Abstract
Gain-of-function mutations in Kir6.2 (KCNJ11), the pore-forming subunit of the KATP channel, cause neonatal diabetes. Many patients also suffer from hypotonia (weak and flaccid muscles) and balance problems. While the diabetes arises from suppressed insulin secretion by overactive KATP channels in pancreatic beta-cells, the source of the motor phenotype is unknown. Using mice carrying a human Kir6.2 mutation (V59M) targeted to either muscle or nerve we show that analogous motor impairments originate in the central nervous system rather than in muscle or peripheral nerves. We also identify locomotor hyperactivity as a feature of KATP channel overactivity. Our findings have important therapeutic implications as they indicate that drugs targeted against neuronal, rather than muscle, KATP channels are needed to treat the motor deficits and that such drugs require high blood-brain barrier permeability.
Heterozygous gain-of-function mutations in the gene encoding the Kir6.2 (KCNJ11) subunit of the KATP channel can give rise to intermediate DEND (iDEND) syndrome, a rare genetic disorder characterised by neonatal diabetes accompanied by muscle hypotonia, delayed speech and motor milestones and balance problems [1]. Hypotonia (lack of muscle tone), which is usually most severe in the lower limbs, may account in part for the gait impairments. KATP channels are widely expressed [2,3], and it is not clear whether the hypotonia in iDEND results from overactive KATP channels in muscle or in nerve. In the former case, muscle excitability would be directly reduced by excess hyperpolarizing KATP conductance, whereas in the latter, neuronal regulation of muscle contraction would be compromised. To distinguish between these possibilities we selectively expressed in mice a human gain-of-function Kir6.2 mutation in either muscle or nerve, and evaluated its effect on muscle strength and motor coordination. We selected the Kir6.2-V59M mutation, which is the most common cause of iDEND (>50% of cases). Mice expressing this mutation selectively in pancreatic beta-cells exhibit severe diabetes but no neurological problems [4].
We used a Cre-lox approach in combination with the muscle creatine kinase promoter (Mck-Cre mice) or the nestin promoter (Nes-Cre mice) to selectively target expression of Kir6.2-V59M to either muscle (m-V59M mice) or nerve (n-V59M mice), respectively [5]. Nestin is expressed in neuronal precursor cells and nestin-Cre is thus expected to target all neurons. However, because KATP channels comprise both Kir6.2 (pore-forming) and SUR (regulatory) subunits [3], and SUR is essential for both proper channel function and plasma membrane targeting [6], only those neurons that endogenously express SUR will express functional transgenic KATP channels. SUR1 mRNA levels were unaffected in mutant mice, suggesting channel density will also be unaltered (Fig.S1).
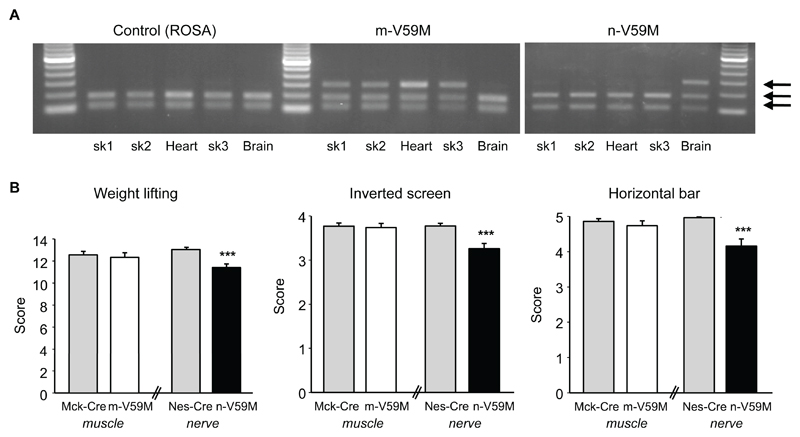
Fig.1A confirms that Kir6.2-V59M mRNA is expressed in muscle but not brain of m-V59M mice, in brain but not muscle of n-V59M mice, and not at all in control mice [5]. Furthermore, endogenous wild-type and introduced mutant Kir6.2 are expressed at comparable levels (Figs.S1, 3D). This establishes our hemizygous mouse as a plausible model for human patients with the V59M mutation, all of whom are heterozygotes [1].
Figure 1. Muscle function is impaired by expression of Kir6.2-V59M in neurons but not in muscle.
