Abstract
In the ventral stream of the primate auditory cortex, cortico-cortical projections emanate from the primary auditory cortex (AI) along 2 principal axes: one mediolateral, the other caudorostral. Connections in the mediolateral direction from core, to belt, to parabelt, have been well described, but less is known about the flow of information along the supratemporal plane (STP) in the caudorostral dimension. Neuroanatomical tracers were injected throughout the caudorostral extent of the auditory core and rostral STP by direct visualization of the cortical surface. Auditory cortical areas were distinguished by SMI-32 immunostaining for neurofilament, in addition to established cytoarchitectonic criteria. The results describe a pathway comprising step-wise projections from AI through the rostral and rostrotemporal fields of the core (R and RT), continuing to the recently identified rostrotemporal polar field (RTp) and the dorsal temporal pole. Each area was strongly and reciprocally connected with the areas immediately caudal and rostral to it, though deviations from strictly serial connectivity were observed. In RTp, inputs converged from core, belt, parabelt, and the auditory thalamus, as well as higher order cortical regions. The results support a rostrally directed flow of auditory information with complex and recurrent connections, similar to the ventral stream of macaque visual cortex.
Keywords: auditory cortex, pathways, rhesus, SMI-32, temporal lobe
Introduction
The auditory cortex of primates has been conceptualized as 2 streams emanating from the primary auditory cortex (AI): a dorsal stream engaged in spatial processing of sound sources, and a ventral stream processing sound quality (Rauschecker 1998; Romanski, Tian, et al. 1999; Rauschecker and Scott 2009). In both New- and Old-world monkeys, anatomical projections of the ventral stream follow 2 axes from AI, one mediolateral and the other caudorostral (Galaburda and Pandya 1983; Hackett et al. 1998a; Hackett 2011). This anatomical division carries functional implications (Bendor and Wang 2008; Kuśmierek and Rauschecker 2009; Kuśmierek et al. 2012), yet comparatively little is known about information flow in the caudorostral dimension. Understanding the flow of information along the caudorostral axis is critical to interpreting the accumulating functional evidence that auditory activation extends to the most rostral regions of the macaque temporal lobe (Poremba et al. 2003, 2004; Petkov et al. 2008; Kikuchi et al. 2010; Ng et al. 2014; Fukushima et al. 2014; Scott et al. 2014).
The auditory cortex in the macaque lies on the supratemporal plane (STP) and the adjacent superior temporal gyrus (STG; Fig. 1) and has been operationally defined (Hackett 2011) as those cortical areas receiving input from the medial geniculate nucleus of the thalamus (MGN). Connectional evidence indicates that auditory cortex is organized as a three-tiered hierarchy of core, belt, and parabelt regions (Kaas and Hackett 1998; Hackett 2011). The core is the primary recipient of thalamic input from the ventral division of the MGN (MGv) and is composed of AI, the rostral auditory area (R), and a third rostrotemporal area (RT). The belt regions surround the core on its lateral, caudal, and medial aspects, each receiving input from the adjacent division of the core. The belt areas thus comprise a secondary level of the hierarchy. Lateral to the belt, a tertiary parabelt region occupies the STG and is connected strongly with the belt. The belt and parabelt regions receive input from other subdivisions of the MGN, but not from MGv (Molinari et al. 1995; de la Mothe et al. 2006b).
Table 2.
Table of abbreviations
| 35 | Area 35 of the perirhinal cortex |
| 36p | Area 36 of the perirhinal cortex, temporo-polar subregion |
| AI | Primary auditory cortex (core) |
| AL | Anterolateral belt |
| cir. s | Circular sulcus |
| CL | Caudolateral belt |
| cl | Claustrum |
| CM | Caudomedial belt |
| CPB | Caudal parabelt |
| Ia | Agranular insula |
| Id | Dysgranular insula |
| ls | Lateral sulcus |
| MGN | Medial geniculate nucleus of thalamus |
| MGv | Ventral division of MGN |
| MGd | Dorsal division of MGN |
| ML | Middle lateral belt |
| MM | Middle medial belt |
| PGa | Sts fundus/dorsal bank area |
| Pi | Parainsular area |
| R | Rostral core |
| Ri | Retroinsula |
| RM | Rostromedial belt |
| RPB | Rostral parabelt |
| rs | Rhinal sulcus |
| RT | Rostrotemporal core |
| RTL | Rostrotemporal-lateral belt |
| RTM | Rostrotemporal-medial belt |
| RTp | Rostrotemporal-polar |
| STGr | Rostral superior temporal gyrus |
| STP | Supratemporal plane |
| sts | Superior temporal sulcus |
| STSd | Sts dorsal bank |
| TAa | Sts dorsal bank area |
| TEa/TEm | Sts ventral bank areas |
| TEav | Ventral subregion of anterior TE |
| TGa | Agranular part of the temporal pole |
| TGdd | Dysgranular part of the dorsal temporal pole |
| TGdg | Granular part of the dorsal temporal pole |
| TGsts | Sts part of the temporal pole |
| TGvd | Dysgranular part of the ventral temporal pole |
| TGvg | Granular part of the ventral temporal pole |
| TPO | Sts dorsal bank area |
| Tpt | Temporo-parietal area |
| wm | White matter |
Figure 1.
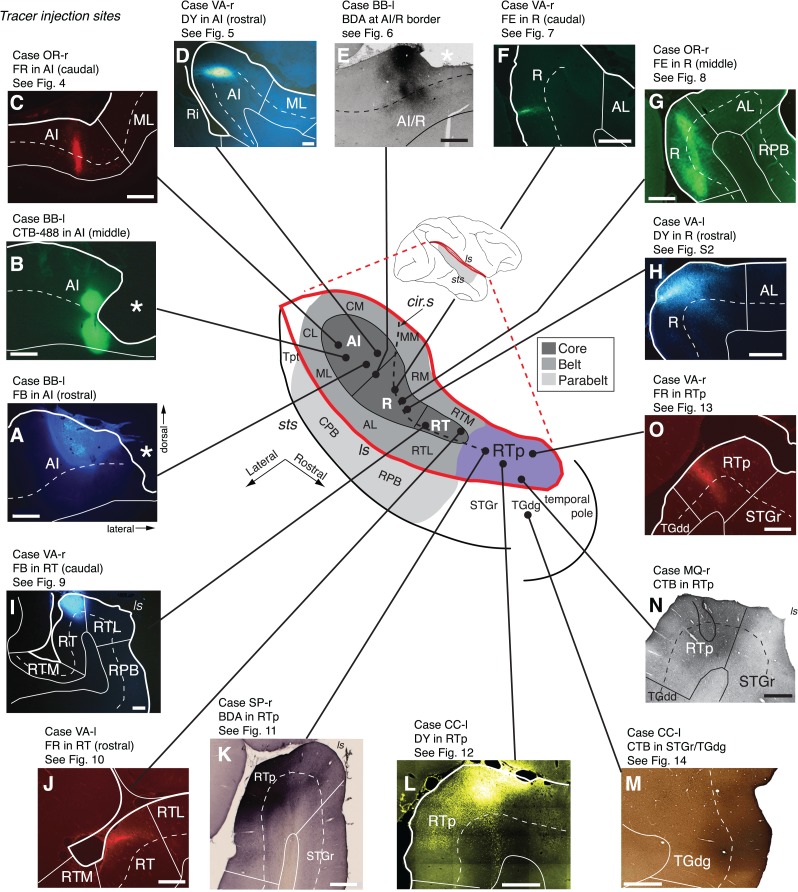
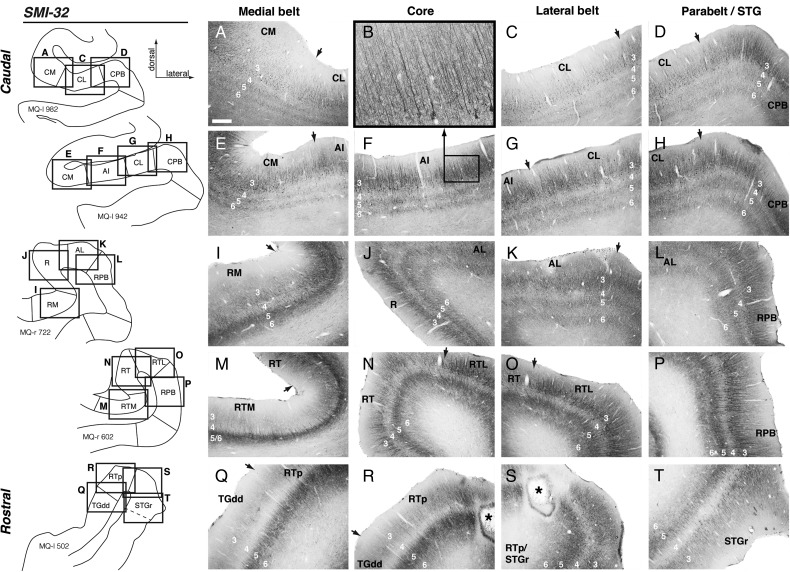
Subdivisions of the auditory cortex in the supratemporal plane (STP) and the superior temporal gyrus (STG), and the sites of the tracer injections in STP. The lateral sulcus (ls), outlined in red on a lateral view of the macaque right hemisphere (inset), is opened to show 3 core areas (dark gray), 4 medial and 4 lateral belt areas (medium gray), and area RTp (purple) on the surface of the STP, illustrated schematically at the center. The rostrocaudal extent of the parabelt areas (light gray) on the STG is also illustrated in the schematic diagram and on the lateral view of the brain. The curved dashed line within the diagram indicates the approximate location of the circular sulcus. Black dots mark tracer injection sites along the caudorostral extent of the STP. A photomicrograph of each injection site from caudal to rostral (AI-R-RT-RTp) is arrayed clockwise from A to H, and counterclockwise from I to O. The laminar involvement of these injection sites is indicated in Table 1. The bold outline indicates the pial surface (in fluorescence photomicrographs only), a solid thin line marks the border between the gray and white matter, and a dashed thin line indicates layer 4. The black outline in N indicates needle damage visible in an adjacent thionine-stained section (not shown). Asterisks in A, B, and E indicate postmortem tissue damage. The text above each photomicrograph indicates: 1) Case identifier, composed of a two-letter subject code followed by “l” or “r” to indicate left or right hemisphere, 2) the tracer and target region, and 3) reference to the corresponding data figure, if applicable. In all figures, images from the left hemisphere are flipped to a right-hemisphere orientation for consistency. See Table 2 for the different auditory areas and sulcus designations. Scale bars = 1 mm.
This serial connectivity in the mediolateral dimension—from core, to belt, to parabelt—has been extensively described (Hackett et al. 1998a; de la Mothe et al. 2006a, 2012), but the connections in the caudorostral dimension are less well documented. This is particularly true for the core regions and the rostral STP, which are located deep within the lateral sulcus and difficult to access for tracer injections (Hackett et al. 2005). The importance of caudal-to-rostral connectivity is underscored by the fact that the predominant model of core, belt, and parabelt occupies only the caudal two thirds of the STP and STG. The remaining territory extending to the rostral STP and dorsal temporal pole is auditory-responsive (Poremba et al. 2003, 2004; Kikuchi et al. 2010) but has not been shown to receive significant thalamic input from the MGN (Molinari et al. 1995; Hackett 2011), implying that this region lies outside the auditory cortex proper and thus depends upon cortico-cortical input from caudal auditory areas. The present data describe these cortico-cortical inputs, but also reveal thalamic inputs (to be detailed in a separate report) from the MGN to the recently identified rostrotemporal polar area (RTp) on the rostral STP (Saleem et al. 2007).
The anatomically defined core corresponds to a region of short-latency, frequency-tuned neural responses that are tonotopically organized along the surface of the STP (Merzenich and Brugge 1973; Morel et al. 1993; Kosaki et al. 1997). The transitions from AI to R, and R to RT, are marked by reversals in the tonotopic gradient, as well as an increase in the mean and variability of the response latency (Recanzone et al. 2000; Petkov et al. 2006; Bendor and Wang 2008; Yin et al. 2008; Scott et al. 2011; Camalier et al. 2012; Fukushima et al. 2012; Baumann et al. 2013). This shift in latency is suggestive of a serial hierarchical organization progressing rostrally along the STP that might engender the more complex and selective tuning of rostral auditory neurons (Kikuchi et al. 2010; Perrodin et al. 2011; Fukushima et al. 2014).
Here we describe the anatomical basis for serial hierarchical processing in the macaque auditory cortex, focusing on the intrinsic connectivity of the core areas, the rostral STP, and the surrounding dorsal temporal pole region. Injections of retrograde and bidirectional neuroanatomical tracers were placed throughout the caudorostral extent of the auditory core, and in area RTp on the rostral STP. Although previous nomenclature grouped RTp and the adjacent rostral STG (STGr) into a single region (Galaburda and Pandya 1983; Cipolloni and Pandya 1989), we find RTp to be distinct from the STGr in both architectonics and connectivity. Taken together, this series of injections identified a rostrally directed serial cascade of connections among the core areas and RTp.
Materials and Methods
Subjects
Six adult rhesus monkeys (Macaca mulatta, 5 males, 5–10 years old) weighing between 5.5 and 13 kg were used. All procedures adhered to the Guide for the Care and Use of Laboratory Animals (National Research Council), and were carried out under a protocol approved by the Institutional Animal Care and Use Committee of the NIMH. Four of the 6 animals (OR, VA, CC, SP) were prepared with a complete commissurotomy in a separate surgery prior to tracer injection, allowing the left and right hemispheres to serve as independent cases. In the remaining 2 animals (BB, MQ), the commissures were intact, and only one hemisphere was injected (only ipsilateral connections are presented here). Each case is indicated by a two-letter animal abbreviation followed by “l” or “r” to indicate the injected hemisphere (e.g., VA-r; see Table 3). For all figures, data are displayed in a right-hemisphere orientation.
Table 3.
Tracer injections in the supratemporal plane
| Case # | Area injected | Tracer | Vol. (μL) | Conc. (%) | Supplier and Catalog # | Figure # |
|---|---|---|---|---|---|---|
| 1 (OR-r) | AI (caudal) | FR | 0.5 | 5 | Molecular Probes D-1817/D-3308 | 4 |
| 2 (BB-l) | AI (mid) | CTB-488 | 0.5 | 1 | Mol. Probes C22841 | |
| 3 (BB-l) | AI (rostral) | FB | 0.5 | 3 | Polysciences 17740 | |
| 4 (VA-r) | AI (rostral) | DY | 0.5 | 3 | Sigma D-0281 | 5 |
| 5 (BB-l) | AI/R border | BDA | 0.5 | 9 | Mol. Probes D1956 | 6 |
| 6 (VA-r) | R (caudal) | FE | 0.5 | 5 | Mol. Probes D-1820 | 7 |
| 7 (OR-r) | R (mid) | FE | 0.5 | 5 | Mol. Probes D-1820 | 8 |
| 8 (VA-l) | R (rostral) | DY | 0.5 | 3 | Sigma D-0281 | Supplementary Fig. 2 |
| 9 (VA-r) | RT (caudal) | FB | 0.5 | 3 | Sigma F5756 | 9 |
| 10 (VA-l) | RT (rostral) | FR | 0.5 | 5 | Mol. Probes D-1817/D-3308 | 10 |
| 11 (SP-r) | RTp | BDA | 1.0 | 10 | Mol. Probes D1956 | 11 |
| 12 (CC-l) | RTp | DY | 0.5 | 3 | Sigma D-0281 | 12 |
| 13 (VA-r) | RTp | FR | 0.5 | 5 | Mol. Probes D-1817/D-3308 | 13 |
| 14 (MQ-r) | RTp (STGr) | CTB | 1.0 | 2 | List Biological 103B | |
| 15 (CC-l) | STGr/TGdg | CTB | 0.5 | 2 | List Biological 103B | 14 |
Tracers
Fourteen tracer injections were placed in the core auditory areas (AI, R, RT) and RTp, spanning approximately 15 mm of the STP (Table 3; Fig. 1). An additional tracer injection was placed in the STGr lateral to RTp. Aqueous solutions of both retrograde and bidirectional tracers were injected in each animal. The volume, concentration, and supplier of the tracers for each injection are indicated in Table 3. We used retrograde tracers Fast blue (FB), Diamidino yellow (DY), and cholera toxin subunit B, either alone (CTB) or conjugated to Alexa-fluor 488 (CTB-488), and bidirectional tracers Fluoro-ruby (FR; dextran-conjugated tetramethylrhodamine), Fluoro-emerald (FE; dextran-conjugated fluorescein), and biotinylated dextran amine (BDA). Note that BDA with 10 kDa molecular weight is primarily an anterograde tracer but is also capable of labeling cells in retrograde transport (Figs 6 and 11). To optimize bidirectional transport of FR, a “cocktail” of both 10 and 3 kDa molecular weights was mixed in equal parts (the former is a more sensitive anterograde tracer, the latter, a more sensitive retrograde tracer). However, FE reliably produced bidirectional transport using only the 10 kDa molecular weight (Figs 7 and 8).
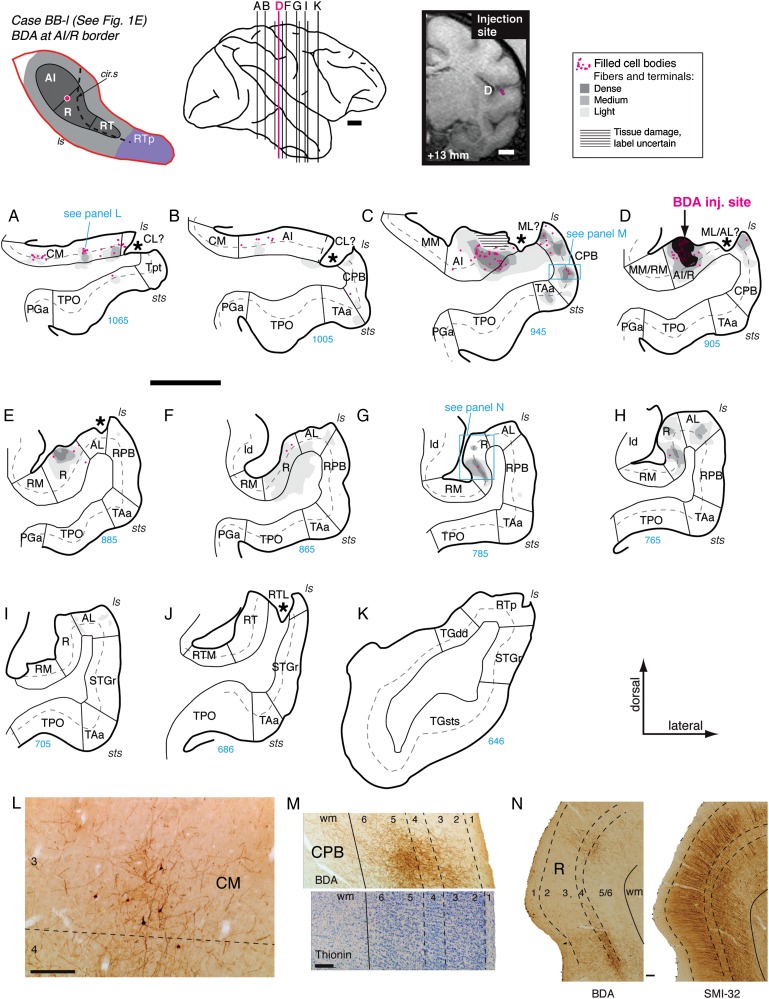
Figure 6.
