Abstract
Background
Variability in imaging protocols and techniques has resulted in a lack of consensus regarding the incorporation of perfusion imaging into stroke triage and treatment. The objective of our study was to evaluate the available scientific evidence regarding the utility of perfusion imaging in determining treatment eligibility in patients with acute stroke and in predicting their clinical outcome.
Methods
We performed a systematic review of the literature using PubMed, Web of Science, and Cochrane Library focusing on themes of medical imaging, stroke, treatment, and outcome (CRD42016037817). We included randomized controlled trials, cohort studies, and case-controlled studies published from 2011 to 2016. Two independent reviewers conducted the study appraisal, data abstraction, and quality assessments of the studies.
Results
Our literature search yielded 13 studies that met our inclusion criteria. In total, 994 patients were treated with the aid of perfusion imaging compared with 1819 patients treated with standard care. In the intervention group 51.1% of patients had a favorable outcome at 3 months compared with 45.6% of patients in the control group (p=0.06). Subgroup analysis of studies that used multimodal therapy (IV tissue plasminogen activator, endovascular thrombectomy) showed a significant benefit of perfusion imaging (OR 1.89, 95% CI 1.43 to 2.51, p<0.01).
Conclusions
Perfusion imaging may represent a complementary tool to standard radiographic assessment in enhancing patient selection for reperfusion therapy, with a subset of patients having up to 1.9 times the odds of achieving independent functional status at 3 months. This is particularly important as patients selected based on perfusion status often included individuals who did not meet the current treatment eligibility criteria.
INTRODUCTION
Stroke is the fourth leading cause of death and the leading cause of chronic disability in the USA.1 Furthermore, the global incidence of stroke has increased to 16.9 million within the last decade.2 Although the mortality rate of stroke has decreased with improvements in stroke recognition and management, the global burden of stroke continues to increase.3 Therefore, how to further enhance stroke treatment to reduce long-term disability remains a significant challenge.
While extensive scientific evidence has shown the benefit of IV tissue plasminogen activator, only 0.6–22% of potentially eligible patients actually receive reperfusion therapy.4,5 This low rate of stroke treatment is largely due to the narrow recommended time window for therapy. Multimodal imaging techniques including perfusion imaging using CT and/or MRI now allow detection of infarcted brain tissue and also potentially salvageable brain tissue. These developments have encouraged further refining and categorization of treatment eligibility. For example, the DEFUSE study highlighted that MRI assessment after the typical 3 hours cut-off allowed selection of patients who would benefit from reperfusion.6 While the potential upside of such technology is evident, considerable variability in the choice of imaging modalities and definition of perfusion status have led to some clinicians raising concerns about premature incorporation of perfusion imaging into standard care.7
The objective of this study was to evaluate the current evidence on the utility of perfusion imaging in treating patients with acute ischemic stroke (AIS). Specifically, we aimed to answer the following research questions: (1) What is the functional outcome of patients with AIS who are treated based on perfusion imaging characteristics in addition to standard clinical and imaging assessment compared with patients treated without perfusion imaging data? (2) What is the ability of perfusion imaging to predict clinical outcome after AIS?
METHODS
Search strategy
A systematic review and meta-analysis were conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. The research protocol was developed a priori and was registered at the International Prospective Register of Systematic Reviews (PROSPERO; CRD42016037817).
We performed our literature search in PubMed, Web of Science, and Cochrane Library for English language publications from 1 January 2011 to 1 January 2016. In collaboration with a medical librarian with expertise in systematic reviews, a broad scope of search terms, related MeSH terms, and their word variants were developed under the themes of medical imaging, stroke, treatment, and outcome (see online supplement). A secondary search was conducted by reviewing citations from previously published literature reviews and major clinical trials along with a search in Google Scholar.
