Abstract
Elevated plasma glucose leads to pancreatic β-cell dysfunction and death in type 2 diabetes. Glycogen accumulation, due to impaired metabolism, contributes to this ‘glucotoxicity’ via dysregulated biochemical pathways promoting β-cell dysfunction. Here, we review emerging data, and re-examine published findings, on the role of glycogen in β-cells in normoglycaemia and in diabetes.
Keywords: glycogen, diabetes, pancreatic β-cell, insulin
Introduction
Glucose metabolism is an essential step in glucose-stimulated insulin secretion from the pancreatic β-cell and metabolic dysfunction is believed to contribute to impaired insulin release in type 2 diabetes. Although glycogen is not normally present in β-cells, substantial amounts are accumulated by β-cells under hyperglycaemic conditions. Nevertheless, its physiological and pathophysiological roles are controversial, and how metabolism drives its accumulation is not fully understood.
Several special features of β-cell metabolism underpin its ability to serve as a glucose sensor and match insulin secretion to the circulating glucose level (Prentki et al., 2013; Schuit et al., 2001). First, glucose uptake by the β-cell is not rate limiting which ensures rapid equalisation of intracellular and extracellular glucose concentrations (Matschinsky and Ellerman, 1968; Thorens, 2015). In rodents, glucose uptake is mediated the high capacity, low affinity glucose transporter GLUT2. Human β-cells express ~100-fold less GLUT2 than rodent β-cells, with GLUT1 and GLUT3 (which have a low Km) being the major glucose transporters (De Vos et al., 1995). Nevertheless, despite these differences in glucose transporters, the rate-limiting step in glucose metabolism in both species is the phosphorylation of glucose to glucose-6-phosphate. This reaction is catalysed by glucokinase, which has a high Km and is not subject to product inhibition (Ashcroft and Randle, 1970; Matschinsky, 1996).
Almost all glucose entering the β-cell is oxidised via glycolysis and mitochondrial oxidative phosphorylation to ATP (Prentki et al., 2013; Schuit et al., 2001). Little is metabolized to lactate because lactate dehydrogenase activity is very low in β-cells (Sekine et al., 1994) and expression of the monocarboxylate transporter MCT1 (Slc16a1) is suppressed (Lemaire et al., 2016; Zhao et al., 2001). The absence of these ‘disallowed’genes is necessary to prevent triggering of insulin secretion during exercise by circulating pyruvate or lactate – indeed, expression of MCT1 in human β-cells (due to a mutation in its promoter) leads to exercise-induced hypoglycaemia (Otonkoski et al., 2003). Under normal conditions, glycogen synthesis accounts for <10% of glucose uptake, the conversion of glucose to lipids and amino acids is of minor quantitative significance, and the sorbitol and pentose phosphate pathways are relatively unimportant (Ashcroft et al., 1972). As a consequence, glucose oxidation closely matches glucose consumption, and ATP levels rise in response to an increase in extracellular glucose (Ashcroft et al., 1973; Detimary et al., 1996). This leads to closure of plasmalemmal KATP channels, which triggers membrane depolarisation, Ca2+-dependent electrical activity, Ca2+-influx and insulin release (Ashcroft, 2007).
Although β-cells have the ability to store glycogen and lipid, both are almost completely absent under normoglycaemic conditions. Nevertheless, it has been known for many years that large amounts of glycogen accumulate in β-cells in diabetes (Toreson, 1951). Why this is the case is not fully established and its functional role is controversial. On the one hand, it has been argued that glycogen metabolism by β-cell is not involved in glucose homeostasis and that its accumulation is not sufficient to trigger β-cell loss or dysfunction (Mir-Coll et al., 2016). On the other, that glycogen accumulation in diabetes may reflect an underlying metabolic dysfunction and lead to a reduction in β-cell mass (Brereton et al., 2016; Malaisse, 2016; Malaisse et al., 1992). Here, we review recent evidence that glycogen storage may contribute to β-cell dysfunction in type-2 diabetes.
Identification of β-Cells as a Glycogen Storing Tissue
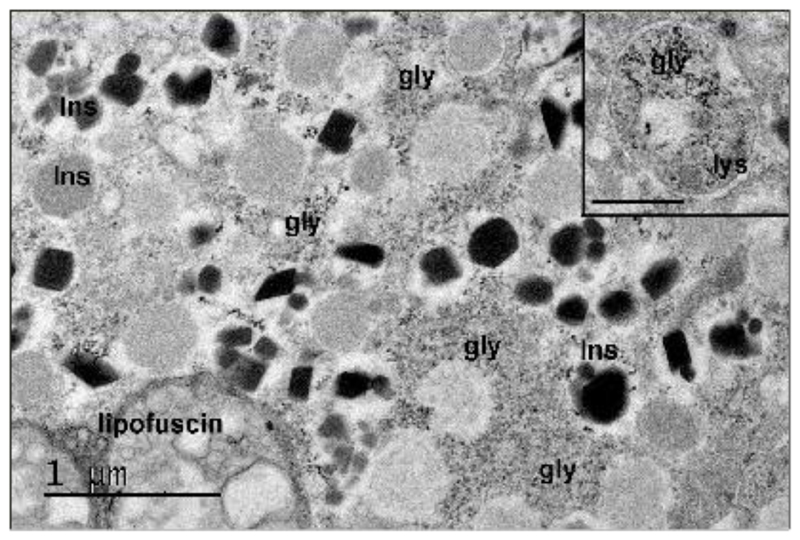
It was discovered over 60 years ago that glycogen accumulates in β-cells of diabetic patients (Toreson, 1951). This gives rise to a characteristic vacuolated appearance of the cytoplasm, that was initially termed ‘hydropic degeneration’ (Weichselbaum and Stangi, 1901). Glycogen was subsequently identified in β-cells in multiple animal models of diabetes or hyperglycaemia (Hellman and Idahl, 1969; Idahl and Hellman, 1968; Lazarus and Volk, 1958; Malaisse et al., 1967; Matschinsky and Ellerman, 1968; Ravelli et al., 2013; Williamson, 1960), and shown to accumulate in β-cell lines and islets isolated from wild-type mice and normoglycaemic human donors when cultured at high glucose (Andersson et al., 2016; Brereton et al., 2016; Malaisse et al., 1992). Glycogen can also be observed in islets from Type 2 diabetic donors (Figure 1). However, it is not found under normoglycaemic conditions (Brereton et al., 2016; Malaisse et al., 1967; Ashcroft et al., 1972).