Panels A–K show the distribution of retrograde and anterograde label in the auditory and adjacent cortical areas after BDA injection into the border between AI and R in case BB-l (section D; see also Fig. 1E). Horizontal hatching in C illustrates an injection site from another tracer that may have obscured label in this case. Asterisks in (A–E) indicate missing tissue; because there was no sign of necrosis or gliosis, this damage is believed to have occurred during postmortem processing of the fixed tissue, due presumably to adhesion to the μECoG arrays implanted in this case (Fukushima et al. 2012). Filled cells and terminals were found in caudal belt area CM (A and L), rostral AI (B–D), and the caudal parabelt (B–D and M). Panel M shows a photomicrograph of the BDA label in CPB (upper half of panel) aligned to an adjacent thionine-stained section (lower half of panel) that was used to determine laminar boundaries. Rostral to the injection site, anterograde label was confined to core area R (E–H) and belt area AL (H). The laminar pattern of anterograde label in R (G) is illustrated in panel N, where a photomicrograph of the BDA label (left panel) is juxtaposed to an adjacent section stained for SMI-32. No label was observed in Tpt, RPB, or STGr. Scale bars = 5 mm in A–K and 0.2 mm in L–N. For other conventions see Figure 4.
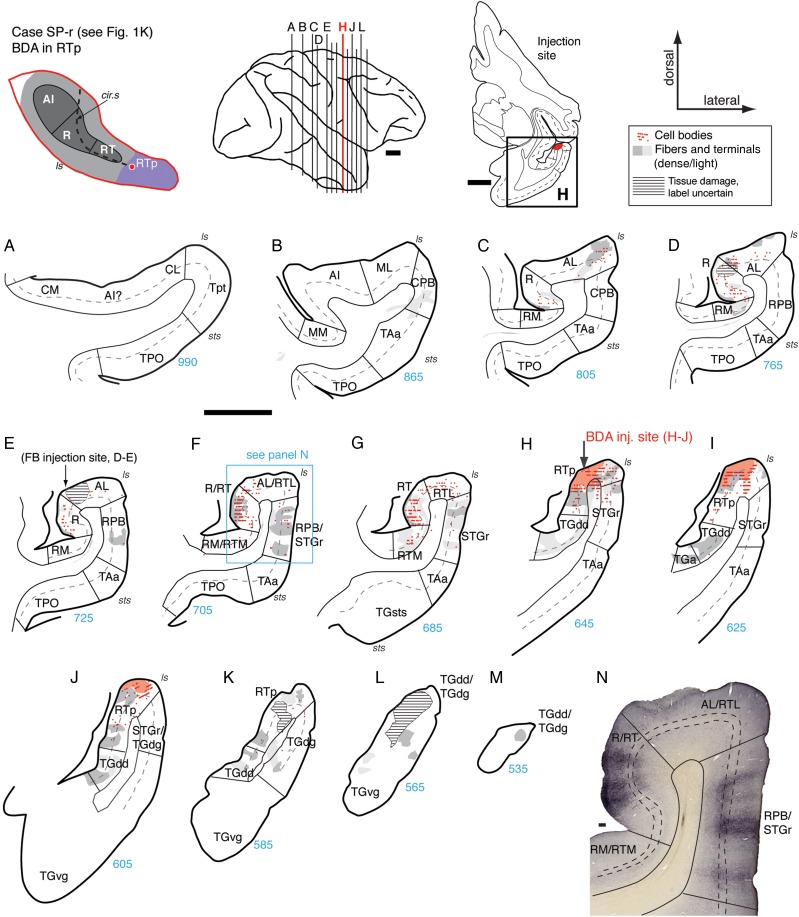
Figure 11.
Connections of area RTp in case SP-r. (A–M) Distribution of retrograde and anterograde label in the STP, STG, and temporal pole areas after BDA injection into the caudal portion of area RTp (section H; see also Fig. 1K). Horizontal hatching in (D and E) marks damage from injections that are not described in the current report. Labeled cells and fibers were found in core area R and belt area AL, with only sparse anterograde label in RM (C–E). Cells and terminals were most dense in area RT (F, G, and panel N). In RPB and STGr, columnar patches of anterograde label spanned layers 1–5 (F, G, N). Retrograde label was located in belt area RTL, and both antero- and retrograde label in RTM (G). Medial and rostral to the injection site, strong anterograde label was found in TGdd (H–K) and the dorsal temporal pole (M). Scale bars = 5 mm in A–M and 0.2 mm in N. For other conventions see Figure 4.
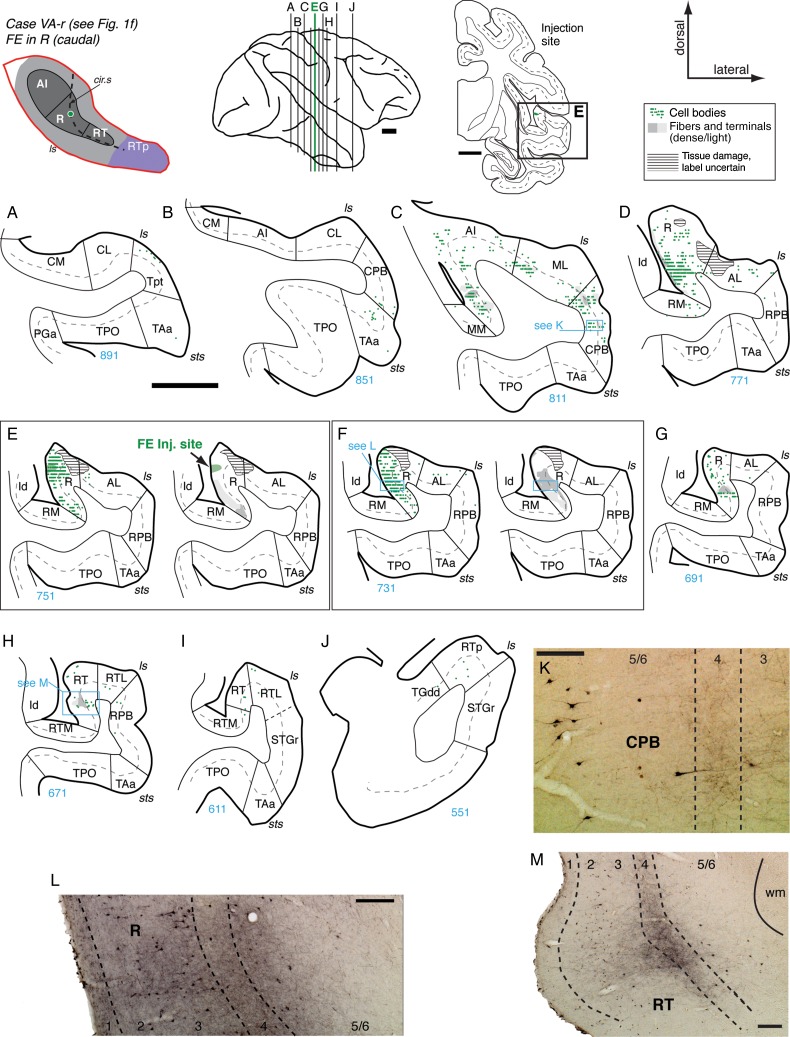
Figure 7.
Connections of area R in case VA-r. (A–J) Distribution of retrograde and anterograde label in the auditory-related areas after FE injection into the caudal portion of area R (section E; see also Fig. 1F). No cells or terminals were found in caudal core or belt (A and B). Labeled cells and patches of anterograde label were located in rostral AI, ML, and CPB (C and K). The strongest label was located within area R (D–F and L); for clarity, retrograde and anterograde label are displayed separately in panels E and F. Relatively few cells were found rostral to the injection site, predominantly within R (G). In RT, anterograde label was concentrated in layer 4 and deep layer 3 of central RT (section H and panel M). Only a few cells were found in RT and RTp (H–J). Scale bars = 5 mm in panels A–J, and 0.2 mm in K–M. For other conventions see Figure 4.
Figure 8.
Connections of area R in case OR-r. (A–L) Distribution of retrograde and anterograde label in the auditory-related areas after FE injection into the middle portion of area R (section G; see also Fig. 1G). Cells and terminals were found in caudal Tpt, parabelt, and TAa/TPO, but not in caudal core or belt (A–C). Both label was strong in rostral AI and ML (D and E), and in caudal R (F). Anterograde label was found in belt area AL and in RPB (F, G, and M). Rostral to the injection site, labeled cells and terminals were found throughout rostral R and area RT, and labeled terminals extended into RTM and RTL (H–J). A dense band of anterograde label was present through layer 4 of rostral RT, with lighter label extending through all layers (K and N). Terminal label was also present in a restricted patch of medial RTp, being densest within layer 4 (L and O). Scale bars = 5 mm in (A–L) and 0.2 mm in (M–O). For other conventions see Figure 4.
Surgery and Injections
Prior to each surgery, the animal was sedated with ketamine (10 mg/kg), intubated, and then maintained at a surgical level of anesthesia using isoflurane (1–4%, to effect). Body temperature was maintained with a heating pad, and the head was fixed in a head-holder. Vital signs (heart and respiration rate, temperature, oxygen saturation, and CO2) were monitored throughout the procedure, and intravenous fluids were provided. For the commissurotomy, unilateral bone and dural flaps were turned to expose the cerebral midline. With the aid of an operating microscope, the corpus callosum, hippocampal commissure, and anterior commissure were visualized and transected with a glass pipette. The dural flap was then replaced, the bone flap sewn in position, and the wound closed in anatomical layers. A prophylactic dose of analgesics and antibiotics was administered, and continued postoperatively in consultation with the facility veterinarian.
In a separate surgery (at least 3 months after the commissurotomy), anatomical tracers were injected by direct visualization of the STP, as follows. The fronto-temporal bone and dural flaps were turned to expose the length of the lateral sulcus and STG in one hemisphere. In 3 cases (VA, CC, SP), the banks of the lateral sulcus were carefully separated with fine forceps and a small glass pipette attached to a vacuum pump. This sulcal separation extended as far medially as the fundus of the inferior limb of the circular sulcus, with special care taken to avoid damaging the pial surface of the STP or compromising blood vessels bridging the lips and banks of the sulcus. Injections were placed at a depth of approximately 1.5 mm below the pial surface, using a Hamilton syringe with a 30-gauge needle for CTB and dextran tracers, or a 26-gauge needle for the more viscous fluorescent tracers (FB and DY). Injection sites on the STP were located in relation to gross anatomical landmarks, with the most rostral site (targeting RTp) located about 3 mm caudal to the temporal pole, and the RT, R, and AI sites spaced at intervals of about 5 mm (spacing was adjusted to avoid blood vessels). RT and R injections were placed medial to the lip of the circular sulcus when possible, as this typically corresponds to the auditory core region (Jones et al. 1995; Hackett et al. 1998a), whereas the more lateral sites on the STP itself correspond to the adjacent belt (RTL and AL). Injections targeting AI were placed caudal to the posterior end of the circular sulcus, near the center of the STP in the mediolateral dimension, often near a small annectant gyrus associated with the primary area (Jones et al. 1995). After completion of injections in one hemisphere, the dural flap was sutured, the bone flap sewn back into place, and the procedure repeated in the opposite hemisphere, allowing for as many as 10 injections per animal. After replacement of the second bone flap, the wound was closed in anatomical layers. Animals were treated with postoperative antibiotics, analgesics, and dexamethasone (0.5–1 mg/kg) to reduce brain swelling.
In the remaining 2 cases (BB-l and MQ-r), micro-electrocorticography (μECoG) arrays had been implanted on the surface of the STP. Across a series of chronic recording sessions, pure tone stimuli were presented to the passive awake monkeys (Fukushima et al. 2012, 2014). Tonotopic maps were constructed based on the local field potential responses across approximately 20 mm of the STP. After the conclusion of the recording experiments, tracer injection sites were chosen in relation to the tonotopic gradients. The surgical procedure for these animals differed from that described above in that injections were placed in only one hemisphere (as the forebrain commissures had not been cut), and the arrays on the surface of the STP were visualized by aspiration of the overlying tissue of the parietal operculum. The arrays are perforated by 0.5-mm diameter holes between the electrode contacts, spaced 1 mm apart (Fukushima et al. 2012, see their Fig. 1A). A Hamilton syringe, positioned in a stereotaxic manipulator for a vertical approach to the STP, was lowered into the cortex through selected holes in the arrays. After the injection of tracer, the needle was left in place for 10 min before being gradually withdrawn.
Histology
After a survival period of approximately 14 days (range: 13–16 days), animals were deeply anesthetized with pentobarbital and perfused transcardially with 0.5 L of saline, followed by 0.5 L of 1% paraformaldehyde and 8 L of 4% paraformaldehyde, both in 0.1 M phosphate buffer (pH 7.4) at room temperature. Brains were then removed from the skull, cryoprotected through a series of glycerols (Rosene et al. 1986), blocked in the coronal plane, and frozen in −80°C isopentane. Sections were cut in the coronal plane on a sliding microtome at a thickness of 40 μm, and sorted into 10 parallel series in each case. Two or three series were immediately mounted on gelatin-coated slides, air-dried, and coverslipped with DPX (Sigma-Aldrich) for the examination of fluorescent tracers (FB, DY, or CTB-488). Other series were processed immunohistochemically with the avidin/biotin immunoperoxidase method for CTB, FR, or FE labeling, or directly with the avidin/biotin method for BDA labeling (see below). Remaining series of sections were processed for thionine, acetylcholinesterase (AChE), or immunohistochemically with antibody against parvalbumin (PV) and a nonphosphorylated epitope of the neurofilament protein (recognized by the SMI-32 antibody). These latter stained sections were used to delineate the cyto- and chemoarchitectonic borders between cortical areas on the plotted sections (see below). The specificity and characterization of antibodies for tracers (CTB, FR, FE), PV, and SMI-32 are shown below.
Antibody Characterization
The antibodies against CTB, FR, and FE were raised against CTB subunit B (http://antibodyregistry.org/AB_10013220 [date last accessed; 6 November 2015]), tetramethylrhodamine (http://antibodyregistry.org/AB_1502299 [date last accessed; 6 November 2015]), and fluorescein, respectively, and the specificity of each antibody was determined by the manufacturer (see Table 4).
Table 4.
Antibodies used
| Antibody | Supplier and Catalog # | Type | Host | Dilution | Immunogen |
|---|---|---|---|---|---|
| Anti-CTB | List Biological #703 | Polyclonal | Goat | 1:3200 | B subunit (choleragenoid) |
| CTB secondary | Vector Labs BA-5000 | IgG (H + L) | Rabbit | 1:50 | Anti-goat |
| Anti-FR | Molecular Probes #A-6397 | IgG fraction | Rabbit | 1:4000 | Tetramethylrhodamine |
| Anti-FE | Molecular Probes #A-6413 | Polyclonal IgG Fab fragment | Rabbit | 1:4000 | Fluorescein |
| FR/FE secondary | Vector Labs BA-1000 | IgG (H + L) | Goat | 1:200 | Anti-rabbit |
| Anti-PV | Sigma #P3088 | Monoclonal IgG1 | Mouse | 1:2000 | PV from purified frog muscle |
| SMI-32 | Sternberger/Covance, #SMI-32R | Monoclonal IgG1 | Mouse | 1:4000 | Rat hypothalamus homogenate |
The anti-PV antibody (http://antibodyregistry.org/AB_477329 [date last accessed; 6 November 2015]) was raised against PV from purified frog muscle and was determined to be specific by immunoblotting (western blot) and to specifically stain the 12-kDa molecular-weight band identified as PV by Ca-binding (Sigma data sheet). PV is a calcium-binding protein associated with a subpopulation of inhibitory interneurons containing gamma-aminobutyric acid (Celio 1986; Hendry et al. 1989). Staining patterns in the current study accord with previous descriptions of staining patterns in the macaque cortex (Jones et al. 1995; Hackett et al. 1998a; Saleem et al. 2007; Saleem and Logothetis 2012).
The SMI-32 antibody (http://antibodyregistry.org/AB_509997 [date last accessed; 6 November 2015]) is specific for a nonphosphorylated epitope in neurofilaments. It was shown to be specific by immunoblot, where it recognizes a double band at 200 and 180 kDa, which merge into a single neurofilament H line on 2D blots (Sternberger and Sternberger 1983; Goldstein et al. 1987). This antibody has been shown to react with nonphosphorylated high-molecular-weight neurofilaments (200 kDa) of most mammalian species, including rats, cats, dogs, monkeys, and humans, and may also show some limited cross-reactivity with nonphosphorylated medium-molecular-weight neurofilaments (Covance/Sternberger). It has been shown to stain the cell bodies and dendrites of a subpopulation of predominantly pyramidal neurons, and the staining patterns in the current study accord with previous descriptions of staining patterns in the macaque cortex (Campbell and Morrison 1989; Saleem et al. 2007; Saleem and Logothetis 2012; Saunders et al. 2012).
Immunohistochemical Procedures
Immunohistochemical (IHC) staining was used to visualize CTB, FR, and FE. Although FR and FE do fluoresce (e.g., Fig. 1C,G), IHC staining provided greater sensitivity in identifying axon fibers and synaptic terminals labeled by anterograde transport.
To visualize CTB, sections were rinsed in phosphate-buffered saline (1X PBS, pH 7.4), washed for 30 min in 0.6% hydrogen peroxide (H2O2) to inhibit endogenous peroxides, washed in PBS, and then incubated for 2 h in blocking serum consisting of 0.3% Triton X-100, 2% bovine serum albumin, and 3.75% normal rabbit serum in PBS. Tissue was then incubated in the primary antibody solution (anti-CTB added to the blocking serum as shown in the previous step; see Table 4) for 60 h at 4° C with agitation. After several washes in PBS, sections were then incubated in the secondary antibody solution (biotinylated anti-goat IgG added to the same blocking serum solution described above; see Table 4) overnight at 4° C with agitation, followed by another wash in PBS. The sections were then processed with the avidin/biotin staining kit (Vector ABC Elite) for 90 min at room temperature, after which sections were washed in PBS and placed in a 0.025% solution of 3,3-diaminobenzidine tetra hydrochloride as chromogen (DAB; Sigma #D5637). After 10 min, approximately 0.0075% of H2O2 was added to initiate the staining reaction. The DAB reaction was stopped when satisfactory contrast was achieved (usually 1–3 min for CTB). After a final rinse in phosphate buffer, sections were mounted on gelatin-coated slides, air-dried, and dehydrated through ascending grades of ethanol concentrations before being cleared in xylenes and coverslipped in DPX.