Study selection and classification
In the initial title and abstract review, articles were included if they involved perfusion imaging related to AIS management. The interventions of interest were multimodal CT scan and MRI performed as a part of stroke assessment for the adult population. We included randomized controlled trials, cohort studies, and case-control studies and excluded case reports, editorials, technical reports, conference abstracts, and books. Studies that met the inclusion criteria were reviewed in full and grouped according to the Provenzale classification of perfusion imaging research, with category 1 studies (perfusion used to guide treatment) and category 2 studies (perfusion used to predict outcome) selected for detailed analyses.8
Data extraction, synthesis and analysis
Two independent reviewers extracted details of the studies grouped as Provenzale category 1 or 2 using a standardized abstraction form developed a priori (see online supplement). The primary outcome for our review was functional status as measured by the modified Rankin Scale (mRS) score at 3-month follow-up categorized in a dichotomous manner (favorable outcome, mRS score 0–2; unfavorable outcome, mRS score 3–6). We used a random effects model for all meta-analyses given the heterogeneity between studies. The summary estimate of functional outcome used ORs with 95% CIs. Subgroup analyses were performed to identify important sources of heterogeneity. Studies examining perfusion imaging as a predictor of outcome were analyzed in a descriptive manner due to the significant heterogeneity of study methodologies.
Quality assessment (QA) was performed based on National Institute of Health (NIH) guidelines for systematic review with standardized NIH Study QA Tools.9 This tool consists of 12–14 questions addressing validity and risk of bias. Two independent reviewers evaluated the overall quality of the studies. Reviewer 1 rated all studies in Provenzale category 1 and 50% of studies in Provenzale category 2, while reviewer 2 rated all studies in Provenzale category 2 and 50% of studies in Provenzale category 1. Discrepancies were resolved through discussion between the reviewers. Begg’s test and Egger’s test were performed to assess publication bias. Statistical analyses were performed using Stata 14 with p<0.05 as the criterion for identifying statistically significant results (StataCorp, Texas, USA).
RESULTS
The initial search yielded 1742 unique publications (see online supplementary figure S1). Of the 154 papers that were reviewed in full, 49 articles were identified as Provenzale category 1 or 2. To answer our first research question, studies in Provenzale category 1 focusing on perfusion imaging-based treatment outcomes were selected for meta-analysis. For our second research question, studies in Provenzale category 2 assessing perfusion imaging characteristics as predictors of outcome were analyzed in a descriptive manner.
Perfusion imaging-based treatment
A total of 13 studies with 3881 patients met our criteria for inclusion under Provenzale category 1 (table 1). Of the 13 studies selected, eight were included for meta-analysis with 994 patients treated based on perfusion imaging characteristics in addition to the standard clinical and imaging assessments (intervention group) compared with 1819 patients treated without perfusion imaging (control group; table 2).10–17 For the majority of the studies, the intervention group was treated with a longer treatment time window (up to 26 hours from stroke onset); three studies treated patients with unknown or unclear stroke onset. The mean patient age between the intervention group and control group differed in two of eight studies, with the intervention group being older. Eight of eight studies had similar baseline NIH Stroke Scale (NIHSS) scores between the two groups.
Table 1.
Characteristics of perfusion imaging-based treatment studies
| CT | MRI | CT or MRI | |
|---|---|---|---|
| No of studies | 9 | 1 | 3 |
| Study design | |||
| Randomized controlled trials | 1 | 0 | 1 |
| Cohort studies | 7 | 1 | 2 |
| Case–control studies | 1 | 0 | 0 |
| Sample | 2952 | 239 | 690 |
| Treatment timing window, hours | 3–24 | N/A | 8 |
| No of studies treating patients with unknown onset time | 2 | 1 | 1 |
| Treatment | |||
| IV tPA only | 4 | 0 | 0 |
| ET only | 0 | 0 | 1 |
| Combined IV tPA and ET | 5 | 1 | 2 |
ET, endovascular thrombectomy; IV tPA, IV tissue plasminogen activator.
Table 2.
