Abstract
Type 2 diabetes is characterized by insulin resistance and a progressive loss of β-cell function induced by a combination of both β-cell loss and impaired insulin secretion from remaining β-cells. Here we review the fate of the β-cell under chronic hyperglycaemic conditions with regard to β-cell mass, gene expression, hormone content, secretory capacity, and the ability to de- or trans-differentiate into other cell types. We compare data from various in vivo and in vitro models of diabetes with a novel mouse model of inducible, reversible hyperglycaemia (βV59M mice). We suggest that insulin staining using standard histological methods may not always provide an accurate estimation of β-cell mass or number. We consider how β-cell identity is best defined, and whether expression of transcription factors normally found in islet progenitor cells, or in α-cells, implies that mature β-cells have undergone dedifferentiation or transdifferentiation. We propose that even in long-standing diabetes, β-cells predominantly remain β-cells – but not as we know them.
Keywords: diabetes, β-cell, insulin content, dedifferentiation, transdifferentiation
Introduction
Type 2 diabetes is characterized by two major pathological abnormalities: insulin resistance in peripheral tissues and impaired insulin secretion from pancreatic β-cells. Both contribute to disease development, although their relative importance varies in different patients and populations. It is generally recognized, however, that diabetes results when the pancreas is no longer able to compensate for insulin resistance by increasing insulin output. Glucagon secretion from pancreatic α-cells is also impaired in diabetes, contributing to the hyperglycaemia.
The origin of the β-cell failure in type 2 diabetes is considered to involve both a partial reduction in β-cell mass (number) and a progressive deterioration of β-cell function – that is, the ability of an individual β-cell to enhance its insulin secretion in response to glucose. The aetiology of these defects is only poorly understood, but it is widely accepted that both are exacerbated by the elevated blood glucose, leading to a vicious cycle in which hyperglycaemia impairs insulin release, and thereby further increases hyperglycaemia. The extent to which these changes in β-cell mass and function can be prevented or reversed by good glycaemic control is debated.
While progressive β-cell loss is currently considered to be central to the development of type 2 diabetes, it is not always clear what is meant by β-cell ‘loss’. Some studies equate β-cell loss with β-cell death, some mean the dedifferentiation or transdifferentiation of β-cells into non β-cells, and in other cases β-cell loss is implicitly taken to mean a loss of β-cell function. Commonly, β-cell loss is used as a blanket term to cover all of these eventualities. Defining what is meant by β-cell loss more precisely is not merely a question of semantics: at its heart is the issue of what happens to the β-cell in diabetes – do β-cells die, lose their ability to respond to glucose, or switch their identity?
It has long been considered that the fate of a fully differentiated pancreatic islet cell is fixed, and that it is unable either to dedifferentiate into an islet progenitor cell or to transdifferentiate into another islet cell type. Over the past decade, however, evidence has accumulated to suggest this dogma is not correct and that mature islet cells show a remarkable plasticity. This evidence principally derives from changes in the expression of genes that encode α-, β- or δ-cell-specific transcription factors and hormones. These findings have led to the proposal that in diabetes β-cells dedifferentiate to some immature ‘progenitor-like’ cell that expresses both insulin and glucagon, and that some of these cells may subsequently transdifferentiate along a different pathway into mature α-cells. Such findings raise the thorny question of what constitutes a β-cell. Is insulin content, for example, sufficient? And does loss of insulin secretion, loss of insulin content or loss of β-cell-specific transcription factors imply a cell is no longer a β-cell?
In this review, we therefore consider what is meant by β-cell identity and how this changes in response to chronic hyperglycaemia.
Models used to determine the β-cell response to hyperglycaemia
The response of the β-cell to hyperglycaemia, or diabetes, is likely to be influenced by the way in which hyperglycaemia is induced, or the animal model or cell line that is used. As many different in vivo, ex vivo and in vitro models have been employed to study the response of the β-cell to hyperglycaemia, we first summarise their relative advantages and disadvantages.
Immortalized insulin-secreting β-cell lines are particularly advantageous for analysing molecular mechanisms underlying β-cell function, as signaling pathways can easily be manipulated using knockdown or overexpression techniques. However, the complex, chronic state of diabetes cannot be mimicked. In addition, β-cell lines suffer from the fact that many are not glucose-responsive in the physiological range [1], lack or overexpress key proteins (e.g. hexokinase, [2], and need to be cultured at high glucose concentrations (typically 11 or 25 mM glucose), which makes studying the effects of different glucose concentrations problematic. The complex microenvironment that defines an islet, namely paracrine interactions, gap junctional coupling, and the presence of a vascular supply, is also absent.
Isolated islets preserve the relationships between different types of islet cells, but have their own disadvantages. As islets may be damaged by the isolation procedure, a recovery period (e.g. 24 hrs of tissue culture) is often employed. However, even short-term culture can alter islet function [3,4]. There is the further problem of which glucose concentration should be used to mimic the in vivo situation - culture at 11mM glucose (the standard islet culture condition) reflects neither the normal nor the diabetic blood glucose level. In addition, culture time is limited by the lack of a blood supply, which leads to hypoxia in the islet core [5] and makes it difficult to separate the effects of hypoxia from those of high glucose.