(A) Kir6.2 expression in tissue isolated from control, m-V59M or n-V59M mice. Wild-type, but not mutant, cDNA is cut by the restriction enzyme BtsCI: two bands thus indicates the presence of the wild-type gene only, and three bands indicates both wild-type and mutant genes. sk1, quadriceps muscle; sk2, triceps muscle; sk3, diaphragm. Data are representative of experiments on 4 ROSA and 4 m-V59M mice done in parallel, and 3 ROSA and 3 n-V59M mice performed in parallel. (Supporting on-line text and additional control data in Fig.S1A).
(B) Weight lifting, inverted screen and horizontal bar tests on 11-12-week old m-V59M (n=42), n-V59M (n=31) and control mice (Mck-Cre, n=35; Nes-Cre, n=53). Mean±SEM. ***, p<0.001 (Kruskal-Wallis One Way Analysis of Variance on Ranks). Additional control data in Fig.S2.
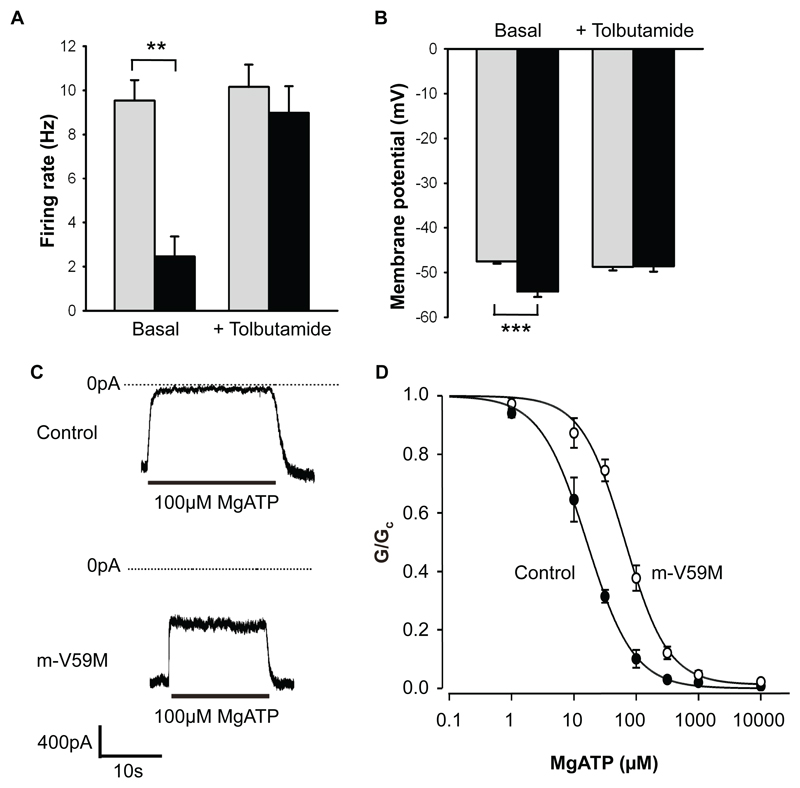
Figure 3. KATP channel activity in Purkinje cells of n-V59M mice and skeletal muscle of m-V59M mice.
(A) Mean (±SEM) action potential frequency of control (grey bars) and n-V59M (black bars) neurons in the absence (control: n=9 mice, 34 neurons. n-V59M: n=3 mice, 26 neurons) and presence (control: n=7 mice, 16 neurons. n-V59M, n=3 mice, 15 neurons) of 500µM tolbutamide. Cell-attached recordings. **, p=0.002 (t-test).
(B) Mean (±SEM) resting membrane potential of control (grey bars, n=17 neurons, 6 mice) and n-V59M neurons (black bars, n=13 neurons, 5 mice) in the absence and then the presence of 200µM tolbutamide. Whole-cell recordings. ***, p<0.001 (t-test).
(C) KATP channel currents recorded at -60mV in inside-out patches from control (above) or m-V59M (below) FDB muscle.
(D) Mean (±SEM) MgATP sensitivity of KATP channels in inside-out patches from control (●, n=4; IC50=15µM) or m-V59M (○, n=4; IC50=66µM) FDB muscle.