Figure 1. Glycogen is present in human β-cells in diabetes.
Representative electron micrograph showing glycogen in a β-cell of a type-2 diabetic organ donor with diet-controlled diabetes (HbA1c, 6.6) for 10 years. Sample prepared and stained for glycogen (Brereton et al 2016). Glycogen particles (gly, electron dense material) were distributed throughout the cytoplasm in most β-cells. Autophagic bodies and lysosomes (inset, lys) also contained glycogen. Insulin granules (Ins) contained either dark crystalline cores (mature) or less electron dense material (immature). Scale bar, 1µM. Scale bar of inset 0.5µM.
Despite its identification many years ago, the presence of glycogen in β-cells is often overlooked. This is because its detection is challenging as it is rapidly degraded and washed out of cells during tissue fixation and processing for histochemistry (Graf and Klessen, 1981). It also quickly disappears if islets (Malaisse et al., 1992) or INS-1 cells (Andersson et al., 2016) exposed to chronic hyperglycaemia are subsequently cultured under normoglycaemic conditions. As a consequence, glycogen-containing β-cells have often been missed. Indeed, it seems likely that β-cells that appear (or are described as) “clear”, “vacuolated” or “empty” in animal models of diabetes (Cinti et al., 2016; Jung et al., 2008; Poudel et al., 2015; Talchai et al., 2012) or in human diabetes (Poudel et al., 2015) may originally have contained glycogen that was subsequently lost during fixation and processing.
In contrast to β-cells, glycogen is not detected in α-cells or δ-cells of islets from diabetic patients, diabetic animal models, or non-diabetic human donors cultured at high glucose (Brereton et al., 2016; Graf and Klessen, 1981). Why this is the case is unknown. It is possible that α-cells take up less glucose than β-cells in response to hyperglycaemia, that they metabolise glucose differently (Schuit et al., 1997) or that they lack some part of the biochemical machinery needed to manufacture and store glycogen.
Regulation of Glycogen Metabolism
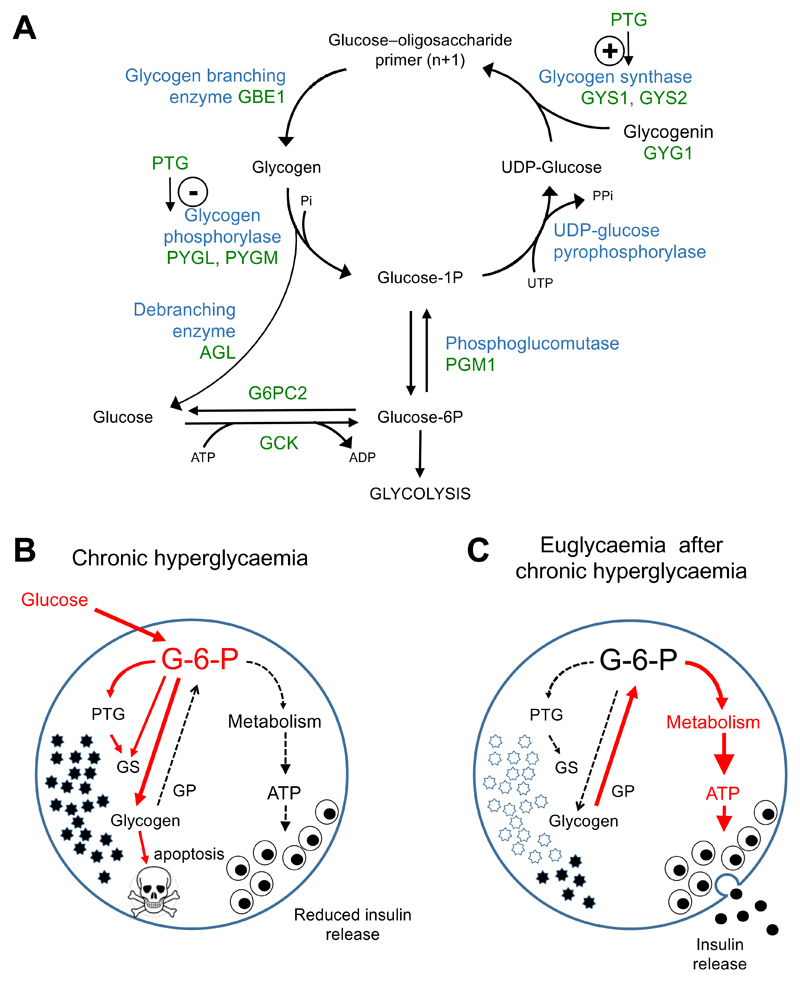
Pancreatic β-cells express many of the key enzymes involved in glycogen synthesis and degradation, including phosphoglucomutase, UDP-pyrophosphorylase, the muscle isoform of glycogen synthase (GS) GYS1, glycogen branching enzyme (Gbe1) and glycogen phosphorylase (GP) (Andersson et al., 2016; Brereton et al., 2016; Brolin and Berne, 1970; Newman, 1977) (Figure 2A). The activity of most of these enzymes in β-cells is relatively low compared to liver and muscle, (Andersson et al., 2016; Brolin and Berne, 1970; Newman, 1977). However, GS is activated (and GP inactivated) when glucose is elevated (Zhang et al., 1994). At least part of this increase is due to allosteric activation by glucose metabolites: glucose-6-phosphate, for example, is a potent activator of glycogen synthase (Bouskila et al., 2010; Villar-Palasi and Guinovart, 1997) (Figure 2A,B).