There are also caveats when using animal models to study diabetes. Many common genetic models of type 2 diabetes, like the leptin-deficient ob/ob mouse [6], the leptin receptor-deficient db/db mouse [7], the New Zealand obese mouse [8] and the Goto-Kakizaki rat model of type 2 diabetes [9], combine insulin resistance with impaired insulin secretion. While these mice may reflect the complex interplay between genes and environment, and the combination of obesity and lipo/glucolipotoxicity typical of human type 2 diabetes, the effects of hyperglycaemia cannot be separated from those of insulin resistance. In some animal models, the underlying aetiology of the disease is also unknown. Thus it is unclear whether the observed phenotypic changes are the direct result of an unknown gene on β-cell function, or a secondary consequence of the hyperglycaemia. A similar caveat applies to animals in which diabetes is produced by β-cell-specific disruption of a known gene.
Chemical induction of diabetes in vivo with toxins such as streptozotocin or alloxan results in death of the majority of endogenous β-cells, thereby producing diabetes [10]. This model is particularly useful for studying pancreatic regeneration. However, it is unclear whether other tissues and/or the remaining β-cells are affected by the toxin, albeit to a lesser extent. Surgical removal of part of the pancreas obviates this problem, and different blood glucose levels can be achieved by removing different numbers of islets [11]. A disadvantage of most animal models of diabetes compared with in vitro studies, however, is that the hyperglycaemia is not easily reversed. Treatment with phloridzin, which blocks glucose reuptake in the kidney, has sometimes been used to normalize glycaemia [12], but risks making the mice dehydrated.
We therefore created a new mouse model of diabetes that enables the effects of hyperglycaemia/hypoinsulinaemia – and their reversal – on pancreatic islets to be studied in vivo [13]. These mice express an inducible β-cell-specific activating KATP channel mutation (βV59M) that is found in patients with neonatal diabetes [14]. When induced by tamoxifen, the mutant version of the channel is expressed and remains constitutively open, thereby hyperpolarising the β-cell and inhibiting glucose-stimulated insulin secretion. This results in hyperglycemia within 48 hours of gene induction. Hyperglycaemia/hypoinsulinaemia can be maintained for 8 weeks, and potentially longer. Importantly, the diabetes can be reversed by subcutaneous implantation of slow-release sulfonylurea pellets. These drugs close KATP channels and rapidly restore normoglycaemia, as they do in neonatal diabetes patients treated with sulfonylureas [15]. βV59M mice are neither obese nor insulin resistant, the diabetes is due to a known genetic defect confined to the β-cell, and diabetes can be rapidly induced or reversed, making them a good model for studying the time course of the effects of hyperglycaemia on β-cells. As with most animal models of diabetes, however, hyperglycaemia is accompanied by hypoinsulinaemia.
Finally, one should not forget that a man is not a mouse, and that the β-cell transcriptome [16], glucose uptake [17,18] and electrophysiological fingerprint [19] differ between humans and mice. Thus all results ultimately need to be verified in humans.
Is insulin staining a good marker of β-cell identity and β-cell mass?
The hallmark of the β-cell is that it expresses insulin and releases it in response to a rise in plasma glucose. In many studies, immunocytochemical detection of insulin is assumed to be sufficient to define a β-cell and cells that do not express measureable insulin are considered to be non β-cells. It is well established that insulin staining and islet insulin content are reduced in animal models of diabetes [13,20,21], and in both isolated islets [22,23] and insulin-secreting cell lines [24] exposed to hyperglycaemia. Insulin gene expression is also markedly downregulated [12,13,20].
The key question is whether the decreased insulin staining represents the loss of β-cells (by death, de- or trans-differentiation) or a reduced insulin content in individual β-cells. The latter idea has received less attention, but a number of recent electron microscopy studies suggest there is a marked reduction in insulin granule number in both diabetic animal models and in type 2 diabetes [13,25–27]. In βV59M mice, for example, chronic diabetes led to a dramatic decrease in insulin staining at the light microscope level, as assessed by both immunohistochemistry and immunofluorescence [13]. At the electron microscope level, it was apparent that this decline in insulin staining was due to a dramatic reduction in the number of insulin granules per β-cell. The few remaining insulin granules could be identified by their characteristic poached egg appearance (with a dense core and pale halo). A reduction in insulin granule number is also seen in other diabetic mouse models [27]. Importantly, this is also true when granule content is assessed by 3-D scanning transmission electron microscopy [27], where the whole insulin granule can be imaged and distance and diameter estimations are less biased.
A similar discrepancy between β-cell mass assessed by immunocytochemistry and electron microscopy was found for islets obtained human donors with type 2 diabetes [26]. Despite a ~25% reduction in the area of the islet that stained for insulin, electron microscopy revealed the percentage of β-cells per islet was only marginally decreased. Marked β-cell degranulation accounted for the difference. Islets from non-diabetic donors cultured at high glucose showed a similar phenomenon. Other studies have also reported a striking decrease in β-cell insulin granule content in type 2 diabetes [25]. However, this is not universally observed [28] perhaps because of differences in glycaemic control in human donors.
These data indicate that if insulin staining is taken as an indicator, the number of β-cells may be considerably underestimated. A similar problem arises when counting islets - as diabetes progresses and β-cell insulin content declines the number of islets may also be underestimated. The method of assessing β-cell number is therefore an important consideration when discussing the loss of β-cell mass in diabetes. We suggest that some of the differences in estimates of islet and β-cell number in type 2 diabetes in the literature may be due to differences in the sensitivity of the method used to detect insulin. In our view, insulin staining is not a good indicator of whether or not the cell in question is indeed a β-cell.
