Abstract
Diabetes is a major global problem. During the past decade, the genetic basis of various monogenic forms of the disease, and their underlying molecular mechanisms, have been elucidated. Many genes that increase type-2 diabetes (T2DM) risk have also been identified but how they do so remains enigmatic. Nevertheless, defective insulin secretion emerges as the main culprit in both monogenic and polygenic diabetes, with environmental and lifestyle factors, via obesity, accounting for the current dramatic increase in T2DM. There also have been significant advances in therapy, particularly for some monogenic disorders. We review here what ails the beta-cell and how its function may be restored.
On 14 September 2011, the International Diabetes Federation announced that 336 million people worldwide now have type-2 diabetes and that the disease is responsible for 4.6 million deaths each year, or one death every seven seconds. It affects ~12% of US adults, and >25% of those over the age of 65. Diabetes is no longer restricted to the Western world, and the greatest increase in disease incidence in the next few decades is expected to be in China and India. These figures serve to emphasize there is currently a fast-growing diabetes pandemic. This is a major health-care problem because diabetes increases the risk of heart disease, stroke and microvascular complications such as blindness, renal failure, and peripheral neuropathy. Consequently, it places a severe economic burden on governments and individuals: the cost of diabetes and its complications amounts to $612 million per day in the USA alone.
Diabetes is characterized by high blood glucose levels as a result of insufficient insulin for the body's needs. It is a heterogeneous disorder with multiple etiologies. Type-1 diabetes (T1DM) is an autoimmune disease that results in beta-cell destruction. It usually presents in childhood, accounts for 5-10% of all diabetes, is associated with the presence of islet-cell antibodies, and patients require lifelong insulin: it will not be considered further here. Type-2 diabetes (T2DM), the most common form of the disease, is influenced by lifestyle factors, such as age, pregnancy and obesity, but has a strong genetic component. Multiple genes are thought to be involved, each producing a small effect on T2DM risk. An increasing number of rare monogenic forms of diabetes have also been identified that result from mutations in a single gene. They amount to 1-2% of all diabetes in Europe and almost all are characterized by reduced insulin secretion. Accumulating evidence also implicates impaired insulin release in T2DM.
The last decade has seen an explosion of new information about the mechanism of insulin secretion and the genetic causes of diabetes, that emphasize the importance of impaired beta-cell function in both polygenic and monogenic disease. This review focuses on these recent findings.
Insulin secretion from the pancreatic beta-cell
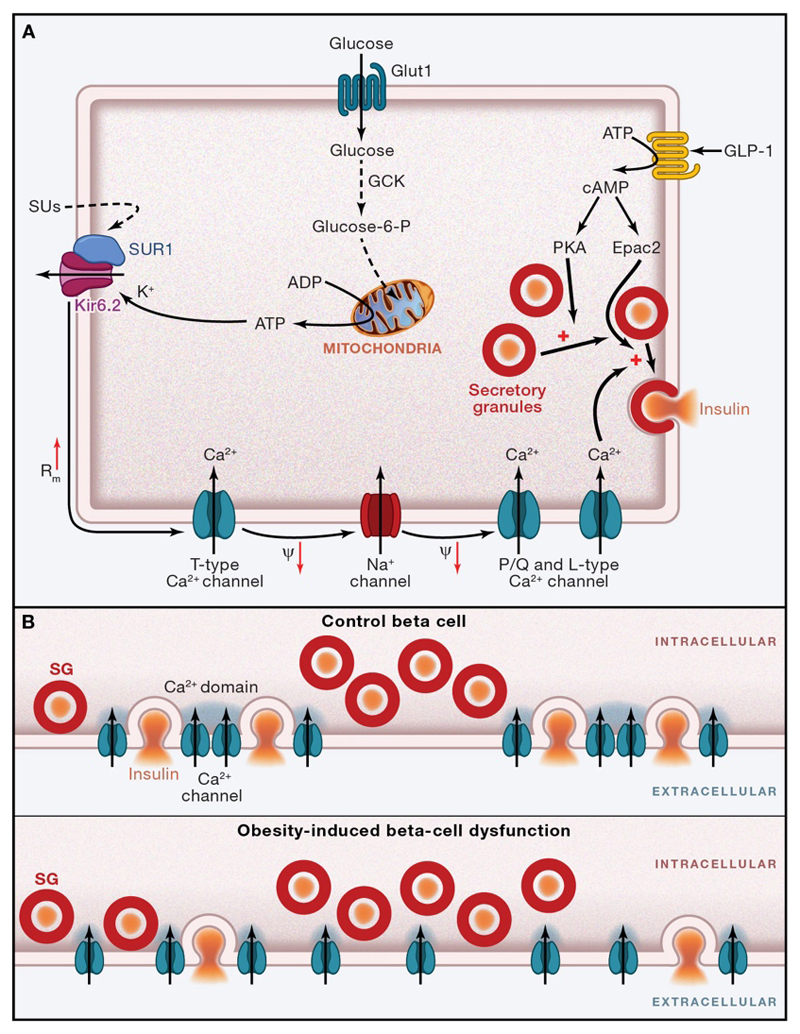
The physiological regulation of insulin secretion from the pancreatic beta-cells is now fairly well understood. Exocytosis of insulin granules requires an increase in intracellular calcium that (at least in the case of glucose-induced insulin secretion) results almost entirely from calcium influx through plasmalemmal voltage-gated calcium channels (Figure 1A). Their opening is controlled by the ATP-sensitive potassium (KATP) channel, which plays a pivotal role in insulin secretion by linking cell metabolism to the membrane potential. At low plasma glucose levels, this channel is open and K+ efflux through the open pore keeps the membrane hyperpolarized, preventing electrical activity, calcium channel opening, calcium influx and insulin secretion. An increase in plasma glucose leads to increased glucose uptake and metabolism by the beta-cell, and thus to a rise in metabolically generated ATP and a concomitant fall in MgADP. These changes in adenine nucleotide concentrations close KATP channels, thereby initiating electrical activity, calcium influx and insulin secretion. Sulphonylurea drugs, such as glibenclamide, also stimulate insulin secretion by closing KATP channels, but they do so by binding directly to the channel, thus bypassing the metabolic steps. These drugs have been used for almost 60 years to treat T2DM and, more recently, certain monogenic forms of diabetes.
Figure 1. Stimulus-secretion coupling in human beta-cells.
A. Glucose is taken up via the glucose transporter Glut1 and phosphorylated by glucokinase (GCK). Further metabolism, especially in the mitochondria (Mitoch) results in generation of ATP at the expense of ADP. This leads to KATP-channel closure. Sulphonylureas (SU) inhibit the channel by direct binding, by-passing metabolism. The increased membrane resistance (Rm↑) resulting from KATP-channel closure allows small background inward currents, such as those associated with spontaneous opening of T-type Ca2+-channels to depolarize the β-cell (Ψ↓). This leads to regenerative activation of voltage-gated L-type and P/Q-type Ca2+-channels and Na+-channels, which produces action potential firing. The associated Ca2+-influx triggers exocytosis of insulin granules (SG). Incretins such as GLP-1 potentiate exocytosis by both protein kinase A (PKA)-dependent and Epac2-dependent mechanisms. Plus signs indicate stimulation, and minus signs inhibition, of the indicated process(es). PM, plasmalemma.
B. Ca2+-channel clustering and extent of [Ca2+]i domains before and after long-term FFA exposure. SG, Secretory granules.