Figure 2. Pathways for glycogen accumulation and its effects in β-cells.
A. Key pathways for glycogen synthesis and degradation. GCK, glucokinase. G6PC2, gucose-6-phosphatase. PGM1, phosphoglucomutase. GYG1, glycogenin. GYS1 GYS2, glycogen synthase. PTG protein targetting to glycogen (a subunit of PKA). GBE1, glycogen branching enzyme. PYLL.M, glycogen phosphorylase muscle (PYGM) and liver (PYGL) types. AGL (amylo-α-1,6-glucosidase, 4-α-glucanotransferase), glycogen debranching enzyme.
B,C. Diagrammatic representation of the effects of glycogen accumulation in β-cells. (B) In chronic hyperglycaemia (as in type 2 diabetes) oxidative metabolism of glucose is reduced, leading to decreased insulin release. Glucose continues to enter the cell and is converted to glucose-6-phosphate (G-6-P) by glucokinase. Elevation of G-6-P leads to allosteric activation of glycogen synthase (GS) and increased expression of Protein Targeting to Glycogen (PTG), resulting in glycogen accumulation. Continued glycogen accumulation can lead to activation of apoptotic mechanisms and cell death. Pathways amplified in chronic hyperglycaemia are indicated in red.
(C) When extracellular glucose is reduced, glycogen is metabolised via glycogen phosphorylase (GP) to G-6-P, which, via restored oxidative metabolism, leads to enhanced ATP production, resulting in inappropriately elevated insulin secretion. Pathways amplified on restoration of blood glucose are indicated in red.
Hyperglycaemia not only increases substrate availability, it also enhances the expression of key genes involved in glycogen synthesis, such as Ppp1r3c, or PTG (protein targeting to glycogen) (Bensellam et al., 2009; Brereton et al., 2016). This protein is a regulatory subunit of protein phosphatase 1 (PP1) and acts as a molecular scaffold that links it to glycogen, thereby activating GS and inhibiting GP. PTG overexpression drives glycogen formation in neurones (Vilchez et al., 2007), sertoli cells (Villarroel-Espindola et al., 2016), adipocytes (Greenberg et al., 2003), cancer cells (Shen et al., 2010) and β-cells (Mir-Coll et al., 2016), and its heterozygous deletion leads to reduced glycogen stores in liver, heart, muscle and adipose tissue (Crosson et al., 2003). Increased PTG mRNA is also associated with increased glycogen content in β-cells from diabetic mice (Bensellam et al., 2009; Brereton et al., 2016; Mir-Coll et al., 2016). Interestingly, a polymorphism in the PPP1R3C gene is associated with a 3.6-fold increased risk of developing type-2 diabetes (Wang et al., 2001).
The extent of glycogen accumulation is both glucose- and time-dependent (Andersson et al., 2016; Brereton et al., 2016; Matschinsky and Ellerman, 1968). In an inducible mouse model of diabetes, 2 weeks of severe hyperglycaemia (>20mM) was required for substantial glycogen stores to form. In vitro, however, 48h exposure to high glucose was sufficient for INS-1 cells, and β-cells in islets isolated from normoglycaemic mice or non-diabetic human donors to accumulate significant glycogen (Andersson et al., 2016; Brereton et al., 2016; Mir-Coll et al., 2016). Thus the rate of glycogen accumulation may vary between the in vitro and in vivo situation. It is also worth noting that the glucose concentration at which human and rodent islets are often cultured (11 to 25mM) is not normoglycaemic, and leads to significant glycogen formation (Brereton et al., 2016; Andersson et al., 2016).
Glycogen accumulation appears to be triggered not only by increased substrate, but also by changes in β-cell metabolism caused by chronic hyperglycaemia. Hyperglycaemia both in vitro and in vivo, is associated with marked changes in the expression of β-cell metabolic genes: expression of enzymes in the Krebs cycle and oxidative phosphorylation are reduced, and glycolytic, gluconeogenic and glycogen metabolising enzymes are increased (Bensellam et al., 2009; Brereton et al., 2016; Brun et al., 2013; Jonas et al., 1999). Expression of mitochondrial carrier proteins, that transfer metabolites across the mitochondrial membrane, is modified, contributing to metabolic dysfunction (Brun and Maechler, 2016). Down-regulation of genes involved in mitochondrial metabolism is also seen in transcriptome analysis of β-cells from type 2 diabetic donors (Marselli et al., 2010; Segerstolpe et al., 2016). Furthermore, the glucose-stimulated increase in ATP production is absent in islets from animal models of diabetes (Brereton et al., 2016) or human donors with type-2 diabetes (Anello et al., 2005). In addition, islets exposed to chronic hyperglycaemia in vitro release insulin in response to tolbutamide but not glucose, consistent with the idea that the impairment in glucose-induced stimulus-secretion coupling lies upstream of KATP channel closure (Henquin et al., 2015).
The trigger for altered β-cell metabolism may not be elevation of glucose per se but rather an increase in one or more of its metabolites. Glycogen accumulation is also found in β-cells from mice expressing an activating mutation (Y214C) in glucokinase (Brereton et al., 2016), which are hypo- not hyper-glycaemic (Tornovsky-Babeay et al., 2014). This mutation is predicted to result in elevation of G-6-P, and thereby contribute to activation of glycogen synthase and glycogenesis (Bouskila et al., 2010).
In summary, the available data suggest that altered β-cell glucose metabolism contributes to dysregulated insulin secretion. Since other pathways for glucose metabolism are limited, the excess glucose entering the β-cell in type 2 diabetes is diverted from oxidative metabolism to glycogenesis. Thus glycogen can be considered a marker for impaired β-cell metabolism.
