Different commercial diets influence markers of autoimmune disease
Keywords: auto-antibody, immune complex, phytoestrogens, systemic lupus erythematosus
Abstract
The course and severity of lupus in spontaneous murine lupus models varies among laboratories, which may be due to variations in diet, housing and/or local environmental conditions. In this study, we investigated the influence of common rodent diets while keeping other factors constant. Female lupus-prone MRL/lpr (MRL/MpJ-Faslpr/J) mice were subjected to the same housing conditions and given one of the three diets: Teklad 7013 containing isoflavone-rich soy and alfalfa, Harlan 2018 isoflavone-rich soy-based diet or Research Diets Inc. D11112226 (RD) purified-ingredients diet containing casein and no phytoestrogens. While the total caloric intake was similar among all three treatment groups, mice fed on the 2018 diet developed higher levels of proteinuria and mice fed on either 7013 or 2018 developed higher levels of glomerular immune complex deposition. Remarkably, mice fed the RD diet had markedly decreased proteinuria with diminished C3, total IgG, IgG1 and IgG3 immune complex deposition, along with reduced CD11b+ cellular infiltration into the glomeruli. The type of diet intake also influenced cytokine production, fecal microbiota (increased Lachnospiraceae in mice fed on 2018), altered microRNAs (miRNAs; higher levels of lupus-associated miR-148a and miR-183 in mice fed on 7013 and/or 2018) and altered DNA methylation. This is the first study to comprehensively compare the cellular, molecular and epigenetic effects of these commercial diets in murine lupus.
Introduction
Autoimmune diseases are characterized by a loss of tolerance and subsequent recognition of self-antigens by dysregulated immune cells. Systemic lupus erythematosus (SLE) is an incurable, multi-systemic autoimmune disease that most often affects women of child-bearing age. Prevalence for SLE throughout the world ranges from 20 to 150 cases per 100000 people, with females being 9–13 times more likely than males to develop the disease symptoms (1–4). Current 10-year survival rates are estimated to be between 70 and 90% with the majority of deaths occurring due to cardiovascular failure, infections or renal failure (5, 6). The full etiology of SLE is unknown at this time, with contributions from genetic, epigenetic, hormonal and environmental factors driving the breakdown of immune cell tolerance, immune attack on target tissues and subsequent development of disease in susceptible individuals (3, 4, 7, 8). In SLE, immune dysregulation is evident in all major immune cell types culminating in the development of auto-antibodies against multiple self-antigens (including auto-antibodies against nuclear components, phospholipids and Sm), increased expression of multiple cytokines, abnormal epigenetics [DNA hypomethylation, altered microRNAs (miRNAs), histone modification and nucleosome remodeling] and altered phenotype and function of innate immune cells, such as dendritic cells (DCs) and neutrophils (9–16).
The prevalence of immune-mediated diseases such as SLE in industrialized countries has been increasing rapidly in the past six decades, which in part may be due to changes in dietary and environmental factors (4, 8). Recently, there has been an increased focus in identifying potential environmental factors that may trigger disease onset in genetically susceptible individuals. The time of onset and severity of expression of lupus in genetically susceptible lupus-prone MRL/lpr mice vary among different laboratories, which is likely attributable to many variables. These include differences in mouse commercial diets, housing conditions (room temperature, dark/light cycle, humidity, bedding, type of endocrine-disrupting containing plastic cages, animal handling), sex and minor alterations in genetics, among other local environmental conditions. To address whether commercial rodent diets have an influence on murine lupus, it is thus imperative to control for the above conditions to the extent possible. Therefore, in this study, we utilized female MRL/lpr mice, a classical mouse model to study immune complex glomerulonephritis that resembles human lupus nephritis, and controlled for the above conditions with one variable—mouse diets. All female MRL/lpr mice were purchased from one vendor and were fed on one of the three diets: (i) a purified-ingredients diet (RD; for a detailed description, see the Methods section) with a casein protein source that lacks phytoestrogens, (ii) a chow diet containing soy and alfalfa (7013) or (iii) a phytoestrogenic isoflavone-rich soy-based chow diet (2018). All mice were housed in the same room and exposed to the same housing and handling conditions. Our studies clearly demonstrate that these diets have a differential regulation on the expression of lupus-associated cellular and molecular parameters, and the type of immune complex glomerulonephritis.
Methods
Mice and rodent diets
Genetically lupus-prone MRL/MpJ-Faslpr/J (MRL/lpr, stock# 000485) breeders were purchased from a single vendor (Jackson Laboratory, ME, USA) and bred in house. All mice were housed in the AAALAC-certified animal facility at the Virginia-Maryland College of Veterinary Medicine (VMCVM), Virginia Tech. Only female MRL/lpr mice were used in this study and fed the following three different diets: (i) Open Standard diet D11112226 purified-ingredients diet (Research Diets, Inc., New Brunswick, NJ, USA; RD), (ii) 7013 NIH-31 Modified 6% Mouse/Rat Sterilizable Diet (Harlan Laboratory, Madison, WI, USA) and (iii) commercial 2018 Teklad Global 18% Protein Rodent Diet (Harlan Laboratory). The RD diet protein source is derived entirely from casein, while diet 7013 is from fish meal, soybean meal and alfalfa meal, and diet 2018 is from soybean meal. These protein sources led to altered phytoestrogen content between diets, with the RD diet having undetectable levels of isoflavones, 7013 having moderate isoflavone content, while the 2018 diet contained the highest level of isoflavones. Isoflavone content of each diet was measured both internally through the toxicology laboratory at VMCVM, as well as through the commercial company Covance (Princeton, NJ, USA). Protein and fat concentrations were comparable between all three diets, with similar levels of vitamin D, choline, thiamine, folate and other B vitamins. The vitamin content of the RD diet was slightly lower in vitamin A, vitamin K, Niacin and pantothenic acid than either 2018 or 7013. Both RD and 7013 had lower concentrations of vitamin E and B vitamins riboflavin, biotin and pyridoxine–HCl compared to diet 2018. Mice were started on each respective diet at weaning (3 weeks of age) until the end of the study at 16 weeks of age. Food consumption was carefully monitored throughout the study, and average caloric ingestion per mouse calculated weekly. We chose to use only females in this study to retain sex-uniformity among groups (and avoid the complication of gender effects). Care was taken to ensure that all three groups were subjected to the same housing, local environment and handling conditions. All animal procedures and experiments were performed in accordance with guidelines of the Institutional Animal Care and Use Committee (IACUC) at Virginia Tech.
Splenocyte preparation and cellular culture
Whole splenocytes were isolated using standard laboratory procedures described in detail previously (10, 11, 17). Briefly, the spleens were dissociated by gently scraping through a steel screen, and the cell suspension was passed through a 70-µm cell strainer to remove undissociated tissue debris. The splenocytes were isolated by lysing red blood cells with Ammonium-Chloride-Potassium–Tris–NH4Cl buffer and then washing with complete RPMI-1640 medium (Mediatech, Inc., Manassas, VA, USA) that was supplemented with 10% charcoal-stripped fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA, USA), 2 mM l-glutamine (HyClone Labs Inc., Logan, UT, USA), 100 IU ml−1 penicillin and 100 µg ml−1 streptomycin (HyClone) and 1% non-essential amino acids (HyClone) before seeding into cell culture plates for treatment.
Multiplex cytokine assay
Ciraplex® Chemiluminescent Assay kits (Aushon Biosystem, Billerica, MA, USA) were used to quantify the levels of IFN-γ, IL-1β, IL-2, IL-6, IL-10, IL-12p70, IL-17 and TNF-α in cell culture supernatants per the manufacturer’s instructions (12). The images of chemiluminescent array plates were captured with the Cirascan image system (Aushon) and the image data were processed with Cirasoft software.
Because of serum sample volume restrictions, a Cytometric Bead Array Th1/Th2/Th17 Cytokine kit (BD Biosciences, San Jose, CA, USA) was used to quantify levels of IL-2, IL-4, IL-6, IFN-γ, IL-17A and IL-10 in serum samples simultaneously per the manufacturer’s instructions. The assay was performed on a BD FACSAria platform.
Assay of serum auto-antibodies
Serum anti-dsDNA auto-antibodies.
The female MRL/lpr mice were aged in our facility and blood was collected by sub-mandibular venipuncture every 2 weeks after they reached 6 weeks of age. The serum anti-dsDNA antibody levels were measured by ELISA per our previous reports (10, 11, 17). Briefly, the Costar 96-well plate was coated overnight with 100 µg ml−1 calf thymus dsDNA (Sigma-Aldrich, St. Louis, MO, USA). After washing, the plate was blocked with PBS and 1% BSA (Fisher, Fairlawn, NJ, USA), incubated with serum samples, followed by incubation with HRP-conjugated goat-anti-mouse IgG-gamma (Sigma), IgG1, IgG2a, IgG2b or IgG3 (Thermo Fisher Scientific, Waltham, MA, USA), and lastly 3,3′,5,5′-tetramethylbenzidine substrate for signal development (KPL, Inc., Gaithersburg, MD, USA). The absorbance was measured by reading the plate at 380 nm with a SpectraMax M5 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Serum anti-cardiolipin.