Characteristics of perfusion imaging-based treatment studies in the meta-analysis
| Study | Design | Sample size | Max time window (hours) | Age | Baseline NIHSS | Time to rescan (hours) | Recanalization (%) | Symptomatic ICH (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||||
| PI | Control | PI | Control | PI | Control | PI | Control | PI | Control | PI | Control | |||
| Bivard et al, 201510 | Prospective cohort | 366 | 396 | 4.5 | 4.5 | 73† | 74† | 14† | 12† | 24 | NR | NR | 5 | 5 |
| García-Bermejo et al, 201211 | Prospective case controlled | 43 | 172 | No limitation (range 4.5–14; 16 patients unknown onset) | 4.5 | 69 | 72 | 9† | 11† | 1, 2, 24 | 65 | 77 | 4.7 | 2.3 |
| Jovin et al, 201112 | Retrospective cohort | 198 | 121 | 8–26; 14 patients unknown onset | 6‡ | 65 | 64 | 15† | 17† | NR | 74 | 66 | 10 | 10 |
| Morelli et al, 201513 | Prospective cohort | 27 | 143 | Unknown onset | 4.5 | 72.2 | 71.3 | 11.9 | 11.4 | NR | NR | NR | 0 | 3.4 |
| Obach et al, 201114 | Prospective cohort | 106 | 262 | 4.5 | 3 | 73.3† | 73† | 9† | 10† | NR | NR | NR | 5 | 7 |
| Prabhakaran et al, 201415 | Retrospective cohort | 76 | 138 | 8 | 8 | 74.4* | 64.4 | 18† | 19† | NR | 62.5* | 47.1 | 5.3 | 12.3 |
| Rai et al, 201316 | Retrospective cohort | 99 | 430 | No limitation (range 3–9) | 3–8§ | 69 | 67 68.1 63.5* |
17 | 20.1*§ 19.1*§ 17.6§ |
NR | 55.6 | 27.7 68 81.6* |
NR | NR |
| Sztriha et al, 201117 | Prospective cohort | 79 | 157 | 3–6 | 3 | 74 | 74 | 13 | 14 | 24 | NR | NR | 4 | 3 |
p<0.05.
Median value reported instead of mean value.
Control from PROACT-II trial patients who were randomized to treatment group.
Control from MERCI, Multi-MERCI, Penumbra pivotal trials, respectively.
ICH, intracranial hemorrhage; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; PI, perfusion imaging group.
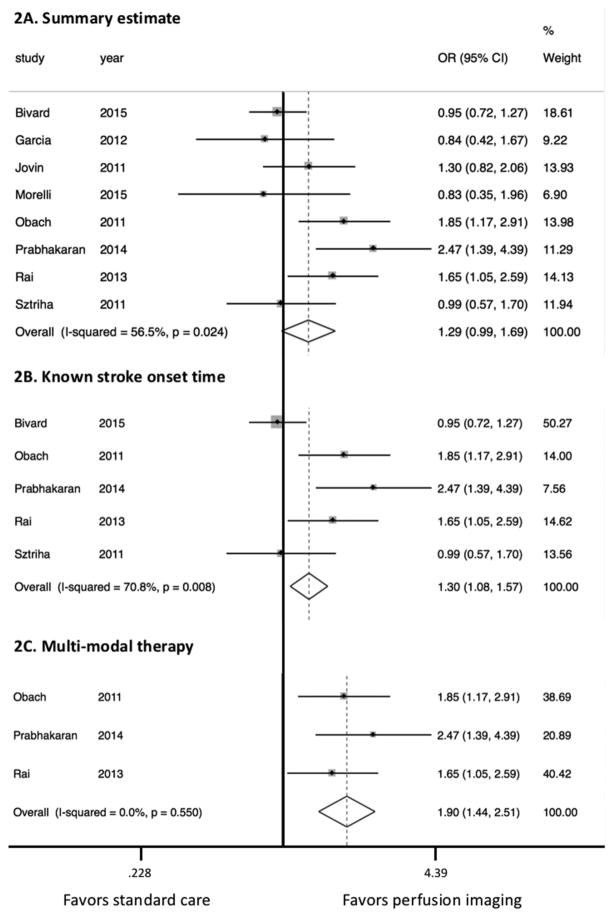
Of the patients who were treated with adjunctive perfusion imaging, 51.1% experienced a favorable clinical outcome at 3-month follow-up compared with 45.6% of patients who were treated with standard care (p=0.06). Based on the random effects model, the summary estimate suggested a trend towards favoring perfusion imaging-based treatment (figure 1A; OR 1.29, 95% CI 0.99 to 1.69; p=0.06). In the subgroup analysis based on studies that included specified stroke onset time there was a significantly higher rate of favorable outcome in the intervention group (figure 1B; OR 1.30, 95% CI 1.08 to 1.57; p<0.01). In contrast, subgroup analysis of studies that included patients with unknown or unclear stroke onset showed similar outcomes between the two groups (OR 1.08, 95% CI 0.76 to 1.53; p=0.66). Studies that used multimodal therapy showed the largest effect size, favoring perfusion imaging (figure 1C; OR 1.89, 95% CI 1.44 to 2.51; p<0.01).