Glucose metabolism also has effects downstream of KATP channel closure and calcium influx, that result in amplification of insulin secretion. The details of the mechanism are still not fully understood but the end result is an increase in the release probability of the secretory granules. Glucose is almost unique among the metabolites in that it has the capacity to initiate insulin secretion. Other fuels such as amino acids and fatty acids enhance glucose-induced insulin secretion but generally have little stimulatory effect on their own. As a consequence, insulin secretion is dependent on glucose metabolism.
Hormones and neurotransmitters also modulate insulin secretion, primarily by influencing the release competence of the insulin granules without elevating intracellular calcium. Current interest is focused on glucagon-like peptide-1 (GLP-1), which is released from gut L-cells in response to the presence of glucose and other nutrients in the gut lumen and potentiates insulin secretion. It stimulates beta-cell production of intracellular cAMP, which acts by PKA-dependent and PKA-independent mechanisms to amplify granule exocytosis, as well as enhancing KATP channel closure, beta-cell electrical activity, and calcium release from intracellular stores (reviewed in (Leech et al., 2011). Most of these effects manifest only at elevated plasma glucose levels, so accounting for the glucose dependence of the insulinotropic action of GLP-1. Release of incretin hormones in the gut, such as GLP-1 and GIP, explains why an oral glucose challenge produces a much larger stimulation of insulin secretion than an intravenous glucose challenge.
The PKA-independent effects of incretins are mediated by the cAMP-sensing protein Epac2 (also known as cAMP-GEFII). Recently, Epac2 was also shown to be a target of sulphonylureas (Zhang et al., 2009): it remains to be determined whether this effect contributes (together with its effect on the KATP-channel) to the stimulation of insulin secretion by these drugs but there is some evidence that sulphonylureas enhance exocytosis in beta-cells (Eliasson et al., 2003). Just as activation of PKA or PKC (e.g. in response to acetylcholine) enhances Ca2+-dependent exocytosis without further elevation of intracellular calcium, other agents (such as adrenaline and somatostatin) inhibit glucose-stimulated insulin secretion even when intracellular calcium is elevated, possibly by activating the protein phosphatase calcineurin (Rorsman and Renstrom, 2003).
Insulin secretion from isolated islets is biphasic, an initial rapid transient peak being followed by a slowly developing second phase of release. In type-2 diabetes, first phase insulin secretion is almost abolished and second phase secretion is strongly reduced (Hosker et al., 1989). It has been proposed that the biphasic nature of insulin secretion reflects the existence of distinct functional pools within the beta-cells. A subset of secretory granules known as the readily releasable pool (by analogy with nerve terminals), situated close to the Ca2+-channels, accounts for first phase secretion. Once this pool has been depleted, exocytosis proceeds at a much lower rate, possibly reflecting the physical translocation of new granules ('newcomer granules') to the release sites, producing second phase release. Like neurotransmitter secretion, insulin granule exocytosis requires the SNARE proteins synaptobrevin, syntaxin and SNAP25 (Rorsman and Renstrom, 2003).
The existence of functionally distinct subsets of secretory granules is supported by FRET-based studies (Takahashi et al., 2010), which demonstrated that beta-cell SNARE complexes exist in different states of ‘pre-assembly’. Granules with pre-assembled SNARE complexes underwent rapid exocytosis upon stimulation. In addition, a time- and Ca2+-dependent assembly of SNARE complexes was observed that preceded a slower phase of exocytosis. It is tempting to equate these two sets of granules with first and second phase release, and to speculate that glucose metabolism may enhance the release competence of the secretory granules by promoting assembly of the SNARE complex. This might explain the elusive amplifying effect of glucose on insulin secretion.
Monogenic Diabetes
An increasing number of clinically and genetically distinct forms of diabetes have been described that result from mutations in single genes (Table 1). Conventionally, monogenic diabetes that develops within the first 6 months of life has been referred to as neonatal diabetes and that which develops in young adults as maturity-onset diabetes of the young (MODY). The latter term is misleading, however, as monogenic diabetes is quite distinct from maturity-onset diabetes (an old name for T2DM). The different genes involved also give rise to clinically distinct types of diabetes that vary in the age of onset, severity of hyperglycemia and risk of complications. In this section, therefore, we refer to the different forms of monogenic diabetes by their gene names and to MODY as young-onset diabetes (Murphy et al., 2008).
Table 1. Monogenic Diabetes Genes.
| Gene | Protein | Type of Diabetes | Comments | References |
|---|---|---|---|---|
| KCNJ11 | Kir6.2 | DEND, PNDM, TNDM, MODY | Mainly autosomal dominant. Reduced β cell function. Most treatable with SU. DEND patients also have developmental delay and epilepsy. Linkage to T2DM. | (Flanagan et al., 2009, Gloyn et al., 2004, Hattersley and Ashcroft, 2005) |
| ABCC8 | SUR1 | DEND, PNDM, TNDM | Genetically heterogenous. Reduced β cell function. Most treatable with SU. | (Ellard et al., 2007, Hattersley and Ashcroft, 2005) |
| INS | Insulin | PNDM, MODY | Increased β cell destruction. Treated with insulin. | (Støy et al., 2010) |
| GCK | Glucokinase | PNDM, MODY2 | PNDM, autosomal recessive. MODY, autosomal dominant. Reduced β cell function. | (Osbak et al., 2009) |
| SCLC2A2 | GLUT2 | PNDM | Autosomal dominant. Reduced β cell function. | (Yoo et al., 2002) |
| PDX1 | Insulin promoter factor 1 | PNDM, MODY4 | PNDM, autosomal recessive.cMODY, autosomal dominant. Pancreatic agenesis. | (Stoffers et al., 1997) |
| GLIS3 | GLIS3 (transcription factor) | Syndromic | Autosomal recessive. Reduced β cell function plus congenital hypothyrodism, glaucoma, liver fibrosis, polycystic kidneys. | (Senée et al., 2006) |
| FOXP3 | Forkhead box P3 | Syndromic | X-linked. Reduced β cell mass (increased β cell destruction) plus immune dysregulation, polyendocrinopathy, enteropathy. | (Bennett et al., 2001) |
| EIF2AK3 | Eukaryotic translation initiation factor 2-alpha kinase 3 | Syndromic | Autosomal recessive. Wolcott-Rallison syndrome. ND plus skeletal abnormalities and liver dysfucntion. | (Delépine et al., 2000) |
| PTF1A | Pancreas transcription factor 1A | Syndromic | Autosomal recessive. Pancreatic and cerebellar agenesis. | (Sellick et al., 2004) |
| Rfx6 | Rfx6 transcription factor | Syndromic | Autosomal recessive. Pancreatic agenesis. Bowel atresia. | (Smith et al., 2010) |
| 6q24 abnormality | TNDM | Abnormality in a region of chromosome 6q24, causing overexpression of ZAC and HYMAI. | (Temple and Shield, 2010) | |
| TCF2 | Hepatic nuclear factor 1β | TNDM, MODY5 | Autosomal dominant, diabetes only. Autosomal recessive, diabetes plus renal cysts, kidney, uterine, and genital developmental disorders. | (Servitja and Ferrer, 2004) |
| HNF4A | Hepatic nuclear factor 4α | MODY1 | Autosomal dominant, diabetes only. Autosomal recessive, diabetes plus lipid abnormalities. Linkage to T2DM. | (Servitja and Ferrer, 2004) |
| TCF1 | Hepatic nuclear factor 1α | MODY3 | Autosomal dominant, diabetes only, treatable with sulphonylureas. Autosomal recessive, diabetes plus extrapancreatic effects. Linkage to T2DM. | (Servitja and Ferrer, 2004) |
| NEUROD1 | Neurogenic differentiation factor 1 | MODY6 | Autosomal dominant. | (Servitja and Ferrer, 2004) |
Considerable advances in our understanding of the genetic causes of monogenic diabetes have been made in the last ten years, largely because of the careful clinical and genetic identification of these subgroups of patients. These have provided valuable insights into beta-cell function, as well as the diagnosis and pathophysiology of the disease. In some instances, this knowledge has also led to a change in therapy for affected patients. Consequently, individuals with neonatal or young-onset diabetes that is not accompanied by islet autoantibodies should be evaluated genetically. This is important, as monogenic diabetes is often misdiagnosed as T1DM (or even T2DM).