End-point serum anti-cardiolipin levels were measured by ELISA. The Costar 96-well plate was coated overnight with 50 µg ml−1 cardiolipin from bovine hearts (Sigma-Aldrich). The plate was blocked with Tris-buffered saline (TBS) with 2% BSA (Fisher), then washed with 1× TBS. The serum samples were diluted 1:100 in TBS with 1% BSA (Fisher) and incubated at 37°C in 0% CO2, washed, followed by incubation with alkaline phosphatase-conjugated goat-anti-mouse IgG (Southern Biotech, Birmingham, AL, USA). Lastly, the plate was incubated for 1 h with 5 mg para-Nitrophenylphosphate (Sigma) dissolved in diethanolamine substrate buffer (Thermo Fisher Scientific), 50 µg per well. The absorbance was measured by reading the plate at 405 nm with a SpectraMax M5 Microplate Reader (Molecular Devices).
Serum anti-SmD1.
The level of anti-Sm protein auto-antibodies in the serum of 16-week-old MRL/lpr mice was measured using the Mouse Anti-SmD1 ELISA Kit (Signosis, Santa Clara, CA, USA). Ninety-six-well plates were pre-coated with SmD1 antigen, samples and controls were added at 1:100 dilution and incubated at room temperature with shaking as per directions. Wells were washed with the provided Assay Buffer, then HRP-conjugated anti-mouse IgG was added to each well and allowed to incubate. The wells were washed and included kit Substrate was added, followed by Stop solution. The absorbance was measured by reading the plate at 450 nm with a SpectraMax M5 Microplate Reader (Molecular Devices).
Measurement of proteinuria
Proteinuria was measured by dipstick analysis using Chemistrip-2GP (Roche Diagnostics Corporation, Indianapolis, IN, USA). The semi-quantitative scale was demonstrated as follows: ‘−’: negative or trace; ‘+’: 30 mg dl−1; ‘++’: 100 mg dl−1 and ‘+++’: ≥500 mg dl−1.
Renal histopathology
As previously described (10), the kidneys from the MRL/lpr mice were collected and fixed in 10% buffered formalin and embedded in paraffin. Five-micron sections were stained with H&E or periodic acid-Schiff (PAS) in the histopathology laboratory at VMCVM, Virginia Tech. The stained renal sections were assessed by Dr Tom Cecere, a board-certified pathologist, in a blinded fashion. A grade of 0–4 (0 = perfect, no change; 1 = minimal; 2 = moderate; 3 = marked; 4 = severe) was given to reflect the glomerular, tubular, interstitial and vessel inflammation and lesions, respectively. By adding the scores together, we derived an overall renal score for the microscopic changes in each sample.
Renal immunofluorescence
Kidneys were embedded in Tissue-Tek O.C.T. (Optimal Cutting Temperature) Compound (Sakura Finetek, Torrance, CA, USA) and flash-frozen in a bath of dry ice and isopentane. Frozen samples were cut to 5-µm sections by the pathology department at VMCVM and unstained slides were stored at −80°C. Frozen slides were warmed to room temperature and allowed to air dry for 30 min, followed by fixation in −20°C cold acetone at room temperature for 10 min. After washing in cold PBS three times, slides were blocked with PBS containing 1% BSA (Fisher) and anti-mouse CD16/32 (eBioscience, San Diego, CA, USA) for 20 min at room temperature. Slides were then incubated with fluorochrome-conjugated antibody mixture for 1 h at room temperature in a dark humid box. Coverslips were mounted with Prolong Gold anti-fade reagent containing DAPI (Invitrogen, Grand Island, NY, USA). The following goat anti-mouse antibodies were used in immunofluorescence analysis: complement C3-PE (Cedarlane, Burlington, NC, USA); IgG-FITC (Sigma); IgG1-Alexa Fluor 568, IgG2a-Alexa Fluor 568, IgG2b-Alexa Fluor 488, IgG3-Alexa Fluor 488 (Thermo Fisher); CD11c-FITC, CD19-FITC, CD4-PE, CD8-FITC (eBioscience); CD11b-PE, Ly6G-PE (BD Biosciences). Kidney sections were examined by fluorescent microscopy. All image parameters including exposure length, magnification and light intensity were kept constant for each antibody tested. Bright-field images were used to identify the basement membrane of each glomerulus. Using the Fiji/ImageJ image processing program (18–20), the basement membranes were traced and the fluorescent intensity of the selected area was measured. The background fluorescence adjacent to each glomerulus was also measured. Background intensity was then subtracted from the glomerular fluorescent intensity to determine the corrected glomerular fluorescent intensity (CGFI) value. Fifteen (15) glomeruli were evaluated per antibody, per sample. The CGFI values are shown in Figs 1–3 along with the representative images for each marker tested.
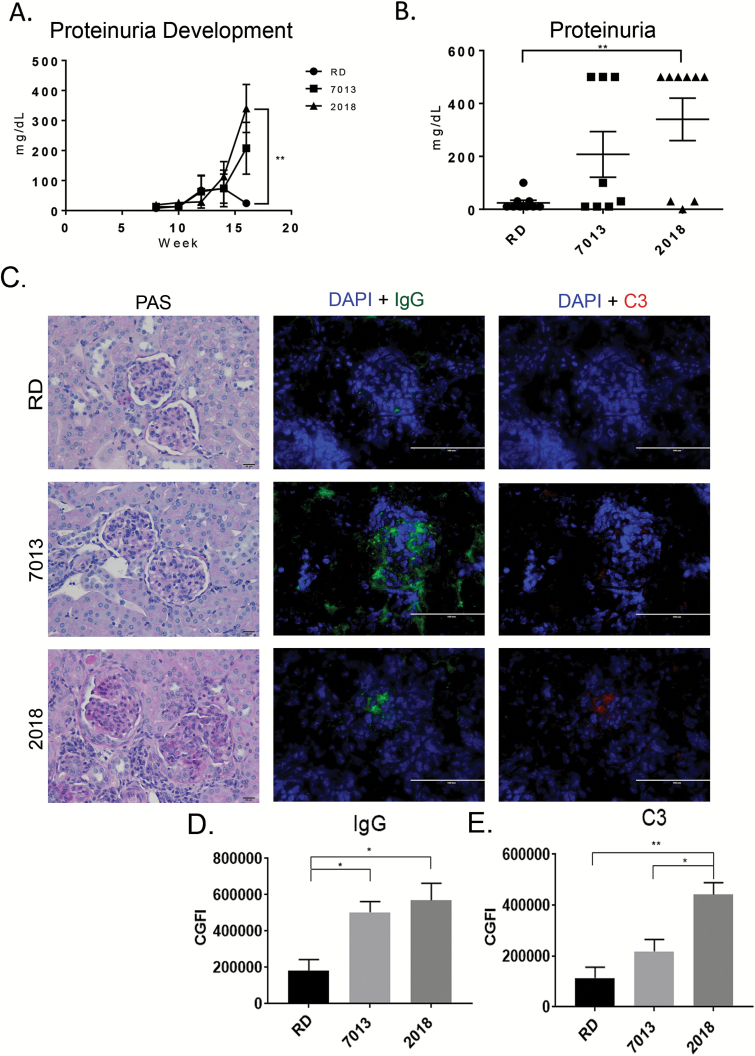
Fig. 1.
Diet source influences proteinuria and immune complex deposition. (A) Mean urine protein over time measured by ChemStix. Urine protein was monitored every other week starting at 8 weeks of age. (B) The graph represents the mean urine protein concentration at the study end-point. Data are shown as mean ± SEM (n = 8 mice for 7013, 9 mice for 2018 and RD groups). (C) Representative micrographs of PAS-stained, formalin-fixed renal sections (bar equals 20 µm, ×20 magnification) and immunofluorescence stain of immune complex deposition in frozen renal sections (bar equals 100 µm, ×20 magnification). IgG immune complex deposition is shown in green, complement C3 protein deposition is shown in red and slides were counterstained with DAPI (blue). (D, E) Mean fluorescence intensity of IgG (D) and C3 (E) per glomerulus determined by Fiji/ImageJ image. Fifteen glomeruli per mouse were analyzed. ns, not significant, *P < 0.05, **P < 0.01, one-way ANOVA. Data are shown as mean ± SEM (n = 4 mice per group).