Figure 1.
Forest plots of studies comparing the effect of perfusion imaging-based acute ischemic stroke treatment versus standard treatment without perfusion imaging. (A) Forest plot of all studies included in the meta-analysis. (B) Forest plot of studies that only included patients with known stroke onset time. (C) Forest plot of studies that treated eligible patients with multimodal reperfusion therapy (IV tissue plasminogen activator and endovascular thrombectomy).
Predictor of patient outcome
A total of 36 studies with 4007 patients met our criteria for inclusion under Provenzale category 2 (table 3).18–53 These studies focused on various measurements derived from perfusion imaging to assess their utility in predicting patient outcome. The primary outcome of interest ranged from early recanalization, final infarct volume, hemorrhagic transformation, and NIHSS score to mRS score (see online supplementary table S1). The most commonly used outcome measure was follow-up mRS score, with 30/36 studies reporting either 30-day or 3-month functional outcome. The imaging characteristics found to be predictive included target mismatch profile, cerebral blood volume (CBV), collateral flow, cerebral blood flow (CBF), CBV–Alberta Stroke Program Early CT score (ASPECTS), CBF–ASPECTS, ischemic core volume, and recanalization status (see online supplementary table S1).
Table 3.
Characteristics of studies examining perfusion imaging as a predictor of outcome.
| CT perfusion | MRI – PWI | CT or MRI | |
|---|---|---|---|
| No. studies | 17 | 12 | 7 |
| Study design | |||
| RCT | 0 | 0 | 1 |
| Prospective cohort | 3 | 1 | 3 |
| Retrospective cohort | 14 | 11 | 3 |
| Sample | 1845 | 1450 | 712 |
| Treatment | |||
| IV tPA only | 8 | 8 | 4 |
| Endovascular Thrombectomy (ET) only | 4 | 0 | 0 |
| Combined IV tPA and ET | 5 | 3 | 3 |
| Conservative Management | 0 | 1 | 0 |
| Primary outcomes | mRS scores; NIHSS score; ICH; recanalization rate | mRS scores; NIHSS score; ICH; final infarct volume | mRS scores; NIHSS score; ICH; final infarct volume |
Quality assessment
The majority of the studies (46 of 49) in our systematic review were of good or fair quality. Initial independent review of QA yielded different ratings in seven studies (κ=0.54); however, discussions between the reviewers led to consensus resolution of all discrepancies. Final inter-rater reliability between the two independent reviewers was strong (κ=1.0). Our assessment identified three studies with concern for bias due to numerous uncertainties in study design including patient recruitment and selection process along with lack of statistical analyses adjusting for potential confounding variables. Analysis of publication bias for Provenzale category 1 studies included in our meta-analysis did not suggest significant asymmetry of study results (see online supplementary figure S2).
DISCUSSION
This systematic review presents an up-to-date evaluation of the utility of perfusion imaging in AIS management. Using the Provenzale classification, we identified 49 studies that focused on perfusion imaging as a clinical decision-making tool (Provenzale category 1) or as a predictor of patient outcome (Provenzale category 2).