An important conclusion from these studies is that while insulin content is clearly decreased in type 2 diabetes (by around 30%; [28,29], the β-cell deficit may be overestimated. This has significant implications for our understanding of the aetiology of the disease. First, it suggests that β-cell death (loss) is unlikely to be the major problem in type 2 diabetes. Second, the dedifferentiation or transdifferentiation of β-cells may also be overestimated, as β-cells cannot have fully switched their identity if they retain some insulin granules, however few they may be. Third, it implies that reduced insulin content and/or impaired stimulus-secretion coupling in β-cells must underlie the impaired insulin secretion in type 2 diabetes.
It seems unlikely that the reduced insulin content is sufficient to account for impaired insulin release in diabetes, as a 95% partial pancreatectomy is required to induce hyperglycemia in rodents and a 50% reduction in β-cell mass in baboons [28]. This may be why normoglycaemia is restored in βV59M mice within 24 hours of commencing sulphonylurea therapy, despite an ~70% reduction in insulin content [13]. The fact that stimulation with GLP-1 or sulphonylureas, or bariatric surgery [30], results in rapid restoration of insulin secretion in patients with type 2 diabetes also argues that sufficient insulin secretory capacity exists to control blood glucose. Rather, the reduced insulin release appears to be a consequence of impaired β-cell metabolism-secretion coupling [28,29].
Why does hyperglycaemia cause such a marked decrease in insulin granule number? Downregulation of insulin gene expression, which is seen in response to chronic hyperglycaemia in numerous mouse models [12,13,31] and in control islets cultured at high glucose (>20mM; [23,24], must be at least part of the reason. Increased insulin release may contribute in the early stages of diabetes, when insulin levels are elevated as a consequence of insulin resistance. Enhanced degradation of insulin granules is another possibility. Indeed, this must be the main mechanism by which insulin granules are removed in β-cells of patients and mice with neonatal diabetes caused by an activating KATP channel mutation [13]. In this case, internal degradation is the only route to reduce the number of insulin granules because exocytosis is prevented by membrane hyperpolarisation.
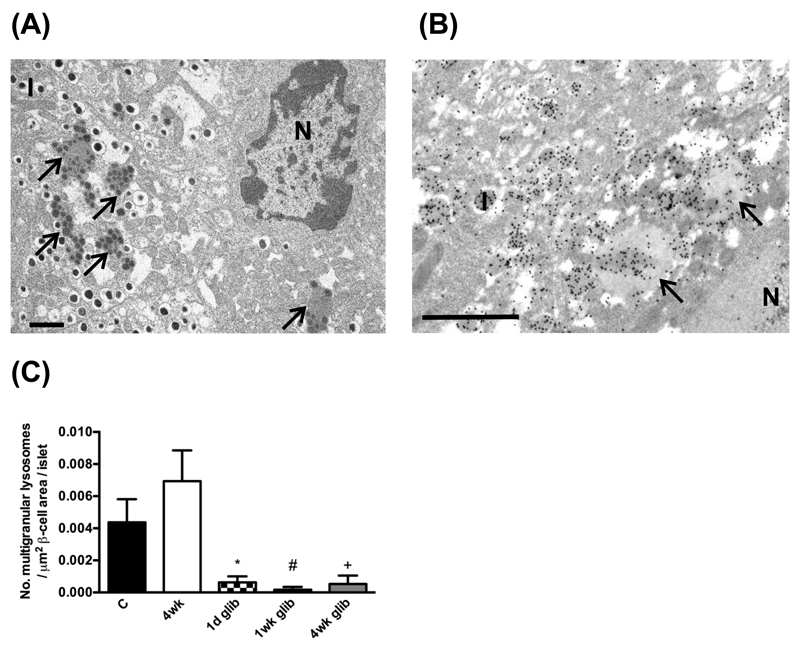
Quantitative electron microscopy supports this idea. Figure 1 shows that diabetes βV59M mice was associated with an increased number of multigranular bodies in β-cells, and that these contain for insulin. Multigranular bodies are large lysosomal related vacuolar compartments that degrade insulin granules in a process known as ‘crinophagy’ [32–34]. When insulin secretion in βV59M mice was restored by sulphonylurea therapy, the number of multigranular bodies in β-cells quickly decreased. It remains to be determined whether enhanced lysosomal degradation of insulin granules also occurs in type 2 diabetes.
Figure 1. Enhanced degradation of insulin in βV59M β-cells.
(A,B) Representative electron micrograph from 4-week diabetic βV59M mice, showing (A) Multigranular bodies (lysosomes) filled with insulin (arrowed) and (B) insulin granules and multivesicular bodies immunolabelled for insulin with immunogold particles. Scale bars 1μm. N, nucleus. I, insulin granule. (C) Mean number of multigranular lysosomes per cm2 of β-cell area per pancreas measured in control mice (C, n=8), βV59M mice that had been diabetic for 4 weeks (4wk, n=4), or βV59M that had been diabetic for 4 weeks and then were treated in vivo with glibenclamide to normalize their blood glucose levels for 24 hours (24hr glib, n=4), 1 week (1wk glib, n=3) or 4 weeks (4wk glib, n=2).
Is diabetes associated with β-cell dedifferentiation and/or transdifferentiation?