Quantification of miRNA expression
Total RNA, containing small RNA, was isolated from whole splenocytes that were either non-stimulated or after 24 h of LPS stimulation, using a miRNeasy Mini Kit (Qiagen, Valencia, CA, USA). On-column DNase digestion with RNase-free DNase (Qiagen) was performed to remove residual amounts of DNA contamination in the isolated RNA. The RNA concentration was quantified using a NanoDrop 2000 (Thermo Fisher Scientific Inc., Wilmington, DE, USA). As we described in detail previously (10, 11), Taqman miRNA assay reagent (Applied Biosystems, Grand Island, NY, USA) was used to quantify the miRNA expression per the manufacturer’s instructions. The expression level of miRNA was normalized to small RNA housekeeping control snoRNA (small nucleolar RNA) 202. The data were shown as relative expression level to an appropriate control by using the 2−ΔΔCt formula (Livak method).
To analyze circulating miRNA in the serum, aliquots (50 µl) of serum were shipped on dry ice to FireflyBioworks (Abcam, Cambridge, MA, USA) for miRNA analysis utilizing a custom Multiplex Circulating miRNA Assay. Probes used targeted miR-18a-5p, miR-20a-5p, miR-21a-5p, miR-31-5p, miR-125a-5p, miR-126a-3p, miR-127-3p, miR-146a-5p, miR-148a-3p, miR-150-5p, miR154-5p, miR155-5p, miR-181a-5p, miR-182-5p, miR-200b-3p, miR-223-3p, miR-379-5p, miR-451a, let-7d-5p, let-7g-5p, let-7i-5p. Analysis of results was performed using Firefly Analysis Workbench software, and signal intensities for each miR probe were normalized to the intensities of the let-7 group.
Microbiota sampling, DNA extraction and PCR
During the study, fecal microbiota samples were obtained by taking individual mice out of their cage and collecting fecal pellets. At the conclusion of the study, colonic microbiota samples were collected within 20 min after euthanasia. To avoid cross-contamination, each microbiota sample was collected using a new pair of sterile tweezers. All samples were stored at −80°C until being processed at the same time. DNA was extracted using the MoBio PowerSoil DNA Isolation kit (MoBio, Carlsbad, CA, USA). Total bacterial DNA was amplified using primers F430 and R514, and Lachnospiraceae DNA was amplified using primers 338F and 491R, and Lactobacillaceae DNA was amplified using primers LabF362 and LabR677. Primer sequences are listed in Supplementary Table 1 (available at International Immunology Online). Relative abundance was evaluated using the 2ΔCt method (21).
RT–qPCR gene expression assays
RNA was extracted from splenic leukocyte cell pellets that were either untreated or stimulated for 24 h with LPS 500 ng ml−1 and stored at −80°C. The kidney tissue extract was protected by RNAlater (Thermo Fisher) then stored at −80°C. RNA was extracted using the miRNEasy Mini Kit (Qiagen). The RNA concentration was quantified using a NanoDrop 2000 (Thermo Fisher Scientific). cDNA was created using the iScript Reverse Transcription Supermix (Bio-Rad). Taqman IFNγ ‘Mm01168134_m1’, IL-6 ‘Mm00446190_m1’, TNF ‘Mm00443258_m1’ and Dnmt1 (DNA methyltransferase 1) assay reagent ‘Mm01151063_m1’ (Thermo Fisher Scientific Inc.) were used to quantify the expression of each gene per the manufacturer’s instructions. The expression level of each mRNA was normalized to Actb2 (actin beta 2). The data were shown as relative expression level to an appropriate control by using the 2−ΔΔCt formula (Livak method).
Combined bisulfite restriction analysis
Combined bisulfite restriction analysis (CoBRA) was used to analyze the promoter region for miR-148a. Bisulfite modification of extracted cellular DNA was carried out as described previously using an Epitect Fast Bisulfite conversion kit (Qiagen). The bisulfite PCR primers were designed to amplify three specific regions within the CpG island region of the miR-148a promoter using MethPrimer (http://www.urogene.org/methprimer/). Digestion of PCR products with BstUI (CGCG) (New England Biolabs Inc., Ipswich, MA, USA) was carried out at 60°C for 60 min prior to visualization on a 2% agarose gel.
Statistical analysis
All values in the graphs are given as means ± SEM, or as otherwise stated in the figure legend. To assess statistical significance, unpaired student’s t-test or one-way ANOVA and the Tukey–Kramer multiple comparisons tests were performed where appropriate using GraphPad Prism (version 6.07 for Windows).
Results
MRL/lpr mice were fed one of the three experimental diets for 13 weeks, from the time of weaning until the date of sacrifice. Changes in the body weight of mice fed diets RD and 2018 were comparable, while mice fed the 7013 diet had significantly lower initial weight gain in the first week (Supplementary Figure 1A, available at International Immunology Online), and then had comparable weight gain for the remainder of the study (Supplementary Figure 1B, available at International Immunology Online). The mice fed the 7013 diet had consistently lower body weights than the mice fed either the 2018 or the RD diet throughout the study because of this initial lower weight gain (Supplementary Figure 1A, available at International Immunology Online). While weekly mass of food ingested per mouse was slightly different between groups (Supplementary Figure 1C, available at International Immunology Online), total caloric ingestion remained similar between groups throughout the study (Supplementary Figure 1D, available at International Immunology Online). The RD diet has undetectable levels of isoflavones, 7013 diet contains about half the isoflavone content of 2018, while diet 2018 had the highest levels of multiple isoflavones as glycosides, daidzin, genistin and glycitin (Table 1). Diet 7013 also contains alfalfa, which may contain other phytoestrogenic compounds.
Table 1.
Isoflavone content per dieta
| Isoflavone | RD (ppm) | 7013 (ppm) | 2018 (ppm) |
|---|---|---|---|
| Daidzein | <10.0 | <10.0 | <10.0 |
| Daidzin | <10.0 | 88.9 | 179 |
| Genistein | <10.0 | <10.0 | <10.0 |
| Genistin | <10.0 | 99.0 | 204 |
| Glycitein | <10.0 | <10.0 | 10.1 |
| Glycitin | <10.0 | 20.7 | 51.9 |
| Total as glucosides | <10.0 | 209 | 451 |
aIsoflavone content of each diet evaluated by LC-MS/MS by Covance Laboratories. The limit of detection for each isoflavone was 10 ppm.
Mice fed RD diet had reduced proteinuria and immune complex deposition in the kidneys
MRL/lpr mice rapidly develop severe glomerulonephritis by 12 weeks of age that is characterized by immune complex deposition, which causes inflammation and damage, leading to proteinuria as a measurable indicator of glomerular damage. Kinetics of proteinuria development revealed that mice fed the RD diet developed the lowest levels of proteinuria, which were significantly lower than the mice fed the 2018 diet during late-stage kidney disease at 16 weeks of age (Fig. 1A). Remarkably, at the end-point of the study (16 weeks), the mice fed the RD diet had minimal evidence of proteinuria compared to mice fed the 2018 diet (Fig. 1B, P < 0.05). Histopathologic evaluation of the kidneys of MRL/lpr mice following PAS staining revealed not only glomerulonephritis but also renal pathological changes in the interstitium, tubules and renal vessels (Fig. 1C). While the glomeruli of mice fed the RD diet appear to have markedly reduced pathologic changes compared to mice fed the 2018 diet, blinded scoring by a board-certified pathologist did not achieve significance (P = 0.09) (Fig. 1C and Table 2).
Table 2.
Renal histopathologic scoresa
| Diet source | Glomerular score | Interstitial score | Vessel score | Tubule score | Kidney scores |
|---|---|---|---|---|---|
| RD | 0.8 ± 0.7 | 1.2 ± 1.0 | 1.8 ± 0.4 | 0.6 ± 0.7 | 4.3 ± 2.3 |
| 7013 | 1.4 ± 0.7 | 1.6 ± 0.7 | 2.1 ± 0.4 | 0.7 ± 0.9 | 5.9 ± 2.0 |
| 2018 | 1.7 ± 1.2 | 2.0 ± 1.0 | 2.1 ± 0.6 | 1.1 ± 1.3 | 6.9 ± 3.7 |
aEvaluation and scoring of formalin-fixed renal sections. H&E- and PAS-stained slides were evaluated by a board-certified veterinary pathologist in a blinded fashion. Individual scores were averaged for each group score for each structure evaluated. Data are shown as mean ± SD (n = 8 mice for 7013, 9 mice for 2018 and RD groups).