Perfusion imaging-based treatment
We identified 13 studies that examined the clinical efficacy of revascularization therapy based on physiologic data. This is significantly different from the state of evidence reported by Provenzale et al8 in 2008, when there was only a single retrospective trial that directly involved perfusion imaging in determining eligibility for reperfusion therapy. The results of our meta-analysis indicate that perfusion imaging leads to better selection of patients who will benefit from reperfusion therapy, with up to 1.9 times the odds of achieving independent functional status at 3 months (p<0.01). This is particularly important as patients selected based on perfusion status often demonstrated longer times from stroke onset and involved patients who would have traditionally been excluded from treatment based on standard eligibility criteria. Even in studies that treated patients with unknown or unclear stroke onset times, patients had similar outcomes compared with the control group without an increased complication rate. These results suggest that current treatment protocols that rely heavily on time from symptom onset may be too conservative, thereby leading to missed opportunities for additional successful treatments. Patient selection that incorporates clinical status with physiologic data may allow more patients to be treated without increasing the risk of futile treatment or complications.
While the findings of our systematic review provide additional support for multimodal imaging, the role of perfusion imaging in current clinical practice remains a topic of contention. Some of the concerns highlighted in previous publications include lack of standardized definitions, inconsistency in imaging technique, and potential delays in treatment from additional imaging.7,54 However, there is an increasing number of randomized clinical trials that incorporate perfusion imaging in their protocol, thus seeking to gain further insight in optimizing stroke treatment. The MR RESCUE trial and DEFUSE 2 studies examined the perfusion imaging characteristics of patients treated with reperfusion therapy.35,55 These studies resulted in mixed conclusions; whereas the MR RESCUE trial did not find greater response to endovascular treatment in patients with penumbra pattern on perfusion imaging, the DEFUSE 2 study reported a significantly higher rate of favorable response to reperfusion in patients with target mismatch.35,55 These two studies, however, did not use perfusion imaging profile as inclusion or exclusion criteria. In contrast, the EXTEND-IA trial specified mismatch characteristics in the selection criteria leading to 25% of patients being excluding based on perfusion imaging findings.56 Unfortunately, EXTEND-IA did not discuss in detail the perfusion imaging characteristics in their patient cohort and the extent of additional benefit that perfusion imaging selection had in their patients’ outcomes. As more clinical studies use perfusion imaging characteristics as part of their inclusion and exclusion criteria, the lack of standardization of mismatch profile and imaging triage must be addressed to improve generalizability of the study results. One important step is to better characterize perfusion imaging findings in the study patient population so that differences in primary outcomes of studies can be further analyzed to optimize and standardize the threshold for inclusion and exclusion criteria. Subsequently, this will translate to reducing both the rates of futile treatment and undertreatment of patients with ischemic stroke. Furthermore, critical evaluation of current evidence is needed to better define the cases where multimodal imaging is most suited for and may play an integral part in clinical decision-making, such as cases of unclear onset of stroke or delayed diagnosis. Currently, two prospective trials (DAWN-NCT02142283 and DEFUSE 3-NCT02586415) are examining the benefit of endovascular therapy in patients beyond 6 hours of stroke onset who are selected based on perfusion imaging characteristics. These studies are estimated to finish in July 2017 and June 2020, respectively. Until the publication of these trials, we believe that this systematic review can assist in critically evaluating the clinical utility of perfusion imaging characteristics in making treatment decisions.
Predictor of patient outcome
The body of evidence has increased significantly from previous published literature reviews on perfusion imaging.8,57 In total, our systematic review identified 36 studies that focused on perfusion imaging as a predictor of patient outcome. While there was significant variability in the methodology of these studies, 35 of the 36 studies reported that physiologic imaging characteristics (ie, perfusion status, ischemic core volume, collateral flow) were noted to be predictors of outcome. However, as Provenzale et al8 noted, studies that focused on perfusion imaging as a predictive tool have a limited capacity in guiding treatment decisions. Given the abundance of such studies, we believe that future research should focus more on prospective validation of the role of perfusion status as an eligibility criterion for treatment.