The IHC process for FR and FE was similar to that for CTB with some modifications. Sections were rinsed in 0.05 M Tris-buffered saline (TBS, pH 7.6), quenched in 0.6% H2O2 for 10 min, then incubated for 1 h in normal blocking serum (as described above, but in TBS). Tissue was incubated in the primary antibody solution (anti-FR or anti-FE, see Table 4) for 3 days at 4° C with agitation. After rinsing in TBS, sections were incubated in the secondary antibody solution (as above, but in TBS; see Table 4) for 90 min at room temperature with agitation. After washing in TBS, the avidin/biotin reaction and DAB staining were carried out as described for CTB staining above.
To visualize PV, sections were rinsed (1X PBS, pH 7.4), quenched in 0.6% H2O2 for 60 min, washed in PBS, and then incubated for 2 h in blocking serum (see CTB staining protocol above). Tissue was incubated in the primary antibody solution (anti-PV, see Table 4) for 3 days at 4°C with agitation. After rinsing overnight in PBS at room temperature with agitation, sections were incubated in the secondary antibody solution for 90 min at room temperature with agitation (biotinylated goat anti-mouse IgG [H + L], Vector #BA-9200). Sections were then washed in PBS, and the avidin/biotin reaction and DAB staining were carried out as described above.
For SMI-32 staining, sections were rinsed in 0.05 M TBS (pH 7.6), quenched in 0.6% H2O2 for 10 min, washed in TBS again, and then incubated for 1 h in blocking serum (as described above, but in TBS). Tissue was incubated in the primary antibody solution (SMI-32, see Table 4) overnight at room temperature with agitation. After rinsing in TBS, sections were incubated in the secondary antibody solution for 60 min at room temperature with agitation (biotinylated goat anti-mouse IgG [H + L], Vector #BA-9200). Sections were then washed in TBS, and the avidin/biotin reaction and DAB staining were carried out as described above.
The BDA series was stained using a streptavidin horseradish peroxidase (HRP) procedure. Sections were washed in 0.05 M TBS (pH 7.6), quenched in H2O2 (0.3% for 30 min), washed in TBS again, then incubated in streptavidin-HRP (0.5 μg/mL; Molecular Probes) overnight at 4° C with agitation. Sections were then put through consecutive washes in TBS at pH 7.6, and then at pH 8.0, after which sections were placed in a 0.025% solution of 3,3-diaminobenzidine tetra hydrochloride (DAB; Sigma) at pH 8.0. The DAB reaction was stopped when satisfactory contrast was achieved (typically 1–3 min). In case SP, DAB staining was intensified with nickel ammonium sulfate to enhance contrast (Fig. 11).
AChE-positive fibers were visualized by a method following that of Tago (Tago et al. 1986; Turchi et al. 2005). To quench endogenous peroxidase activity, sections were placed in 0.1% H2O2 for 30 min and rinsed in 0.05 M maleate buffer (pH 6.0). Sections were then incubated for 90 min in a solution of 15 mg acetylthiocholine iodide, 0.75 mL of 0.1 M sodium citrate, 1.5 mL of 30 mM cupric sulfate, and 1.5 mL of 5 mM potassium ferricyanide in 200 mL of maleate buffer (pH 6.0). Sections were rinsed in 30 mM Tris buffer (pH 7.6) before a second incubation in a medium composed of 0.05 g of diaminobenzidine and 3.75 g of nickel ammonium sulfate in 125 mL of 30 mM Tris buffer (solution pH 6.4). After 10 min of incubation, 12 drops of 0.1% H2O2 were added to this solution, and sections remained in solution for 4–5 min (adjusted to effect) before a final rinsing in 3 mM Tris buffer. Sections were then mounted on gelatin-coated slides, air-dried, and dehydrated through an ascending series of ethanol concentrations before being placed in xylenes and coverslipped with DPX.
Data Analysis
Sections were examined with a Zeiss Imager Z.1 microscope, and images were captured by a Zeiss Axiocam MRc5 or MRm camera. Digital images were adjusted for brightness and contrast using Adobe Photoshop CS. In bright-field images, the background outside the pial surface was masked. Fluorescence images of cells labeled by DY (Fig. 5J,K) were further adjusted by selective color replacement: the color of an exemplary DY cell was replaced with a brighter yellow, and cells stained by FB (which is excited at the same wavelength) were masked by replacing their color with that of the background. Cortical laminae were drawn on photomicrographs by alignment to an adjacent section stained for thionine or SMI-32 (this is shown explicitly in Fig. 6M,N).
Figure 5.
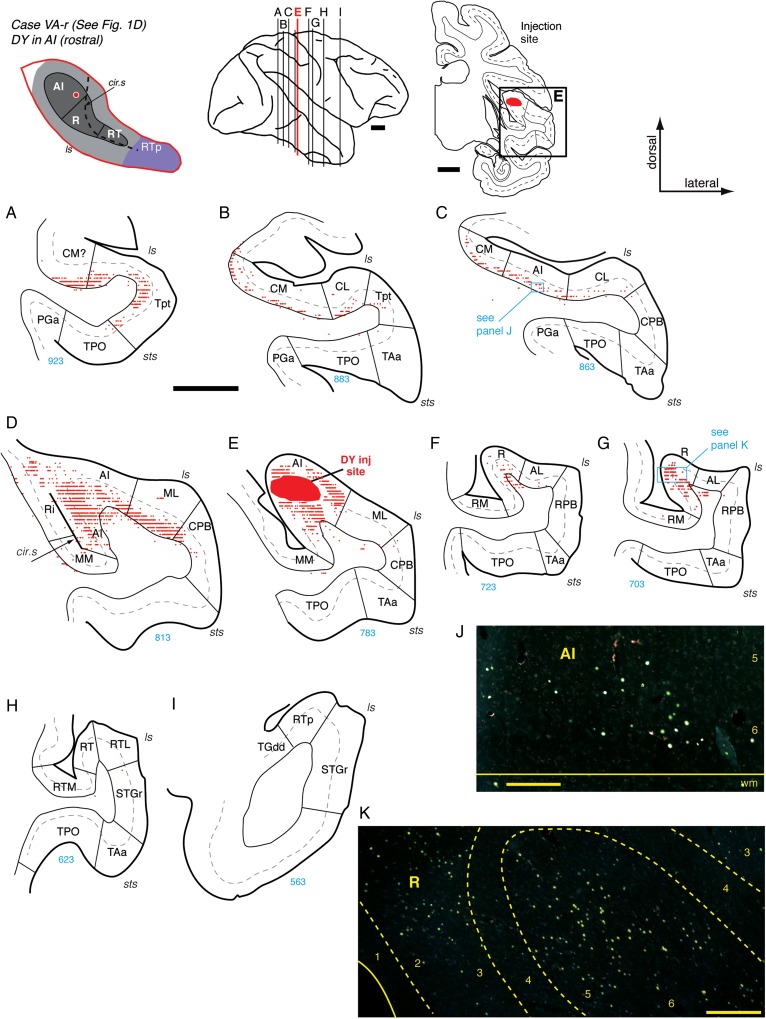
Connections of AI in case VA-r. (A–I) Distribution of labeled cells after injection of retrograde tracer DY into the rostral part of AI (section E; see also Fig. 1D). In this case, the temporal cortex at the level of AI is fused with the posterior insula in both hemispheres (panel 5D), a feature found in 15–20% of hemispheres in this macaque species (Barks et al. 2014). This injection resulted in extensive label through the infragranular layers of area Tpt and caudal belt (A and B), extending through AI and lateral belt (C, D, and J). Labeled cells were found in core area R (F, G, and K), but not in parabelt or in areas rostral to R (H and I). Scale bars = 5 mm in (A–I) and 0.2 mm in (J and K). For other conventions see Figure 4.
Sections were plotted at a sampling interval of 0.4 or 0.8 mm using either a Zeiss Axiophot microscope fitted with an MDplot digitizer and software (AccuStage, Shoreview, MN, USA) or a Zeiss Imager Z.1 fitted with the Neurolucida system (MBF Bioscience, Williston, VT, USA). The outline of the brain surface was traced, and the individual cells retrogradely labeled by different tracers were plotted. For the anterograde labeling, the individual fibers or terminals were not plotted; instead the distribution of labeling was outlined and represented with 2 or 3 relative thresholds of staining intensity (e.g., the shades of gray in Fig. 4). The plotted sections were exported to Adobe Illustrator CS, where the traced outline of the brain surface was aligned with digital images of corresponding adjacent sections stained for thionine and SMI-32. The border between the gray and white matter, cortical layer 4, and the outlines of subcortical structures were traced digitally. Borders between cortical areas were determined by examination of thionine, PV, AChE, and SMI-32 staining patterns (described below). When these borders were drawn on the plotted sections, all tracer label was hidden in the Adobe Illustrator file so as not to bias the placement of the borders. In all figures, the plotted sections were cropped to show only the relevant regions of the temporal lobe. Connections to other brain regions will be described in a separate report.
Figure 4.
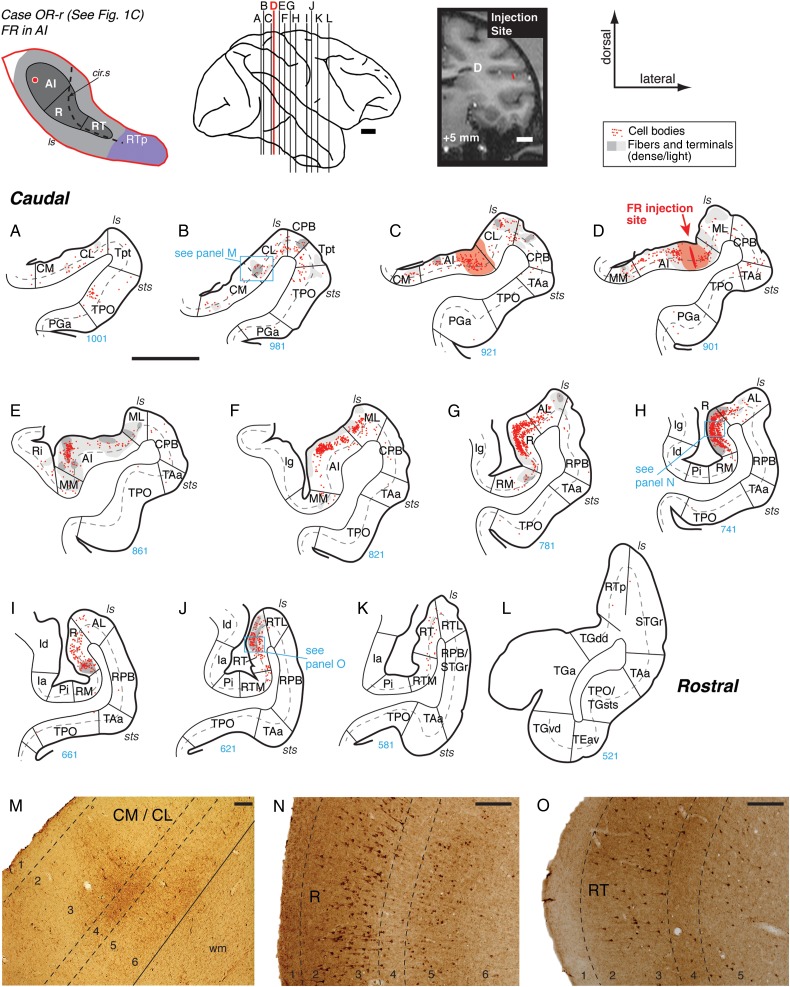
Connections of primary auditory area AI in case OR-r. (A–L) Distribution of labeled cells and terminals in the auditory and neighboring temporal cortical areas after a bidirectional tracer (FR) injection into caudolateral AI (section D; see also Figs 1C and 2B). The schematic diagram at the top left indicates the location of the AI injection site on the STP (same as in Fig. 1). Each red dot represents one cell labeled by retrograde transport of FR, and gray shading marks the fields of axonal fibers and synaptic terminals labeled by anterograde transport (dark and light gray indicate dense and light label, respectively). Red shading in (C and D) indicates the halo around the injection site where background staining was high and anterograde label could not be reliably identified (C and D). In this and other figures, only those portions of the coronal section are depicted that include the STP (core/belt/RTp), STG (parabelt/STGr), dorsal bank of the sts, temporal pole, and adjacent parts of the insula. The corresponding caudorostral location of each section is illustrated on the lateral view of the brain at the top center; the bold red line indicates the section in which the injection site was located (AP +5; arrow in D). The injection site is also indicated on the corresponding coronal MRI slice from the same animal at the top right. As in Figure 1, thick lines in A–L indicate the pial surface, thin lines indicate the border between gray and white matter, dashed gray lines indicate layer 4, and radial lines mark the boundaries between cortical areas. The section number is provided under each panel (in blue), allowing the distance between any 2 sections to be calculated from the section thickness of 40 µm. Blue boxes on plotted sections indicate corresponding photomicrographs at the bottom of the figure (e.g., label in section B is illustrated in panel M). This injection identified reciprocal connectivity with the belt areas caudal and lateral to AI (panels A–F), as well as weaker connections with medial belt and caudal parabelt area CPB (B–E and M). The strongest connection of AI was with area R, where cells and terminal label were dense throughout the supragranular layers and layer 5 (G, H, and N). A similar but weaker distribution of filled cells was evident in the caudal portion of area RT (J and O). No significant label was observed in RTp. See Table 2 for area and sulcus designations. Scale bars = 5 mm in panels A–L and in the lateral brain view and coronal MRI at the top, and 0.2 mm in panels M–O.
To summarize the strength of connections, retrogradely labeled cells were counted in both supragranular and infragranular layers of each cortical area (Table 1, left panel). All plotted sections were examined, not only those selected for inclusion in figures. The highest cell count in any one section was used to assign a connection strength of 1, 2, or 3 asterisks, corresponding to a sparse, moderate, or dense connection (a minimum of 5, 15, or 30 labeled cells), respectively. To best capture the relative connection strength among areas and across cases, this scale was adjusted to account for weak retrograde labeling by BDA (1, 5, or 15 cells in case BB-l, and 3, 10, or 20 cells in SP-r) and the higher sensitivity of FB or CTB (15, 50, or 100 cells in VA-r, CC-l, and MQ-r). Anterograde label in the supragranular, middle, and infragranular layers was categorized as a sparse, moderate, or dense connection by subjective visual criteria (Table 1, right panel).
Table 1.
Tracer injection summary
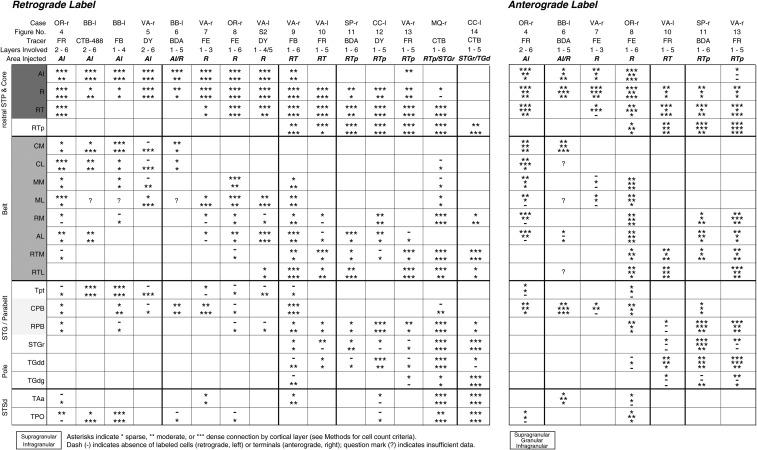 |
Results
Fourteen tracer injections were placed in the core auditory cortex and rostral STP, and a 15th injection was placed in the STGr (Fig. 1). Interpretation of connectional data based on these injections hinges on accurate delineation of cortical areal boundaries in and around the STP. In the first section, we describe and differentiate the auditory cortical areas using SMI-32 immunostaining in relation to previously established cyto- and chemoarchitectonic criteria. In the second section, we describe the connectivity of the different core areas (AI, R, RT) and rostral STP (area RTp).
Part 1: Cyto- and Chemoarchitectonic Delineation of Auditory Areas
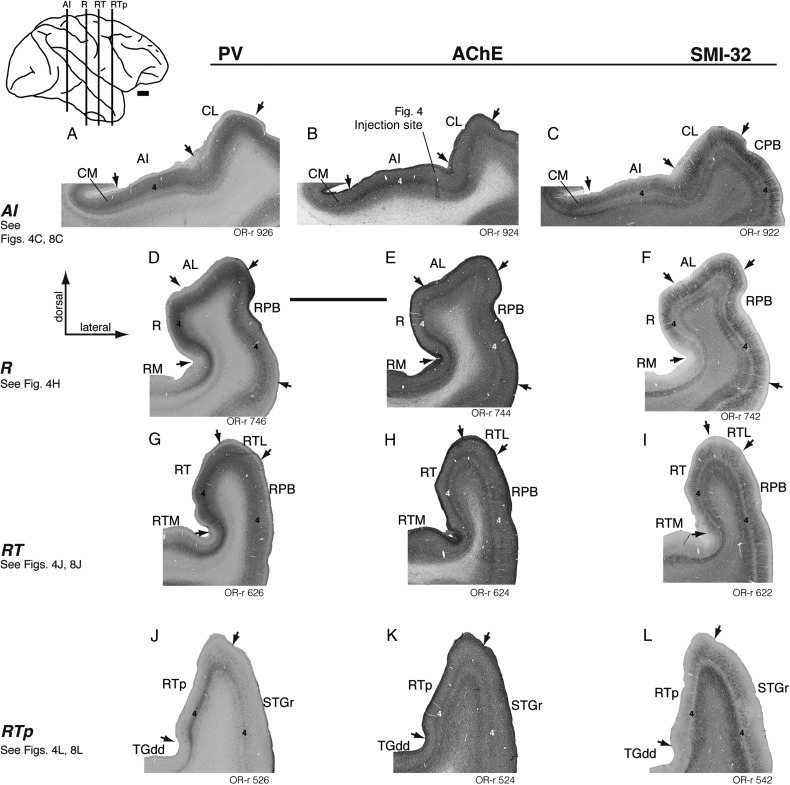
The divisions of auditory cortex can be distinguished by cytoarchitectonic criteria and neurochemical markers (Jones et al. 1995). The granular cytoarchitecture of the auditory core has long been recognized (Brodmann 1905; Walker 1937; Pandya and Sanides 1973; Galaburda and Pandya 1983), and this region of koniocortex is co-extensive with dense staining for AChE, PV, and the metabolic marker cytochrome oxidase (Morel et al. 1993; Jones et al. 1995; Hackett et al. 1998a, 2001). In the present study, the density of AChE staining, the immunoreactivity of the neuropil for PV, and the cytoarchitectonic organization of the cortex were generally consistent with prior descriptions (Fig. 2) and served to indicate the transitions between auditory core, belt, and parabelt. As schematized in Figure 1, the elongated core region occupies the center of the caudal STP and extends rostrally, tapering as it descends ventromedially into the circular sulcus. At the level of the caudal core, the STP is wide and flat, and AI is evident as a thickened band of staining for PV and AChE in layer 4 and the lower part of layer 3 (Fig. 2A,B). The same staining is evident rostral to AI in core area R, which occupies the medial portion of the STP and twists rostrally onto the lateral wall of the circular sulcus (Fig. 2D–F). Rostral to AI and R, the density of PV and AChE staining wanes and their extent narrows, marking the transition into the third and smallest core region, RT (Hackett et al. 1998a). Rostral to the core, area RTp is evident as a weakened extension of the PV immunoreactivity in RT, unaccompanied by denser AChE reactivity that distinguishes the core (Fig. 2J,K). The intensity of staining for all of these markers decreases with each transition from core, to belt, to parabelt (Hackett et al. 1998a), and is weakest in STGr and the dorsal temporal pole (Jones et al. 1995).