In addition to PAS staining of kidneys, we also evaluated for the IgG immune complex and complement protein 3 (C3) deposition within the glomeruli, as the deposition of these proteins, or protein complexes, in the glomeruli leads to further cellular infiltration and damage. Immunofluorescent microscopy revealed striking differences in IgG and C3 deposition among the mice fed on different diets. It is noteworthy that mice fed on the RD diet, consistent with reduced proteinuria, also had minimal C3 and IgG deposition (Fig. 1C). Mice fed the 7013 or 2018 diets had a much higher degree of glomerular IgG deposition compared to mice fed the RD diet (P < 0.05) (Fig. 1C and D). Mice fed on the 2018 diet had a significantly higher level of C3 deposition within the glomeruli compared to mice fed the other 2 diets (P < 0.05) (Fig. 1C and E).
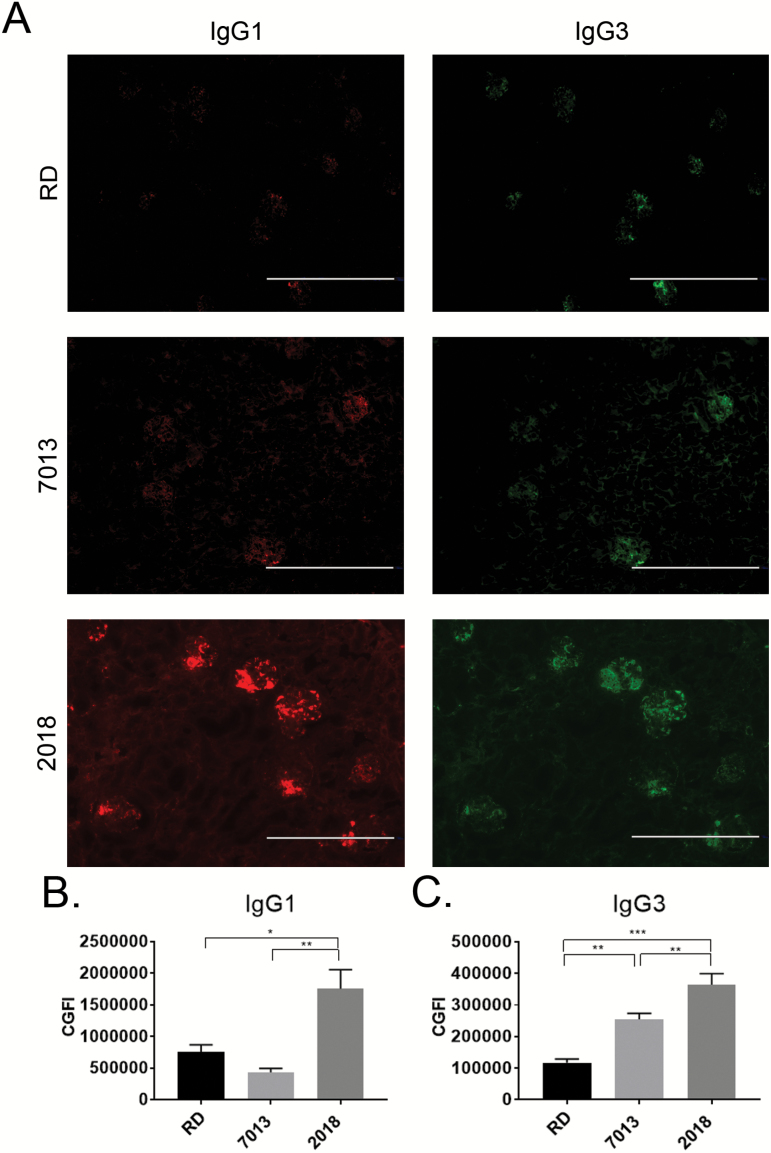
It has been previously reported that while IgG3 is a small fraction of the total circulating IgG, it is the primary IgG subclass that is deposited within the glomeruli of MRL/lpr mice, contributing to glomerular damage and progression of glomerulonephritis (22, 23). To further determine the cause of the different levels of IgG deposition among diet groups, we performed immunofluorescence imaging to identify specific IgG subclasses deposited within the glomeruli. Mice fed on 2018 had significantly increased IgG1 (Fig. 2A and B), and mice fed on the 7013 or 2018 diets both had higher levels of IgG3 deposition (Fig. 2A and C). In contrast, mice fed on the RD diet had noticeably decreased deposition of IgG1 and IgG3 compared to mice fed 2018 (Fig. 2A–C). Interestingly, deposition of IgG2a and IgG2b was similar among all diet groups (Supplementary Figure 2, available at International Immunology Online).
Fig. 2.
Diets 7013 and 2018 increased IgG subclass glomerular deposition. (A) Representative immunofluorescence micrographs of 5 µm frozen renal sections to show IgG1 (red) and IgG3 (green) protein deposition within glomeruli (bar = 400 µm, ×10 magnification). (B, C) Mean fluorescence intensity of IgG1 (B) and IgG3 (C) per glomerulus determined by Fiji. Fifteen glomeruli per mouse were analyzed (n = 4 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA. Data are shown as mean ± SEM.
Infiltrating cellular phenotype in the glomeruli of MRL/lpr mice is influenced by diet
As shown in the PAS-stained histopathologic micrographs (Fig. 1C), the degree of cellular infiltration of glomeruli among diet groups was altered. This disparity led us to investigate potential differences in infiltrating immune cell phenotypes that are commonly implicated in lupus pathogenesis. These include CD11b+ [monocytes, macrophages, conventional DCs (cDCs)], CD19+ (B cells), CD4+ (T helper, CD4+DCs), CD8+ (T cytotoxic, CD8+DCs), CD11c+ (plasmacytoid DCs, cDCs, some macrophages) and Ly6G+ (neutrophils). Of the cells investigated, no diet group had identifiable glomerular infiltration of CD11c+, CD8+ T cells or CD19+ B cells (data not shown). The casein-based RD diet-fed mice had low, but identifiable, amounts of CD11b+ staining, along with minimal CD4+ and Ly6G+ stained cells (Fig. 3A–D). The group fed the soymeal and alfalfa meal-based diet 7013 had no statistically different levels of cellular infiltration compared to the RD diet group, while CD4+ cells were slightly more abundant (Fig. 3A and C, P = 0.085). Consumption of soymeal-based diet 2018 led to the highest level of infiltration of CD11b+ cells with similar levels of CD4+ and Ly6G+ cells to the other two diet groups (Fig. 3B–D).
Fig. 3.
Glomerular leukocyte infiltration phenotypes. (A) Frozen renal sections (5 µm thick) were stained for immunofluorescent analysis with CD11b+ cells (red, CD11b-PE), CD4+ T cells (red, CD4-PE) or neutrophils (red, Ly6G-PE) and counterstained using DAPI. Images presented are representative images from three separate experiments for each group (bar equals 200 µm, ×20 magnification). (B–D) Mean fluorescence intensity of CD11b+ (B), CD4+ (C) and Ly6G (D), per glomerulus determined by Fiji. Fifteen glomeruli per mouse were analyzed (n = 4 mice per group). **P < 0.01, ***P < 0.001, one-way ANOVA. Data are shown as mean ± SEM.