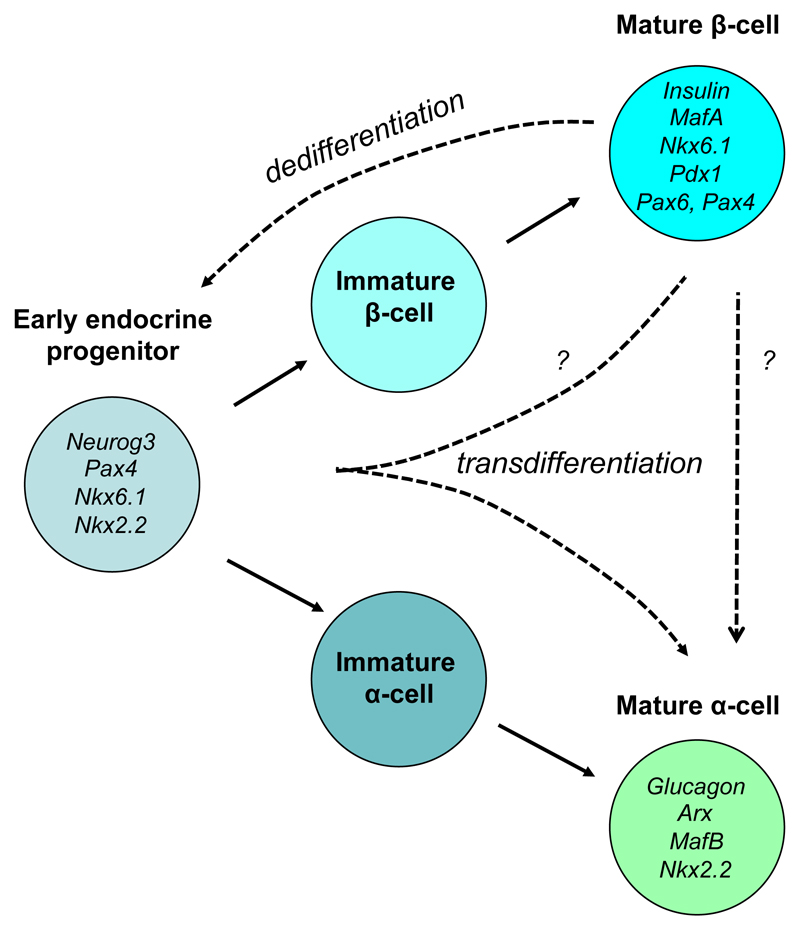
During development, α- and β-cells differentiate from a common endocrine progenitor cell that is characterized by expression of the transcription factor neurogenin 3 (Ngn3) [35]. Subsequently, activation of different patterns of transcription factors leads to the differentiation of mature α- and β-cells (Figure 2) [35]. β-Cell specific transcription factors include FoxO1, Pdx1, MafA, Pax6 and Nkx6.1, with Pdx-1, NeuroD1 and MafA being especially important for insulin gene transcription. The α-cell-specific transcription factors MafB and Arx1 are required for glucagon expression.
Figure 2. Schematic of α- and β-cell differentiation and dedifferentiation.
A number of transcription factors associated with early endocrine progenitor cells, and mature α-cells and β-cells are indicated. The dashed arrows indicate possible pathways for dedifferentiation and transdifferentiation.
Recent studies suggest that chronic hyperglycaemia, or genetic deletion of key β-cell proteins, may induce mature β-cells to express markers of islet progenitor cells, or even α-cells. As a consequence, they progressively lose the ability to produce insulin and may start to express glucagon. For example, lineage tracing revealed genetic deletion of FoxO1 in mice caused β-cells to express the pancreatic progenitor cell marker Ngn3, and the pluripotency markers Oct4, Nanog, and L-myc [36]. Likewise, in two inducible mouse models of neonatal diabetes, induction of diabetes in adult mice led to enhanced expression of Ngn3 in β-cells, a reduction in expression of β-cell-specific transcription factors and a marked decrease in insulin content [13,21]. Similar results were found using a partial pancreatectomy mouse model in which 85-95% of β-cells were surgically removed leaving the remaining β-cells exposed to chronically high blood glucose [12]. In this study, elevation of blood glucose to >8.5mM for 4 weeks led to a 50% reduction in islet insulin gene expression. The β-cell-specific transcription factors (Pdx1, Nkx6.1 and Pax6) were also downregulated. Many other diabetic mouse models also exhibit a dramatic loss of β-cell transcription factors and insulin content in their β-cells. For example, db/db mice lose the ability to express insulin [36], and the progressive development of diabetes in these mice is associated with an age-dependent decline in β-cell specific genes, such as Nkx6.1 and Pdx1 [37].
In summary, such studies indicate that β-cells exposed to chronic hyperglycaemia may adopt some of the characteristics of immature β-cells, as indicated by expression of Ngn3, loss of β-cell transcription factors and, as a consequence, reduced insulin content. Whether this dedifferentiation is simply a reversal of the normal β-cell differentiation pathway is unclear. Equally unknown, is how far back β-cells can dedifferentiate, and how the process is induced by hyperglycaemia.
Even more fascinating is that in many of these studies a small percentage of β-cells were found to coexpress insulin and glucagon, or even glucagon alone. That this occurred in response to genetic ablation of β-cell-specific transcription factors such as FoxO1 or NeuroD1 is perhaps not so surprising [36,38]. However, it has also been seen in several animal models of diabetes [12,13] and in human type 2 diabetes [39,40], suggesting it is a consequence of hyperglycaemia. This has led to the suggestion that β-cells can not only dedifferentiate to ‘progenitor-like’ cells but may then transdifferentiate to α-cells (Figure 2), and that this change in β-cell identity might account not only for β-cell ‘loss’ but also the hyperglucagonaemia seen in type 2 diabetes.
However, the percentage of cells that expressed both insulin and glucagon is usually relatively small. In islets isolated from human type 2 diabetes donors, coexpression of insulin and glucagon was seen in only 1-5% of islet cells [39,40]. Likewise, lineage tracing in βV59M mice indicated that only about 7% of β-cells expressed both insulin and glucagon and only 8% expressed glucagon alone, as visualised by immunofluorescence of FAC-sorted cells [13]. Increased expression of the α-cell-specific transcription factors MafB and Arx in FAC-sorted β-cells explains the elevated glucagon content [5].