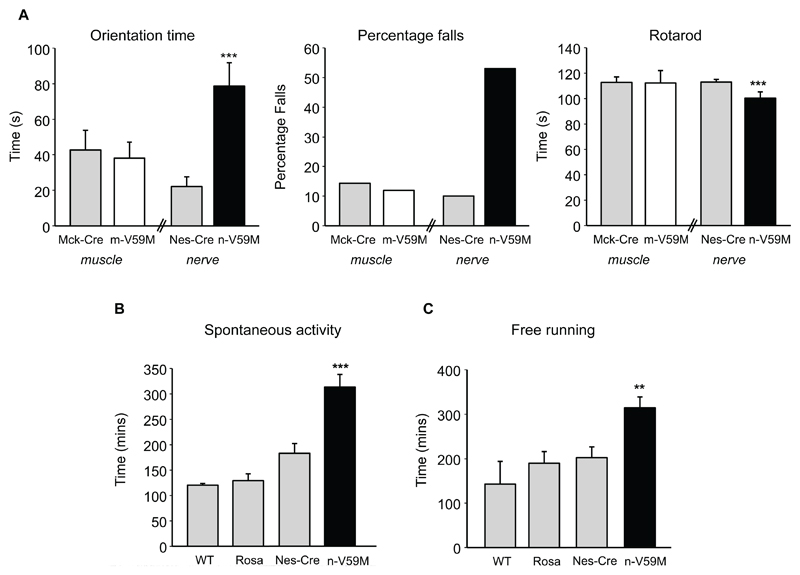
On three tests of muscle strength [5], mice selectively expressing the Kir6.2-V59M mutation in muscle performed equally well to control mice (Figs.1B, S2). Likewise, their motor control and balance were unaffected ([5], Figs.2A, S3). In contrast, mice in which Kir6.2-V59M was targeted to neurons performed less well in all tests of muscle function: they were unable to lift weights as effectively or to hang as long from an inverted screen or horizontal bar (Figs.1B, S2). n-V59M mice also showed impaired balance control (Fig.2A, S3). For example, they took 3-fold longer than controls to turn around on a thin rod suspended above the ground, and many more fell off whilst attempting to do so (Movie S1, S2). They also fell off a rotating rod sooner.
Figure 2. Motor coordination and locomotor activity are impaired by expression of Kir6.2-V59M in neurons but not in muscle.
(A) Orientation time and percentage of falls on the static rod test, and time before falling from a rotating rod, for m-V59M, n-V59M and control mice. Same mice as in Fig.1. (B,C) Duration of time spent in spontaneous physical activity (B) or using a free-running wheel (C) over a 23-hr period for 12-week old n-V59M (n=13), Nes-Cre (n=14), ROSA (n=14) and WT (n=4) littermates. Mean±SEM. **, p<0.01. ***, p<0.001 (One-way ANOVA). Additional control data in Fig.S3.
These results demonstrate that n-V59M mice are impaired in muscle strength, balance and motor coordination. Their motor problems could either be due to reduced excitability of central nervous system (CNS) neurons or to decreased transmitter release at the neuromuscular junction. Studies of isolated nerve-muscle preparations [5] rule out the latter possibility, as no differences were observed in the amplitude and frequency of miniature endplate potentials, or evoked endplate potentials between control and n-V59M mice (Fig.S4). There was also no difference in the muscle resting membrane potential. Similar results were found for m-V59M mice (Fig.S5). This suggests the motor difficulties experienced by the mice originate in the CNS.
The n-V59M mice also display hyperactivity, spontaneously moving around more frequently (Fig.2B) and staying significantly longer than controls in free-running wheels [5] (Figs.2C, S6). Although hyperactivity is not stated as a feature of iDEND syndrome [1], several reports indicate that once iDEND children have learnt to walk they exhibit pronounced hyperactivity [7–9]. Our results suggest this is caused by KATP channel overactivity and should be considered a characteristic of iDEND.