Dietary source has minimal effect on serologic lupus parameters in MRL/lpr mice
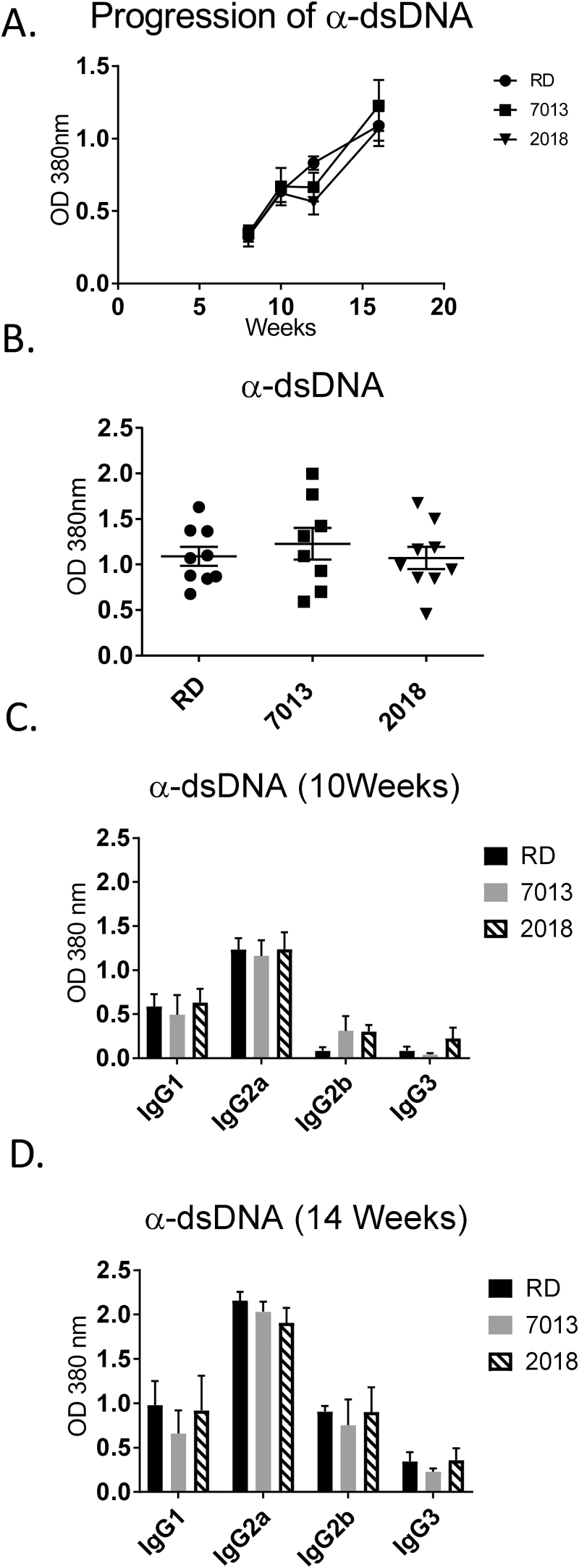
Given that MRL/lpr mice fed 2018 or 7013 manifested greater glomerular immune complex deposition than mice fed the RD diet, we next assessed whether similar alterations in lupus-associated autoimmune antibodies (anti-dsDNA, anti-cardiolipin and anti-Sm auto-antibodies) are also evident. There was no measurable difference in the circulating levels of any of the three auto-antibodies among diet groups, except for anti-dsDNA at week 11 of age (Fig. 4A and B; Supplementary Figure 3, available at International Immunology Online). It has been previously reported that IgG2a is the predominant circulating IgG subclass (23, 24). To further evaluate the effects of the diets on circulating anti-dsDNA, we measured the levels of anti-dsDNA IgG subclasses IgG1, IgG2a, IgG2b and IgG3. While our data confirm that IgG2a is the predominant circulating subclass, there were no differences found among diet groups, either in early disease stage at 10 weeks of age, or in late-stage disease at 14 weeks of age (Fig. 4C and D).
Fig. 4.
Effects of diet source on auto-antibody production. (A) Serum anti-dsDNA antibody levels were monitored sequentially every 2 weeks in individual mice by ELISA. (B) Serum anti-dsDNA antibody levels at 16 weeks of age when the mice were terminated. (C, D) Serum anti-dsDNA IgG subclass (IgG1, IgG2a, IgG2b, IgG3) levels were measured by ELISA at early onset of disease (10 weeks of age) and late-stage disease (14 weeks of age). Data are shown as mean ± SEM (n = 8–9 mice per group).
Systemic cytokine production was not influenced by diet source without a secondary stimulus
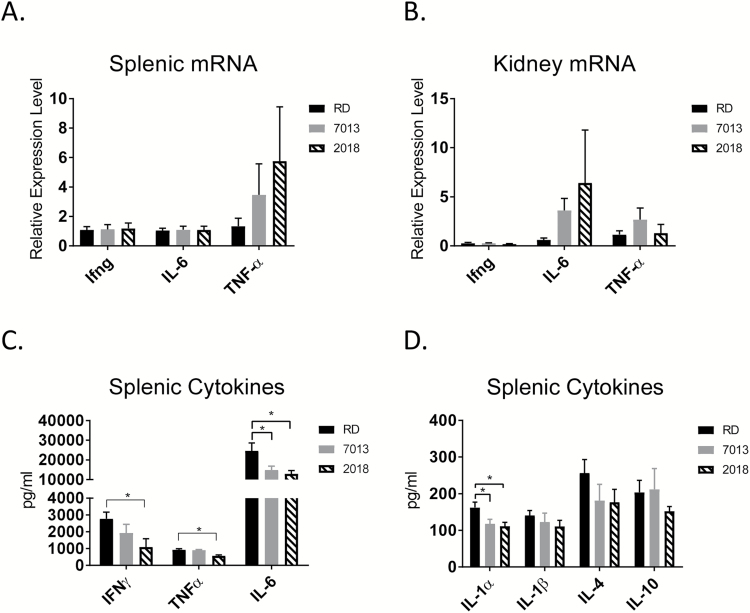
SLE is associated with changes in pro-inflammatory cytokines (IFN-γ, IL-6 and TNF-α) in the secondary lymphoid organs, including the spleen and lymph nodes. mRNA levels of IFN-γ, IL-6 and TNF-α were analyzed in both unstimulated splenocytes and kidney tissues. No differences were found in cytokine mRNA expression among diet groups (Fig. 5A and B). In past experiences, we did not see detectable levels of cytokine proteins in non-stimulated splenocytes, so we evaluated the cytokine production from splenocytes and mesenteric lymphoid cells after 24 h of stimulation with LPS.
Fig. 5.
Dietary-mediated changes to cytokine production. (A, B) qRT–PCR analysis of the cytokine mRNA expression in spleen and kidney. mRNA expression levels in 7013 and 2018 diet shown as relative expression level compared to RD diet expression levels, using comparative 2-ΔΔCt. ACTB2 was used as the endogenous control gene. One-way ANOVA. Data are shown as mean ± SEM (n = 8 mice per group). (C, D) Splenocytes were treated with LPS for 24 h at 500 ng ml−1. Culture supernatant was evaluated using Aushon cytokine Multiplex ELISA. *P < 0.05, one-way ANOVA. Data are shown as mean ± SEM (n = 8 mice per group).
There were no statistically significant changes found in cytokine levels of the mesenteric lymphocytes, or in circulation, among diet groups (data not shown). Changes to cytokine levels were only observed following splenic leukocyte in vitro stimulation with LPS. Mice fed diets 7013 or 2018, compared to the RD group, had a reduction in the levels of multiple cytokines from LPS-activated splenocytes. Compared to the RD diet-fed mice, mice fed the 7013 diet had reduced IL-12p70, and mice fed the 2018 diet had reduced IFN-γ. Mice fed either 7013 or 2018 had reduced IL-1α, IL-6 and TNF-α (Fig. 5C and D). These data suggest that reduced immune complex deposition in mice fed the RD diet is not due to generalized immunosuppression. These data also support that mice fed the RD diet are not experiencing systemically higher levels of cytokine production, rather, the changes seen are organ specific following LPS stimulation.
Immune cell phenotype in secondary lymphoid organs was not significantly affected by diet sources in MRL/lpr mice
To determine if the changes in cytokine levels were due to altered cellular populations, we analyzed the cellular composition of different tissues by flow cytometry. We assessed multiple cell populations that have been implicated in lupus pathology, including CD4+ and CD8+ T cells, T regulatory cells, Th17 cells and B-cell populations in the spleen and mesenteric lymph node (mLN) both before, and after, stimulation. There were no statistically significant changes in any of the T-cell sub-populations in either the spleen or mLN among any of the diet groups (Supplementary Figure 4, available at International Immunology Online). We identified a trend in changes of the B-cell populations in the spleens and mLN among diet groups, with mice fed the RD diet having the lowest number of CD19+ B cells before and after stimulation with anti-CD3/CD28, though these values did not reach statistical significance.
Dietary effect on fecal microbiota
A recent report has shown lupus disease severity in the MRL/lpr mouse model correlates with the relative abundance of specific colonic bacterial groups, with Lachnospiraceae abundance having a positive correlation in lupus severity while Lactobacillaceae has a negative correlation (21). Because of the interactions of intestinal microbiota with dietary components, we wanted to determine if there were alterations in the relative abundance of colonic bacterial groups shown to be important in lupus development. Bacterial DNA was isolated from fecal samples; both 1 week after weaning and at the end of the study. qPCR was performed with primers specific for total bacteria, Lachnospiraceae and Lactobacillaceae, to amplify the isolated DNA and evaluate relative abundance. The relative amount of total bacterial DNA was not different among diet groups (Supplementary Figure 5, available at International Immunology Online). Abundance of Lachnospiraceae was shown to be higher in the mice fed the 2018 diet compared to the mice fed the RD diet at both time points, consistent with previous reports of higher Lachnospiraceae abundance in more severe lupus disease (Fig. 6A). Interestingly, Lactobacillaceae DNA was amplified at only negligible levels among all diet groups at both time points, suggesting a lack of Lactobacillaceae species within the MRL/lpr mice used in this study (Fig. 6B).
Fig. 6.
Effects of dietary source on fecal microbiota. (A, B) Real-time PCR analysis of (A) Lachnospiraceae or (B) Lactobacillaceae relative abundance in feces from mice 1 week after weaning and at termination. Family-specific gene amplification was compared to total bacterial DNA amplification to determine relative abundance. *P < 0.05, one-way ANOVA. Data shown are representative of three independent experiments. Data are shown as mean ± 95% confidence interval (n = 8 mice for 7013, 9 mice for 2018 and RD groups).