The presence of both insulin and glucagon in the same cell has led to the suggestion that these cells may represent a dedifferentiated cell, or a β-cell in transitional state, on its way to becoming an α-cell. An alternative view is that such cells remain essentially β-cells but now also express glucagon. To some extent this depends on how β-cell identity is defined. The crucial issue, however, is not a semantic one, but whether such double-positive cells will release the hormone(s) they contain at low glucose (like an α-cell) or when blood glucose rises (as a β-cell normally does). A similar question arises for cells of β-cell lineage that now express glucagon alone -how is their hormone secretion regulated? If glucagon is released from cells of β-cell lineage when blood glucose rises, as is the case for a normal β-cell, this would result in elevation of glucagon at glucose levels that would normally be inhibitory and might explain the hyperglucagonaemia found in diabetes [41–43]. However, this has not been proven and given the small percentage of ‘β-cells’ that express glucagon it is unclear if it would be quantitatively sufficient to explain the impaired glucagon response.
Diabetes induces changes in expression of β-cell ion channels and transporters
Conversion of one islet cell into another is not just simply a matter of changing the hormone it expresses. It is necessary to alter the entire stimulus-secretion coupling mechanism. Insulin secretion is initiated (in rodent β-cells) at ~6mM glucose, and increases further as glucose is raised until it reaches a maximum at ~30mM glucose. In contrast, glucagon is secreted continuously at low plasma glucose levels and its secretion is inhibited by glucose levels above ~5mM.
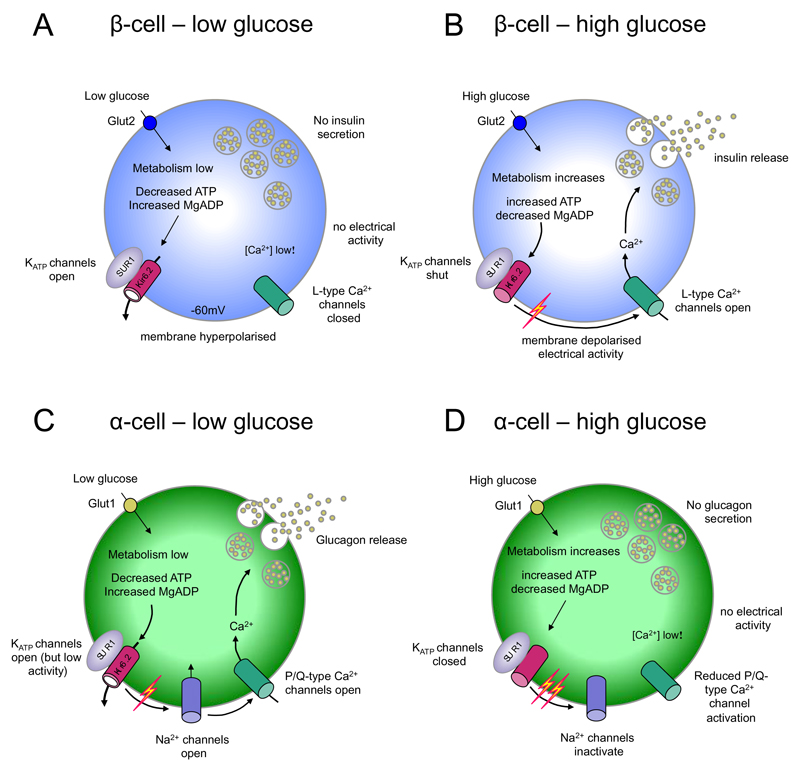
In both α- and β-cells, hormone secretion is triggered by action potential firing [42] (Figure 3). This leads to the influx of calcium (Ca2+) ions through voltage-gated Ca2+ channels and a rise in the intracellular Ca2+ concentration that activates secretory granule exocytosis. Glucose metabolism is required to trigger glucose-stimulated hormone secretion in both α- and β-cells, but it has opposite effects on their electrical activity. α-Cells are electrical active at low glucose and fall silent as glucose is increased, accounting for the ability of glucose to inhibit glucagon release. Conversely, β-cells are electrically silent at low glucose and increase their electrical activity and secretion as glucose is increased. Differences in the complement of ion channels and transporters expressed by the two cell types underlie these very different responses to glucose [44]. Crucially, they possess different types of voltage-gated ion channels and exhibit very different levels of basal KATP channel activity [45] (Figure 3). Therefore, the entire electrophysiological fingerprint needs to alter if a β-cell is to transdifferentiate into a fully mature α- (or δ-) cell.
Figure 3. Glucose modulation of electrical activity in α-cells and β-cells.
Insulin secretion is inhibited at low glucose concentrations (A) and stimulated at high glucose levels (B). Conversely, glucagon secretion is stimulated at low glucose levels (C) and inhibited at high glucose levels (D). (A,B) At low (<5 mM) glucose, ATP levels are sufficiently low that KATP channels are active, which keeps the β-cell membrane hyperpolarized and prevents electrical activity and insulin release. When plasma glucose levels rise, glucose is taken up mainly via GLUT2 (in rodents) leading to an increase in intracellular glucose and enhanced ATP generation. This closes KATP channels, causing membrane depolarization and activation of electrical activity and Ca2+ influx through voltage-gated Ca2+ channels. The resulting rise in [Ca2+]i triggers insulin granule exocytosis. (C,D) At low glucose (<5mM), α-cell KATP currents are smaller than in β-cells, depolarizing the membrane sufficiently to activate voltage-gated Na+ channels and Ca2+ channels, which leads to Ca2+ influx and glucagon release. Increasing glucose levels to 6mM enhances glucose uptake (via GLUT1) and closes KATP channels completely, further depolarizing the membrane. This increases action potential frequency but also reduces α-cell spike amplitude, due to inactivation of voltage-gated Na+ channels. In turn, this results in less activation of the P/Q-type Ca2+ channels linked to exocytosis and thus to reduced glucagon secretion. At higher glucose levels, paracrine secretion of insulin, zinc and GABA from adjacent β-cells contributes to inhibition of α-cell electrical activity and glucagon secretion.