Limitations
This study has a number of limitations that must be noted. Given the nature of the systematic review, there is variability in the methodology of included studies such as treatment protocol, imaging processing, and measures of perfusion status. Future research in clarifying and standardizing perfusion imaging techniques will be important to allow broader generalizability of published reports. Another limitation is that only a subset of studies in our systematic review was included in the meta-analysis. The inclusion criteria for the meta-analysis were defined a priori to promote homogeneity in the data for analysis. Despite the exclusion of some studies from the meta-analysis, our summary estimate consisted of 2813 patients, which is larger than other studies published to date.
CONCLUSION
Advanced neuroimaging focusing on real-time physiologic status of patients represents a potential solution for addressing one of the major treatment limitations in stroke. Specifically, our systematic review of the literature and meta-analysis show that perfusion imaging may allow better patient selection leading to a greater number of patients receiving reperfusion therapy with higher rates of successful functional outcomes. The ongoing generation of perfusion-based clinical trials will move us closer to a definitive answer.
Supplementary Material
Acknowledgments
Funding This work was supported by National Institutes of Health grant numbers 5R01EB012031 and 5R01CA194533, Alberta Innovates Health Solution Clinician Fellowship (no grant number), University of Calgary Surgeon Scientist Program Scholarship (no grant number), and University of Calgary Clinician Investigator Program (no grant number).
Footnotes
Contributors WHAR: Conception, design of the work; acquisition, analysis, interpretation of data; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MBA: acquisition and interpretation of data; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. ND: Conception, design of the work; interpretation of data; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. IEA: Analysis and interpretation of data; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SWH: Conception, design of the work; analysis, interpretation of data; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests SWH: co-investigator in DAWN-NCT02142283; DEFUSE 3-NCT02586415.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/neurintsurg-2016-012751).
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–55. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evers SM, Struijs JN, Ament AJ, et al. International comparison of stroke cost studies. Stroke. 2004;35:1209–15. doi: 10.1161/01.STR.0000125860.48180.48. [DOI] [PubMed] [Google Scholar]
- 4.Chapman SN, Mehndiratta P, Johansen MC, et al. Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag. 2014;10:75–87. doi: 10.2147/VHRM.S39213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000;283:1151–8. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology. 2013;266:16–21. doi: 10.1148/radiol.12112134. [DOI] [PubMed] [Google Scholar]
- 8.Provenzale JM, Shah K, Patel U, et al. Systematic review of CT and MR perfusion imaging for assessment of acute cerebrovascular disease. AJNR Am J Neuroradiol. 2008;29:1476–82. doi: 10.3174/ajnr.A1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Heart Lung Blood Institute. [accessed 13 Mar 2016];NIH Study Quality Assessment Tools. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools.