Select epigenetic markers of lupus are significantly affected by diet source in MRL/lpr mice
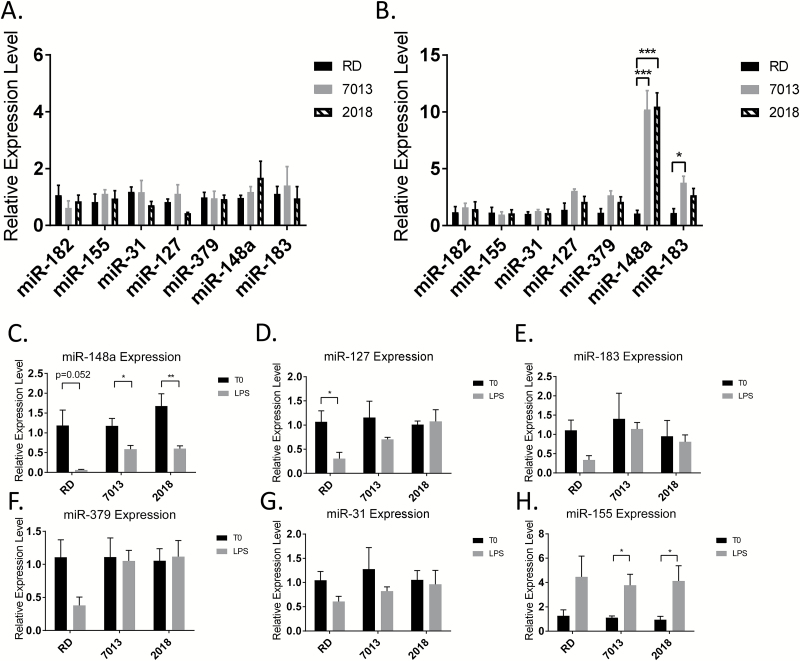
qPCR analysis of the expression of selected lupus-related miRNAs (miR-182, miR-155, miR-31, miR-127, miR-379, miR-148a, miR-183) revealed that diet did not influence the level of above miRNAs in unstimulated splenocytes of MRL/lpr mice (Fig. 7A). To further our understanding of the impact of diet source on the disease development, we next asked if dietary source augments the epigenetic response following activation of splenocytes by LPS. Following 24 h of stimulation with LPS, there was a higher expression level of miR-148a in mice fed either the 7013 or 2018 diet, and an increased level of expression in miR-183 in mice fed the 7013 diet when compared to mice fed the RD diet (Fig. 7B). Further evaluation revealed that LPS stimulation significantly reduced miR-148a expression in splenocytes of mice from all three diet groups (Fig. 7C). The suppression effect is more profound in the RD diet group than that from 7013 and 2018, which contributes to the observation of increased miR-148a in LPS-activated splenocytes from 7013 and 2018 diet when compared to RD diet. Similar to miR-148a, the trends of increased miR-127, miR-183, miR-379 and miR-31 in LPS-activated splenocytes from 7013 and 2018 are also attributed to LPS-induced suppression in the RD diet group (Fig. 7D–G). miR-155, which is a highly LPS-sensitive miRNA (25), was, not surprisingly, up-regulated in LPS-activated splenocytes from all three diet groups to a similar level (Fig. 7H). This observation suggested that different diets may differentially affect splenic cell immune response to secondary stimuli by altering specific miRNA expression at different levels.
Fig. 7.
Dietary source alters miRNA expression in LPS-stimulated splenocytes from MRL/lpr mice. (A, B) Taqman miRNA assays were performed to quantify lupus-associated miRNA expression in splenocytes. (A) miRNA expression in non-stimulated splenocytes. (B) miRNA expression in splenocytes after 24 h of 500 ng ml−1 LPS stimulation. Expression is shown as relative expression level to mice fed the RD diet. (C–H) miRNA expression in splenocytes without stimulation (T0) and after 24 h of LPS stimulation (LPS). Data are expressed relative to the non-stimulated mean. The graphs represent mean + SEMs (n = 4 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001, (A, B) one-way ANOVA, (C–H) t-test.
Previous work in our laboratory has shown a connection between estrogen-regulated miRNA and lupus disease progression as well (10–12). Because of the potential estrogenic activity of exogenous estrogens found in diets 7013 and 2018, we evaluated the serum levels of both lupus-associated miRNA and estrogen-regulated miRNA, with no significant changes found, though miR-155 approached significance (P = 0.07) (data not shown).
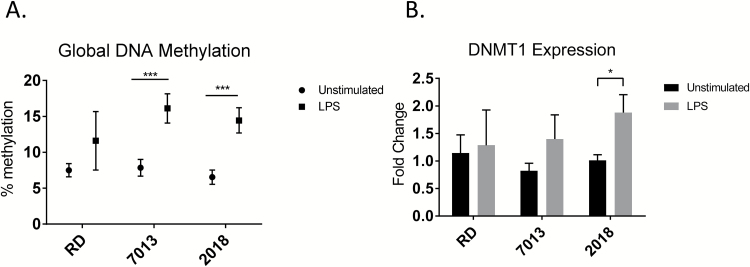
To further our understanding of the contribution of diet source to epigenetic modulation, we also evaluated the global DNA methylation value of non-stimulated and LPS-stimulated splenocytes. As indicated in Fig. 8A, there was no difference in the global DNA methylation level in splenocytes (either unstimulated or LPS-stimulated) among different diet groups. LPS stimulation significantly increased global DNA methylation levels, compared to unstimulated cells, in splenocytes of mice fed the 7013 or 2018 diets (Fig. 8A). Correspondingly, we also observed a significant increase of Dnmt1 in LPS-activated splenocytes of mice fed with 2018 when compared to unstimulated control (Fig. 8B). While it is not significant, there is also a trend of increased Dnmt1 gene expression in LPS-activated splenocytes of mice fed the 7013 diet (Fig. 8B).
Fig. 8.
Global DNA methylation and Dnmt1 expression in splenocytes increased after LPS stimulation in MRL/lpr mice based on diet source. (A) 5-mC DNA methylation ELISA. ELISA was performed on DNA extracted from splenocytes that were non-stimulated or stimulated for 24 h with LPS. (B) Dnmt1 mRNA expression. RNAs were extracted from splenocytes with and without LPS stimulation for 24 h. The Dnmt1 expression was quantified using the Taqman gene expression system. The graphs represent mean ± SEMs. *P < 0.05, ***P < 0.001, one-way ANOVA (A), t-test (B) (n = 4 mice per group).
miR-148a has been reported to regulate DNA methylation by targeting DNMT1 (26). On the other hand, the expression of miR-148a is reciprocally regulated by the DNA methylation level at its promoter region (27). With the finding of increased global DNA methylation and reduction of miR-148a expression in LPS-activated splenocytes of different diet groups, we next asked if the miR-148a promoter region is differentially methylated between diet groups. We performed CoBRA analysis on three distinct CGCG sites located at the CpG island region of the miR-148a promoter. No methylation change was detected on these three CGCG sites in either unstimulated or LPS-stimulated splenocytes of different diet groups (data not shown). Further investigation of other CpG sites in the promoter region or gene body is necessary to determine if LPS-induced changes in miR-148a expression among diets are due to altered miR-148a methylation status.
Discussion
The influence of diet in autoimmune diseases has long been established (28–31). Genetically prone autoimmune mice such as MRL/lpr mice have been extensively used in many laboratories across the world. However, although all laboratories have consistently reported the autoimmune nature of the disease, there have been notable differences in the time of onset, course and severity of the disease including proteinuria. This implies differences in environmental factors such as diet and housing conditions. Since the level of phytoestrogens in diet can influence the lupus disease, in this study, we utilized a diet that was devoid of phytoestrogens (RD) and compared with two commonly fed diets, one based on soy (2018) and the other based on soy and alfalfa (7013). While many of the nutritional parameters of the three diets were comparable, the RD diet had a higher concentration of carbohydrates, contributing to a higher energy density. Unlike the RD diet, which is strictly controlled with purified ingredients, 7013 and 2018 are grain-based diets, and thus subject to variations in not only grains, but also could include pesticides and other contaminants.