In rodents, glucose entry into the β-cell is principally mediated by the low affinity (high Km, 17 mM), high Vmax glucose transporter Glut2, which ensures that the cytoplasmic glucose concentration tracks the circulating glucose level [46]. In contrast, α-cells express only Glut1 [47], a high affinity glucose transporter (with a low Km of <3mM glucose)[48]. Expression of Glut2 is reduced in ‘dedifferentiated’ β-cells [36] and in many models of hyperglycaemia [12]. However, in 4-week diabetic βV59M islets, cells expressing both insulin and glucagon retained both Glut2 mRNA and protein [13]. This argues that despite the presence of glucagon, these cells are not α-cells. Whether other metabolic enzymes differ between α- and β-cells is unclear.
Voltage-gated potassium and calcium channels also vary between α- and β-cells [44]. In α-cells, the upstroke of the action potential is due to opening of voltage-gated Na+ channels whereas these channels appear to have no physiological role in β-cells [49]. This is because α-cells predominantly express Nav1.3 channels whereas β-cells express Nav1.7, which are largely inactivated at physiological membrane potentials and thus unable to participate in electrical activity. Interestingly, cells expressing both insulin and glucagon in diabetic βV59M islets retained sodium channels with β-cell-like properties [13]. This again argues that the presence of glucagon is not sufficient to identify a given cell as an α-cell.
The regulation of secretion by hormones and neurotransmitters also differs in α- and β-cells. Glucagon secretion is inhibited by glucagon-like peptide-1 (GLP-1) and stimulated by adrenaline, whereas the opposite is true for β-cells. For a β-cell to become a fully mature α cell it must therefore also alter its receptors and/or their signaling pathways.
Data on hyperglycaemia-induced changes in β-cell ion channels, receptors and metabolic enzymes is sparse, especially for β-cells that now express glucagon. A full transcriptome analysis of single cells that express both insulin and glucagon would be invaluable in this respect, but has not yet been reported.
Reversal of diabetes-induced changes when euglycaemia is restored
Another way to address the issue of β-cell identity in diabetes is to examine what happens when euglycaemia is restored. A time course of the onset and reversal of the hyperglycaemia-induced changes would also be useful, as events that occur earlier are more likely to be causative. Several studies have addressed this issue in vivo. In partially pancreatectomized mice, normalization of blood glucose with phloridzin reversed the changes in insulin content and gene expression caused by hyperglycaemia within 2 weeks [12]. Similarly, in diabetic βV59M mice blood glucose levels reversed within 24hrs of sulphonylurea therapy [13]. If β-cells have indeed transdifferentiated to mature α-cells, this implies they must be able to reprogramme back to α-cells very quickly indeed.
After near-total loss of β-cells, a small number of α-cells in adult mice [50], and δ-cells in juvenile mice [51], are reprogrammed to produce insulin. These insulin-positive cells also express a number of β-cell-specific transcription factors. In animals treated with insulin to control their blood glucose levels, it took 4 months to regenerate just 23% of normal age-matched β-cell mass from δ-cells [51]. One month after near-total β-cell ablation 90% of cells containing insulin also still contained glucagon. Thus while there is no doubt that mature α-cells can convert to β-cells, the time scale on which they do so seems too slow to explain the very rapid restoration of glycaemia in mouse models of diabetes. Far more likely is that chronic hyperglycaemia does not cause β-cells to fully transdifferentiate into α-cells or that if this should occur it happens only in a minority of cells.
Clearly, if β-cells remain, they can be quickly repopulated with insulin granules when normoglycaemia is restored. This is expected to occur far more rapidly than the production of new β-cells or the transdifferentiation of α-cells into β-cells. The rapid restoration of glucose stimulated insulin release in patients with type 2 diabetes following bariatric surgery [30], intensive insulin treatment [52,53], or a very low calorie diet [54] argues that their β-cells have not died or de/trans-differentiated. Likewise, some neonatal diabetes patients have responded to sulphonylurea therapy within days, despite years of diabetes, (although their ability to do so gradually declines with diabetes duration;[55]). Similarly, sulphonylureas normalize blood glucose levels in diabetic βV59M mice within 24 hours of commencing therapy [13]. This timeline seems too short for generation of new β-cells, but is in good agreement with that needed for insulin biosynthesis (4-5hrs; Steiner [56].
Conclusions : a β-cell – but not as we know it!
In summary, we conclude that cells of β-cell lineage that no longer express insulin but instead express glucagon and/or α-cell transcription factors are not necessarily fully differentiated α-cells. Indeed, the fact that diabetic βV59M β-cells still express Glut2 and β-cell-like Na+ channels indicate this is not the case. In our view, these cells are certainly not (yet) α-cells, and whether they will eventually become so remains uncertain. Likewise, the rapid repopulation of β-cells with insulin granules and re-expression of β-cell specific genes when glycaemia is restored in diabetic animal models is incompatible with the time scale of α-cell to β-cell reprogramming (or, indeed, β-cell regeneration). Taken together, the available evidence suggests that diabetes does not result in full-scale transdifferentiation of β-cells to α-cells. The β-cells remain but insulin gene expression is markedly downregulated. A small percentage (<20%) of the total number of β-cells also begin to express glucagon. Whether these cells release glucagon in a β-cell-like or an α-cell-like manner remains to be determined and likely depends on their electrophysiological fingerprint.