Figure 2.
Regions of auditory cortex as distinguished by staining for parvalbumin (PV, left), acetylcholinesterase (AChE, center), and SMI-32 (right) in case OR-r. (Tracer injections in this case are presented in Figs 4 and 8; a needle track in AI is evident in panel B.) In each column of Figure 2, a representative coronal section is shown at the level of AI, R, RT, and RTp. The caudorostral level of these sections is indicated on the lateral view of the brain inset at the top left. Arrows mark architectonic boundaries between areas as determined from a consensus of the 3 stains, and the standard thionine stain (not shown). Layer 4 is indicated in the core and STG regions of each section. Both PV and AChE distinguish core auditory cortex (AI and R) by dense staining of the neuropil in the middle layers (panels A, B, D, and E). The intensity of staining tapers off in the belt regions medially and laterally. For example, at the level of area R (second row) staining in lateral belt area AL is often only slightly lighter than that in the core, whereas the difference between core and medial belt area RM is more stark (panels D and E). Area RT is considered a core region in part because of its dense immunoreactivity to PV, but AChE does not distinguish this area as well as it does the more caudal core (panels G and H). Both PV and AChE staining are progressively weaker rostral to the core; although PV still serves to define RTp, AChE is effectively uniform across the cortex at this level (panels J and K). The delineation of auditory areas based on SMI-32 staining is described in detail in the main text and (for another case) in Figure 3. Scale bars = 5 mm.
Within each coronal section, SMI-32 reactivity provided an additional criterion for distinguishing cortical areas, often with greater clarity than that provided by AChE or PV (Fig. 2, right column). Prior surveys of SMI-32 reactivity in the macaque cortex discussed the auditory areas only briefly (Lewis and Van Essen 2000; Saleem and Logothetis 2012), or describe only 1 or 2 areas in detail (Campbell and Morrison 1989; Saleem et al. 2007). Here we provide the first comprehensive description of SMI-32 staining in the auditory areas of the macaque temporal lobe (see next section and Fig. 3).
Figure 3.
Patterns of SMI-32 immunoreactivity distinguish different auditory areas of the superior temporal cortex in case MQ. Each row of photomicrographs is from a single coronal section (rectangles on the left), highlighting changes in the laminar distribution and cytoarchitecture of SMI-32-labeled pyramidal neurons between (from left to right) medial belt, core, lateral belt, and parabelt, arrayed from caudal (top) to rostral (bottom). There is no core region at the level of the first row; in panel B, a high-magnification image of AI (from panel F) highlights the radial structure of the long apical dendrites among layer 3 pyramidal neurons in the core. Note that the core/belt classifications do not strictly apply in the last row (Q–T). Rows A–H are from the left hemisphere (reversed for consistency), and rows I–T are from the right. The asterisk in panels R and S indicates the CTB injection site in RTp, near the border of STGr, in case MQ-r. Scale bar = 0.5 mm in A (applies to C–T); 0.125 mm in B.
Core Areas
In sections stained with the SMI-32 antibody, areas AI and R are characterized by a moderate to strong concentration of labeled pyramidal neurons and their dendrites in layer 3, as well as lighter, distinct bands in layers 5 and 6 (Fig. 3F,J). Cells in layers 3 and 5 are small and densely packed, with radially oriented apical processes (Fig. 3B). In area RT, layer 6 is marked only by a light band of neuropil staining that is contiguous with the band in layer 5 (Fig. 3N); the 3 distinct bands in layers 3, 5, and 6 of AI are no longer apparent in RT (compare Fig. 3N with 3F). The dominant feature of SMI-32 immunoreactivity in AI and R is the dense staining of pyramidal neurons in layer 3, with lighter bands in layers 5 and 6; in contrast, the relative intensities of the supra- and infragranular staining are equivalent in RT. It should be noted that the case in Figure 3 (MQ-r) is one in which a tonotopic map was obtained (Fukushima et al. 2012, 2014), confirming that the observed shifts in cytoarchitecture within the core correspond to reversals in the tonotopic gradient along the caudorostral axis.
Belt and Parabelt Areas
The auditory belt regions surround the core on its caudal, lateral, and medial aspects, and each belt area derives its name from its location relative to the adjacent core area (Fig. 1). The caudolateral (CL) and caudomedial (CM) belt areas flank caudal AI; these fields are contiguous and share relatively similar cytoarchitecture (discussed below). The middle lateral (ML) and middle medial (MM) belt areas lie lateral and medial to AI, respectively. Similarly, the anterolateral (AL) and rostromedial (RM) belt areas flank R, and the rostrotemporal-lateral (RTL) and rostrotemporal-medial (RTM) areas flank RT. Lateral to the belt, the parabelt extends ventrally onto the STG. The border between the caudal parabelt (CPB) and the rostral parabelt (RPB) is indistinct, but, roughly speaking, CPB abuts CL and ML, and RPB abuts AL and RTL (Fig. 1).
In thionine-stained coronal sections, the transition from AI to caudal belt (CM or CL) is marked by a diminished granular layer 4, and larger and more sparsely distributed cell bodies in layer 3, where the columnar structure visible in layers 2/3 of the core is reduced (data not shown; see also Jones et al. 1995; Hackett et al. 1998a). Accordingly, in SMI-32-stained sections CM and CL are characterized by larger and more widely spaced cells in layer 3, with a less well-organized radial structure among their dendritic processes than is seen in the core (Fig. 3E,F,G). Apical dendrites in CM are shorter and more lightly stained than those in AI; apical dendrites in CL stain darkly but are short and convoluted (Fig. 3C,G), as opposed to the long, straight, thin processes in AI. As in layer 3, the stained processes in layer 6 of both CM and CL are less radially oriented than they are in the core.
Caudal to the belt and parabelt, the temporo-parietal area (Tpt) occupies the most posterior portion of the STP and STG near the terminus of the lateral sulcus (Fig. 1). Tpt is distinguished from CM and CL by a more columnar cytoarchitecture, with larger pyramidal neurons in layer 5 (Galaburda and Pandya 1983; their Fig. 4) and less PV immunoreactivity in layers 3 and 4 (Smiley et al. 2007). In SMI-32, layer 3 pyramidal neurons in Tpt lack the long apical dendrites and strong radial structure of CPB (Fig. 3D,H), and staining deep to layer 3 is relatively weak.
Farther rostral at the level of AI, belt areas MM and ML were not notably different from CM and CL, respectively, in their SMI-32 immunoreactivity, and thus can be distinguished from AI by the same criteria described above. Lateral to CL or ML, near the lip of the lateral sulcus, the transition to CPB is indicated by a strong radial columnar structure in layers 3, 5, and 6 (Fig. 3H); in this regard CPB is similar to AI, but pyramidal cells within parabelt are larger and more widely spaced than those in the core. Moving ventrally along the STG, this radial structure breaks down at the transition to area TAa, near the dorsal lip of the superior temporal sulcus (sts). The multisensory areas of the dorsal bank of the sts (Baylis et al. 1987) were delineated into TAa, TPO, and PGa by previously established criteria (Seltzer and Pandya 1978; Smiley et al. 2007; Saleem and Logothetis 2012).
At the level of area R, SMI-32 staining distinguishes medial belt area RM by a thin layer of darkly stained neuropil in the infragranular layers, and a relative absence of stained pyramidal cells in layer 3 (Fig. 3I). The transition from RM to R is marked by the emergence of dark staining in the neuropil and sparse stained cells in layer 3 (typically near the lateral edge of the floor of the insula; see Figs 2F and 3I). The transition from R to AL is much like that between core and lateral belt at the level of AI: the long radially oriented apical dendrites in R are shorter and less clearly organized in AL, and the cell bodies less densely packed (Fig. 3J,K). At the level of RT, the borders of RTM and RTL with the core are distinguished by the same criteria as those described for RM and AL, respectively (Fig. 3M,N,O), though staining of layer 3 pyramidal cells in RTM is slightly weaker than that in RM (Fig. 3I,M). Note that the patterns of SMI-32 reactivity in belt areas CM and CL are quite similar (Fig. 3E,G), but the medial and lateral belt areas flanking the rostral core (RM and AL) are distinct in their architecture, particularly in the degree of immunoreactive pyramidal neurons within layer 3 (Fig. 3I,K). Laterally, the transition from AL or RTL to RPB is similar to that between CL and CPB, as long straight dendrites and radial structure emerge on the surface of the STG (Fig. 3L,P) and continue as far ventral as the border of TAa. Immunoreactivity for SMI-32 does not indicate a clear distinction between CPB and RPB, as was also the case with other cytoarchitectonic and neurochemical markers (Hackett et al. 1998a), confirming a structural homogeneity along the caudal-rostral extent of the parabelt region.
Rostral Superior Temporal Cortex
Area RTp was first identified as a continuation of weak PV immunoreactivity rostral to area RT on the superior aspect of the rostral STP (Fig. 2J; Saleem et al. 2007; Saleem and Logothetis 2012). The overall intensity of SMI-32 staining is lighter in RTp than in RT, and the laminar balance relative to the caudal core is reversed: cells in layer 3 are labeled only sparsely, and the predominant feature is a dark band in layer 5 that is continuous with lighter staining extending into layer 6 (Figs 2L and 3R; see also Saleem et al. 2007). Medial to RTp, the transition to the dysgranular portion of the dorsal temporal pole (area TGdd) is evident in lighter SMI-32 immunoreactivity in the neuropil of layers 3 and 5; stained pyramidal cells in TGdd are largely restricted to the upper part of layer 5 (Fig. 3Q; Saleem et al. 2007, their Fig. 8). As in the core/belt transition, the processes of stained pyramidal cells in RTp are more radially oriented than those in TGdd. Lateral to RTp, the border of the STGr is marked by a zone of transition with features similar to those seen in lateral belt areas more caudally. The apical dendrites of layer 3 cells in the STGr stain darkly but are short, convoluted, and more widely spaced than they are in RTp (Fig. 3R,S,T). Because of these similar features, the border between the lateral belt and parabelt/STGr is sometimes difficult to discern around the caudorostral level of RTL and is indicated by dashed lines where necessary (e.g., Figs 7I, 9H and 13G). The STGr is distinguished from caudally adjacent RPB by a strong reduction in immunostained pyramidal neurons in layer 3, and an absence of the strong radial structure evident in apical dendrites of the parabelt (Fig. 2, right column; compare Fig. 3T with 3P). This transition typically occurs in coronal sections around the level of the amygdala. At the temporal pole, rostral to RTp and STGr, thionine staining suffices to distinguish TGdd from the laterally adjacent granular area TGdg, which do not differ appreciably in SMI-32 staining.
Figure 9.
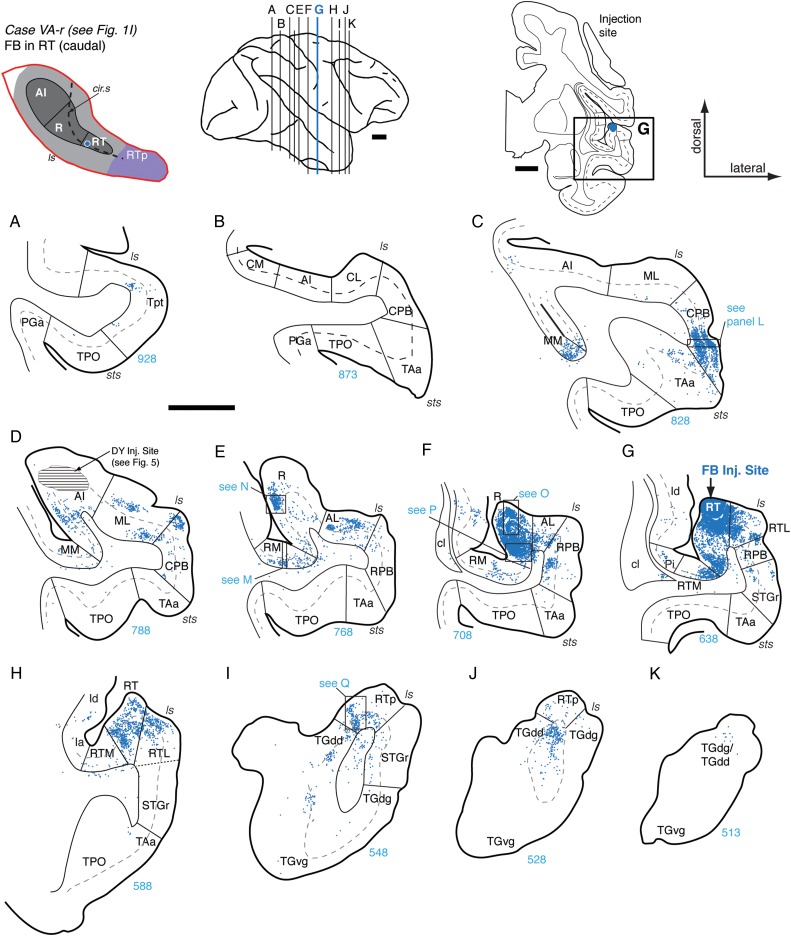
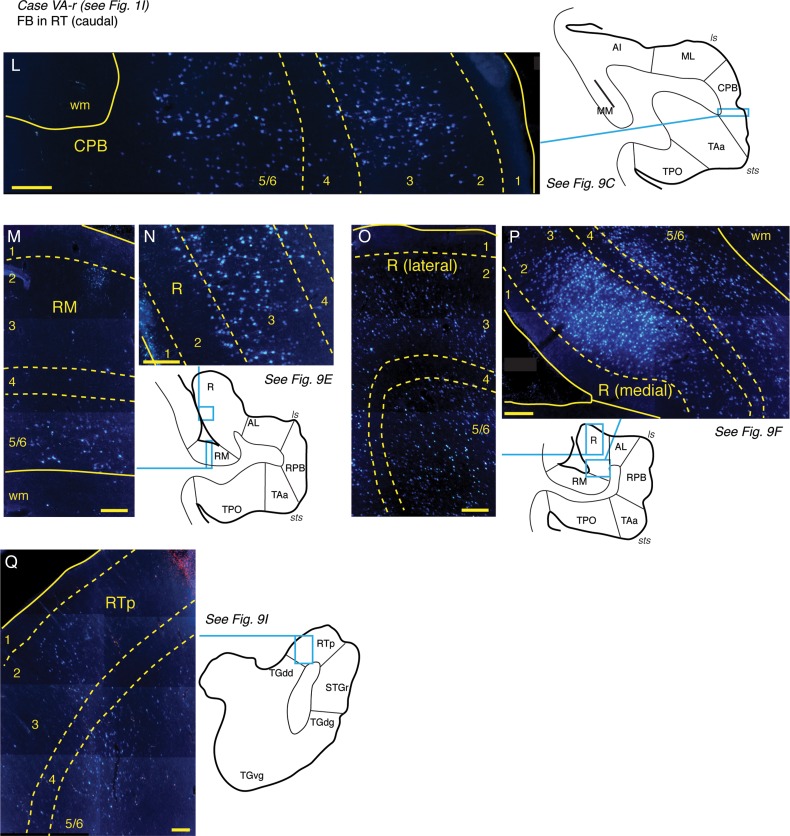
Connections of area RT in case VA-r. (A–K) Distribution of labeled cells in the STP, STG, and temporal pole after FB injection into the caudal part of area RT (section G; see also Fig. 1I). Labeled cells were observed in Tpt (A) and in medial belt area MM (C), though none were observed in caudal belt areas CM, CL, or caudal AI (B). A moderate to dense concentration of cells was observed in the ventral portion of the CPB (section C and panel L), rostral AI and the adjacent lateral belt (D and E), caudal R (E, and panel N), and area RM (E, and panel M). The strongest concentration of label was within rostral R (F), particularly in the medial portion (see panels O and P). Cells were located in the belt areas medial and lateral to the injection site, extending into the RPB and STGr (F and G). Cells were found throughout the more rostral portion of RT and RTL (H), and RTp, with some label in the dorsal temporal pole areas TGdd/TGdg, and STGr (sections I, J, K, and panel Q). A sparse concentration of cells was present in the dorsal temporal pole (K). Scale bars = 5 mm in (A–K) and 0.2 mm in (L–Q). For other conventions see Figure 4.
Figure 13.
Connections of area RTp in case VA-r. (A–J) Distribution of retrograde and anterograde label in the STP, STG, and temporal pole areas after FR injection into the rostral part of area RTp (section H; see also Fig. 1O). Filled cells and terminal label were found throughout area RT (E–G and K, L), as well as RTL and RTM (F and G), though more caudal regions of the medial belt contained primarily anterograde label (RM in section D; RTM in section E). Filled cells and moderate anterograde label were also found within RPB lateral to RT (E and F). Rostral to the injection, both anterograde and retrograde label were found in TGdd and TGdg (I and J). Scale bars = 5 mm in (A–J) and 0.2 mm in (K and L). For other conventions see Figure 4.