For convenience, we consider neonatal diabetes and young-onset diabetes separately, although it is now clear that mutations in the same gene may manifest their effects at different ages.
Neonatal Diabetes
Neonatal diabetes (ND), usually defined as diabetes diagnosed within the first six months of life, was once considered a rare variant of T1DM that presented unusually early. Within the last decade, however, it has become clear that ND is a monogenic disorder that is caused by mutations in genes that play key roles in beta-cell function or development, including glucokinase, the KATP channel and insulin itself. The incidence of ND is low (around 1 in 200,000 live births). It may either be permanent (PNDM), transient, or follow a remitting-relapsing time course. Rarely, a few individuals may present with diabetes between 6-9 months of age (Rubio-Cabezas et al., 2011). The disease is usually associated with intrauterine growth retardation and a lower birth weight, in keeping with the role of insulin as a growth factor. In marked contrast to T1DM, islet-cell antibodies are absent.
Gain-of-function mutations in the genes encoding the Kir6.2 (KCNJ11) and SUR1 (ABCC8) subunits of the KATP channel are the most common cause of PNDM, accounting for around 50% of cases (Flanagan et al., 2009; Hattersley and Ashcroft, 2005). All ND mutations impair the ability of metabolism to close the channel, by enhancing either ATP block at Kir6.2 or channel activation by Mg-nucleotides (MgADP/MgATP) at SUR1 (McTaggart et al., 2010). This leads to an increased KATP current, which prevents beta-cell depolarization in response to glucose metabolism and consequently impairs insulin secretion. Most mutations in KCNJ11 occur spontaneously and patients are heterozygotes. ABCC8 mutations are genetically more heterogeneous with dominant, recessive, uniparental disomy and compound heterozygous inheritance being described (Ellard et al., 2007).
In addition to diabetes, around 20% of patients with KATP channel mutations have a constellation of neurological symptoms (Hattersley and Ashcroft, 2005). A minority (3%) have ‘DEND syndrome’, which is characterized by severe mental and motor developmental delay, epilepsy and neonatal diabetes, as well as muscle hypotonia, hyperactivity and balance problems. However, most have an intermediate disorder (iDEND syndrome) that resembles DEND syndrome but where epilepsy is usually lacking.
The spectrum of symptoms found in iDEND and DEND reflects the widespread tissue distribution of KATP channels, which link cell metabolism to plasmalemmal electrical activity in muscle, heart and brain as well as pancreatic beta-cells. Mouse models indicate the motor problems originate in the central nervous system rather than muscle, which may be related to the different SUR isoforms in these tissues (SUR1 in neurons, SUR2A in muscle) (Clark et al., 2010). There is a good correlation between genotype and phenotype, with those mutations that have the greatest functional effect on ATP sensitivity producing a more severe clinical phenotype (McTaggart et al., 2010).
ND patients were originally treated with insulin as they were assumed to have an unusually early form of T1DM but the discovery of the causal role of KATP channels in 2004 has enabled ~90% of patients (>500 to date) to switch to sulphonylurea therapy. These drugs block their open KATP channels, thereby stimulating insulin secretion. This results in a significant improvement in diabetes control: fluctuations in glucose homeostasis are reduced and HbA1C levels fall, which should translate into a reduced risk of diabetic complications (Pearson et al., 2006; Zung et al., 2004). In some patients the accompanying neurological problems also show some improvement (Mlynarski et al., 2007; Slingerland et al., 2006).
There is a good correlation between the efficacy of sulphonylureas at blocking KATP channel activity and the ability of patients with the same mutation to transfer to oral drug therapy. Those with the most severe mutations, which cause DEND syndrome, are often unable to transfer because their mutant channels are not blocked sufficiently by glibenclamide (McTaggart et al., 2010). The efficacy of sulphonylurea therapy also shows some interesting differences between patients with ND and those with T2DM. In ~50% of patients with T2DM, sulphonylureas cease to be effective at controlling blood glucose levels within a few years of beginning therapy. This phenomenon is known as secondary failure and its mechanism is unknown. Because drug therapy has been implemented so recently, it is not clear if patients with KCNJ11 or ABCC8 mutations will continue to respond to sulphonylureas in the long term. Nevertheless, this seems likely as a few patients have been successfully treated with these drugs for many years and, to date, no desensitization to glibenclamide has been observed (Iafusco et al., 2011).
The ability of some adult ND patients to transfer to sulphonylurea therapy implies that their beta-cells are fully functional, despite many years of suboptimal glucose control. They often need much higher drug doses, relative to body weight, than T2DM patients (Pearson et al., 2006), probably because their KATP channels are more active at ambient glucose levels and thus require (at least initially) a higher drug dose to close them to the same extent as a lower dose would in 'normal' beta-cells. Some mutant channels are also less sensitive to sulphonylureas.
Recent studies indicate that heterozygous mutations in the insulin (INS) gene account for around 20% of cases of PNDM. Most coding mutations are de novo mutations and are inherited in a dominant manner (Stoy et al., 2007). One of these is identical to that found in the Akita mouse, where it acts to disrupt insulin biosynthesis, causing the protein to be degraded so that few insulin granules are produced, despite one Ins2 and two Ins1 alleles remaining functional (Wang et al., 1999). By analogy, the human mutations are predicted to prevent normal protein folding and induce the unfolded protein response, leading to ER stress and beta-cell death. Recessively acting mutations have also been reported, which reduce insulin synthesis (Garin et al., 2010). Insulin therapy is essential for patients with INS mutations.
Homozygous loss-of-function mutations in the glucokinase gene (GCK) can cause permanent neonatal diabetes, but are extremely rare (Njolstad et al., 2001). Glucokinase catalyzes the first step in glucose oxidation - phosphorylation of the sugar - and has been termed the glucose sensor of pancreatic beta-cells (Matschinsky, 2009). Thus the complete absence of insulin secretion in these patients reveals that ATP production from glucose is essential for insulin secretion and cannot be substituted by ATP produced by protein or fat metabolism. The importance of glucokinase for insulin secretion is illustrated by the finding that the sulphonylurea glibenclamide reversibly increased both basal and glucagon-stimulated insulin secretion in a patient with a homozygous GCK mutation, leading to a reduced insulin dose and lower HBA1c level (Turkkahraman et al., 2008). However, insulin therapy could not be stopped, perhaps because ATP generation was not sufficient to support the amplifying effect of glucose on insulin secretion.
Transient neonatal diabetes accounts for ~50% of cases of ND. Diabetes presents within the first few weeks after birth but remits within several months or years, only to often return in adolescence or early adult life. It. The most common cause (70%) is an abnormality in the imprinted region of chromosome 6q24 that leads to overexpression of at least two imprinted genes, ZAC and HYMAI (Flanagan et al., 2007; Temple and Shield, 2010). It remains unclear how this results in diabetes, or why the diabetes is transient, although a mouse model that overexpressed these two genes recapitulates the clinical phenotype (Ma et al., 2004). Most other cases of TNDM are due to activating mutations in KCNJ11 and ABCC8 and can be treated with sulphonylureas. Considerable overlap exists with PNDM, with the same mutation sometimes causing PNDM in some family members and TNDM in others. Why the disease follows a remitting-relapsing time course is also an enigma.