With well-controlled genetics and housing conditions, in this study, we reported the effects of various diets on the development of lupus-like disease by using a genetically susceptible mouse model carrying the Faslpr mutation, MRL/lpr. We showed that mice fed a casein-based purified ingredient diet had decreased renal deposition of IgG and C3 (Fig. 1C–E), including reduced IgG1 and IgG3 deposition (Fig. 2A–C). This reduction in immune complex deposition is supported by alterations in the level of glomerular CD11b+ immune cell infiltration depending on diet consumed (Fig. 3A and B). Consistent with the decreased IgG deposition in the kidneys, there was a trend of decreased (albeit not statistically significant) splenic B cells in mice fed the RD diet (Supplementary Figure 4, available at International Immunology Online). Cytokine profiles, both at the mRNA and protein levels, were largely unchanged for multiple organs, including unstimulated spleen, mesenteric lymph nodes, kidney and LPS-stimulated mLNs (Fig. 5A and B; and data not shown). The exception to this was that in splenic leukocytes stimulated with LPS for 24 h, multiple cytokines were reduced at the protein level (Fig. 5C and D). Evaluation of epigenetic factors associated with lupus, including lupus-associated miRNA expression and global DNA methylation, revealed that miR-148a, miR-127, miR-183, miR-379 and Dnmt1 expression is influenced by dietary source followed by an inflammatory stimulus LPS (Fig. 7B–G and 8A and B).
Circulating auto-antibody levels were comparable among groups at the majority of time points evaluated. Our data support that most of the circulating anti-dsDNA IgG is composed of the IgG2a subclass. It is noteworthy that even though the IgG3 subclass constitutes a small percentage of total IgG, previous studies have shown that IgG3 is deposited in the glomeruli of MRL/lpr mice and is considered to be pathogenic (22, 23). Our studies also show IgG3 glomerular deposition in mice fed on 2018, which had the highest glomerulonephritis. This supports the view that while there are minimal differences in the levels of total circulating IgG, IgG3 can preferentially deposit in the glomeruli of mice fed the diet that promoted the highest levels of glomerulonephritis. Possible explanations for the higher levels of IgG deposition within the glomeruli of mice fed diets 2018 and 7013 include altered intracellular signaling due to the binding of phytoestrogens to estrogen receptor isotypes or alterations in cellular activation profiles due to differences within the microbiota that could not be controlled for. More work needs to be done to determine the mechanism by which immune complex deposition is altered by specific dietary components, which is beyond the scope of the present study.
The presence of immunoglobulin deposition in the glomeruli was not the only change found among diet groups, as our results showed a disparity in the level of CD11b+ cell infiltration in the glomeruli among diet groups at the time of sample collection. The changes in cell infiltration by diet may be due to differences in the number of circulating immune cells, alterations in cell signaling and the presence of deposited inflammatory proteins within the glomeruli.
While the mice fed the casein-based RD diet had the lowest level of glomerulonephritis, these mice also had the highest levels of cytokine production in in vitro LPS-activated splenocytes. It is important to note that while many of these cytokines have conventionally been classified as ‘pro-inflammatory’, current knowledge supports that cytokine activity is context specific. IL-17 and IFN-γ are generally considered to be pro-inflammatory; however, IL-17 can exhibit anti-inflammatory activity in specific contexts (32), as can IFN-γ (33). Changes in tissue cytokine response were not a result of differences with T-, B- or MHC II+ cell numbers between diet groups. Our data support that the RD diet did not lead to reduced glomerulonephritis through immunosuppression. This is consistent with previous in vitro reports in which genistein treatment led to a reduction in LPS-induced TNF-α and IL-6 in RAW 264.7 macrophages (34). There may be altered cell numbers in cell populations that were not explored in the scope of this study that may contribute to the higher cytokine production in splenocytes of mice fed the RD diet.
Previous studies have linked the gut microbiota to regulation of multiple autoimmune diseases, ranging from diabetes to multiple sclerosis to SLE (35–41). Since dietary nutrients influence microbiota signatures, we investigated the effects of the three diets on two groups of bacteria identified to be important to SLE parameters in the MRL/lpr mouse model. Our data showing higher levels of Lachnospiraceae 1 week after weaning, as well as during late-stage disease (Fig. 6A), in mice fed the 2018 diet support a role for Lachnospiraceae influencing glomerulonephritis development and severity. It has been widely reported that dietary fiber, both soluble and insoluble, is able to modulate the gut microbiota, altering the relative abundance between Firmicutes and Bacteroidetes (42, 43). The dietary fiber sources are different among diets, with the fiber in the RD diet exclusively consisting of soluble fiber inulin, and the chow diets 7013 and 2018 containing a mixture of plant-derived soluble and insoluble fibers. These alterations in fiber source and content may also contribute to differences in relative abundance of Lachnospiraceae seen between the RD diet and the 2018 diet. Finding negligible relative abundance of Lactobacillaceae sp. in all three groups was unexpected (Fig. 6B). It is possible that over time, these mice derived from our breeding colonies have had reductions in Lactobacillaceae abundance, leading to negligible levels of these bacteria in our breeding colony. Caution should be taken when evaluating microbiota signatures and effects on disease between mice from multiple facilities.
We have previously shown that exogenous administration of 17-β estradiol (estrogen) induced signature miRNA changes in the spleens of both C57BL/6 and lupus-prone NZB/WF1 (New Zealand Black/White F1 progeny) mice (44). In this study, we show that diet source has the ability to modify the splenic lupus-associated miRNA expression after LPS stimulation, with no changes in baseline or serum miRNA expression profiles in the MRL/lpr mouse model. miR-148a was shown to contribute to lupus CD4+ T-cell hypomethylation through direct down-regulation of Dnmt1 (26). While this may not lead to a change in numbers of CD4+ T cells, hypomethylation can have a profound impact on cellular signaling and function. miR-183, together with miR-96 and miR-182 (members of the miR-183-96-182 cluster), have been shown to be up-regulated in multiple mouse models of lupus, potentially leading to down-regulation of Foxo1/3 in T cells and MITF in B cells. This may result in subsequent breakdown of T-cell tolerance, spontaneous B- and T-cell activation, enhanced auto-antibody production and secretion and enhanced T-helper cell activation (10, 45). It is noteworthy that while miR-183 was significantly higher in mice fed the 7013 diet compared to the RD diet, miR-182 was not changed between diet groups.
Reports have shown that the isoflavone genistein can influence the methylation of the ERβ (estrogen receptor β) promoter region in prostate cancer cells (46). Because of the reduced miR-148a expression and increased global DNA methylation in LPS-activated splenocytes, we wanted to determine if diet source can influence the methylation levels of the miR-148a promoter region. Evaluation of the methylation status of the CpG island region of the miR-148a promoter revealed no evidence of methylation at the three regions of interest. Soy isoflavones have been shown to regulate Dnmt1 expression in breast cancer cells (47, 48), which may in turn regulate multiple miRNA expression levels. Our data showed that consumption of diet 2018 led to increased Dnmt1 expression following LPS stimulation (Fig. 8B). Other epigenetic changes, such as histone modification or methylation of other regulatory protein genes, may contribute to alterations in miRNA expression due to dietary ingestion. Further studies are warranted to determine the exact epigenetic mechanisms by which dietary components can exert exacerbating or ameliorating effects on SLE in mouse models.
These results show that the serum miRNA levels do not necessarily correlate with the splenic tissue miRNA levels of lupus-associated miRNAs. Our results support that when evaluating patients with autoimmune disease, as well as mouse models of disease, it is vitally important to evaluate each organ system individually, as what is seen in the serum or secondary lymphoid organs is not necessarily representative of what is occurring in other disease target organs elsewhere in the body.
While the three standard rodent diets were chosen without modification for their variations in phytoestrogen content and protein source, we recognize that the diets are not balanced in all nutrients, and these variables may have contributed to our results. Control must be taken when extrapolating dietary findings in mouse models to human disease influence, as only 30% of the human population has a gut microbiota that allows for the conversion of daidzein to its metabolite equol, while almost all mice have this ability (49). Equol has been shown to have wide ranging effects, including reduced DNA damage after UV exposure and alterations to the immune system (49, 50). Our results show that, while controlling for housing, sex and handling conditions, diet alone can have an impact on immune complex glomerulonephritis, renal cellular infiltrates, microbiota and molecular behavior of cells after LPS activation. Additionally, our studies on the RD diet (in which dietary formulations are meticulously controlled) provided base line effects on lupus nephritis in MRL/lpr mice. It will now thus be possible to incorporate an exogenous compound of interest (e.g. endocrine-disrupting chemicals) in the RD diet formulation to investigate the effect of this compound. Our ongoing studies are utilizing this approach to understand the impact of oral exposure to estrogenic chemicals. The results of this study contribute to a better understanding of the role of commercial dietary sources and how diet choice can influence disease phenotype variability in the field of autoimmune lupus.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
This work was supported by the Virginia-Maryland College of Veterinary Medicine (VMCVM) Intramural Research Competition (IRC) Grant (grant number 175185); Interdepartmental funds to S.A.A. and by the National Institutes of Health T32 training grant (grant number 5T32OD010430-09).