Part 2: Connectivity of the Core and Rostral Supratemporal Plane
Connections of AI
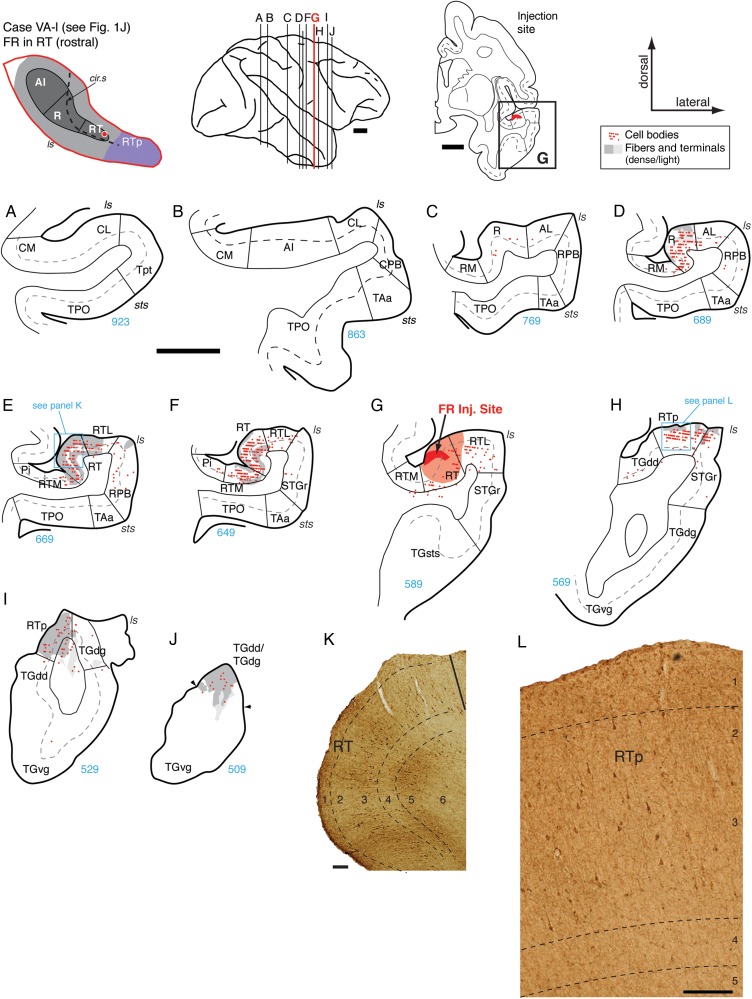
Data from 4 tracer injections into core area AI were analyzed (Fig. 1A–D; see the left 4 columns in Table 1; this table also indicates the laminar involvement of all injection sites). Injections into AI showed strong local connectivity with its adjacent belt areas and a focused connection with the rostral core areas. The constellation of connections of AI is exemplified in the flagship case OR-r, in which a bidirectional tracer (FR) was placed into the caudolateral part of AI (Figs 1C, 2B, and 4). Caudal to the injection, both retrogradely labeled cells and anterogradely labeled axonal varicosities (terminals) were found in the caudal belt areas CM and CL on the STP, in Tpt and CPB on the STG, and in area TPO within the dorsal bank of the superior temporal sulcus (STSd; Fig. 4A–C,M). In these caudal belt and parabelt areas, both types of label were found in the supra- and infragranular layers, but the cells were predominantly distributed in the infragranular layers 5 and 6 (see also the AI case in Fig. 5A–C, discussed below). Both retrograde and anterograde label were dense within area AI, particularly in the supragranular layers (Fig. 4C–F). Bidirectional connectivity with the belt fields flanking AI was evident in the overlapping distribution of filled cells and labeled terminals in ML and, to a lesser extent, in MM (Fig. 4D–F). Rostral to the injection, a very strong connection was evident with core area R, where filled cells and labeled terminals formed a dense bi-laminar distribution in layers 2/3 and 5 (Fig. 4G,H,N). Labeled cells and terminals were present in the belt areas flanking caudal R, with more label in AL than in RM (Fig. 4G,H). Rostral to R, a moderately strong distribution of retrograde and anterograde label was evident in the caudal portion of RT (Fig. 4J,K). As in R, filled cells were found in layers 2/3 and 5, but the distribution of anterograde label was different from that in R: fibers and terminals extended from layers 2/3 into layer 4 (compare layer 4 in Fig. 4N,O). Very little label was observed in the belt fields flanking RT (RTM/RTL; Fig. 4J,K) or at the level of RTp (Fig. 4L).
Three additional injections of retrograde tracer in AI (Fig. 1A,B,D) confirmed a pattern of inputs primarily from adjacent areas of the belt, and a restricted connection to core area R and belt area AL as shown above. An injection of DY into rostral AI (Figs 1D and 5) labeled cells in the infragranular layers of areas Tpt, CM, CL, and caudal AI (Fig. 5A–C,J). Very few cells were found in the CPB or TPO (Fig. 5A,D,E). Immediately caudal to the injection site, filled cells were dense within AI and in the infragranular layers of lateral belt area ML (Fig. 5D). Rostral to the injection site, labeled cells were confined to the core area R, where they were found throughout layers 2/3 and 5/6 (Fig. 5F,G,K). Two additional retrograde injections in central and rostral AI (Fig. 1A,B; data not shown but see Table 1) produced a pattern similar to that in Figure 5, with 2 exceptions: Labeled cells in caudal belt areas CM and CL and in Tpt (both the planar and gyral regions) were not exclusively within the infragranular layers, and some labeled cells were found in area TPO.
Connections of R
Four cases were examined for the connections of core area R (Table 1). In the first case, a BDA injection was located at the border between AI and R (Figs 1E and 6). In 2 of the cases, injections of a bidirectional tracer (FE) were localized in the caudal and middle regions of R, and in the last case, a retrograde tracer (DY) was localized in the rostral part of R (Figs 1F–H, 7 and 8, and Supplementary Fig. 2).
The BDA injection at the AI/R border revealed reciprocal connectivity with caudal core, belt, and parabelt areas, as well as anterograde projections to rostral core and lateral belt. Most filled cells were found caudal to the injection, particularly in the adjacent region of AI (Fig. 6C) and a few distinct clusters in CM (Fig. 6A). Anterograde labeling was typically co-localized with filled cells, indicating that these connections with caudal areas were reciprocal. Filled cells in CM and caudal AI were predominantly found in layer 3, whereas patches of anterograde label extended from layers 3 to 6 (Fig. 6A,B,L). A similar result was evident in CM/CL following the injection in caudal AI, as described above (Fig. 4M). However, the laminar location of filled cells differed from the pattern seen in the rostral AI injection case, in which labeled cells caudal to the injection were found almost exclusively in the infragranular layers (Fig. 5A–C). Although the BDA injection site was apparently confined to the core (Fig. 6D), clear label was present in the CPB, including patches of fiber and terminal stain across layers 3, 4, and 5 (Fig. 6C,M). Retrograde label was sparse rostral to the injection, but focused patches of anterograde label targeted areas R and, to a lesser extent, AL; although light anterograde label is seen through all layers in area R, staining was densest within layer 4 (Fig. 6G,H,N).
The FE injection in the caudal part of R (Fig. 7) showed reciprocal connections with the rostral part of AI, the CPB, and a predominantly anterograde projection to area RT. Labeled cells were located throughout the mediolateral extent of rostral AI in both supra- and infragranular layers, but a few patches of labeled terminals were mainly concentrated in the medial part of this core area, with similar laminar distribution (Fig. 7C). Labeled cells were found in the infragranular layers of lateral belt area ML, near the AI border, but almost no label was evident in any part of the medial belt area MM (Fig. 7C). In caudal parabelt area CPB, anterograde label was mainly distributed in layers 3 and 4, with filled cells in both supra- and infragranular layers, particularly in layer 6 (Fig. 7C,K). No label was observed in the rostral parabelt area RPB lateral to the injection (Fig. 7D–H) or in more rostral area STGr (Fig. 7I,J), but a few scattered cells were found in the caudal STG area Tpt (Fig. 7A). Labeled cells and fibers were predominantly located within area R, where filled cells were distributed through both the supra- and infragranular layers (Fig. 7D–F) but were particularly numerous in layer 3 (Fig. 7L). Immediately caudal and medial to the injection, anterograde label was confined to the superficial layers (Fig. 7D,E), but, rostral to the injection, this label extended from layer 1 through layer 5 (Fig. 7F,L). Filled cells were sparse in the areas rostral to R (Fig. 7H,I,J), but a concentrated patch of anterograde label was localized to layer 4 and the lower part of layer 3 in area RT (Fig. 7H,M). This rostrally directed projection focused on layer 4 is similar to that resulting from the injection at the AI-R border (Fig. 6), but terminal label in that case did not extend past area R; by contrast, label in this injection into caudal R extended into RT.
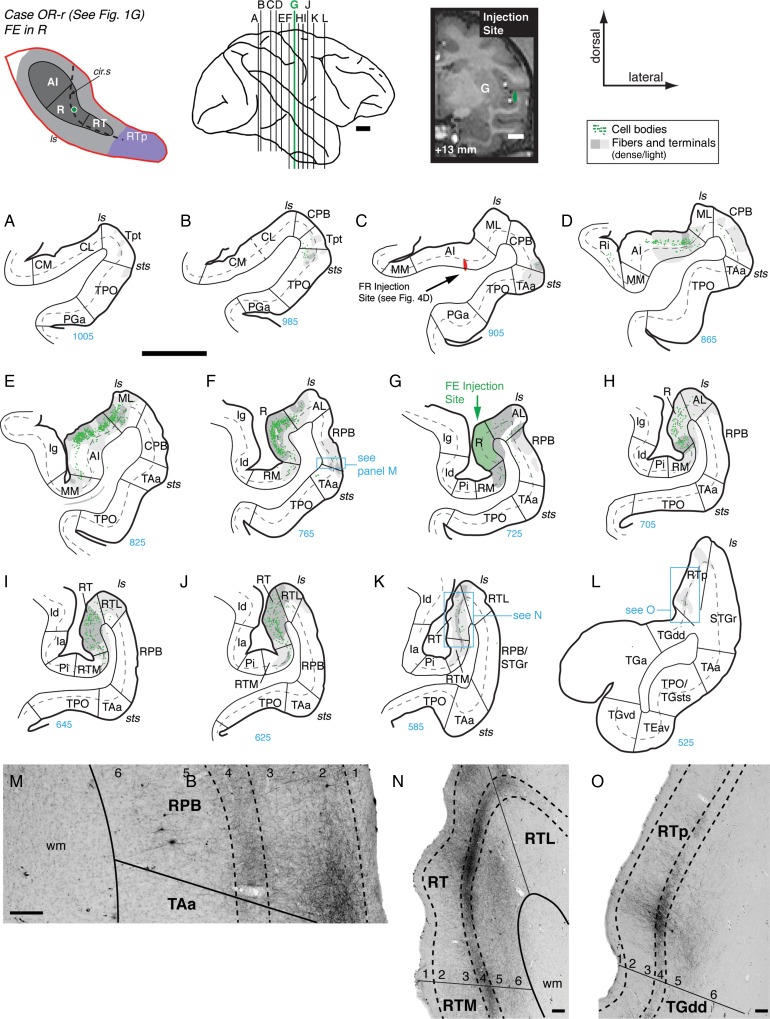
The extent of the connections identified in the preceding case may have been limited by the small size of the injection, which involved primarily the supragranular layers (Figs 1F and 7E). A larger but well-localized injection of FE into the middle of area R in case OR-r (Fig. 1G) confirmed the connections in the previous case and also revealed additional connections to the medial belt, RPB, and area RTp (Fig. 8). Caudal to the injection, a few retrogradely filled cells and modest terminal labeling were found in Tpt, CPB, and area TPO near the lip of the sts, but none in caudal belt or caudal AI (Fig. 8A–C). Filled cells were most dense within the supragranular layers of rostral AI, within R, and lateral belt area ML, and were largely co-extensive with the anterograde terminals (Fig. 8D–F). Terminal label was strong in the belt areas flanking R (AL and RM), though filled cells were less numerous than they were within the core (Fig. 8F–H). In the parabelt, anterograde label was present in the supragranular layers of CPB, and a sparse array of filled cells was found primarily in the infragranular layers (Fig. 8C,D). Anterograde label was also found in RPB (predominantly in the supragranular layers), including a small dense patch of fibers and terminals in layers 2/3 and a weaker band of terminals in layer 4 (Fig. 8F,G,M). Rostral to the injection, labeled cells were largely confined to core areas R and RT (in both the supragranular and infragranular layers), with a few filled cells in the infragranular layers of the belt areas RTM and RTL (Fig. 8I,J). Anterograde label was found through all layers of area RT, forming a particularly dense band within layer 4 across the full mediolateral extent of the core (Fig. 8K,N). In contrast to the previous 2 cases, anterograde label in this case extended beyond RT to include a small patch within medial RTp (Fig. 8L,O). Labeled fibers and terminals in RTp spanned layers 2/3 to 5 but were densest within layer 4 (Fig. 8O).
An injection of DY into the rostral part of area R (Supplementary Fig. 2) largely replicated the cases above. Filled cells were found in rostral AI, lateral belt area ML, and Tpt, predominantly in the infragranular layers (Supplementary Fig. 2A,C,D). Labeled cells were found in all layers of area R immediately caudal to the injection site, and in lateral belt area AL (Supplementary Fig. 2E,F). Rostral to the injection, cells were found only within core area RT, in both the supra- and infragranular layers (Supplementary Fig. 2H,I,K). The overall pattern of connectivity was similar in AI and R, in that each area was widely interconnected with core and belt areas caudal to the injection, but more selectively connected with the core region rostral to the injection. In addition, injections in AI and R identified limited direct projections to the parabelt.
Connections of RT
Two injection cases were analyzed for the connections of area RT (Table 1). In the first case, the retrograde tracer FB was centered at the caudolateral part of RT close to the RTL border (Figs 1I and 9), and in the second case, a bidirectional tracer FR was localized at the rostromedial part of RT (Figs 1J and 10). The distribution of labeling after RT injections shifted rostrally to include areas in the anterior STP, temporal pole, and a broader array of belt and parabelt areas (Figs 9 and 10).
Figure 10.
Connections of area RT in case VA-l. (A–J) Distribution of both retro- and anterograde labeling in the STP, STG, and temporal pole areas after FR injection into the rostral part of area RT (section G; see also Fig. 1J). No label was observed in AI, caudal belt, or Tpt (A and B) and only a few filled cells were found in caudal R (C). Cells and terminals were observed in rostral R (D), near the border with RT. Label was most dense within RT caudal to the injection site (E, F, and K). Labeled cells and fibers were dense in RTL and RTM (E and F), but sparse in RPB (D and E). Label rostral to the injection was restricted to area RTp (H, I, and panel L) and the dorsal temporal pole (J). Scale bars = 5 mm in (A–) and 0.2 mm in (K and L). For other conventions see Figure 4.
The injection of FB into the caudolateral part of RT produced a localized cluster of labeled cells in the infragranular layers of Tpt and in all layers of medial belt area MM (Fig. 9A,C). A strong concentration of labeled cells was present in the ventral portion of the CPB, particularly in layers 3 and 5/6, with some spillover into area TAa at the dorsal lip of the sts (Fig. 9C,L). Cells in AI were restricted to its rostral portion closest to R and the adjacent lateral belt area ML (Fig. 9D). In the caudal extent of area R, label was particularly strong in layer 3 (Fig. 9E,N), whereas cells in the adjacent medial belt area RM were clustered in layers 5/6 (Fig. 9E,M). Labeled cells were also located in the lateral belt area AL but tapered off at the transition into RPB (Fig. 9E). The strongest concentration of labeled cells was within rostral R (Fig. 9F), where cells were found throughout layers 2/3 and 5/6, particularly in the medial region (Fig. 9O,P). Labeled cells were located in the belt areas medial and lateral to the injection site (Fig. 9G), extending into the RPB and STGr (Fig. 9F,G). Rostral to the injection site, cells were found within the rostral part of RT and lateral belt area RTL, but only a few in medial belt area RTM. (Fig. 9H). In addition, a dense concentration of cells was observed in RTp and its flanking fields in the dorsal temporal pole (TGdd) and STGr (Fig. 9I–K,Q). Labeling was found in both the supra- and infragranular layers in these anterior cortical areas.
An injection of FR into the rostromedial portion of RT (Fig. 10) revealed both anterograde and retrograde transport extending rostrally to RTp and the temporal pole but produced no label in any area caudal to R (unlike the injection in caudal RT described above; compare the 2 RT cases in Table 1). Filled cells and patches of labeled terminals were localized to the most rostral portion of R, adjacent to RT, with only a few filled cells in caudal R or AL (Fig. 10C,D). Within the more caudal region of RT, labeled cells extended throughout layers 2/3 and 5, and moderate to dense patches of anterograde label were present throughout all layers, but were generally strongest in layers 1–3 and 5 (Fig. 10E,F,K). Both cells and terminals extended to adjacent lateral and medial belt areas RTL and RTM, respectively (Fig. 10E,F), in contrast to the label at the level of area R, which was mainly confined to the core (compare RM and AL in Fig. 10D to RTL and RTM in Fig. 10E,F). Only a sparse array of filled cells was present in the RPB and STGr (Fig. 10D,E). This stands in contrast to the caudal RT injection that labeled both RPB and, particularly, CPB; the discrepancy may stem from the high sensitivity of FB, and the placement of that injection near the border of the lateral belt. Rostral to the injection site, retrograde and anterograde label was largely confined to area RTp and extended to the most dorsal aspect of the temporal pole (Fig. 10H,I,J). Fibers and terminals within RTp spanned all cortical layers, but filled cells were predominantly within layer 2/3 (Fig. 10H,L).
Connections of RTp
Data from 4 tracer injections in area RTp were analyzed (Fig. 1K,L,N,O; Table 1). These experiments demonstrated a pattern of connections different from that seen following more caudal injections in AI, R, and RT. The rostral injections show that area RTp has extensive and divergent connections with the rostral core region, particularly RT and its flanking belt areas, as well as the parabelt areas, STGr, and the temporal pole (compare summary diagrams in Fig. 15).
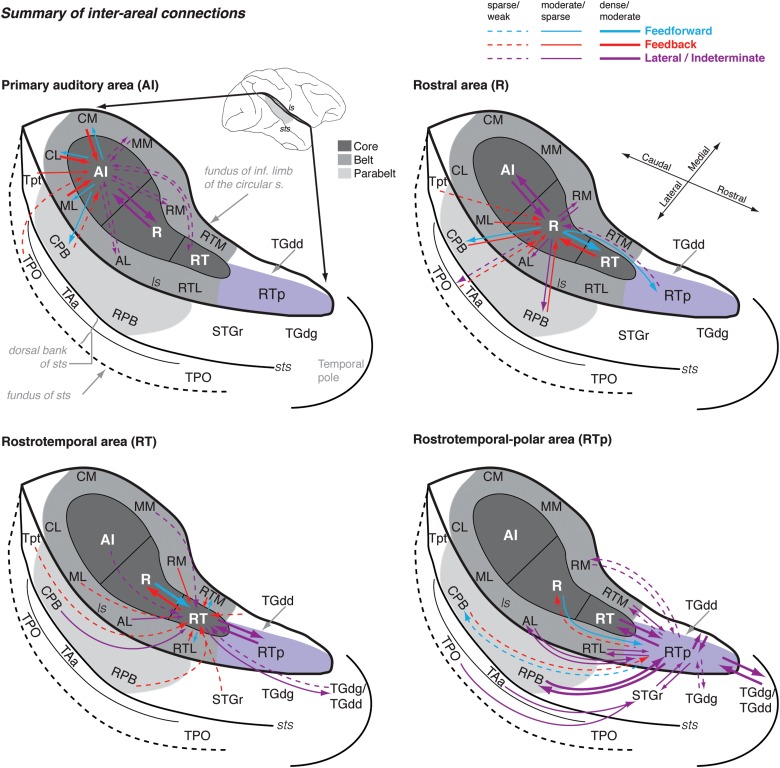
Figure 15.