Do β-cells that no longer express insulin, or express both insulin and glucagon constitute de-differentiated β-cells? This is more contentious as what constitutes as dedifferentiated β-cell is ill defined and the term means different things to different people [57]. It has variously been taken as loss of insulin content, expression of Ngn3, loss of a range of β-cell transcription factors - or all of these together. Whether this phenotype is the same as that of an islet progenitor cell, which has the capacity to differentiate into either an α-cell or a β-cell is unclear, as the transcriptome of islet progenitor cells and diabetic β-cells is not sufficiently well known to be able to reach a firm conclusion. However, the fact that insulin production and secretion is so rapidly reversible suggests that these cells are essentially β-cells and have not fully de-differentiated to an islet progenitor cell. In our view, they are β-cells – but not as we know them.
Callouts.
β-cell degranulation in response to hyperglycaemia means insulin staining does not provide an accurate estimate of β-cell mass or a good marker of β-cell identity.
Although chronic hyperglycaemia may result in β-cell expression of islet progenitor cell markers, and altered expression of transcription factors, it remains unclear if β-cells that undergo these changes have fully altered their identity.
The number of cells with β-cell lineage that express glucagon following exposure to chronic hyperglycaemia may be too few to account for the hyperglucagonaemia found in diabetes.
Reversal of diabetes when euglycaemia is restored is probably too fast to be explained by transdifferentiation of β-cells from α-cells or regeneration of new β-cells.
Acknowledgements
We thank Anne Clark for help with the electron microscope studies shown in Figure 3 and the Wellcome Trust (grant nos. 884655, 089795) and the European Union (ERC Advanced grant 322620) for support. FMA holds an ERC Advanced Investigatorship and a Royal Society Research Wolfson Merit Award. MFB holds a Wellcome Trust OXION Training Fellowship. MR is supported by a Novo Nordisk postdoctoral fellowship run in partnership with the University of Oxford.
Footnotes
Conflict of Interest Statement
All authors declare they have no conflicts of interest
References
- [1].Efrat S. Regulation of insulin secretion: insights from engineered beta-cell lines. Annals of the New York Academy of Sciences. 2004;1014:88–96. doi: 10.1196/annals.1294.009. [DOI] [PubMed] [Google Scholar]
- [2].Skelin M, Rupnik M, Cencic A. Pancreatic beta cell lines and their applications in diabetes mellitus research. Altex. 2010;27:105–113. doi: 10.14573/altex.2010.2.105. [DOI] [PubMed] [Google Scholar]
- [3].Gilon P, Jonas JC, Henquin JC. Culture duration and conditions affect the oscillations of cytoplasmic calcium concentration induced by glucose in mouse pancreatic islets. Diabetologia. 1994;37:1007–1014. doi: 10.1007/BF00400464. [DOI] [PubMed] [Google Scholar]
- [4].Szollosi A, Nenquin M, Henquin JC. Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. The Journal of biological chemistry. 2007;282:14768–14776. doi: 10.1074/jbc.M701382200. [DOI] [PubMed] [Google Scholar]
- [5].Zheng X, Zheng X, Wang X, et al. Acute hypoxia induces apoptosis of pancreatic beta-cell by activation of the unfolded protein response and upregulation of CHOP. Cell death & disease. 2012;3:e322. doi: 10.1038/cddis.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [7].Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- [8].Bielschowsky M, Bielschowsky F, Lindsay D. A new strain of mice with a high incidence of mammary cancers and enlargement of the pituitary. British journal of cancer. 1956;10:688–699. doi: 10.1038/bjc.1956.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. The Tohoku journal of experimental medicine. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- [10].Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- [11].Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. The Journal of clinical investigation. 1983;71:1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jonas JC, Sharma A, Hasenkamp W, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. The Journal of biological chemistry. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- [13].Brereton MF, Iberl M, Shimomura K, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nature communications. 2014;5:4639. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- [15].Pearson ER, Flechtner I, Njolstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- [16].Benner C, van der Meulen T, Caceres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].De Vos A, Heimberg H, Quartier E, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. The Journal of clinical investigation. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Molecular genetics and metabolism. 2011;104:648–653. doi: 10.1016/j.ymgme.2011.08.026. [DOI] [PubMed] [Google Scholar]
- [19].Rorsman P, Braun M. Regulation of Insulin Secretion in Human Pancreatic Islets. Annual review of physiology. 2012 doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- [20].Girard CA, Wunderlich FT, Shimomura K, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. The Journal of clinical investigation. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic beta Cell Dedifferentiation in Diabetes and Redifferentiation following Insulin Therapy. Cell metabolism. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andersson A, Asplund K, Larkins R. Insulin production by pancreatic islets of obese-hyperglycemic mice cultured for one week in different glucose concentrations. Acta physiologica Scandinavica. 1978;104:377–385. doi: 10.1111/j.1748-1716.1978.tb06294.x. [DOI] [PubMed] [Google Scholar]