Glygogen Rapidly Disappears on Euglycaemia
β-cell glycogen stores are rapidly dissipated when euglycaemia is restored, via both metabolic degradation and glycophagy. The former, which takes place in the cytoplasm, involves the enzymes glycogen phosphorylase and glycogen debranching enzyme, whereas the latter is mediated via the autosomal/lysosomal route. In isolated islets, the rate of glycogenolysis is related to the glycogen content (Malaisse et al., 1992), and in mice with an activating KATP channel mutation that led to substantial glycogen accumulation, glycogen stores vanished within 24h of establishing euglycaemia in vivo (Brereton et al., 2016). In this model, the anti-diabetic sulphonylurea drug glibenclamide was more effective than insulin (Brereton et al., 2016), perhaps because, in addition to normalising blood glucose, it stimulated Ca2+-dependent electrical activity. The enhanced Ca2+ influx would facilitate glycogen degradation by stimulating glycogen metabolising enzymes (Denton, 2009; Liu et al., 2008).
Glycogen can also be degraded by autophagy in a process known as ‘glycophagy’ (Kondomerkos et al., 2005; Kotoulas et al., 2006; Mellor et al., 2014). Glycophagy is a route for glycogen degradation in both mouse and human β-cells, as substantial numbers of glycogen particles can be found within autophagosomes and lysosomes (Figure 1) (Brereton et al., 2016). It may be significant that the number of autophagosomes does not change during hyperglycaemia, but is markedly enhanced following restoration of normoglycaemia, when glycogen stores are dissipated (Brereton et al., 2016).
In the liver, glycogen breakdown results in the release of glucose into the circulation. In contrast, muscle does not metabolise glycogen to free glucose as it lacks the enzyme glucose-6-phosphatase (G6Pase). β-cells do express G6Pase (Ashcroft et al., 1970; Brereton et al., 2016; Segerstolpe et al., 2016), but it is a different isoform and its activity is substantially less than that in liver (Ashcroft and Randle, 1968; Matschinsky and Ellerman, 1968). Its function in β-cells is largely unknown. Knockout of G6pc2 (the major islet G6Pase) in mice leads to enhanced basal insulin secretion and reduced fasting blood glucose (Pound et al., 2013), and a polymorphism in the human G6PC2 gene is associated with elevated fasting plasma glucose (Bouatia-Naji et al., 2008). Expression of G6pc2 is downregulated by hyperglycaemia (Brereton et al., 2016).
Interestingly, there is a paradoxical transient (~5min) increase in insulin release from both rodent and human islets previously incubated at high glucose, immediately upon glucose reduction (Henquin et al., 2015; Malaisse et al., 1993; Malaisse et al., 1967; Malaisse et al., 1977). This has been attributed to a rapid increase in glycogenolysis, and the duration of increased insulin secretion has been proposed to reflect the time taken to dissipate the glycogen stores (Malaisse et al., 1993) (Figure 2C). Whereas in normal islets, a fall in extracellular glucose is accompanied by a parallel fall in glycolytic flux, this was not the case in islets isolated from hyperglycaemic rats despite a marked decrease in the contribution of exogenous glucose to metabolism (Malaisse et al., 1993). This suggests that glycogenolysis may interfere with glycolysis, and provide an intracellular substrate for metabolism that enhances KATP channel closure and insulin secretion. However, this interesting idea requires further testing under controlled conditions of intra and extra-cellular glucose and measurement of glycogen flux.
An Emerging Role for Glycogen in the Pathophysiology of Diabetes
Type-2 diabetes mellitus is characterised by inappropriate insulin release from the pancreatic β-cell, which results in chronically elevated blood glucose levels. This further impairs β-cell function, exacerbating the diabetes, and ultimately leads to complications such as retinopathy, nephropathy, neuropathy and macro- and micro-vascular disease. This phenomenon is referred to as ‘glucotoxicity’. Why hyperglycaemia is detrimental to the β-cell and to other tissues is not clearly established. Given that β-cells accumulate glycogen in diabetes, often in very substantial amounts, a salient question is what impact glycogen storage has on β-cell function and whether it is protective or deleterious.
In astrocytes, a substantial fraction of glucose is normally metabolised via glycogen production and breakdown (Walls et al., 2009). Similarly, although neurones do not use glycogen as an energy store, they synthesize and break down tiny amounts of it continuously and upregulation of this process in hypoxia is important for neuronal survival (Saez et al., 2014). Whether glycogen shunting exists in β-cells is not established. It seems unlikely to be important in β-cells under physiological conditions, as knockout of GS had no effect on glucose homeostasis in non-diabetic mice (Mir-Coll et al., 2016). Nevertheless, it remains possible that it plays a role in hypoxia by promoting β-cell survival, as in neurones. Under hyperglycaemic conditions, inhibition of oxidative metabolism may activate the same pathway and precipitate glycogen accumulation. Initially, this may help protect the cell from glucose-induced generation of reactive oxygen species, as has been suggested for various types of cancer cell (Zois and Harris, 2016). Whether this is actually the case, however, requires investigation.
Excessive glycogen accumulation, however, appears to be detrimental. Mouse pancreatic β-cells containing substantial glycogen stores showed increased apoptosis, whereas β-cells that did not contain glycogen were protected (Brereton et al., 2016). Likewise, α-cells, which did not contain glycogen, were protected. β-cell apoptosis appears to result from the enhanced glycogen content, rather than hyperglycaemia per se, as manipulations that lower glycogen levels prevent caspase 3 activation (one of the first steps in apoptosis), despite the elevated glucose (Brereton et al., 2016). No apoptotic changes were found in mouse β-cells in vivo following overexpression of PTG (Mir-Coll et al., 2016). However, these mice were normoglycaemic and glycogen was only elevated 3.5-fold, suggesting that glycogen must reach a higher concentration before it has adverse effects. This raises the question of whether excessive glycogen accumulation might lead to β-cell death in type-2 diabetes and contribute to the reduced β-cell mass found in some patients. If this proves to be the case, ways to prevent its accumulation might provide a novel target for therapeutic drugs. In this respect, it may be significant that metformin decreases glycogen accumulation in INS-1 cells exposed to hyperglycaemia (Brereton et al., 2016).