Summary of the interareal connections of the STP. The schematic diagram of the STP and surrounding auditory and auditory-related areas is the same as in Figure 1, except that it has been modified to include the dorsal bank of the sts (TAa and TPO) and to represent the temporo-polar areas adjacent to RTp (TGdg and TGdd). Different color arrows reflect likely hierarchical relationships between areas: Blue, feedforward (ascending) connections; red, feedback (descending) connections; and purple, connections that are lateral (i.e., do not traverse a hierarchical level) or in which no clear hierarchical pattern is evident in the data. Thick, thin, and dashed lines indicate strong, moderate, and weak connection strength, respectively. The injections have been split into 4 figures, representing the connections of AI, R, RT, and RTp. In each division of the core (AI, R, and RT), connections are primarily with adjacent areas of the core and belt. However, this arrangement is not strictly the case: one AI case showed a connection with RT; R shows weak connections with area RTp and parabelt; and the connections of RT range from area Tpt to the temporal pole. This pattern of more heterogeneous connectivity at more rostral locations reaches its zenith in RTp, which is broadly interconnected with rostral core and belt, parabelt, STGr, and the temporal pole. Input from the dorsal bank of the sts (TPO and TAa) is shown as terminating lateral to RTp, as this connection was not found consistently for injections well confined to RTp and is more characteristic of STGr and the temporal pole area TGdg (Fig. 14).
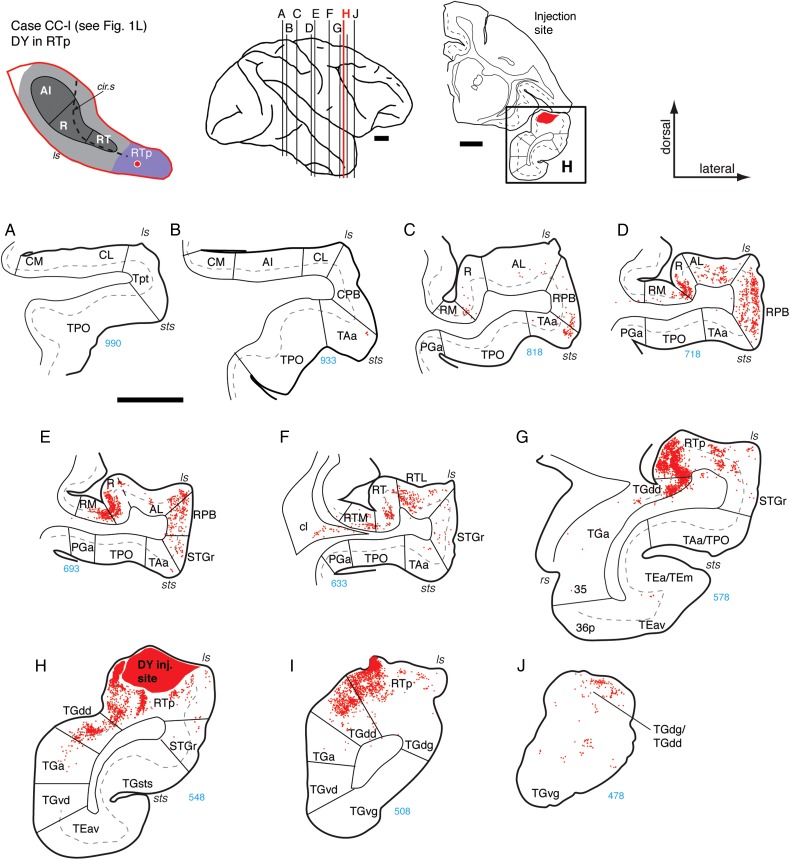
These wide-ranging connections were evident after an injection of BDA into the caudal part of RTp, just rostral to core area RT (Figs 1K and 11). This injection produced anterograde label that extended caudally into CPB and rostrally to the tip of the dorsal temporal pole (Fig. 11B,M). Moderate anterograde and retrograde label was observed in caudal regions of area R, predominantly within the supragranular layers (Fig. 11C,D,E). A dense patch of labeled cells and terminals was observed in the supragranular layers of lateral belt area AL, but only sparse anterograde label was seen in the medial belt area RM (Fig. 11C,D). Filled cells were concentrated in the supragranular layers of area RT (Fig. 11F,G), accompanied by anterograde label that was very strong in layers 1–3 but moderate to weak in layers 4 and 5 (Fig. 11F,N). In both RPB and STGr, columnar patches of moderate to strong anterograde label spanned layers 1–5, with weaker label in layer 6 (Fig. 11F,G,N). In the rostral belt areas, retrograde label was located in RTL, and both antero- and retrograde label was observed in RTM (Fig. 11G). Medial and rostral to the injection site, strong anterograde label was found in TGdd (Fig. 11H–K), but label in STGr was only present caudal to the injection (compare Fig. 11F,G with I,J). Patches of anterograde label reached the most rostral extent of the dorsal temporal pole (Fig. 11K,L,M). In this case, and in all 4 RTp injections, little or no connection was evident to the caudal core or belt areas at the level of AI (Fig. 11A,B).
In the second case, an injection of DY into the mid to caudal part of RTp (Figs 1L and 12) revealed bilaminar retrograde labeling in the RPB that was complementary to the anterograde projection observed in the BDA case above. The most caudal connections were at the level of area R, where filled cells were denser in the core and parabelt areas R and RPB, respectively, than in the belt areas AL and RM (Fig. 12D). At the level of area RT, core and belt areas contained more filled cells than did the STGr, which was labeled only sparsely relative to the RPB (Fig. 12F). The same was true at the level of RTp, where labeled cells were much denser medially, in the supra- and infragranular layers of TGdd, than laterally in STGr (Fig. 12G,H,I). Complementing the anterograde projections revealed by BDA in the previous case, DY filled retrograde-labeled cells in TGdd/TGdg, confirming that the connection of RTp with the dorsal temporal pole is reciprocal (Figs 12J and 11K–M).
Figure 12.
Connections of area RTp in case CC-l. (A–J) Distribution of labeled cells in the STP, STG, and temporal pole areas after DY injection into the mid to caudal part of area RTp (section H; see also Fig. 1L). Label was absent in AI and caudal belt (A and B), and sparse in caudal R and AL (C). Labeled cells were observed in the rostral portion of area R and in RT, as well as their flanking belt areas (RM and AL, sections D and E; RTM and RTL, section F). Additional cells were found in RPB (D and E), throughout RTp, and in the dorsal temporal pole areas TGdg/TGdd (G–J). Scale bars = 5 mm. For other conventions see Figure 4.
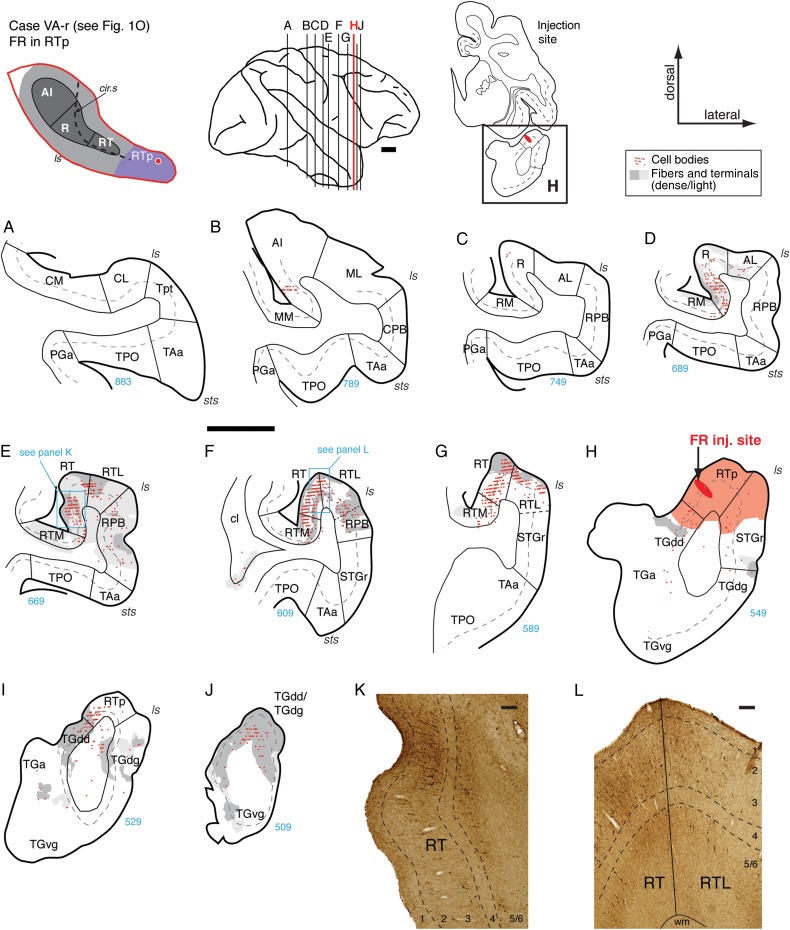
A third injection in rostral RTp, about 2.5 mm caudal to the temporal pole (Figs 1O and 13), again revealed retrograde labeling concentrated in the core areas caudal to RTp and a reciprocal projection to the dorsal temporal pole. Only few filled cells were located in rostral AI or caudal R (Fig. 13B,C), whereas a moderate to dense concentration of cells was present in both rostral R and RT (Fig. 13D–G). A similar caudorostral distribution was evident in the belt, where very few cells were found in AL or RM, but the filled cells in RT extended into the supragranular layers of RTM and into both the supra- and infragranular layers of RTL (Fig. 13F,G). Fibers and terminals were mostly co-extensive with areas of retrograde labeling. Within area RT, anterograde label was strongest in 2 bands covering layers 1–3 and 5, and weak or absent in layers 4 and 6 (Fig. 13E,K and F,L). Dense anterograde label was found medial to RTp in all layers of temporal pole area TGdd, and patches of label were observed lateral to RTp in TGdg (Fig. 13H–J). Both cells and terminals were present in the dorsal pole, with patches of anterograde label evident ventrally as well.
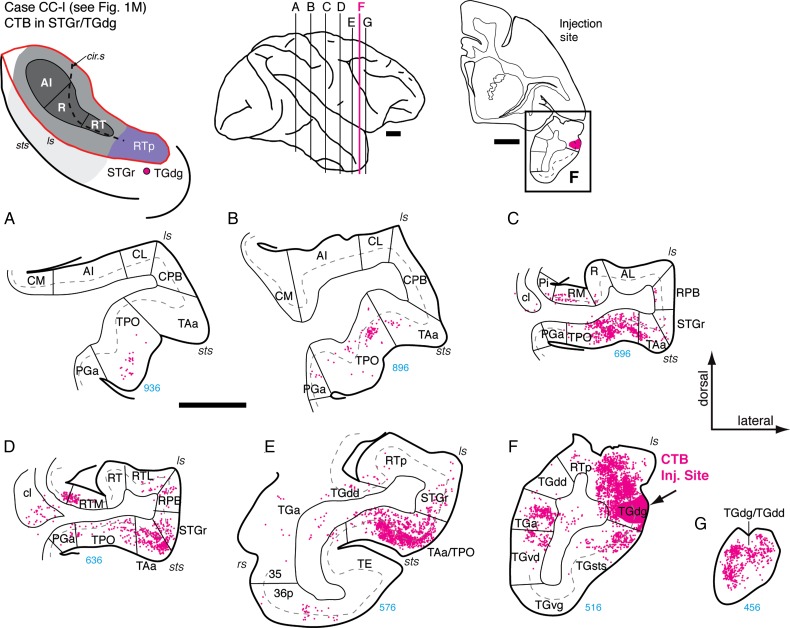
A fourth injection into rostro-lateral RTp (case MQ-r, data not shown) corroborated the connections described above, but also indicated a strong input from the dorsal bank of the sts, including the supra- and infragranular layers of areas TAa and caudal TPO. The injection in this case was quite close to the lateral border of RTp and may have included the STGr (Fig. 1N; also visible in Fig. 3R,S). Helping to resolve this discrepancy are data from an injection of CTB placed just ventral to the lip of the lateral sulcus (Figs 1M and 14), 1.2 mm rostral to the RTp injection in the same case (Fig. 12H). This lateral injection near the rostral border of STGr and TGdg resulted in extensive label in areas TPO and TAa in the dorsal bank of the sts, but none in the rostral core. From this, it seems likely that RTp proper, on the dorsomedial aspect of the STP, does not have direct connections with the dorsal bank of the sts, but instead connects to these regions indirectly via the STGr.
Figure 14.
Connections of area TGdg/STGr in case CC-l. (A–G) Distribution of labeled cells in the superior temporal cortex after CTB injection in the dorsal temporal pole area TGdg (near the border with STGr; section F; see also Fig. 1M). This injection, ventral to the lip of the lateral sulcus, was intended for comparison with those into RTp on the adjacent STP (e.g., Fig. 12, in the same case). Filled cells were located throughout the caudal-rostral extent of the dorsal bank of the sts, including areas TPO and TAa (A–C), being most dense rostrally (D and E). Cells were also found in RM and RTM, as well as in RPB, but not in any part of the core (R or RT) or lateral belt (aside from a few cells in RTL; panel D). Labeled cells were dense medial and rostral to the injection site in temporal pole areas TGa and TGdd/TGdg (F and G). Scale bars = 5 mm. For other conventions see Figure 4.
Thalamic Inputs to the Core and RTp
The subcortical connections of the core and rostral STP are outside the focus of this report, but the thalamic inputs to these areas may bear on our interpretation of their cortico-cortical connections. Within the core, AI and R received input primarily from the MGv (as expected), whereas RT received a mix of inputs from the ventral and dorsal divisions (MGv and MGd). Results across RTp cases were variable, but all except one (BDA in case SP-r) revealed retrograde labeling in the MGN. In cases CC-l and MQ-r, labeled cells were found within the MGv, and in case VA-r, a small number of filled cells were found within MGv and MGd. The former 2 cases had relatively large injections of sensitive retrograde tracers DY and CTB, whereas the latter case had a smaller injection of FR. Relative to the core, RTp received stronger inputs from thalamic nuclei outside the MGN, particularly the suprageniculate and medial pulvinar. Lateral to RTp, the STGr (case CC-l) received input from these 2 thalamic regions, along with the magnocellular division of MGN (MGm); no input was evident from MGv or MGd.
Discussion
A series of tracer injections spanning the core auditory cortex and rostral STP revealed a rostrally directed, step-wise connectivity in the ventral auditory stream (Fig. 15). In the caudal core, the primary auditory area AI is strongly interconnected with the rostral core area R. The connections of areas R, RT, and RTp show a common pattern in which inputs arise from the caudally adjacent area of the core, and the output of each area targets the rostrally adjacent region of the STP (i.e., R to RT, RT to RTp, and RTp to the dorsal temporal pole). The data broadly support a serial hierarchical architecture, but several aspects of the current findings suggest a more complex arrangement of parallel and recurrent connections akin to that described in the ventral visual pathway (for review see Kravitz et al. 2013). Although AI was predominantly connected with its neighboring fields (including caudal belt and Tpt), there was evidence for a direct projection from AI to RT, and from R to RTp in the caudorostral dimension, and from AI and R to parabelt in the mediolateral dimension (see Table 1; Fig. 15). Connections of more rostral areas of the STP are progressively more wide-ranging than those of the caudal areas. This pattern culminated in the broad connectivity of area RTp, which spans the tissue from the CPB to the temporal pole, including core, belt, parabelt, and the STGr (Fig. 15).
Cyto- and Chemoarchitecture of the Auditory Cortex
Previous studies have established criteria by which to partition the auditory areas of the macaque cortex based on cytoarchitecture (thionine staining), myeloarchitecture, and histochemical stains, for example, AChE, PV, and cytochrome oxidase (Pandya and Sanides 1973; Morel et al. 1993; Jones et al. 1995; Hackett et al. 1998a). The evidence from several of these techniques (including PV, AChE, and myelin) suggests that the transition in staining density between core and belt is a gradual one rather than a sharp border (Hackett et al. 1998a, p. 478), a characterization confirmed in the present study (Fig. 2). Caudal-to-rostral transitions, like those within the core or within the lateral belt, can be particularly difficult to discern. However, immunostaining for the neurofilament protein SMI-32 provided an additional cytoarchitectonic criterion by which to identify borders between cortical areas with considerable precision, in both the mediolateral dimension and the caudorostral dimension (Fig. 3). Staining with the SMI-32 antibody has previously been applied to the auditory cortex of cats (Mellott et al. 2010), rats (Ouda et al. 2012), gerbils (Budinger et al. 2000), and ferrets (Bajo et al. 2007), and such staining could be of considerable utility in future studies of auditory cortex in macaques.
The trilaminar distribution of SMI-32 immunoreactive pyramidal neurons in layers 3, 5, and 6 of macaque AI was initially described by Campbell and Morrison (1989), and this was contrasted to that in the anterolateral STG, where cell bodies were larger and more widely spaced than in AI, and located mainly in layers 3 and 5. “Anterolateral STG” in that study likely corresponds to the RPB, where we observed a similar pattern of staining. The dominant band of SMI-32 staining in layer 3 was later cited as a defining feature of AI and R, which served to distinguish the core from the more lightly stained caudal belt (Lewis and Van Essen 2000). In the rostral extent of the core, particularly RT, the intensity of layer 3 staining is diminished, and the light staining in layer 6 is continuous with the darker band in layer 5. This transition from a trilaminar architecture in AI to a bilaminar architecture in RT confirms earlier observations (Saleem and Logothetis 2012, p. 18). The transition from core to belt was marked by a transition from small, densely packed pyramidal cells with radially oriented apical dendrites to larger and more widely spaced cells with a less well-organized radial structure.
Outside the core, SMI-32 immunoreactivity highlighted 2 features of cortical structure that are worth noting. The first is the corroboration of the recently proposed area RTp, identified as an extension of weak PV immunoreactivity rostral to RT confined to the medial or central aspect of the STP (Fig. 2). In SMI-32-stained sections, RTp was characterized by a dark band of stained cells and processes in layer 5 (Saleem et al. 2007). These characteristics distinguish RTp from the adjacent STGr, which had previously been grouped as a single area (Ts2) in studies relying on staining for cell bodies (e.g., Galaburda and Pandya 1983). Second, SMI-32 highlighted the difference in architectonics between the medial and lateral belt, particularly the regions flanking area R. Caudally, areas CM and CL were qualitatively similar, and from CL, extending through AL and RTL, the lateral belt had a similar distribution of labeled cells and processes. However, the medial belt areas RM and RTM differed markedly from AL and RTL; labeled pyramidal cells were nearly absent in the supragranular layers of the medial belt, which was characterized by a single dark band of staining in layers 5/6. These laminar patterns are broadly consistent with the cytoarchitectonic observations of Galaburda and Pandya (1983), who identified the medial belt (root) in thionine-stained sections by its strong layer 5, but grouped the core and lateral belt as architectonically similar.