- [23].Eizirik DL, Korbutt GS, Hellerstrom C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. The Journal of clinical investigation. 1992;90:1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. The Journal of clinical investigation. 1992;90:320–325. doi: 10.1172/JCI115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of beta-cell Dedifferentiation in Human Type 2 Diabetes. The Journal of clinical endocrinology and metabolism. 2015 doi: 10.1210/jc.2015-2860. jc20152860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Marselli L, Suleiman M, Masini M, et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia. 2014;57:362–365. doi: 10.1007/s00125-013-3098-3. [DOI] [PubMed] [Google Scholar]
- [27].Xue Y, Zhao W, Du W, et al. Ultra-structural study of insulin granules in pancreatic beta-cells of db/db mouse by scanning transmission electron microscopy tomography. Protein & cell. 2012;3:521–525. doi: 10.1007/s13238-012-2937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Clark A, Jones LC, de Koning E, Hansen BC, Matthews DR. Decreased insulin secretion in type 2 diabetes: a problem of cellular mass or function? Diabetes. 2001;50(Suppl 1):S169–171. doi: 10.2337/diabetes.50.2007.s169. [DOI] [PubMed] [Google Scholar]
- [29].Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes, obesity & metabolism. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- [30].Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- [31].Korsgren O, Jansson L, Sandler S, Andersson A. Hyperglycemia-induced B cell toxicity. The fate of pancreatic islets transplanted into diabetic mice is dependent on their genetic background. The Journal of clinical investigation. 1990;86:2161–2168. doi: 10.1172/JCI114955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Halban PA. Structural domains and molecular lifestyles of insulin and its precursors in the pancreatic beta cell. Diabetologia. 1991;34:767–778. doi: 10.1007/BF00408349. [DOI] [PubMed] [Google Scholar]
- [33].Orci L, Ravazzola M, Amherdt M, et al. Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. The Journal of cell biology. 1984;98:222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Skoglund G, Ahren B, Lundquist I. Biochemical determination of islet lysosomal enzyme activities following crinophagy-stimulating treatment with diazoxide in mice. Diabetes research. 1987;6:81–84. [PubMed] [Google Scholar]
- [35].Servitja JM, Ferrer J. Transcriptional networks controlling pancreatic development and beta cell function. Diabetologia. 2004;47:597–613. doi: 10.1007/s00125-004-1368-9. [DOI] [PubMed] [Google Scholar]
- [36].Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes. 2005;54:2755–2763. doi: 10.2337/diabetes.54.9.2755. [DOI] [PubMed] [Google Scholar]
- [38].Gu C, Stein GH, Pan N, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell metabolism. 2010;11:298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Spijker HS, Song H, Ellenbroek JH, et al. Loss of beta-Cell Identity Occurs in Type 2 Diabetes and Is Associated With Islet Amyloid Deposits. Diabetes. 2015;64:2928–2938. doi: 10.2337/db14-1752. [DOI] [PubMed] [Google Scholar]
- [40].White MG, Marshall HL, Rigby R, et al. Expression of mesenchymal and alpha-cell phenotypic markers in islet beta-cells in recently diagnosed diabetes. Diabetes Care. 2013;36:3818–3820. doi: 10.2337/dc13-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–283. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- [42].Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine reviews. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- [43].Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. The Journal of clinical endocrinology and metabolism. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- [44].Rorsman P, Ramracheya R, Rorsman NJ, Zhang Q. ATP-regulated potassium channels and voltage-gated calcium channels in pancreatic alpha and beta cells: similar functions but reciprocal effects on secretion. Diabetologia. 2014;57:1749–1761. doi: 10.1007/s00125-014-3279-8. [DOI] [PubMed] [Google Scholar]
- [45].Zhang Q, Ramracheya R, Lahmann C, et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell metabolism. 2013;18:871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–232. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- [47].Heimberg H, De Vos A, Pipeleers D, Thorens B, Schuit F. Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. The Journal of biological chemistry. 1995;270:8971–8975. doi: 10.1074/jbc.270.15.8971. [DOI] [PubMed] [Google Scholar]
- [48].Augustin R. The protein family of glucose transport facilitators: It's not only about glucose after all. IUBMB life. 2010;62:315–333. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- [49].Zhang Q, Chibalina MV, Bengtsson M, et al. Na+ current properties in islet alpha- and beta-cells reflect cell-specific Scn3a and Scn9a expression. The Journal of physiology. 2014;592:4677–4696. doi: 10.1113/jphysiol.2014.274209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Thorel F, Nepote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chera S, Baronnier D, Ghila L, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- [53].Kramer CK, Choi H, Zinman B, Retnakaran R. Determinants of reversibility of beta-cell dysfunction in response to short-term intensive insulin therapy in patients with early type 2 diabetes. American journal of physiology Endocrinology and metabolism. 2013;305:E1398–1407. doi: 10.1152/ajpendo.00447.2013. [DOI] [PubMed] [Google Scholar]
- [54].Steven S, Hollingsworth KG, Al-Mrabeh A, et al. Very-Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiologic Changes in Responders and Nonresponders. Diabetes Care. 2016 doi: 10.2337/dc15-1942. [DOI] [PubMed] [Google Scholar]
- [55].Babiker T, Vedovato N, Patel K, et al. Successful transfer to sulfonylureas in KCNJ11 neonatal diabetes is determined by the mutation and duration of diabetes. Diabetologia. 2016 doi: 10.1007/s00125-016-3921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dodson G, Steiner D. The role of assembly in insulin's biosynthesis. Current opinion in structural biology. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- [57].Weir GC, Aguayo-Mazzucato C, Bonner-Weir S. beta-cell dedifferentiation in diabetes is important, but what is it? Islets. 2013;5:233–237. doi: 10.4161/isl.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]