Dissipation of glycogen stores on return to euglycaemia might also contribute to inappropriately elevated fasting insulin levels and so aggravate hypoglycaemia (Malaisse et al., 1993). In type-2 diabetes fluctuating blood sugar concentrations are common, with sustained periods of hyperglycaemia alternating with normal or low blood glucose levels. The experimental data suggests that endogenous insulin secretion may be greater than expected when sustained hyperglycaemia is reduced (by insulin or drugs); at the low glucose concentration, glycogen mobilisation to intracellular G-6-P would act as a substrate for increased metabolism and insulin secretion, thereby predisposing to hypoglycaemia (Figure 2C).
Many tissues affected by the secondary complications of diabetes also both accumulate glycogen and undergo apoptosis. This phenomenon is observed in several animal models of diabetes and affected cells include nephrons, cardiomyocytes, retinal epithelia, and neurones (Gatica et al., 2015; Hernandez et al., 2014; Kang et al., 2005; Reichelt et al., 2013; Vallon and Komers, 2011). Thus it is possible that excessive glycogen accumulation induced by hyperglycaemia, leading to dysfunction and death, is a common pathophysiology that adversely affects many tissues in diabetes.
Lessons from Glycogen Storage Disorders
Glycogen accumulation is also found in a number of rare genetic disorders caused by defects in enzymes involved in its synthesis or degradation (Hicks et al., 2011; see also www.OMIM.org). More than 12 different types of glycogen storage disease (GSD) are known. The effects of these disorders are manifested in different organs dependent upon the different enzyme isoforms present (for review, see Hicks et al., 2011), and their differential regulation in different tissues (Zois and Harris, 2016).
Glycogen storage disease Type 1 (von Gierke’s disease) is most commonly due to a mutation in G6PC1 (glucose-6-phosphatase 1), which catalyses the terminal step of gluconeogenesis. Consequently, its loss leads to glycogen accumulation, primarily in the liver. This gene is not expressed at any significant level in β-cells, which explains why they are unaffected in this disease. Nevertheless, it is of interest that downregulation of the β-cell isoform G6PC2 occurs in diabetes (Brereton et al., 2016), which is also expected to enhance glycogen accumulation.
Pompe’s disease (GSD2) results from a deficiency of the enzyme acid alpha-glucosidase (GAA), which is required for lysosomal degradation of glycogen. This leads to accumulation of structurally normal glycogen in lysosomes of affected cells, which is toxic. Accumulation of insoluble, hyperphosphorylated and poorly branched glycogen occurs in Lafora disease, which is caused by mutations in laforin or malin. It is proposed this leads to impaired autophagy and thereby induces neuronal apoptosis (Duran et al., 2014). Glycogen is also observed in lysosomes of hyperglycaemia mice and patients with type 2 diabetes (Brereton et al., 2016) (Figure 1). However, it is not yet clear whether this represents impaired glycophagy or simply reflects the presence of excess glycogen in the cell.
Both Andersen’s disease (GSD4) and Cori’s disease (GSD3) are characterised by large amounts of abnormal glycogen in the cell. GSD3 results from mutations in the glycogen debranching enzyme AGL, resulting in accumulation of abnormal glycogen with short outer chains. GSD4 is due to a deficiency in glycogen debranching enzyme (Gbe1) and leads to the massive build-up of unbranched glycogen, which results in lethal liver cirrhosis. Expression of Gbe1 mRNA is enhanced in diabetic mouse islets (Brereton et al., 2016), suggesting accumulation of unbranched glycogen probably does not occur in diabetes. However, as downregulation of enzymatic activity cannot be excluded, this idea needs to be confirmed with a careful analysis of glycogen branching in both rodent and human islets.
Other forms of GSD are caused by inhibition of glycogen breakdown. McArdle's disease (GSD5) is due to loss-of-function mutations in the muscle type of glycogen phosphorylase (Pygm), which results in muscle weakness on exercise. Hers disease results from mutations in the liver isoform of glycogen phosphorylase (Pygl, GSD6) or phosphorylase kinase (GSD8), and has a relatively benign phenotype. Both Pygm and Pygl are expressed in β-cells (Andersson et al., 2016; Brereton et al., 2016) but there is no evidence of a β-cell phenotype in GSD5 or GSD6. It seems possible that this is because glycogen only accumulates in β-cells under hyperglycaemic conditions.
Fanconi-Bickel syndrome (GSD11) is caused by mutations in SLC2A2 (GLUT2) and these patients display disturbances of hepatic and renal glucose metabolism but, interestingly, no defects in insulin secretion. This may be because of the dominance of GLUT 1 and GLUT3 in human β-cells. Other types of glycogen storage disease result from mutations in glycolytic enzymes, such as phosphofructokinase (GSD7, in muscle) and aldolase A (GSD12, in muscle and red blood cells). The isoforms involved are cell specific and do not lead to diabetes. However, they demonstrate that inhibition of glucose metabolism may lead to glycogen accumulation. This supports the view that impaired β-cell metabolism (due to hyperglycaemia) may lead to glycogen accumulation in diabetes.
A common feature of glycogen storage diseases is that excessive glycogen accumulation is often fatal for the cell. Why glycogen is toxic to cells, and the threshold concentration for impaired viability, remain unresolved. Similarly, the mechanism(s) underlying glycogen-induced dysfunction and cell death are unclear. Suggestions include impaired autophagy (Duran et al., 2014), aberrant mitochondrial metabolism (Villarroel-Espindola et al., 2016) and accumulation of abnormally branched glycogen (Duran et al., 2012; Valles-Ortega et al., 2011). Reduced cellular ATP levels might also contribute, as glycogen synthesis consumes two molecules of ATP, and futile cycling between glycogen synthesis and breakdown may also occur. Potentially, this might contribute to the failure of glucose to elevate ATP levels in diabetic β-cells (Anello et al., 2005; Brereton et al., 2016). Impairment of normal autophagic and lysosomal processes is also apparent in glycogen-containing islets (Brereton et al., 2016) and loss of autophagy in mouse β-cells induced by deletion of Atg7 enhanced apoptosis and induced ‘vacuolar’ changes that likely reflect glycogen accumulation (Jung et al., 2008). Why glycogen causes β-cell toxicity, and how long and how high glucose must be elevated to produce toxic levels of glycogen in β-cells, is a matter for future investigation.