Discussion of the present results in relation to the existing literature requires bridging the shift in nomenclature from that of Pandya and coworkers (e.g., Galaburda and Pandya 1983) to that of Hackett et al. (1998a), which we have further modified for the STGr (Saleem and Logothetis 2012). Supplementary Figure 1 juxtaposes these schemes and proposes equivalencies between the 2 nomenclatures. The historical summary of Hackett et al. (1998a, their Fig. 10) is also useful in this regard.
Connectivity of the Core Areas and RTp
Primary Auditory Cortex
Tracer injections into AI and the AI/R border identified reciprocal connections to belt areas caudal and mediolateral to AI, and a rostral projection focused within the core; the belt areas flanking R were only moderately connected, if at all (Fig. 15; Table 1). Prior studies using retrograde tracers have also demonstrated that AI is locally connected to the caudal belt and selectively connected to the rostral portion of area R (Morel et al. 1993; Jones et al. 1995). This pattern of predominantly local connectivity corroborates earlier observations that “connections between nonadjacent fields are, at best, sparse” (Cipolloni and Pandya 1989). However, in at least one case in the present study (Fig. 4), we identified a projection beyond R, spanning approximately 12 mm along the STP from AI to putative RT. We cannot rule out that this projection is still within area R (there is no tonotopic map available for this case), but the degree to which connectivity between auditory areas is “local” may depend on individual differences and the tracers employed. Studies in New World monkeys generally confirm a local connectivity in which AI connects to R, but weakly (if at all) to RT (Fitzpatrick and Imig 1980; Morel and Kaas 1992; de la Mothe et al. 2006a). In accord with our data, AI in marmoset is connected most strongly with area CM, but is connected also with CL, ML, and the belt fields lateral to R (de la Mothe et al. 2006a).
An unexpected observation following 3 of 4 AI injections was the presence of labeled cells in the caudal parabelt area CPB (discussed below) and, particularly, the multisensory area TPO in the dorsal bank of the sts. A connection to TPO had been observed following injections that straddled AI and the lateral belt (Morel et al. 1993, their Figure 9), leaving their interpretation ambiguous. Area TPO is known to have connections with belt areas CM and CL, as well as CPB (Smiley et al. 2007; Hackett et al. 2014). If our injection sites encroached on the belt, then the labeled cells in TPO may reflect this secondary connection of AI, but the present data with restricted injections within AI (Fig. 4) suggest that this core area may receive a direct, if modest, input from TPO.
Although AI was found to be predominantly connected with the adjacent core and belt, a consistent exception to that rule is area Tpt (Figs 4 and 5), an auditory-related multisensory region at the most caudal extent of the STP/STG (Leinonen et al. 1980; Hackett et al. 2007). Injections of retrograde tracers in Tpt have not been shown to label AI (Smiley et al. 2007), suggesting that any input to Tpt from AI arrives indirectly via CM and CL. The present data based on the laminar distribution of cells in Tpt suggest that this area may send direct feedback to AI (Figs 5B and 6A), which would provide a pathway for the multisensory convergence between touch and sound that has been observed in AI (Brosch et al. 2005; Kayser et al. 2005; Lakatos et al. 2007). A complementary pathway has been described in the visual cortex, in which Tpt projects directly to primary visual area V1, but the opposite projection (from V1 to Tpt) is indirect, via secondary area V2 (Rockland and Ojima 2003).
Area R
The connectivity of R followed a pattern similar to that of AI, being broadly connected with caudally adjacent core and belt areas, and more selectively connected with the core area rostral to the injection (Fig. 15, Table 1). Area R was strongly interconnected with both of its neighboring subdivisions of the core, AI and RT, as well as the belt areas lateral to AI and R (ML and AL). Only one case showed evidence for a projection from R to RTp (Case OR-r, Fig. 8).
A similar pattern was evident in the data of Jones et al. (1995): 3 injections of retrograde tracer in area R (their “RL”) labeled cells in AI, the lateral and/or medial belt, and a small rostral region that may correspond to RT (their “A-m”; equivalence to previous nomenclature is discussed below and in Supplementary Fig. 1). No labeled cells were found farther rostral on the STP (Jones et al. 1995), which is consistent with the present data (the R to RTp connection in Fig. 8 was only evident in anterograde transport). In an older study, a large injection of tritiated amino acid covering much of the koniocortex (both AI and R) resulted in terminal label throughout what is likely area RT (“paAr,” Galaburda and Pandya 1983; Supplementary Fig. 1), as well as medial and lateral belt; label did not extend into RTp or STGr. Data from New World monkeys indicate a step-wise connectivity in which AI connects to R, and R to RT, with no direct connection from AI to RT (Morel and Kaas 1992) or only a weak one (de la Mothe et al. 2006a). Most of our cases are consistent with this scheme, with the exception of the AI–RT connection in Figure 4.
Core and Parabelt
Surprisingly, injections into AI, the AI/R border, and R (Figs 4 and 6–8) revealed direct reciprocal connectivity between core and caudal parabelt (CPB), and in one case RPB as well (Fig. 8). The retrograde label in parabelt indicates a feedback connection to area R, which is consistent with earlier observation in macaques (Pandya and Rosene 1993) and marmosets (de la Mothe et al. 2006a). However, the anterograde label in layer 4 of CPB (e.g., Fig. 7K) and RPB (Fig. 8M) would not be predicted from previous studies, and appears to contradict a strictly serial model of information flow from core, to belt, to parabelt. (Note that these injection sites do not appear to encroach on the belt, particularly the one in Fig. 7.) The extent and density of the terminal fields in CPB was modest relative to that in the lateral belt, and may be complementary to parabelt injections in 2 prior studies that resulted in retrograde labeling within area R: In one case very sparse labeling (Hackett et al. 1998a, their Figs 4–6); in the other, surprisingly extensive labeling (Markov et al. 2014, their case 23).
Area RT
The third, rostrotemporal subdivision of the auditory core was first identified as RT in owl monkeys (Morel and Kaas 1992). Later recognized in macaques, RT is more prominent in the rhesus (M. mulatta, used in this study) than in other species (e.g., M. nemestrina; Hackett et al. 1998a). Because RT was identified later than were AI and R, comparisons to the prior literature are complicated by differences in organizational schemes and nomenclature. Galaburda and coworkers recognized a small rostral appendage to the koniocortex in M. mulatta that they termed paAr, which likely corresponds to RT (Galaburda and Pandya 1983; Cipolloni and Pandya 1989). Morel et al. (1993) refer to RT in M. fascicularis, but do not map its borders; Jones et al. (1995) recognized an antero-medial belt field A-m in M. fuscata, which may correspond to area RT in the rhesus monkey.
Relative to area R, the connections of RT shifted rostrally to include area RTp and the dorsal temporal pole (Fig. 15; Table 1). Between the 2 cases presented, one identified a network of connections that was predominantly restricted to the adjacent core and belt, whereas the other labeled a broader array of areas including rostral and caudal parabelt, rostral AI, and area Tpt. In the latter case, the injection was located more caudally within RT, a very sensitive retrograde tracer (FB) was used, and the injection site may have impinged on the lateral belt (Fig. 9G); any combination of these factors could have contributed to the more widespread label in this case. The closest example of an RT injection in the literature may be an injection of DY into the STP rostral to R in M. fuscata (area “A-m,” Jones et al. 1995). That injection indicated connections to the STP rostral to the injection site (possibly corresponding to RTp), area R, the belt areas medial and lateral to R, and rostral AI, consistent with the current study; however, labeled cells in that case extended into caudal AI (Jones et al. 1995, their Figure 12C), which may reflect a subtle difference in species or injection site.
Area RTp
The architectonics and connectivity of area RTp represent the culmination of several trends evident in the transitions from AI to RT: Staining for PV grows progressively lighter (Fig. 2); the distribution of immunoreactivity for SMI-32 becomes more strongly confined to the infragranular layers (Figs 2 and 3); and the pattern of connectivity shifts rostrally to exclude AI (Fig. 15; Table 1). Area RTp draws its inputs from the rostral core, particularly area RT and its flanking belt areas (RTM and RTL), and the dorsal temporal pole. This pattern of connections echoes that seen following an injection of retrograde tracer into the rostral STP of M. fuscata by Jones et al. (1995; their Fig. 12C), including a few labeled cells in the medial portion of R and rostral AI (see also Figs 12 and 13 of the current study). Area RTp is also strongly connected to the RPB and, to a lesser extent, the STGr. It is likely via this route that RTp connects indirectly to the multisensory areas TAa and TPO in the dorsal bank of the sts (Fig. 14; Cipolloni and Pandya 1989; Morel et al. 1993; Hackett et al. 1998a). An additional route to the STGr is afforded via the dense connections between RTp and TGdd, on the medial aspect of the temporal pole, which is strongly connected to STGr (Moran et al. 1987; Kondo et al. 2003).
Under a longstanding model of temporal lobe organization (Pandya and Sanides 1973; Jones and Burton 1976; Galaburda and Pandya 1983), the auditory association area Ts2 comprised both the rostral STP and an adjacent strip extending ventrally and caudally along the STG. The portion of Ts2 on the STP (RTp in the present study) was distinguished from the adjacent STG by a peninsula of PV immunoreactivity extending rostrally from RT (Fig. 2J; Saleem et al. 2007; Saleem and Logothetis 2012). The present data confirm that the cytoarchitecture and connectivity of area RTp differ from those of the STGr (Figs 2 and 3) and that these areas lie at different hierarchical levels: Only RTp receives input from the rostral core and the MGN of the thalamus, and only STGr is interconnected with the multimodal areas in the dorsal bank of the sts (Figs 11–14). This difference in connectivity was first noted by Cipolloni and Pandya (1989), who suggested over 25 years ago that the planar and gyral regions of area Ts2 should be divided into separate components.
Laminar Patterns and Hierarchical Connectivity
The hierarchical relationship between cortical areas can be related to patterns of laminar specificity in the origin and termination of cortico-cortical projections (Rockland and Pandya 1979; Felleman and Van Essen 1991). Forward, or ascending, projections originate in the supragranular layers or in both the supra- and infragranular layers, and terminate primarily in layer 4. Feedback, or descending, projections originate in the infragranular layers or both supra- and infragranular layers, and terminate in layer 1 or in layers 1 and 6. A third pattern corresponds to lateral connections between areas at an equivalent stage of the hierarchy, in which connections originate in the supra- and infragranular layers and terminate in a columnar fashion across all layers. These patterns highlight a limitation of retrograde tracers, in that a projection of bilaminar origin (i.e., supra- and infragranular layers) may indicate a forward, lateral, or feedback connection. For this reason, discerning hierarchical structure is greatly facilitated by the inclusion of anterograde tracers.
The hierarchical model was initially proposed in the primate visual cortex, and has been applied to the primate auditory cortex as well (Galaburda and Pandya 1983; Hackett et al. 2014). As discussed below (see Conclusions), this model may not completely capture the complexity of the anatomical hierarchy in visual cortex, or map directly onto the functional hierarchy among visual areas (Hegde and Felleman 2007). The same caveats apply to the organization of auditory cortex, which should not be presumed to follow that of the visual system; however, the continued prominence of the hierarchical model (Markov and Kennedy 2013) merits considering our data within this framework.
AI and R
A clear example of these laminar patterns identifies the caudal belt as lying at a higher hierarchical level than AI: Projections from AI terminated in and around layer 4 of CM and CL (Figs 4M and 6L), characteristic of a feedforward projection; and projections to AI arose from the infragranular layers of the belt (CM, CL, ML) and Tpt, characteristic of feedback projections (Fig. 5). The relationship between AI and R is less clear-cut, and is best interpreted as a lateral connection between areas at an equivalent stage of the hierarchy. The FR injection in AI produced terminal label in the supragranular layers of R, and in layer 5 but not in layer 4 (Fig. 4N), a pattern most consistent with a lateral projection. The BDA injection at the border of AI and R (Fig. 6) resulted in a concentrated band of terminal label in layer 4 of rostral R, with lighter label extending across the full depth of cortex; this pattern is intermediate between a forward and a lateral projection (Hackett et al. 2014).
Despite the strong connectivity between AI and R, anatomical and physiological data do not support a hierarchical relationship between the two. Studies placing anterograde tracers into AI have failed to identify a classic forward projection to layer 4 of R (our Fig. 4; de la Mothe et al. 2006a; but see Fitzpatrick and Imig 1980). Aspiration of AI failed to abolish tone responses in R (Rauschecker et al. 1997), because AI and R receive independent input from the ventral subdivision of the auditory thalamus (MGv; Molinari et al. 1995; Rauschecker et al. 1997; de la Mothe et al. 2006b, 2006a). However, although responses in R persisted after aspiration of AI, neuronal firing rates and frequency tuning in R were affected (Rauschecker et al. 1997, their Figs 4 and 6), consistent with a modulatory interaction in which AI inputs affect at least some physiological aspects of responses in R. For example, the longer response latency and slower temporal resolution of neurons in R may reflect both parallel thalamocortical inputs (that differ in their temporal properties) as well as cortico-cortical inputs arriving serially from AI (Recanzone et al. 2000; Bendor and Wang 2008; Scott et al. 2011; Smith 2011).
R and RT
Unlike the connections between AI and R, the projection from R to RT conforms to the classic feedforward pattern, with dense terminal label in layer 4 of RT; filled cells in the infragranular layers of RT indicate a reciprocal feedback projection from RT to R. This projection was quite specific following the injection in caudal R (Fig. 7H,M), which was small and involved mostly the supragranular layers (Fig. 1F). Terminal label in RT was more broadly distributed across layers after the larger injection in central R (Figs 1G and 8), but still focused predominantly in layer 4 (Fig. 8N). The retrograde injection into RT resulted in strong labeling in layer 3 of R (Fig. 9E,N)—a unilaminar, supragranular origin typical of ascending projections (Felleman and Van Essen 1991). Consistent with this, anterograde label resulting from an injection in rostral RT revealed a feedback projection to the supra- and infragranular layers of R (Fig. 10D–F). The laminar pattern of connections between R and RT suggest that the 3 areas of the core do not represent a unitary hierarchical level, but that RT may lie at a higher stage than AI and R.
Medial and Lateral Belt
The medially and laterally directed connections of the core were not necessarily symmetric: The core regions, particularly AI and R, were more strongly connected with the lateral belt than with the medial belt, whereas RT and RTp were strongly connected to both. Coupled with the architectonic differences between the medial and lateral belt at the level of R and RT (discussed above), and with the recent observation that RM receives feedforward inputs from ML and CPB (Hackett et al. 2014), the belt cortex medial to R and RT is a site of widespread convergence that is not clearly at the same hierarchical level as are the opposing lateral belt areas. Unfortunately, the functional properties of the rostral core and belt do little to clarify the hierarchical relationships suggested by the anatomy: For example, neural response latencies and spectral selectivity have not been shown to differ between R, RM, and AL (Kuśmierek and Rauschecker 2009; Camalier et al. 2012).
Rostrotemporal polar field
Following an injection in RT, anterograde label was present through all layers of RTp, and vice versa (Figs 10I, 11N, and 13K; Table 1). These distributions are not characteristic of a classical feedforward or feedback projection, but more suggestive of a lateral connection between RTp and the rostral core. Similarly, projections from RTp to the RPB and STGr spanned all layers, including strong label within layer 4 (Fig. 11N), consistent with a lateral projection that does not traverse hierarchical levels. This casts doubt on the utility of laminar patterns for assigning RTp to a hierarchical level, in that the distribution of label does not establish a clear distinction between RTp and RT, or between RTP and parabelt.
The patchy, columnar-like distribution of anterograde labeling in the rostral core and parabelt following the BDA injection into RTp (Fig. 11N) is similar to patterns of sound-evoked metabolic activity in these areas as measured by autoradiography (Poremba et al. 2003). In that study, ipsilateral auditory input activated columns of cortical tissue 300–600 μm wide; the dimensions of those activated columns are consistent with the patches of ipsilateral cortico-cortical input identified in this case, suggestive of a columnar organization in which adjacent bands in the parabelt and STG receive different amounts of input (strong, weak, or none) from RTp.
Is Area RTp Part of Auditory Cortex? Anatomical and Functional Consideration
Given the anatomical evidence presented above, where in the auditory cortical hierarchy should RTp be considered to lie? In most cases, RTp received thalamic input from the MGN, indicating that RTp may be considered a part of the auditory cortex (as opposed to the auditory-related areas). The mix of inputs from ventral, dorsal, and magnocellular divisions of the MGN (along with suprageniculate and pulvinar) is not characteristic of the core, but is not inconsistent with patterns of input to the belt or parabelt (Hackett et al. 1998b); the strength of MGv input to RTp was greater than that observed in STGr, or that reported in the temporal pole (Markowitsch et al. 1985). The staining of the neuropil for PV suggests that RTp is a continuation of the core area RT, but AChE staining does not (Fig. 2; see also Saleem et al. 2007). Architectonically, RTp is no more similar to RT than it is to RTL, so interpreting RTp as a rostral belt area capping RT is equally plausible. Until further evidence clarifies the issue, we will consider RTp to be sui generis, not clearly classified as core, belt, or parabelt, but rather a point of convergence between hierarchical levels.
The connectivity of area RTp with the rostral core, belt, and parabelt establishes a framework for integration of auditory information across multiple levels of the cortical hierarchy. From the caudal core, auditory information flows along 2 primary axes (as discussed above): the mediolateral flow of information is thought to correspond to widening integration of spectral inputs, as neurons in lateral belt show preferences for band-pass noise and complex harmonic stimuli over pure tones (Rauschecker et al. 1995; Rauschecker and Tian 2004; Petkov et al. 2006; Kikuchi et al. 2014); the orthogonal caudorostral stream is thought to correspond to widening windows of temporal integration, as evidenced by longer response latencies and more sluggish responses to rapidly modulated sounds (Recanzone et al. 2000; Bendor and Wang 2008; Scott et al. 2011; Camalier et al. 2012). Functionally, the convergence of inputs in area RTp provides a basis for the complex stimulus selectivity evident in physiological responses recorded from neurons of the anterior STP (Kikuchi et al. 2010). Auditory responses in this region evince heightened selectivity for macaque vocalizations compared with other classes of sounds (Petkov et al. 2008; Perrodin et al. 2011, 2014). Neural discrimination among vocalizations in this region has been shown to require conjoined spectral and temporal features (Fukushima et al. 2014).