To confirm that neurons are indeed affected by the Kir6.2-V59M mutation, we recorded the electrical activity of cerebellar Purkinje cells in acute brain slices [5]. KATP channels are highly expressed in multiple brain regions including those involved in movement (cortex, cerebellum) [10,11], but we selected Purkinje cells because of their importance for motor control. In both cell-attached (Figs.3A-B, S7) and whole-cell (Fig.S7C) recordings, action potential frequency was substantially lower in Purkinje cells of n-V59M mice than controls. Tolbutamide, a specific inhibitor of KATP channels, increased the firing rate of n-V59M cells to control levels, but had no effect on control cells (Fig.3A). The resting membrane potential of n-V59M Purkinje cells was also more hyperpolarised than in controls and was restored by tolbutamide (Fig.3B). This suggests that in n-V59M mice, an increased KATP current in Purkinje cells suppresses firing and may thereby impair motor function. Neurons other than Purkinje cells are also likely to contribute to the motor phenotype.
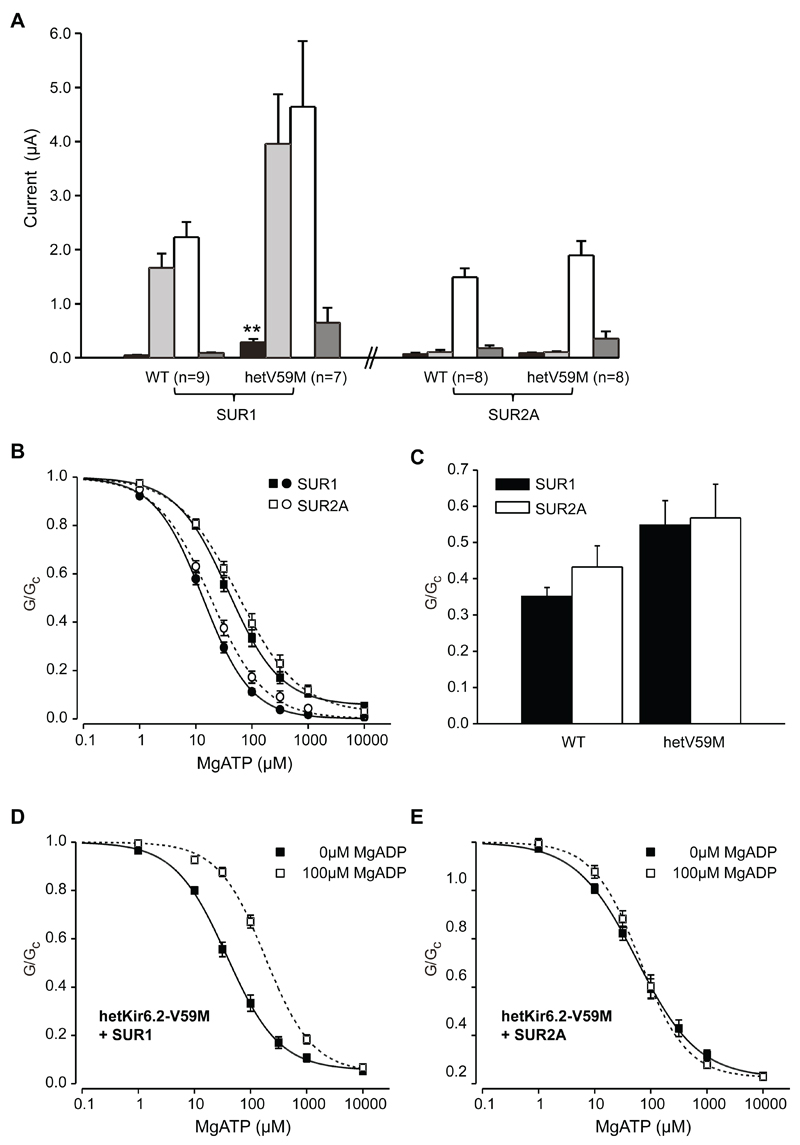
Patch-clamp recordings of isolated muscle fibres from m-V59M mice revealed that the ATP sensitivity of the native muscle KATP channel is significantly reduced compared to control mice (Fig.3C,D, TableS1). So why should the Kir6.2-V59M mutation fail to affect the muscle membrane potential yet hyperpolarise neurons? We reasoned this might reflect differences in tissue metabolism (and thus [ATP]i) or, alternatively, that it may be due to the different SUR isoforms that comprise muscle (SUR2A) and nerve (SUR1) KATP channels [3]. To distinguish between these possibilities we compared the responses of muscle and neuronal types of KATP channel heterologously expressed in the same cell type (Xenopus oocytes) [5]. Channels composed of wild-type Kir6.2 and either SUR1 or SUR2A were closed under resting conditions due to the high oocyte [ATP]i. Lowering [ATP]i by metabolic inhibition activated large whole-cell Kir6.2/SUR1 currents but had no effect on Kir6.2/SUR2A channels (Fig.4A). The Kir6.2-V59M mutation enhanced the resting Kir6.2/SUR1 current but had no effect on Kir6.2/SUR2A currents, which remained closed even when metabolism was inhibited. Thus differences in SUR isoforms explain why muscle is unaffected by the Kir6.2-V59M mutation whilst neuronal electrical activity is reduced.