Conclusions and Future Perspectives
It is clear that whilst β-cells do not store glycogen under normoglycaemic conditions, they can accumulate substantial amounts in response to hyperglycaemia. This appears to require a triad of stressors: elevated plasma glucose (or G-6-P), upregulation of PTG and impaired oxidative metabolism.
Understanding why glycogen is accumulated, and how it impacts on β-cell viability and insulin secretion is now a priority. This knowledge also has implications for emerging type-2 diabetes therapies including glucokinase activators (Matschinsky, 2009) and glycogen phosphorylase inhibitors (Praly and Vidal, 2010), which would promote glycogen formation and may thereby impact β-cell function. A detailed understanding of how β-cell metabolism is altered in diabetes is also crucial.
Acknowledgements
We thank Patrik Rorsman for much valuable discussion. We are very grateful to Patrick McDonald, Jocelyn Manning Fox and James Lyon of the Alberta Diabetes Institute, Edmonton, Canada for supplying the pancreatic islets isolated from a type 2 diabetic donor illustrated in Figure 1. Work in our own laboratory referred to here was supported by the Wellcome Trust (grant nos 884655, 089795), and the European Research Council (ERC Advanced grant 322620). MFB held a Wellcome Trust OXION Training fellowship. MR was supported by a Novo Nordisk postdoctoral fellowship run in partnership with the University of Oxford. FMA held a Royal Society/Wolfson Merit Award and an ERC Advanced Investigatorship.
References
- Andersson LE, Nicholas LM, Filipsson K, Sun J, Medina A, Al-Majdoub M, Fex M, Mulder H, Spegel P. Glycogen metabolism in the glucose-sensing and supply-driven β-cell. FEBS Lett. 2016;590:4242–4251. doi: 10.1002/1873-3468.12460. [DOI] [PubMed] [Google Scholar]
- Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48:282–289. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. The Walter B. Cannon Physiology in Perspective Lecture, 2007. ATP-sensitive K+ channels and disease: from molecule to malady. Am J Physiol Endocrinol Metab. 2007;293:E880–889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- Ashcroft SJH, Randle PJ. Glucose 6-phosphatase activity of mouse pancreatic islets. Nature. 1968;5156:857–858. doi: 10.1038/219857a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft SJH, Randle PJ. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem J. 1970;119:5–15. doi: 10.1042/bj1190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft SJH, Weerasinghe LCC, Bassett JM, Randle PJ. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972;126:525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft SJ, Weerasinghe LC, Randle PJ. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973;132:223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensellam M, Van Lommel L, Overbergh L, Schuit FC, Jonas JC. Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia. 2009;52:463–476. doi: 10.1007/s00125-008-1245-z. [DOI] [PubMed] [Google Scholar]
- Bouatia-Naji N, Rocheleau G, Van Lommel L, Lemaire K, Schuit F, Cavalcanti-Proenca C, Marchand M, Hartikainen AL, Sovio U, De Graeve F, et al. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science. 2008;320:1085–1088. doi: 10.1126/science.1156849. [DOI] [PubMed] [Google Scholar]
- Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, van Diepen JA, Voshol PJ, Jensen J, Sakamoto K. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab. 2010;12:456–466. doi: 10.1016/j.cmet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Brereton MF, Rohm M, Shimomura K, Holland C, Tornovsky-Babeay S, Dadon D, Iberl M, Chibalina MV, Lee S, Glaser B, et al. Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat Commun. 2016;7:13496. doi: 10.1038/ncomms13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brolin SE, Berne C. On the glycolytic conversion of triosephosphates in the pancreatic islets and acini of NZO mice. Acta Endocrinol (Copenh) 1970;63:533–538. doi: 10.1530/acta.0.0630533. [DOI] [PubMed] [Google Scholar]
- Brun T, Maechler P. Beta-cell mitochondrial carriers and the diabetogenic stress response. Biochim Biophys Acta. 2016;1863:2540–2549. doi: 10.1016/j.bbamcr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Brun T, Scarcia P, Li N, Gaudet P, Duhamel D, Palmieri F, Maechler P. Changes in mitochondrial carriers exhibit stress-specific signatures in INS-1Ebeta-cells exposed to glucose versus fatty acids. PLoS One. 2013;8:e82364. doi: 10.1371/journal.pone.0082364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P, et al. Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson SM, Khan A, Printen J, Pessin JE, Saltiel AR. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J Clin Invest. 2003;111:1423–1432. doi: 10.1172/JCI17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology. 1996;137:4671–4676. doi: 10.1210/endo.137.11.8895332. [DOI] [PubMed] [Google Scholar]
- Duran J, Gruart A, Garcia-Rocha M, Delgado-Garcia JM, Guinovart JJ. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum Mol Genet. 2014;23:3147–3156. doi: 10.1093/hmg/ddu024. [DOI] [PubMed] [Google Scholar]
- Duran J, Tevy MF, Garcia-Rocha M, Calbo J, Milan M, Guinovart JJ. Deleterious effects of neuronal accumulation of glycogen in flies and mice. EMBO Mol Med. 2012;4:719–729. doi: 10.1002/emmm.201200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatica R, Bertinat R, Silva P, Kairath P, Slebe F, Pardo F, Ramirez MJ, Slebe JC, Campistol JM, Nualart F, et al. Over-expression of muscle glycogen synthase in human diabetic nephropathy. Histochem Cell Biol. 2015;143:313–324. doi: 10.1007/s00418-014-1290-2. [DOI] [PubMed] [Google Scholar]