Although connectivity and physiology define RTp as a predominantly auditory area, a substantial fraction of single units in this region was found to exhibit multisensory influences, in that the response to a vocalization was affected by the simultaneous visual presentation of a face (Perrodin et al. 2014). Visual influences were less common and less specific in the anterior STP (an area largely coextensive with our “RTp”) than in the upper bank of the sts (Perrodin et al. 2014), a known site of multisensory convergence (Bruce et al. 1981; Baylis et al. 1987; Barraclough et al. 2005). We found that RTp is not directly connected with the sts (i.e., areas TAa and TPO), but it is strongly interconnected via the STGr. Anterior TPO is therefore a likely source of visual input to RTp (Baylis et al. 1987), though this information may arrive via the thalamus as well (e.g., pulvinar or suprageniculate nucleus). Functional microstimulation of RTp has been shown to engage a network of anterior auditory fields (Petkov et al. 2015) consistent with the direct anatomical pathways identified here. Microstimulation also activated the anterior sts (Petkov et al. 2015), a finding most easily reconciled with the current results if the stimulating electrode were slightly lateral on the STP and activation spread to the adjacent STGr or temporal pole area TGdg, which connect directly with the sts (Fig. 14; see also (Seltzer and Pandya 1978). It is unknown whether this rostral multisensory network is distinct from that of caudal TPO, which receives independent auditory input from the caudal belt and parabelt (Seltzer et al. 1996; Hackett et al. 1998a, 2014), may project directly back to AI (discussed above), and shows functional interactions with AI during the processing of multimodal (face/voice) stimuli (Ghazanfar et al. 2008).
In addition to its connections with the anterior auditory fields, RTp displayed a range of connections to structures outside the superior temporal lobe including the claustrum, amygdala, striatum, medial pulvinar, and the prefrontal cortex, as has been demonstrated for the gyral component of Ts2 in prior tracing studies (e.g., Petrides and Pandya 1988, 2007; Yeterian and Pandya 1998; Romanski, Bates, et al. 1999; Yukie 2002; Barbas et al. 2005; Saleem et al. 2008). These connections lie outside the scope of the present report, but strongly imply a role for the anterior STP, including both RTp and the dorsal temporal pole, in auditory aspects of higher order behavioral and cognitive functions (Munoz-Lopez et al. 2010, 2015; Ng et al. 2014; Scott et al. 2014).
Conclusions
Hierarchical architecture is believed to be fundamental to sensory processing (Felleman and Van Essen 1991), including that underlying speech perception in the human auditory cortex (Okada et al. 2010; DeWitt and Rauschecker 2012; Cammoun et al. 2015). In the simplest interpretation of the prevailing model of auditory cortical organization, the core, belt, and parabelt correspond to primary, secondary, and tertiary levels of cortical processing, respectively. In the present study, placement of anterograde tracer injections within the core demonstrated rostrally directed feedforward hierarchical connectivity, possibly even within the core itself. Although AI and R cannot be said to lie at different hierarchical levels, RT appears to represent a higher stage, despite the architectonic features and MGv input that define it as “core.” In this regard, RT is a mirror image of area CM, which receives some MGv input but is considered a belt region (Mesulam and Pandya 1973; Burton and Jones 1976; Hackett et al. 2007; Kuśmierek and Rauschecker 2009). Area RT then projects to RTp, which also receives input from the belt and parabelt, forming a point of convergence between the laterally directed hierarchy from core to belt to parabelt, and the caudorostrally directed hierarchy along the STP.
Although we have presented the auditory ventral stream as a serial step-wise progression, the connections described diverge from this simple model in several respects. Connections were shown to bypass intermediate stages in both the mediolateral dimension (e.g., from core directly to parabelt) and in the caudorostral dimension (e.g., from AI to RT, and from R to RTp). Although most connections were reciprocal, several unidirectional feedback connections were evident (including projections to AI from Tpt and TPO). As has been argued with regard to the ventral visual pathway (Kravitz et al. 2013), violations of a strictly serial, step-wise flow of visual information are evident from the earliest stages of the cortical hierarchy, and the assignment of cortical areas to hierarchical levels is straightforward only through the level of early visual area V4 (Hegde and Felleman 2007). Even setting aside brainstem and midbrain circuitry, the connectivity of the auditory system is arguably even more complex. Multiple regions of the core receive independent thalamic input (Rauschecker et al. 1997), and projections to prefrontal cortex are present at every level beyond the core (Hackett et al. 1999; Romanski, Bates, et al. 1999; Saleem et al. 2008, 2014). In this regard, the ventral auditory stream of the nonhuman primate appears to be a “recurrent and interactive network” similar to that of the ventral visual stream, incorporating multiple parallel pathways, both direct and indirect, through which sound stimuli are processed.
Supplementary Material
Supplementary material can be found here.
Funding
This work was supported by the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health (Project Number ZIAMH001101-23).
Supplementary Material
Notes
We thank D. Yu, A. Cummins, H. Vinal, A. Kloth, R. Reoli, C. Blackwell, A. Doyle, and M. Muñoz-Lopez for technical assistance and advice, and 4 anonymous reviewers for their feedback on the manuscript. Conflict of Interest: None declared.
References
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. 2007. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex. 17:475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. 2005. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 15:1356–1370. [DOI] [PubMed] [Google Scholar]
- Barks SK, Bauernfeind AL, Bonar CJ, Cranfield MR, de Sousa AA, Erwin JM, Hopkins WD, Lewandowski AH, Mudakikwa A, Phillips KA et al. 2014. Variable temporoinsular cortex neuroanatomy in primates suggests a bottleneck effect in eastern gorillas. J Comp Neurol. 522:844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough NE, Xiao D, Baker CI, Oram MW, Perrett DI. 2005. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. J Cogn Neurosci. 17:377–391. [DOI] [PubMed] [Google Scholar]
- Baumann S, Petkov CI, Griffiths TD. 2013. A unified framework for the organization of the primate auditory cortex. Front Syst Neurosci. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET, Leonard CM. 1987. Functional subdivisions of the temporal lobe neocortex. J Neurosci. 7:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, Wang X. 2008. Neural response properties of primary, rostral, and rostrotemporal core fields in the auditory cortex of marmoset monkeys. J Neurophysiol. 100:888–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. 1905. Beitrage zur histologischen Lokalisation der Grosshirnrinde. III. Die Rindenfelder der niederen. Affen J Psychol Neurol. 4:177–226. [Google Scholar]
- Brosch M, Selezneva E, Scheich H. 2005. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 25:6797–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce C, Desimone R, Gross CG. 1981. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 46:369–384. [DOI] [PubMed] [Google Scholar]
- Budinger E, Heil P, Scheich H. 2000. Functional organization of auditory cortex in the Mongolian gerbil (Meriones unguiculatus). III. Anatomical subdivisions and corticocortical connections. Eur J Neurosci. 12:2425–2451. [DOI] [PubMed] [Google Scholar]
- Burton H, Jones EG. 1976. The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol. 168:249–301. [DOI] [PubMed] [Google Scholar]
- Camalier CR, D'Angelo WR, Sterbing-D'Angelo SJ, de la Mothe LA, Hackett TA. 2012. Neural latencies across auditory cortex of macaque support a dorsal stream supramodal timing advantage in primates. Proc Natl Acad Sci USA. 109:18168–18173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammoun L, Thiran JP, Griffa A, Meuli R, Hagmann P, Clarke S. 2015. Intrahemispheric cortico-cortical connections of the human auditory cortex. Brain Struct Funct. 220:3537–3553. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Morrison JH. 1989. Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol. 282:191–205. [DOI] [PubMed] [Google Scholar]
- Celio MR. 1986. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 231:995–997. [DOI] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN. 1989. Connectional analysis of the ipsilateral and contralateral afferent neurons of the superior temporal region in the rhesus monkey. J Comp Neurol. 281:567–585. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. 2012. Cortical connections of auditory cortex in marmoset monkeys: lateral belt and parabelt regions. Anat Rec (Hoboken). 295:800–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. 2006. a. Cortical connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 496:27–71. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. 2006. b. Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 496:72–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt I, Rauschecker JP. 2012. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA. 109:E505–E514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1:1–47. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KA, Imig TJ. 1980. Auditory cortico-cortical connections in the owl monkey. J Comp Neurol. 192:589–610. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Saunders RC, Leopold DA, Mishkin M, Averbeck BB. 2014. Differential coding of conspecific vocalizations in the ventral auditory cortical stream. J Neurosci. 34:4665–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Saunders RC, Leopold DA, Mishkin M, Averbeck BB. 2012. Spontaneous high-gamma band activity reflects functional organization of auditory cortex in the awake macaque. Neuron. 74:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Pandya DN. 1983. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol. 221:169–184. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Chandrasekaran C, Logothetis NK. 2008. Interactions between the superior temporal sulcus and auditory cortex mediate dynamic face/voice integration in rhesus monkeys. J Neurosci. 28:4457–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein ME, Sternberger LA, Sternberger NH. 1987. Varying degrees of phosphorylation determine microheterogeneity of the heavy neurofilament polypeptide (Nf-H). J Neuroimmunol. 14:135–148. [DOI] [PubMed] [Google Scholar]
- Hackett TA. 2011. Information flow in the auditory cortical network. Hear Res. 271:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, de la Mothe LA, Camalier CR, Falchier A, Lakatos P, Kajikawa Y, Schroeder CE. 2014. Feedforward and feedback projections of caudal belt and parabelt areas of auditory cortex: refining the hierarchical model. Front Neurosci. 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, De La Mothe LA, Ulbert I, Karmos G, Smiley J, Schroeder CE. 2007. Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol. 502:924–952. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Karmos G, Schroeder CE, Ulbert I, Sterbing-D'Angelo SJ, D'Angelo WR, Kajikawa Y, Blumell S, de la Mothe L. 2005. Neurosurgical access to cortical areas in the lateral fissure of primates. J Neurosci Methods. 141:103–113. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Preuss TM, Kaas JH. 2001. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol. 441:197–222. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. 1999. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Res. 817:45–58. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. 1998. a. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 394:475–495. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. 1998. b. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 400:271–286. [DOI] [PubMed] [Google Scholar]
- Hegde J, Felleman DJ. 2007. Reappraising the functional implications of the primate visual anatomical hierarchy. Neuroscientist. 13:416–421. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Emson PC, Lawson DE, Heizmann CW, Streit P. 1989. Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res. 76:467–472. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. 1976. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol. 168:197–247. [DOI] [PubMed] [Google Scholar]
- Jones EG, Dell'Anna ME, Molinari M, Rausell E, Hashikawa T. 1995. Subdivisions of macaque monkey auditory cortex revealed by calcium-binding protein immunoreactivity. J Comp Neurol. 362:153–170. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. 1998. Subdivisions of auditory cortex and levels of processing in primates. Audiol Neurootol. 3:73–85. [DOI] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Augath M, Logothetis NK. 2005. Integration of touch and sound in auditory cortex. Neuron. 48:373–384. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Horwitz B, Mishkin M. 2010. Hierarchical auditory processing directed rostrally along the monkey's supratemporal plane. J Neurosci. 30:13021–13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Horwitz B, Mishkin M, Rauschecker JP. 2014. Processing of harmonics in the lateral belt of macaque auditory cortex. Front Neurosci. 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. 2003. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 465:499–523. [DOI] [PubMed] [Google Scholar]
- Kosaki H, Hashikawa T, He J, Jones EG. 1997. Tonotopic organization of auditory cortical fields delineated by parvalbumin immunoreactivity in macaque monkeys. J Comp Neurol. 386:304–316. [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. 2013. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci. 17:26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśmierek P, Ortiz M, Rauschecker JP. 2012. Sound-identity processing in early areas of the auditory ventral stream in the macaque. J Neurophysiol. 107:1123–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśmierek P, Rauschecker JP. 2009. Functional specialization of medial auditory belt cortex in the alert rhesus monkey. J Neurophysiol. 102:1606–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. 2007. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 53:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen L, Hyvarinen J, Sovijarvi AR. 1980. Functional properties of neurons in the temporo-parietal association cortex of awake monkey. Exp Brain Res. 39:203–215. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. 2000. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J Comp Neurol. 428:79–111. [DOI] [PubMed] [Google Scholar]
- Markov NT, Ercsey-Ravasz MM, Ribeiro Gomes AR, Lamy C, Magrou L, Vezoli J, Misery P, Falchier A, Quilodran R, Gariel MA et al. 2014. A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb Cortex. 24:17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Kennedy H. 2013. The importance of being hierarchical. Curr Opin Neurobiol. 23:187–194. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B. 1985. Cortical and subcortical afferent connections of the primate's temporal pole: a study of rhesus monkeys, squirrel monkeys, and marmosets. J Comp Neurol. 242:425–458. [DOI] [PubMed] [Google Scholar]
- Mellott JG, Van der Gucht E, Lee CC, Carrasco A, Winer JA, Lomber SG. 2010. Areas of cat auditory cortex as defined by neurofilament proteins expressing SMI-32. Hear Res. 267:119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Brugge JF. 1973. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res. 50:275–296. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Pandya DN. 1973. The projections of the medial geniculate complex within the sylvian fissure of the rhesus monkey. Brain Res. 60:315–333. [DOI] [PubMed] [Google Scholar]
- Molinari M, Dell'Anna ME, Rausell E, Leggio MG, Hashikawa T, Jones EG. 1995. Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. J Comp Neurol. 362:171–194. [DOI] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM. 1987. Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol. 256:88–103. [DOI] [PubMed] [Google Scholar]
- Morel A, Garraghty PE, Kaas JH. 1993. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 335:437–459. [DOI] [PubMed] [Google Scholar]
- Morel A, Kaas JH. 1992. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol. 318:27–63. [DOI] [PubMed] [Google Scholar]
- Munoz-Lopez M, Insausti R, Mohedano-Moriano A, Mishkin M, Saunders RC. 2015. Anatomical pathways for auditory memory II: information from rostral superior temporal gyrus to dorsolateral temporal pole and medial temporal cortex. Front Neurosci. 9:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Lopez MM, Mohedano-Moriano A, Insausti R. 2010. Anatomical pathways for auditory memory in primates. Front Neuroanat. 4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CW, Plakke B, Poremba A. 2014. Neural correlates of auditory recognition memory in the primate dorsal temporal pole. J Neurophysiol. 111:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Rong F, Venezia J, Matchin W, Hsieh IH, Saberi K, Serences JT, Hickok G. 2010. Hierarchical organization of human auditory cortex: evidence from acoustic invariance in the response to intelligible speech. Cereb Cortex. 20:2486–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouda L, Druga R, Syka J. 2012. Distribution of SMI-32-immunoreactive neurons in the central auditory system of the rat. Brain Struct Funct. 217:19–36. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Rosene DL. 1993. Laminar termination patterns of thalamic, callosal, and association afferents in the primary auditory area of the rhesus monkey. Exp Neurol. 119:220–234. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Sanides F. 1973. Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. Z Anat Entwicklungsgesch. 139:127–161. [DOI] [PubMed] [Google Scholar]
- Perrodin C, Kayser C, Logothetis NK, Petkov CI. 2014. Auditory and visual modulation of temporal lobe neurons in voice-sensitive and association cortices. J Neurosci. 34:2524–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrodin C, Kayser C, Logothetis NK, Petkov CI. 2011. Voice cells in the primate temporal lobe. Curr Biol. 21:1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, Kayser C, Augath M, Logothetis NK. 2006. Functional imaging reveals numerous fields in the monkey auditory cortex. PLoS Biol. 4:e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, Kayser C, Steudel T, Whittingstall K, Augath M, Logothetis NK. 2008. A voice region in the monkey brain. Nat Neurosci. 11:367–374. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Kikuchi Y, Milne AE, Mishkin M, Rauschecker JP, Logothetis NK. 2015. Different forms of effective connectivity in primate frontotemporal pathways. Nat Commun. 6:6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 1988. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 273:52–66. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2007. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 27:11573–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. 2004. Species-specific calls evoke asymmetric activity in the monkey's temporal poles. Nature. 427:448–451. [DOI] [PubMed] [Google Scholar]
- Poremba A, Saunders RC, Crane AM, Cook M, Sokoloff L, Mishkin M. 2003. Functional mapping of the primate auditory system. Science. 299:568–572. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. 1998. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 3:86–103. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. 2009. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 12:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. 2004. Processing of band-passed noise in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 91:2578–2589. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. 1995. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 268:111–114. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Pons T, Mishkin M. 1997. Serial and parallel processing in rhesus monkey auditory cortex. J Comp Neurol. 382:89–103. [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, Phan ML. 2000. Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. J Neurophysiol. 83:2315–2331. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Ojima H. 2003. Multisensory convergence in calcarine visual areas in macaque monkey. Int J Psychophysiol. 50:19–26. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. 1979. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179:3–20. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Bates JF, Goldman-Rakic PS. 1999. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 403:141–157. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. 1999. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 2:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. 1986. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 34:1301–1315. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. 2008. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 506:659–693. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. 2012. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates, 2nd edition with horizontal, coronal and sagittal series. San Diego: (CA: ): Elsevier/Academic Press. [Google Scholar]
- Saleem KS, Miller B, Price JL. 2014. Subdivisions and connectional networks of the lateral prefrontal cortex in the macaque monkey. J Comp Neurol. 522:1641–1690. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Price JL, Hashikawa T. 2007. Cytoarchitectonic and chemoarchitectonic subdivisions of the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 500:973–1006. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Vann SD, Aggleton JP. 2012. Projections from Gudden's tegmental nuclei to the mammillary body region in the cynomolgus monkey (Macaca fascicularis). J Comp Neurol. 520:1128–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BH, Malone BJ, Semple MN. 2011. Transformation of temporal processing across auditory cortex of awake macaques. J Neurophysiol. 105:712–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BH, Mishkin M, Yin P. 2014. Neural correlates of auditory short-term memory in rostral superior temporal cortex. Curr Biol. 24:2767–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Cola MG, Gutierrez C, Massee M, Weldon C, Cusick CG. 1996. Overlapping and nonoverlapping cortical projections to cortex of the superior temporal sulcus in the rhesus monkey: double anterograde tracer studies. J Comp Neurol. 370:173–190. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1978. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 149:1–24. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE. 2007. Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol. 502:894–923. [DOI] [PubMed] [Google Scholar]
- Smith EH. 2011. Temporal processing in the auditory core: transformation or segregation? J Neurophysiol. 106:2791–2793. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. 1983. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 80:6126–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. 1986. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 34:1431–1438. [DOI] [PubMed] [Google Scholar]
- Turchi J, Saunders RC, Mishkin M. 2005. Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proc Natl Acad Sci USA. 102:2158–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE. 1937. The projection of the medial geniculate body to the cerebral cortex in the macaque monkey. J Anat. 17:319–331. [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. 1998. Corticostriatal connections of the superior temporal region in rhesus monkeys. J Comp Neurol. 399:384–402. [PubMed] [Google Scholar]
- Yin P, Mishkin M, Sutter M, Fritz JB. 2008. Early stages of melody processing: stimulus-sequence and task-dependent neuronal activity in monkey auditory cortical fields A1 and R. J Neurophysiol. 100:3009–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukie M. 2002. Connections between the amygdala and auditory cortical areas in the macaque monkey. Neurosci Res. 42:219–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.