Figure 4. Kir6.2/SUR1 is more sensitive to metabolism than Kir6.2/SUR2A due to differential nucleotide sensitivity.
(A) Mean (±SEM) steady-state whole-cell KATP currents evoked by a voltage step from -10 to -30 mV before (black bars) and after (pale-gray bars) application of 3mM azide for oocytes injected with Kir6.2 (WT), or 1:1 mixture of Kir6.2 and Kir6.2-V59M (hetV59M mRNA, plus either SUR1 or SUR2A mRNAs. White bars, 3mM azide plus 300µM diazoxide (SUR1) or 100µM pinacidil (SUR2A). Dark-grey bars, 3mM azide, 300µM diazoxide and 0.5mM tolbutamide (SUR1) or 3mM azide, 100µM pinacidil and 10µM glibenclamide (SUR2A). The number of oocytes is indicated. **, p<0.001 with respect to wild-type (t-test).
(B) Mean (±SEM) MgATP sensitivity of Kir6.2/SUR1 (●, n=15), Kir6.2/SUR2A (○, n=15), hetKir6.2-V59M/SUR1 (■, n=12) and hetKir6.2-V59M/SUR2A (□, n=16) channels. KATP conductance (G) is expressed relative to that in the absence of nucleotides (GC). The curves are the best fit of the Hill equation to the mean data (Supporting on-line text, Table S1).
(C) MgADP activation of channels composed of wild-type Kir6.2 (left) or Kir6.2-V59M (right) plus either SUR1 (black bars) or SUR2A (white bars). KATP conductance (G) in the presence of 100µM MgADP is expressed relative to the maximal conductance in the absence of MgADP (GC). Mean±SEM.
(D,E) Mean (±SEM) MgATP sensitivity of hetKir6.2-V59M/SUR1 (D) and hetKir6.2-V59M/SUR2A (E) channels in the absence (■; SUR1, n=12; SUR2A, n=16) and presence (□; SUR1, n=14; SUR2A, n=7) of 100µM MgADP. (Supporting on-line text, TableS2).
Adenine nucleotides inhibit KATP channels by binding to Kir6.2 and (when Mg2+ is present) activate them via interaction with SUR [12,13]. MgATP block (Fig.4B) and MgADP activation in the absence of MgATP (Fig.4C) were similar for Kir6.2/SUR1 and Kir6.2/SUR2A channels, whether wild-type or mutant. By contrast, whereas MgADP activated SUR1-containing channels when MgATP was present, markedly shifting the ATP dose-response curve (Fig.4D), this was not the case for SUR2A-containing channels (Fig.4E). This difference probably underlies the different sensitivities of mutant Kir6.2/SUR1 and Kir6.2/SUR2A channels to metabolic inhibition.
Our results demonstrate that hemizygous mice carrying the Kir6.2-V59M mutation provide a faithful model for the muscle weakness, balance problems and hyperactivity seen in iDEND children. They also confirm that analogous problems in human patients are not a secondary consequence of diabetes (or of insulin-induced hypoglycaemia), because our mice have normal blood glucose levels (Fig.S8). Importantly, the data indicate that the motor deficits result from impairment of neuronal, not muscle, function.
Over the past four years, sulphonylurea therapy has become the treatment of choice for neonatal diabetes and iDEND [14]. These drugs stimulate insulin secretion by blocking open KATP channels [15]. They also improve the neurological problems in many (but not all) iDEND patients. Some children begin to walk or talk for the first time shortly after starting therapy [16]; in most cases hypotonia and fine motor control are improved [17], and hyperactivity is reduced [9,10]. Our data suggest that these improvements reflect sulphonylurea block of inappropriately active KATP channels in brain neurons.