Recessive mutations in a number of other genes cause a range of very rare syndromes that include neonatal diabetes as one component of a multi-system disorder (Table 1).
Young-onset diabetes
Familial young-onset diabetes is typically diagnosed outside the neonatal period but before 25 years of age. Mutations in the glucokinase and HNF1A genes account for about 70% of cases. Heterozygous loss-of-function mutations in the glucokinase gene (GCK) lead to lifelong, mild, stable fasting hyperglycemia from birth. However, this is often only detected later in life, such as during routine screening in pregnancy (Osbak et al., 2009). GCK mutations act by decreasing glucose-dependent ATP production, which results in impaired KATP closure and reduced insulin secretion. Treatment is rarely needed and ~85% of patients can be managed by diet alone. Microvascular complications are also rare, reflecting the fact that glucose homeostasis is not severely impaired.
Five genes associated with familial young-onset diabetes are transcription factors that interact in a complex network to regulate gene transcription (Table 1). They are needed both for correct functioning of adult beta-cells and for beta-cell development. The most common mutations are found in the homeobox genes HNF1A and HNF4A: mutations in HNF1B, IPF1 and NEUROD1 are more rare. Most mutations cause a loss of function and are inherited in an autosomal dominant manner. They lead to a progressive deterioration of beta-cell function, increasing hyperglycemia and eventual diabetes. Studies of mouse models suggest the impaired insulin section may be a result of defective metabolism (Dukes et al., 1998). While heterozygous mutations cause a phenotype that is largely confined to the beta-cell, homozygous mutations in these transcription factors cause a range of additional effects, reflecting the role of these proteins in extra-pancreatic tissues. For example, HNF1A is associated with a low renal glucose threshold, HNF4A with altered lipids and lipoproteins and HNF1B with renal cysts and uterine and genital developmental disorders. Patients are at risk of microvascular complications due to poor glucose control.
Patients with HNF1A mutations are highly sensitive to sulphonylureas and can transfer from insulin to low dose oral drug therapy without deterioration of glycemic control (Pearson et al., 2003). This indicates that despite diabetes of long duration they possess sufficient beta-cell mass, and thus that HNF1A likely controls beta-cell function.
It is evident from the above discussion that the age at which monogenic diabetes presents is variable and depends on the functional severity of the mutation. Patients with more severe mutations, or a greater gene dosage (homozygous rather than heterozygous), tend to develop diabetes earlier in life, whatever the gene involved. In many cases, they may also exhibit extra-pancreatic symptoms. Furthermore, common variants in the same genes that cause monogenic disease may predispose to T2DM in later life.
Polygenic diabetes
Genetics of T2DM
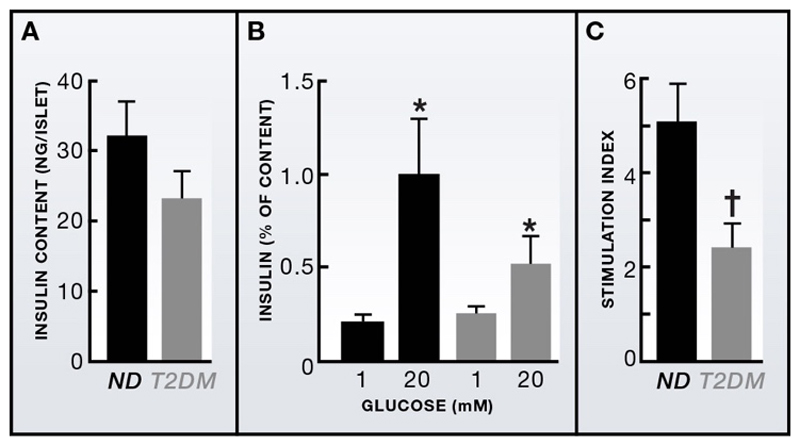
Perhaps one of the most radical changes in the field in the past decade has been widespread recognition that reduced beta-cell function is the key problem in T2DM. Ten years ago, many leading groups still continued to regard T2DM as largely a disease of insulin resistance: today, this is not the case. In part, this paradigm shift has come about because studies of human islets have shown reduced glucose-dependent insulin secretion in T2DM even when allowance is made for the reduction of insulin content (Figure 2). In part, it derives from linkage studies and genome-wide association studies (GWAS), which have identified more than 40 genes associated with increased risk of T2DM over the past 5-6 years. Here we discuss a selection of these genes; for a more comprehensive summary, see (Bonnefond et al., 2010; McCarthy, 2010).
Figure 2. Impaired glucose-induced insulin secretion cannot be accounted for by reduced insulin content.
(A) Insulin content in islets from non-diabetic (ND) and age- and BMI-matched T2DM organ donors). (B) Insulin secretion, normalized to islet insulin content, evoked by 1mM and 20mM glucose in ND and T2DM islets. Mean±s.e.m. (C) Stimulation index (insulin secretion at 20mM glucose divided by that at 1mM) in ND and T2DM islets *p<0.05 versus 1mM glucose; †p<0.05 vs. ND islets. From: Walker et al. (2011)
Most gene associations were inferred from SNPs within non-coding regions of the gene and in many cases the annotation is not yet conclusive. Thus it remains uncertain if diabetes is linked to the gene within whose intron the SNP residues, or to genes that lie close by, or are co-regulated. With few exceptions, the genes identified were unexpected based on existing knowledge and precisely how they predispose to T2DM is not yet understood. However, many are believed to be important for beta-cell function, beta-cell development or the regulation of beta-cell mass (Bonnefond et al., 2010; McCarthy, 2010). Others, such as FTO, predispose toward obesity and thus indirectly to T2DM: when corrected for body mass index the association with T2DM vanishes.
The most important diabetes susceptibility gene identified to date is TCF7L2, which increases diabetes risk 1.7-fold. It is involved in WNT signaling and functional studies suggest that it influences insulin secretion, although, there is disagreement about whether its expression is increased (Lyssenko et al., 2007) or unaltered (Kirkpatrick et al., 2010) in T2DM. However, down-regulation of TCF7L2 in mouse or human islets causes reduced insulin secretion and exocytosis (Shu et al., 2008).
Several GWAS genes are implicated in cell cycle regulation and thus suggested to act by influencing beta-cell mass during development. This is not entirely unexpected since a reduced beta-cell mass might predispose to T2DM by reducing the capacity to cope with an increased demand, such as that imposed by age and/or obesity.
The functional effects of other genes are less clear. The zinc transporter, Znt8 (SLC30A8), is necessary for the uptake of zinc into the insulin granules and formation of Zn-complexed insulin crystals (Sladek et al., 2007). Thus one might expect it to have an effect on insulin storage or release: however, deleting the gene in mice has only weak and variable effects (Lemaire et al., 2009). KCNQ1 (Yasuda et al., 2008) encodes a voltage-sensitive K-channel and a gain-of-function mutation in this gene might be expected to reduce action potential duration and thereby calcium influx and insulin secretion. However, it is uncertain whether KCNQ1 is present in human beta-cells, and if the intronic SNP influences its expression. A common polymorphism in Kir6.2 (E23K) predisposes to T2DM (Gloyn et al., 2003). Although the increase in disease risk is small (odds ratio, 1.2), the population risk is highly significant because ~65% of people carry at least one K allele. The E23K polymorphism is found in strong linkage disequilibrium with another variant, S1369A, in the adjacent SUR1 gene, which means either variant could cause the increased disease risk. Precisely how the polymorphism increases disease risk remains controversial, as functional effects on KATP channel activity are very small and variable.
