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.