Conflicts of interest statement: The authors declared no conflicts of interest.
Supplementary Material
Acknowledgements
We thank Melissa Makris for assistance with flow cytometry results. We thank Caroline Moon for her technical assistance with DNA extraction and DNA methylation ELISA work. We thank Dr Nicole Lindstrom, Ms Karen Hall, Betsy S. Midkiff and other animal care staff members at VMCVM, Virginia Tech.
References
- 1. Osio-Salido E. and Manapat-Reyes H. 2010. Epidemiology of systemic lupus erythematosus in Asia. Lupus 19:1365. [DOI] [PubMed] [Google Scholar]
- 2. Jakes R. W., Bae S. C., Louthrenoo W., Mok C. C., Navarra S. V. and Kwon N. 2012. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res. (Hoboken) 64:159. [DOI] [PubMed] [Google Scholar]
- 3. Selmi C. and Tsuneyama K. 2010. Nutrition, geoepidemiology, and autoimmunity. Autoimmun. Rev. 9:A267. [DOI] [PubMed] [Google Scholar]
- 4. Shapira Y., Agmon-Levin N. and Shoenfeld Y. 2010. Defining and analyzing geoepidemiology and human autoimmunity. J. Autoimmun. 34:J168. [DOI] [PubMed] [Google Scholar]
- 5. Gordon C. 2002. Long-term complications of systemic lupus erythematosus. Rheumatology (Oxford) 41:1095. [DOI] [PubMed] [Google Scholar]
- 6. Yap D. Y., Tang C. S., Ma M. K., Lam M. F. and Chan T. M. 2012. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol. Dial. Transplant. 27:3248. [DOI] [PubMed] [Google Scholar]
- 7. Mackay I. R. 2009. Clustering and commonalities among autoimmune diseases. J. Autoimmun. 33:170. [DOI] [PubMed] [Google Scholar]
- 8. Chiu Y. M. and Lai C. H. 2010. Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus 19:1250. [DOI] [PubMed] [Google Scholar]
- 9. Ippolito A., Wallace D. J., Gladman D. et al. . 2011. Autoantibodies in systemic lupus erythematosus: comparison of historical and current assessment of seropositivity. Lupus 20:250. [DOI] [PubMed] [Google Scholar]
- 10. Dai R., McReynolds S., Leroith T., Heid B., Liang Z. and Ahmed S. A. 2013. Sex differences in the expression of lupus-associated miRNAs in splenocytes from lupus-prone NZB/WF1 mice. Biol. Sex Differ. 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dai R., Zhang Y., Khan D. et al. . 2010. Identification of a common lupus disease-associated microRNA expression pattern in three different murine models of lupus. PLoS ONE 5:e14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai R., Lu R., Ansar Ahmed S. 2016. The upregulation of genomic imprinted DLK1-Dio3 miRNAs in murine lupus is associated with global DNA hypomethylation. PLoS ONE 11:e0153509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pons-Estel G. J., Serrano R., Plasín M. A., Espinosa G. and Cervera R. 2011. Epidemiology and management of refractory lupus nephritis. Autoimmun. Rev. 10:655. [DOI] [PubMed] [Google Scholar]
- 14. Rubtsov A. V., Rubtsova K., Kappler J. W. and Marrack P. 2010. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun. Rev. 9:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Somers E. C. and Richardson B. C. 2014. Environmental exposures, epigenetic changes and the risk of lupus. Lupus 23:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou X., Jeker L. T., Fife B. T. et al. . 2008. Selective miRNA disruption in Treg cells leads to uncontrolled autoimmunity. J. Exp. Med. 205:1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan D., Dai R., Karpuzoglu E. and Ahmed S. A. 2010. Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur. J. Immunol. 40:2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schindelin J., Arganda-Carreras I., Frise E. et al. . 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schneider C. A., Rasband W. S. and Eliceiri K. W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schindelin J., Rueden C. T., Hiner M. C. and Eliceiri K. W. 2015. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol. Reprod. Dev. 82:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H., Liao X., Sparks J. B. and Luo X. M. 2014. Dynamics of gut microbiota in autoimmune lupus. Appl. Environ. Microbiol. 80:7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenspan N. S., Lu M. A., Shipley J. W. et al. . 2012. IgG3 deficiency extends lifespan and attenuates progression of glomerulonephritis in MRL/lpr mice. Biol. Direct 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mannoor K., Matejuk A., Xu Y., Beardall M. and Chen C. 2012. Expression of natural autoantibodies in MRL-lpr mice protects from lupus nephritis and improves survival. J. Immunol. 188:3628. [DOI] [PubMed] [Google Scholar]
- 24. Takahashi S., Nose M., Sasaki J., Yamamoto T. and Kyogoku M. 1991. IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J. Immunol. 147:515. [PubMed] [Google Scholar]
- 25. Taganov K. D., Boldin M. P., Chang K. J. and Baltimore D. 2006. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl Acad. Sci. USA 103:12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan W., Zhu S., Yuan M. et al. . 2010. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 184:6773. [DOI] [PubMed] [Google Scholar]
- 27. Li S., Chowdhury R., Liu F. et al. . 2014. Tumor-suppressive miR148a is silenced by CpG island hypermethylation in IDH1-mutant gliomas. Clin. Cancer Res. 20:5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hong Y., Wang T., Huang C., Cheng W. and Lin B. 2008. Soy isoflavones supplementation alleviates disease severity in autoimmune-prone MRL-lpr/lpr mice. Lupus 17:814. [DOI] [PubMed] [Google Scholar]
- 29. Hsieh C. C. and Lin B. F. 2011. Dietary factors regulate cytokines in murine models of systemic lupus erythematosus. Autoimmun. Rev. 11:22. [DOI] [PubMed] [Google Scholar]
- 30. Kamen D. L. 2014. Environmental influences on systemic lupus erythematosus expression. Rheum. Dis. Clin. North Am. 40:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pons-Estel G. J., Alarcón G. S., Scofield L., Reinlib L. and Cooper G. S. 2010. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 39:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ke Y., Liu K., Huang G. Q. et al. . 2009. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J. Immunol. 182:3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nicoletti F., Di Marco R., Zaccone P. et al. . 2000. Dichotomic effects of IFN-gamma on the development of systemic lupus erythematosus-like syndrome in MRL-lpr / lpr mice. Eur. J. Immunol. 30:438. [DOI] [PubMed] [Google Scholar]
- 34. Ji G., Zhang Y., Yang Q. et al. . 2012. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS ONE 7:e53101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Markle J. G., Frank D. N., Mortin-Toth S. et al. . 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084. [DOI] [PubMed] [Google Scholar]
- 36. Berer K., Mues M., Koutrolos M. et al. . 2011. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479:538. [DOI] [PubMed] [Google Scholar]
- 37. Hevia A., Milani C., López P. et al. . 2014. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio 5:e01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee Y. K., Menezes J. S., Umesaki Y. and Mazmanian S. K. 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108(Suppl. 1):4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathis D. and Benoist C. 2012. The influence of the microbiota on type-1 diabetes: on the threshold of a leap forward in our understanding. Immunol. Rev. 245:239. [DOI] [PubMed] [Google Scholar]
- 40. Wen L., Ley R. E., Volchkov P. Y. et al. . 2008. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yurkovetskiy L., Burrows M., Khan A. A. et al. . 2013. Gender bias in autoimmunity is influenced by microbiota. Immunity 39:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parnell J. A. and Reimer R. A. 2012. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 107:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parnell J. A. and Reimer R. A. 2012. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dai R., Phillips R. A., Zhang Y., Khan D., Crasta O. and Ahmed S. A. 2008. Suppression of LPS-induced Interferon-gamma and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: a novel mechanism of immune modulation. Blood 112:4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dambal S., Shah M., Mihelich B. and Nonn L. 2015. The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res. 43:7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahmoud A. M., Al-Alem U., Ali M. M. and Bosland M. C. 2015. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J. Steroid Biochem. Mol. Biol. 152:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pudenz M., Roth K. and Gerhauser C. 2014. Impact of soy isoflavones on the epigenome in cancer prevention. Nutrients 6:4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie Q., Bai Q., Zou L. Y. et al. . 2014. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes. Chromosomes Cancer 53:422. [DOI] [PubMed] [Google Scholar]
- 49. Matthies A., Loh G., Blaut M. and Braune A. 2012. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal Slackia isoflavoniconvertens in gnotobiotic rats. J. Nutr. 142:40. [DOI] [PubMed] [Google Scholar]
- 50. Weaver C. M. and Legette L. L. 2010. Equol, via dietary sources or intestinal production, may ameliorate estrogen deficiency-induced bone loss. J. Nutr. 140:1377S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.