What these examples serve to show is that it is not easy to determine how the various SNPs predispose to T2DM. Given that genes found in GWAS studies only cause a small increase in disease risk and that their effects manifest only later in life or in the face of obesity, disease-associated SNPs may only have small effects on beta-cell function that will be hard to measure in human studies or even in vitro. As might be expected, disease risk is enhanced for individuals who carry multiple risk-associated SNPs (McCarthy, 2010). Even taking this into consideration, GWAS genes collectively appear to explain only 5-10% of T2DM. This has led to the suggestion that there may be a large number of far less common mutations, private to each individual, which carry a greatly enhanced disease risk (Bonnefond et al., 2010). Much current effort is devoted to identifying these rare variants.
Over the last five years, a vast amount of information about the genetics of T2DM has been obtained. What is lacking is an understanding of the functional roles of many these genes and how they contribute to T2DM. Addressing this problem will require considerable effort, substantial funding and a multidisciplinary approach - incorporating studies of human islets, generation and analysis of mouse models (lacking or overexpressing the gene of interest), molecular and cellular studies, and, perhaps most importantly, in vivo studies in humans carrying these different variants. But before beginning this task it will be essential to determine precisely which gene(s) is affected by any intronic T2DM susceptibility SNP of interest.
What can rodent models teach us?
Not all T2DM genes identified in the last decade were found by GWAS or linkage studies. Some were found by analysis of genes that cause glucose intolerance or diabetes in rodents. For example, the discovery that the alpha2-adrenoreceptor gene contributes to diabetes in the GK rat, led to identification of a common variant of this gene that decreases insulin secretion in humans (Rosengren et al., 2010). In fact, deletion of a number of different genes in mice impairs glucose tolerance. This indicates that glucose homeostasis is finely balanced, involves many pathways, and that perturbation of any of these can cause glucose intolerance.
As much work on the mechanism of insulin secretion has involved rodents, particularly genetically modified mice, it is pertinent to consider the extent to which rodents provide a valid model of human insulin secretion. In some instances, mouse models faithfully recapitulate the human phenotype as in the case of ND due to KCNJ11 mutations (Clark et al., 2010; Girard et al., 2009). Nevertheless, it is now evident that human and mouse beta-cells are far from identical: e.g., they express different complements of ion channels and membrane transporters (Braun et al., 2008; De Vos et al., 1995)) and have a different architecture. Thus while mouse models and studies of mouse islets are very valuable, it is important to be cautious when extrapolating from mice to humans.
Is T2DM associated with reduced Beta-cell mass?
There is an ongoing debate about the extent to which beta-cell mass is reduced in T2DM and its precise role in the etiology of T2DM remains controversial. Over the last decade, several investigators have come to favor the hypothesis that a reduced number of beta cells rather than impaired beta-cell function is a major factor in the development of T2DM. A decrease in beta-cell mass of up to 60% has been reported in T2DM (Butler et al., 2003), which parallels the extent of reduction in glucose-induced insulin secretion (Del Guerra et al., 2005). However, others have found considerably lower decrements (Rahier et al., 2008). Because no longitudinal studies of beta-cell mass in man exist, it is unclear whether individuals with T2DM started with a lower beta-cell mass or if this developed as a consequence of sustained hyperglycemia.
How much beta-cell mass is needed to maintain glycaemia is also uncertain. Simple calculations based on the rate of insulin release each day in a non-diabetic individual suggest as few as 40% of beta-cells would be sufficient for adequate glucose control. This number is supported by the fact that removal of half the pancreas has only a small effect on glucose tolerance (Menge et al., 2008). Collectively, these findings suggest that while beta-cell mass plays some role in T2DM, it is not the only, or even the most important, factor. The finding that insulin secretion from T2DM islets is reduced even when allowance is made for a slight reduction of insulin content (Fig. 2) implicates beta-cell function rather than beta-cell number in the etiology of T2DM. Indeed, the rapid reversal of diabetes and the increase in insulin secretion provoked by GLP-1, demonstrate that even in established T2DM there is sufficient, albeit latent, beta-cell capacity.
Recent observations suggest that unlike islet cells in young rodents, adult human beta-cells are largely senescent, post-mitotic and long-lived (Cnop et al., 2010; Kohler et al., 2011), so aberrant cellular turnover is unlikely to contribute to T2DM. However, ageing processes (e.g. reduced mitochondrial oxidation) in long-lived endocrine cells could impair secretory function. Amyloid formation in pancreatic islets, from islet amyloid polypeptide (IAPP), is a hallmark of T2DM but because the severity of amyloidosis is low in most patients, it is likely to be a consequence rather than a cause of the disease (Clark and Nilsson, 2004).
Environmental and lifestyle factors
Although this review focuses on the role of the beta-cell in diabetes, it is important to consider its role in relation to insulin resistance briefly. The impact of obesity on T2DM risk is truly dramatic and the catastrophic increase in T2DM over the past 50 years is largely due to the concomitant increase in obesity - diabetes would be reduced to a manageable problem if only obesity could be controlled.
Obesity is a key risk factor for T2DM as it lowers insulin sensitivity in peripheral tissues. The beta-cells compensate for this by upregulating insulin secretion (Bergman, 2005), and the degree to which they are able to do so determines whether or not the individual develops diabetes. This, in turn, is influenced by their genetic constitution. This explains why most obese people, and those with other insulin-resistant states, do not develop T2DM: their beta-cells are able to compensate. A dramatic illustration of the interplay between diabetes, obesity and genetics is provided by the report that infusion of triglyceride emulsion led to a decrease in insulin secretion in people who had first-degree relatives with T2DM, but not in those who did not (Kashyap et al., 2003). All individuals were non-diabetic in the absence of elevated plasma triglyceride emphasizing that it is only in the presence of elevated fat that genetic differences become obvious.
Lipids have complex effects on beta-cell function. In the short term, free fatty acids (FFA) potentiate glucose-induced insulin secretion. This contributes to the increased insulin secretion following a mixed meal and enables storage of excess calories as fat. It has also been proposed to account for the compensatory upregulation of beta-cell function in response to insulin resistance (Stefanovski et al., 2011). In the long-term, however, FFAs suppress glucose-induced insulin secretion. The mechanism by which this occurs is still hotly debated. It has been suggested to involve impaired glucose metabolism, reduced insulin biosynthesis and beta-cell loss (Poitout and Robertson, 2008; Yaney and Corkey, 2003). A further idea has recently been proposed, based on the lack of any change in Ca2+-currents or global [Ca2+]i transients in the presence of physiological levels of FFA during fasting, and the observation that Ca2+ channels become more diffusely distributed in the plasmalemma (Hoppa et al., 2009). The beta-cell has a low density of calcium channels which normally colocalize with the secretory granules, ensuring that even if calcium channels open only briefly (as during a brief action potential), Ca2+ entry is sufficient to trigger exocytosis (Figure 1B). Loss of this co-localization means that although the calcium channels continue to open, the resulting increase in [Ca2+]i occurs in the ‘wrong’ place and fails to evoke secretion (Hoppa et al., 2009).