- Graf R, Klessen C. Glycogen in pancreatic islets of steroid diabetic rats. Carbohydrate histochemical detection and localization using an immunocytochemical technique. Histochemistry. 1981;73:225–232. doi: 10.1007/BF00493022. [DOI] [PubMed] [Google Scholar]
- Greenberg CC, Meredith KN, Yan L, Brady MJ. Protein targeting to glycogen overexpression results in the specific enhancement of glycogen storage in 3T3-L1 adipocytes. J Biol Chem. 2003;278:30835–30842. doi: 10.1074/jbc.M303846200. [DOI] [PubMed] [Google Scholar]
- Hellman B, Idahl LA. Presence and mobilization of glycogen in mammalian pancreatic β cells. Endocrinology. 1969;84:1–8. doi: 10.1210/endo-84-1-1. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Dufrane D, Kerr-Conte J, Nenquin M. Dynamics of glucose-induced insulin secretion in normal human islets. Am J Physiol Endocrinol Metab. 2015;309:E640–650. doi: 10.1152/ajpendo.00251.2015. [DOI] [PubMed] [Google Scholar]
- Hernandez C, Garcia-Ramirez M, Garcia-Rocha M, Saez-Lopez C, Valverde AM, Guinovart JJ, Simo R. Glycogen storage in the human retinal pigment epithelium: a comparative study of diabetic and non-diabetic donors. Acta Diabetol. 2014;51:543–552. doi: 10.1007/s00592-013-0549-8. [DOI] [PubMed] [Google Scholar]
- Hicks J, Wartchow E, Mierau G. Glycogen storage diseases: a brief review and update on clinical features, genetic abnormalities, pathologic features, and treatment. Ultrastruct Pathol. 2011;35:183–196. doi: 10.3109/01913123.2011.601404. [DOI] [PubMed] [Google Scholar]
- Idahl LA, Hellman B. Microchemical assays of glucose and glucose-6-phosphate in mammalian pancreatic β-cells. Acta Endocrinol (Copenh) 1968;59:479–486. doi: 10.1530/acta.0.0590479. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, et al. Loss of autophagy diminishes pancreatic β cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Kang J, Dai XS, Yu TB, Wen B, Yang ZW. Glycogen accumulation in renal tubules, a key morphological change in the diabetic rat kidney. Acta Diabetol. 2005;42:110–116. doi: 10.1007/s00592-005-0188-9. [DOI] [PubMed] [Google Scholar]
- Kondomerkos DJ, Kalamidas SA, Kotoulas OB, Hann AC. Glycogen autophagy in the liver and heart of newborn rats. The effects of glucagon, adrenalin or rapamycin. Histol Histopathol. 2005;20:689–696. doi: 10.14670/HH-20.689. [DOI] [PubMed] [Google Scholar]
- Kotoulas OB, Kalamidas SA, Kondomerkos DJ. Glycogen autophagy in glucose homeostasis. Pathol Res Pract. 2006;202:631–638. doi: 10.1016/j.prp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Lazarus SS, Volk BW. Pathogenesis of glycogen infiltration of the pancreas in diabetes. Diabetes. 1958;7:15–20. doi: 10.2337/diab.7.1.15. [DOI] [PubMed] [Google Scholar]
- Lemaire K, Thorrez L, Schuit F. Disallowed and Allowed Gene Expression: Two Faces of Mature Islet Beta Cells. Annu Rev Nutr. 2016;36:45–71. doi: 10.1146/annurev-nutr-071715-050808. [DOI] [PubMed] [Google Scholar]
- Liu W, Priddy TS, Carlson GM. Physicochemical changes in phosphorylase kinase associated with its activation. Protein Sci. 2008;17:2111–2119. doi: 10.1110/ps.037895.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse WJ. Role of glycogen metabolism in pancreatic islet β cell function. Diabetologia. 2016;59:2489–2491. doi: 10.1007/s00125-016-4092-3. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Maggetto C, Leclercq-Meyer V, Sener A. Interference of glycogenolysis with glycolysis in pancreatic islets from glucose-infused rats. J Clin Invest. 1993;91:432–436. doi: 10.1172/JCI116219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse WJ, Malaisse-Lagae F, Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967;46:1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse WJ, Marynissen G, Sener A. Possible role of glycogen accumulation in β-cell glucotoxicity. Metabolism. 1992;41:814–819. doi: 10.1016/0026-0495(92)90160-c. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Sener A, Koser M, Ravazzola M, Malaisse-Lagae F. The stimulus-secretion coupling of glucose-induced insulin release. Insulin release due to glycogenolysis in glucose-deprived islets. Biochem J. 1977;164:447–454. doi: 10.1042/bj1640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, Marchetti P, Weir GC. Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One. 2010;5:e11499. doi: 10.1371/journal.pone.0011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM, Ellerman JE. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968;243:2730–2736. [PubMed] [Google Scholar]
- Mellor KM, Varma U, Stapleton DI, Delbridge LM. Cardiomyocyte glycophagy is regulated by insulin and exposure to high extracellular glucose. Am J Physiol Heart Circ Physiol. 2014;306:H1240–1245. doi: 10.1152/ajpheart.00059.2014. [DOI] [PubMed] [Google Scholar]
- Mir-Coll J, Duran J, Slebe F, Garcia-Rocha M, Gomis R, Gasa R, Guinovart JJ. Genetic models rule out a major role of β cell glycogen in the control of glucose homeostasis. Diabetologia. 2016;59:1012–1020. doi: 10.1007/s00125-016-3871-1. [DOI] [PubMed] [Google Scholar]
- Newman ME. Glycogen metabolism and cyclic AMP levels in isolated islets of lean and genetically obese mice. Horm Metab Res. 1977;9:358–361. doi: 10.1055/s-0028-1093527. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Kaminen N, Ustinov J, Lapatto R, Meissner T, Mayatepek E, Kere J, Sipila I. Physical exercise-induced hyperinsulinemic hypoglycemia is an autosomal-dominant trait characterized by abnormal pyruvate-induced insulin release. Diabetes. 2003;52:199–204. doi: 10.2337/diabetes.52.1.199. [DOI] [PubMed] [Google Scholar]