Most iDEND patients are currently treated with glibenclamide, which interacts with both SUR1 and SUR2A. Our finding that their muscle problems are neuronal in origin means it may also be possible to use SUR1-specific sulphonylureas such as tolbutamide, gliclazide, or nateglinide [14]. Indeed, there is one report that gliclazide can enhance movement control in a patient [17].
The choice of sulphonylurea used for type 2 diabetes therapy has long been debated [18–20]. In particular, there has been concern that these drugs may interact adversely with cardiac (SUR2A-containing) KATP channels. KATP channel activation plays an important role in cardiac ischemic preconditioning in the heart [3], and this effect is prevented by glibenclamide [21]. Neonatal diabetes patients routinely require much higher sulphonylurea doses than type 2 diabetes patients and they may be expected to take the drugs far longer. Our demonstration that the motor problems in iDEND are neuronal in origin indicates it is unnecessary to use blockers of muscle (SUR2A-containing) KATP channels to ameliorate the motor problems. Thus it may be worth considering whether iDEND patients would be better treated with SUR1-specific drugs to avoid potential cardiac cross-reactivity.
Finally, in the light of our results, the success of sulphonylureas in treating the muscle impairments of iDEND children indicates that these drugs gain access to the brain. This means that efforts to improve sulphonylurea therapy for motor problems in iDEND patients should focus on identifying those that have high blood-brain barrier permeability.
Supplementary Material
One sentence summary.
A gain-of-function mutation in the KATP channel (Kir6.2-V59M) that causes muscle weakness, balance problems and locomotor hyperactivity in humans does so by changing neuronal excitability not muscle function.
Acknowledgments
We thank Dr C. Girard for helping make the ROSA mouse [4] and help with some early experiments, and Dr R. Deacon for teaching us the mouse behavioural tests and for the use of the OXION mouse behaviour facility. We thank the Wellcome Trust, the Medical Research Council, the Royal Society, the European Union (LHSB-CT-2004-005137, HEALTH-F4-2007-201924), the Muscular Dystrophy Campaign and Myasthenia Gravis Association for support. RHC holds an OXION Wellcome Trust prize studentship and JSM an MRC studentship. FMA is a Royal Society University Research Professor
References
- 1.Hattersley AT, Ashcroft FM. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM. Am J Physiol. 2007;293:E880–889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 3.Nichols CG. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 4.Girard CA, et al. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Materials and methods are available as supporting material on Science Online
- 6.Zerangue N, Schwappach B, Jan YN, Jan LN. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 7.Sagen JV, et al. Diabetes. 2004;53:2713–8. doi: 10.2337/diabetes.53.10.2713. [DOI] [PubMed] [Google Scholar]
- 8.Slingerland AS, et al. Diabet Med. 2008;25:277–281. doi: 10.1111/j.1464-5491.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 9.Mlynarski W, et al. Nat Clin Pract Neurol. 2007;3:640–645. doi: 10.1038/ncpneuro0640. [DOI] [PubMed] [Google Scholar]
- 10.Karschin C, Ecke C, Ashcroft FM, Karschin A. FEBS Letts. 1996;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- 11.Mourre C, Widmann C, Lazdunski M. Brain Res. 1990;519:29–43. doi: 10.1016/0006-8993(90)90057-i. [DOI] [PubMed] [Google Scholar]
- 12.Tucker SJ, et al. Nature. 1997;387:179–181. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 13.Nichols CG, et al. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 14.Pearson ER, et al. New Eng J Med. 2006;355:467–77. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 15.Gribble FM, Reimann F. Diabetologia. 2003;46:875–91. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 16.Slingerland AS, Nuboer R, Hadders-Algra M, Hattersley AT, Bruining GJ. Diabetologia. 2006;49:2559–2563. doi: 10.1007/s00125-006-0407-0. [DOI] [PubMed] [Google Scholar]
- 17.Koster JC, et al. J Clin Endocrinol Metab. 2008;93:1054–61. doi: 10.1210/jc.2007-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quast U, et al. Diabetes. 2004;53:S156–164. doi: 10.2337/diabetes.53.suppl_3.s156. [DOI] [PubMed] [Google Scholar]
- 19.Simpson SH, et al. CMAJ. 2006;174:169–174. doi: 10.1503/cmaj.050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UKPDS Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 21.Ghosh S, Standen NB, Galiñianes M. J Am Coll Cardiol. 2001;37:711–8. doi: 10.1016/s0735-1097(00)01161-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.