Obesity and high-fat feeding mimic the effects of long-term incubation of islets with FFA on insulin secretion and Ca2+-channel distribution (Collins et al., 2010). All correlate with an increased amount of fat within the islets (Hoppa et al., 2009) and the surrounding exocrine pancreas (Pinnick et al., 2008). Moreover, in Zucker diabetic rats fed a high-fat diet, an increase in islet triglyceride content precedes hyperglycemia (Lee et al., 1994). There is also an inverse correlation between the amount of fat in the human pancreas and glucose-induced insulin secretion, with glucose tolerance and insulin secretion improving in parallel with a reduction in pancreatic fat (Tushuizen et al., 2007). This raises the interesting possibility that intrapancreatic/intraislet fat depositions provide a long-term local source of FFA that adversely affects beta-cell function.
How do studies of monogenic diabetes inform our understanding of T2DM?
Recent years have seen considerable advances in our understanding of the genetic origin and associated phenotype of several types of monogenic diabetes. The confounding effects of obesity are less important in these disorders, suggesting it may be possible to glean information on the etiology of T2DM by comparing it with monogenic diabetes.
Current evidence favors the idea that insufficient glucose-stimulated insulin secretion is primarily responsible for T2DM. As discussed above, estimates of beta-cell mass in T2DM vary but do not appear sufficient to account for the disease. Moreover, the ability of GLP-1, or bariatric surgery, to restore glucose-induced insulin secretion and plasma glucose in patients with T2DM to normal levels argues that neither beta-cell mass nor insulin content are severely reduced. This implicates defective beta-cell function as the cause of T2DM.
Where does this defect occur? As T2DM is not associated with impaired secretion from other endocrine organs, metabolic and exocytotic pathways common to all endocrine cells cannot be adversely affected: it must be some process unique to the beta-cell. Given the key role of KATP channels and the ability of sulphonylureas to enhance insulin secretion in T2DM, it is tempting to attribute the impaired insulin secretion to slight overactivity of KATP channels. However, any such overactivity cannot be as great as in ND patients with KATP channel mutations because agents such as arginine and GLP-1, which work only when KATP channels are largely closed, are still effective secretagogues in T2DM but not in ND. These considerations suggest that T2DM may be associated with mild impairment of metabolism and/or the KATP channel itself, leading to a small reduction in KATP channel closure when blood glucose increases. A key question is whether this is sufficient to explain the diabetes.
Reduced activity of glucokinase cannot be solely responsible for T2DM as the phenotype of the disease does not mimic that of patients with either heterozygous or homozygous GCK mutations. Impaired mitochondrial metabolism has been postulated to contribute to T2DM (Maechler and Wollheim, 2001), and an age-dependent decline in mitochondrial function might help explain why T2DM develops later in life (in lean individuals). However, if impaired metabolic regulation of KATP channel activity were the only problem in T2DM, then the phenotype would resemble that of patients with ND or GCK mutations, which it does not.
This suggests additional processes, downstream of KATP channel closure, which regulate the amplifying response to glucose or exocytosis itself, also contribute to T2DM. Together with a slight deterioration of metabolic function and insulin content with age, these might lead to impaired glucose tolerance, thus setting in progress a vicious cycle in which slight hyperglycemia produces down-regulation of critical genes, impairs the amplifying effects of glucose and leads to a progressive decline in beta-cell function (Weir and Bonner-Weir, 2004). Obesity, via insulin resistance, would place an increased functional demand on the beta-cell and accelerate beta-cell failure.
Therapies for T2DM
As T2DM progresses, blood glucose levels rise and β-cell function progressively declines. Treatment options are complex, with patients starting on oral anti-diabetic agents, but most soon proceeding to insulin injections; many also take drugs that reduce insulin resistance. Almost all of the current therapeutic interventions lead to weight gain, an increased risk of hypoglycemia and have little if any impact on disease progression.
Recent years have seen the introduction of the incretin-based therapies. GLP-1 has a half-life of one minute in the plasma and is rapidly degraded by the enzyme dipeptidyl peptidase 4 (DPP4) so it cannot be used itself (Deacon et al., 2008). However, inhibitors of DPP4, or stable GLP-1 analogues, are very effective at enhancing glucose-stimulated insulin secretion. Their efficacy demonstrates that even in people with diabetes of long duration, sufficient beta-cell capacity exists to maintain normoglycemia, supporting the idea that impaired beta-cell function is the prime cause of the disease. In rodents, GLP-1 also increases islet cell functional mass (Xu et al., 1999), although it is uncertain whether this applies to human islets (Parnaud et al., 2008). Plasma GLP-1 levels are lower in T2DM, but the extent to which a diminished incretin effect contributes to disease progression is unknown (Holst, 2007). Most recently, agonists against novel G-protein coupled receptors, including GPR119, GPR40, GPR120 and GPR43 (Kebede et al., 2009), that have the potential to stimulate not only pancreatic beta-cells but also the cells in the gut that release incretin hormones, have emerged as possible treatments for diabetes. In addition, the use of anti-inflammatory agents such as interleukin-1-receptor antagonists, that improve glycemic control and beta cell function, are entering trial programs (Mandrup-Poulsen et al., 2010).
Diabetes is usually considered to be a chronic disease, associated with a progressive reduction in beta-cell mass and irreversible beta-cell failure that cannot be cured and requires lifelong therapy. However, over the past 10 years, two independent sets of studies have challenged this view. First, severe energy restriction was able to reverse established T2DM within one week (Lim et al., 2011). Second, patients who undergo bariatric surgery often show remission of diabetes long before there is any substantial weight loss (Karra et al., 2010).
The purpose of bariatric surgery is to enhance weight loss in morbid obesity. Two different types of gastric bypass are routinely employed: gastric banding and Roux-en-Y by-pass (RYGB) (Karra et al., 2010). Intriguingly, both methods result in a rapid resolution (within days) of type-2 diabetes (Karra et al., 2010). Meta-analysis indicated that diabetes resolves in 70% of patients after RYGB, and somewhat fewer after gastric banding (Buchwald et al., 2009). The rapid improvement of glucose tolerance cannot be accounted for by the weight loss, which is much slower, but is associated with increased insulin sensitivity and often (but not always) increased insulin secretion (Falken et al., 2011; Isbell et al., 2010). The mechanisms involved have not yet been elucidated but proposed explanations include increased release of the incretin hormone GLP-1, an altered gut microbiotic environment, and caloric restriction. Bariatric surgery is so effective that in 2011 it was approved as an appropriate therapy for morbidly obese patients with T2DM by the International Diabetes Federation.
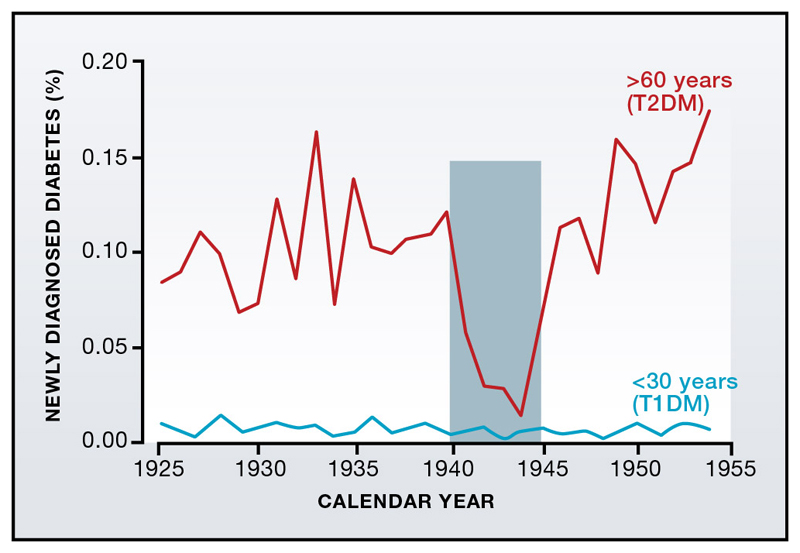
Interestingly, the effects of bariatric surgery can be mimicked by a very low calorie-diet (600 kcal/day) (Lim et al., 2011), which normalized plasma glucose levels within 4-7 days. In itself, this is not so remarkable. During WWII, the number of people with newly diagnosed T2DM in several European countries crashed, only to rise after the end of food rationing (despite the restricted diet, people were not starving) (Figure 3; (Westlund, 1966)). In the most recent Balkan war, glycemic control significantly improved in T2DM patients, as did obesity (Kulenovic et al., 1996). However, there have been few studies of how reduced caloric intake leads to diabetes remission. The significance of the study by Lim and colleagues (Lim et al., 2011) is that it illustrates just how fast diabetes resolves. This correlates with a marked fall in hepatic glucose output and insulin resistance, which normalizes glycemia. The underlying mechanism is unclear. There is also a gradual restoration of glucose-induced biphasic insulin secretion that mirrors a reduction in pancreatic fat content. This suggests a factor derived from intrapancreatic fat depots may exert a negative effect on beta-cell function. The identity of this factor remains unknown but local release of FFAs is one of several possible candidates.