- Poudel A, Savari O, Striegel DA, Periwal V, Taxy J, Millis JM, Witkowski P, Atkinson MA, Hara M. Beta-cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine. 2015;49:693–702. doi: 10.1007/s12020-015-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound LD, Oeser JK, O'Brien TP, Wang Y, Faulman CJ, Dadi PK, Jacobson DA, Hutton JC, McGuinness OP, Shiota M, et al. G6PC2: a negative regulator of basal glucose-stimulated insulin secretion. Diabetes. 2013;62:1547–1556. doi: 10.2337/db12-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praly JP, Vidal S. Inhibition of glycogen phosphorylase in the context of type 2 diabetes, with focus on recent inhibitors bound at the active site. Mini Rev Med Chem. 2010;10:1102–1126. doi: 10.2174/1389557511009011102. [DOI] [PubMed] [Google Scholar]
- Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Ravelli RB, Kalicharan RD, Avramut MC, Sjollema KA, Pronk JW, Dijk F, Koster AJ, Visser JT, Faas FG, Giepmans BN. Destruction of tissue, cells and organelles in type 1 diabetic rats presented at macromolecular resolution. Sci Rep. 2013;3:1804. doi: 10.1038/srep01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt ME, Mellor KM, Curl CL, Stapleton D, Delbridge LM. Myocardial glycophagy - a specific glycogen handling response to metabolic stress is accentuated in the female heart. J Mol Cell Cardiol. 2013;65:67–75. doi: 10.1016/j.yjmcc.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Saez I, Duran J, Sinadinos C, Beltran A, Yanes O, Tevy MF, Martinez-Pons C, Milan M, Guinovart JJ. Neurons have an active glycogen metabolism that contributes to tolerance to hypoxia. J Cereb Blood Flow Metab. 2014;34:945–955. doi: 10.1038/jcbfm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in β cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50:1–11. doi: 10.2337/diabetes.50.1.1. [DOI] [PubMed] [Google Scholar]
- Segerstolpe A, Palasantza A, Eliasson P, Andersson EM, Andreasson AC, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab. 2016;24:593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, Girotti M, Marie S, MacDonald MJ, Wollheim CB, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- Shen GM, Zhang FL, Liu XL, Zhang JW. Hypoxia-inducible factor 1-mediated regulation of PPP1R3C promotes glycogen accumulation in human MCF-7 cells under hypoxia. FEBS Lett. 2010;584:4366–4372. doi: 10.1016/j.febslet.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–232. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- Toreson WE. Glycogen infiltration (so-called hydropic degeneration) in the pancreas in human and experimental diabetes mellitus. Am J Pathol. 1951;27:327–347. [PMC free article] [PubMed] [Google Scholar]
- Tornovsky-Babeay S, Dadon D, Ziv O, Tzipilevich E, Kadosh T, Schyr-Ben Haroush R, Hija A, Stolovich-Rain M, Furth-Lavi J, Granot Z, et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in beta cells. Cell Metab. 2014;19:109–121. doi: 10.1016/j.cmet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Valles-Ortega J, Duran J, Garcia-Rocha M, Bosch C, Saez I, Pujadas L, Serafin A, Canas X, Soriano E, Delgado-Garcia JM, et al. Neurodegeneration and functional impairments associated with glycogen synthase accumulation in a mouse model of Lafora disease. EMBO Mol Med. 2011;3:667–681. doi: 10.1002/emmm.201100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Komers R. Pathophysiology of the diabetic kidney. Compr Physiol. 2011;1:1175–1232. doi: 10.1002/cphy.c100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medrano-Fernandez I, Dominguez J, et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Villar-Palasi C, Guinovart JJ. The role of glucose 6-phosphate in the control of glycogen synthase. Faseb j. 1997;11:544–558. [PubMed] [Google Scholar]
- Villarroel-Espindola F, Tapia C, Gonzalez-Stegmaier R, Concha II, Slebe JC. Polyglucosan Molecules Induce Mitochondrial Impairment and Apoptosis in Germ Cells Without Affecting the Integrity and Functionality of Sertoli Cells. J Cell Physiol. 2016;231:2142–2152. doi: 10.1002/jcp.25315. [DOI] [PubMed] [Google Scholar]
- Walls AB, Heimburger CM, Bouman SD, Schousboe A, Waagepetersen HS. Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience. 2009;158:284–292. doi: 10.1016/j.neuroscience.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Wang G, Qian R, Li Q, Niu T, Chen C, Xu X. The association between PPP1R3 gene polymorphisms and type 2 diabetes mellitus. Chin Med J (Engl) 2001;114:1258–1262. [PubMed] [Google Scholar]
- Weichselbaum A, Stangi E. Zur Kenntniss der feineren Verindermgen des Pankreas bei Diabetes mellitus. Wien klin Wchnschr. 1901;14:968–972. [Google Scholar]
- Williamson JR. Electron microscopy of glycogenic changes in β cells in experimental diabetes. Diabetes. 1960;9:471–480. doi: 10.2337/diab.9.6.471. [DOI] [PubMed] [Google Scholar]
- Zhang TM, Maggetto C, Malaisse WJ. Hexose metabolism in pancreatic islets: glycogen synthase and glycogen phosphorylase activities. Biochem Med Metab Biol. 1994;51:129–139. doi: 10.1006/bmmb.1994.1017. [DOI] [PubMed] [Google Scholar]
- Zhao C, Wilson MC, Schuit F, Halestrap AP, Rutter GA. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes. 2001;50:361–366. doi: 10.2337/diabetes.50.2.361. [DOI] [PubMed] [Google Scholar]
- Zois CE, Harris AL. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J Mol Med (Berl) 2016;94:137–154. doi: 10.1007/s00109-015-1377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]