Figure 3. Diet affects diabetes incidence.
New cases of diabetes diagnosed in Norway during the years 1925-1955 (expressed as a percentage of the population). Diabetes incidence is higher in individuals >60 years (i.e. with T2DM) than those <30 years (most of whom will have T1DM). While the incidence of T1DM remains unchanged, T2DM decreases by 85% during the 1940-45 German occupation. From Westlund (1966).
Conclusions
The last ten years have seen major advances both in the scientific understanding and treatment of diabetes. Permanent neonatal diabetes has been recognized as a monogenic disorder, separate from T1DM, with which it was previously confused. The understanding of the molecular basis of neonatal diabetes, and the introduction of a novel therapy (oral sulphonylureas) for patients with KATP channel or HNF1A mutations constitute a real breakthrough. Importantly, tailoring therapy to an individual's genetic constitution in this way has led to improved glycemic control and a far better quality of life.
There has also been unprecedented progress in identification of genes that are highly significantly associated with T2DM. Like any discovery, their identification precipitates a plethora of new questions and heralds a daunting amount of new functional work. But it also provides an opportunity for gaining important new insights into beta-cell function. Although it was always appreciated that T2DM is a polygenic disease, few would probably have guessed how many genes would be involved, or which ones they would turn out to be. Indeed, the 40 genes identified already are enough that each diabetic could have their own unique combination of gene variants underlying their disease. At present, known SNPs are of little or no value in predicting an individual's risk of T2DM, clinical translation has so far been minimal and it remains to be seen whether they will be of therapeutic value. Rather, their importance lies in the insights they provide into the pathophysiology of disease and potential therapeutic targets. Unraveling their roles will be challenging but rewarding.
The idea that multiple genes and multiple etiologies underlie T2DM suggests that there will be an associated multiplicity of phenotypes. One lesson from monogenic diabetes studies is that different genotypes are associated with distinct phenotypes, a finding of considerable significance for disease diagnosis, choice of therapy and an understanding of disease mechanism. This suggests it will be important to phenotype T2DM patients more fully. A valuable approach might be to focus on lean type-2 diabetic patients, where the confounding effects of obesity and insulin resistance are minimal.
Finally, we point out that although there is a strong genetic predisposition to T2DM, shared by many of the population, most people are not doomed to become diabetic. The massive increase in diabetes incidence in recent years is not due to genetic changes but a consequence of the rise in obesity. It is clear that weight control is highly beneficial in T2DM, and recent studies show that it is even possible to reverse established disease by bariatric surgery and caloric restriction (at least in patients who have had diabetes for only a few years). The big problem is that dietary compliance is very difficult to achieve and is rarely perfect. Thus it may be appropriate to consider radical measures such as taxing high-fat foods, as has recently been implemented in Denmark: the examples of cigarette smoking and alcohol intake suggest that such a tax might be an effective deterrent to excess consumption.
Acknowledgements
We apologize to colleagues whose work we were unable to include, or may have overlooked due to reasons of space. It is also, by necessity, a highly personal perspective. We thank Drs Anne Clark, Fiona Gribble and our many other colleagues and students for stimulating discussion and reviewing the manuscript; and the Wellcome Trust, the MRC, the Royal Society and Diabetes UK for financial support.
References
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Bergman RN. Minimal model: perspective from 2005. Horm Res. 2005;64(Suppl 3):8–15. doi: 10.1159/000089312. [DOI] [PubMed] [Google Scholar]
- Bonnefond A, Froguel P, Vaxillaire M. The emerging genetics of type 2 diabetes. Trends Mol Med. 2010;16:407–416. doi: 10.1016/j.molmed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256 e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47:157–169. doi: 10.1007/s00125-003-1304-4. [DOI] [PubMed] [Google Scholar]
- Clark RH, McTaggart JS, Webster R, Mannikko R, Iberl M, Sim XL, Rorsman P, Glitsch M, Beeson D, Ashcroft FM. Muscle dysfunction caused by a KATP channel mutation in neonatal diabetes is neuronal in origin. Science. 2010;329:458–461. doi: 10.1126/science.1186146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, Gunter JH, de Koning EJ, Walls GV, Gray DW, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–330. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- Collins SC, Hoppa MB, Walker JN, Amisten S, Abdulkader F, Bengtsson M, Fearnside J, Ramracheya R, Toye AA, Zhang Q, et al. Progression of diet-induced diabetes in C57BL6J mice involves functional dissociation of Ca2(+) channels from secretory vesicles. Diabetes. 2010;59:1192–1201. doi: 10.2337/db09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF, Carr RD, Holst JJ. DPP-4 inhibitor therapy: new directions in the treatment of type 2 diabetes. Front Biosci. 2008;13:1780–1794. doi: 10.2741/2799. [DOI] [PubMed] [Google Scholar]
- Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–735. doi: 10.2337/diabetes.54.3.727. [DOI] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- Dukes ID, Sreenan S, Roe MW, Levisetti M, Zhou YP, Ostrega D, Bell GI, Pontoglio M, Yaniv M, Philipson L, et al. Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice. J Biol Chem. 1998;273:24457–24464. doi: 10.1074/jbc.273.38.24457. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121:181–197. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;81:375–382. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Clauin S, Bellanne-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, Ellard S. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin I, Edghill EL, Akerman I, Rubio-Cabezas O, Rica I, Locke JM, Maestro MA, Alshaikh A, Bundak R, del Castillo G, et al. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci U S A. 107:3105–3110. doi: 10.1073/pnas.0910533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin I, Edghill EL, Akerman I, Rubio-Cabezas O, Rica I, Locke JM, Maestro MA, Alshaikh A, Bundak R, del Castillo G, et al. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci U S A. 2010;107:3105–3110. doi: 10.1073/pnas.0910533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P, Abdulkader F, Clark A, Ball V, Zubcevic L, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, Johnson P, Ashcroft FM, Rorsman P. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab. 2009;10:455–465. doi: 10.1016/j.cmet.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosker JP, Rudenski AS, Burnett MA, Matthews DR, Turner RC. Similar reduction of first- and second-phase B-cell responses at three different glucose levels in type II diabetes and the effect of gliclazide therapy. Metabolism. 1989;38:767–772. doi: 10.1016/0026-0495(89)90064-4. [DOI] [PubMed] [Google Scholar]
- Iafusco D, Bizzarri C, Cadario F, Pesavento R, Tonini G, Tumini S, Cauvin V, Colombo C, Bonfanti R, Barbetti F. No beta cell desensitisation after a median of 68 months on glibenclamide therapy in patients with KCNJ11-associated permanent neonatal diabetes. Diabetologia. 2011;54:2736–2738. doi: 10.1007/s00125-011-2273-7. [DOI] [PubMed] [Google Scholar]
- Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra E, Yousseif A, Batterham RL. Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab. 2010;21:337–344. doi: 10.1016/j.tem.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab. 2009;11(Suppl 4):10–20. doi: 10.1111/j.1463-1326.2009.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick CL, Marchetti P, Purrello F, Piro S, Bugliani M, Bosco D, de Koning EJ, Engelse MA, Kerr-Conte J, Pattou F, et al. Type 2 diabetes susceptibility gene expression in normal or diabetic sorted human alpha and beta cells: correlations with age or BMI of islet donors. PLoS ONE. 2010;5:e11053. doi: 10.1371/journal.pone.0011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CU, Olewinski M, Tannapfel A, Schmidt WE, Fritsch H, Meier JJ. Cell cycle control of beta-cell replication in the prenatal and postnatal human pancreas. Am J Physiol Endocrinol Metab. 2011;300:E221–230. doi: 10.1152/ajpendo.00496.2010. [DOI] [PubMed] [Google Scholar]
- Kulenovic I, Robertson A, Grujic M, Suljevic E, Smajkic A. The impact of war on Sarajevans with non-insulin-dependent diabetes mellitus. European Journal of Public Health. 1996;6:252–258. [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, Genieser HG, Holz GG. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic beta cells. Prog Biophys Mol Biol. 2011;107:236–247. doi: 10.1016/j.pbiomolbio.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci U S A. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjogren M, Ling C, Eriksson KF, Lethagen AL, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007;117:2155–2163. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Shield JP, Dean W, Leclerc I, Knauf C, Burcelin RR, Rutter GA, Kelsey G. Impaired glucose homeostasis in transgenic mice expressing the human transient neonatal diabetes mellitus locus, TNDM. J Clin Invest. 2004;114:339–348. doi: 10.1172/JCI19876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:158–166. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- McTaggart JS, Clark RH, Ashcroft FM. The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J Physiol. 2010;588:3201–3209. doi: 10.1113/jphysiol.2010.191767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes. 2008;57:142–149. doi: 10.2337/db07-1294. [DOI] [PubMed] [Google Scholar]
- Mlynarski W, Tarasov AI, Gach A, Girard CA, Pietrzak I, Zubcevic L, Kusmierek J, Klupa T, Malecki MT, Ashcroft FM. Sulfonylurea improves CNS function in a case of intermediate DEND syndrome caused by a mutation in KCNJ11. Nat Clin Pract Neurol. 2007;3:640–645. doi: 10.1038/ncpneuro0640. [DOI] [PubMed] [Google Scholar]
- Murphy R, Ellard S, Hattersley AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:200–213. doi: 10.1038/ncpendmet0778. [DOI] [PubMed] [Google Scholar]
- Njolstad PR, Sovik O, Cuesta-Munoz A, Bjorkhaug L, Massa O, Barbetti F, Undlien DE, Shiota C, Magnuson MA, Molven A, et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med. 2001;344:1588–1592. doi: 10.1056/NEJM200105243442104. [DOI] [PubMed] [Google Scholar]
- Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, Gloyn AL. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009;30:1512–1526. doi: 10.1002/humu.21110. [DOI] [PubMed] [Google Scholar]
- Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, Bruun C, Mandrup-Poulsen T, Billestrup N, Halban PA. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 2003;362:1275–1281. doi: 10.1016/S0140-6736(03)14571-0. [DOI] [PubMed] [Google Scholar]
- Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring) 2008;16:522–530. doi: 10.1038/oby.2007.110. [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, Nagaraj V, Reinbothe TM, Tuncel J, Eliasson L, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327:217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- Rubio-Cabezas O, Flanagan SE, Damhuis A, Hattersley AT, Ellard S. K(ATP) channel mutations in infants with permanent diabetes diagnosed after 6 months of life. Pediatr Diabetes. 2011 doi: 10.1111/j.1399-5448.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- Senee V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec JC, Charon C, Nicolino M, Boileau P, Cavener DR, et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38:682–687. doi: 10.1038/ng1802. [DOI] [PubMed] [Google Scholar]
- Servitja JM, Ferrer J. Transcriptional networks controlling pancreatic development and beta cell function. Diabetologia. 2004;47:597–613. doi: 10.1007/s00125-004-1368-9. [DOI] [PubMed] [Google Scholar]
- Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes. 2008;57:645–653. doi: 10.2337/db07-0847. [DOI] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Slingerland AS, Nuboer R, Hadders-Algra M, Hattersley AT, Bruining GJ. Improved motor development and good long-term glycaemic control with sulfonylurea treatment in a patient with the syndrome of intermediate developmental delay, early-onset generalised epilepsy and neonatal diabetes associated with the V59M mutation in the KCNJ11 gene. Diabetologia. 2006;49:2559–2563. doi: 10.1007/s00125-006-0407-0. [DOI] [PubMed] [Google Scholar]
- Smith SB, Qu HQ, Taleb N, Kishimoto NY, Scheel DW, Lu Y, Patch AM, Grabs R, Wang J, Lynn FC, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775–780. doi: 10.1038/nature08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovski D, Richey JM, Woolcott O, Lottati M, Zheng D, Harrison LN, Ionut V, Kim SP, Hsu I, Bergman RN. Consistency of the disposition index in the face of diet induced insulin resistance: potential role of FFA. PLoS ONE. 2011;6:e18134. doi: 10.1371/journal.pone.0018134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy J, Steiner DF, Park SY, Ye H, Philipson LH, Bell GI. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev Endocr Metab Disord. 2010;11:205–215. doi: 10.1007/s11154-010-9151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Hatakeyama H, Okado H, Noguchi J, Ohno M, Kasai H. SNARE conformational changes that prepare vesicles for exocytosis. Cell Metab. 2010;12:19–29. doi: 10.1016/j.cmet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Temple IK, Shield JP. 6q24 transient neonatal diabetes. Rev Endocr Metab Disord. 2010;11:199–204. doi: 10.1007/s11154-010-9150-4. [DOI] [PubMed] [Google Scholar]
- Turkkahraman D, Bircan I, Tribble ND, Akcurin S, Ellard S, Gloyn AL. Permanent neonatal diabetes mellitus caused by a novel homozygous (T168A) glucokinase (GCK) mutation: initial response to oral sulphonylurea therapy. J Pediatr. 2008;153:122–126. doi: 10.1016/j.jpeds.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16–21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- Westlund K. Incidence of diabetes mellitus in Oslo, Norway, 1925-1954. Brit J Prev Soc Med. 1966;20:105–116. [Google Scholar]
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- Yaney GC, Corkey BE. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia. 2003;46:1297–1312. doi: 10.1007/s00125-003-1207-4. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008;40:1092–1097. doi: 10.1038/ng.207. [DOI] [PubMed] [Google Scholar]
- Yoo HW, Shin YL, Seo EJ, Kim GH. Identification of a novel mutation in the GLUT2 gene in a patient with Fanconi-Bickel syndrome presenting with neonatal diabetes mellitus and galactosaemia. Eur J Pediatr. 2002;161:351–353. doi: 10.1007/s00431-002-0931-y. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–610. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- Zung A, Glaser B, Nimri R, Zadik Z. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004;89:5504–5507. doi: 10.1210/jc.2004-1241. [DOI] [PubMed] [Google Scholar]