- 10.Bivard A, Levi C, Krishnamurthy V, et al. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. 2015;138:1919–31. doi: 10.1093/brain/awv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Bermejo P, Calleja AI, Pérez-Fernández S, et al. Perfusion computed tomography-guided intravenous thrombolysis for acute ischemic stroke beyond 4. 5 hours: a case-control study. Cerebrovasc Dis. 2012;34:31–7. doi: 10.1159/000338778. [DOI] [PubMed] [Google Scholar]
- 12.Jovin TG, Liebeskind DS, Gupta R, et al. Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: retrospective multicenter analysis of 237 consecutive patients. Stroke. 2011;42:2206–11. doi: 10.1161/STROKEAHA.110.604223. [DOI] [PubMed] [Google Scholar]
- 13.Morelli N, Rota E, Immovilli P, et al. Computed tomography perfusion-based thrombolysis in wake-up stroke. Intern Emerg Med. 2015;10:977–84. doi: 10.1007/s11739-015-1299-0. [DOI] [PubMed] [Google Scholar]
- 14.Obach V, Oleaga L, Urra X, et al. Multimodal CT-assisted thrombolysis in patients with acute stroke: a cohort study. Stroke. 2011;42:1129–31. doi: 10.1161/STROKEAHA.110.605766. [DOI] [PubMed] [Google Scholar]
- 15.Prabhakaran S, Soltanolkotabi M, Honarmand AR, et al. Perfusion-based selection for endovascular reperfusion therapy in anterior circulation acute ischemic stroke. AJNR Am J Neuroradiol. 2014;35:1303–8. doi: 10.3174/ajnr.A3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rai AT, Raghuram K, Domico J, et al. Pre-intervention triage incorporating perfusion imaging improves outcomes in patients undergoing endovascular stroke therapy: a comparison with the device trials. J Neurointerv Surg. 2013;5:121–7. doi: 10.1136/neurintsurg-2011-010189. [DOI] [PubMed] [Google Scholar]
- 17.Sztriha LK, Manawadu D, Jarosz J, et al. Safety and clinical outcome of thrombolysis in ischaemic stroke using a perfusion CT mismatch between 3 and 6 hours. PLoS ONE. 2011;6:e25796. doi: 10.1371/journal.pone.0025796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn SH, d’Esterre CD, Qazi EM, et al. Occult anterograde flow is an under-recognized but crucial predictor of early recanalization with intravenous tissue-type plasminogen activator. Stroke. 2015;46:968–75. doi: 10.1161/STROKEAHA.114.008648. [DOI] [PubMed] [Google Scholar]
- 19.Alawneh JA, Jones PS, Mikkelsen IK, et al. Infarction of “non-core-non-penumbral” tissue after stroke: multivariate modelling of clinical impact. Brain. 2011;134(Pt 6):1765–76. doi: 10.1093/brain/awr100. [DOI] [PubMed] [Google Scholar]
- 20.Albers GW, Goyal M, Jahan R, et al. Relationships between imaging assessments and outcomes in solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke. Stroke. 2015;46:2786–94. doi: 10.1161/STROKEAHA.115.010710. [DOI] [PubMed] [Google Scholar]
- 21.Bivard A, Spratt N, Levi C, et al. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain. 2011;134:3408–16. doi: 10.1093/brain/awr257. [DOI] [PubMed] [Google Scholar]
- 22.Bivard A, Stanwell P, Levi C, et al. Arterial spin labeling identifies tissue salvage and good clinical recovery after acute ischemic stroke. J Neuroimaging. 2013;23:391–6. doi: 10.1111/j.1552-6569.2012.00728.x. [DOI] [PubMed] [Google Scholar]
- 23.Borst J, Berkhemer OA, Roos YB, et al. Value of computed tomographic perfusion-based patient selection for intra-arterial acute ischemic stroke treatment. Stroke. 2015;46:3375–82. doi: 10.1161/STROKEAHA.115.010564. [DOI] [PubMed] [Google Scholar]
- 24.Campbell BCV, Christensen S, Parsons MW, et al. Advanced imaging improves prediction of hemorrhage after stroke thrombolysis. Ann Neurol. 2013;73:510–19. doi: 10.1002/ana.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Wu B, Liu N, et al. Using standard first-pass perfusion computed tomographic data to evaluate collateral flow in acute ischemic stroke. Stroke. 2015;46:961–7. doi: 10.1161/STROKEAHA.114.008015. [DOI] [PubMed] [Google Scholar]
- 26.Cho T-H, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke. 2015;46:1582–9. doi: 10.1161/STROKEAHA.114.007964. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa de Rueda M, Parrilla G, Manzano-Fernández S, et al. Combined multimodal computed tomography score correlates with futile recanalization after thrombectomy in patients with acute stroke. Stroke. 2015;46:2517–22. doi: 10.1161/STROKEAHA.114.008598. [DOI] [PubMed] [Google Scholar]
- 28.Fargen KM, Chaudry I, Turner RD, et al. A novel clinical and imaging based score for predicting outcome prior to endovascular treatment of acute ischemic stroke. J Neurointerv Surg. 2013;5(Suppl 1):i38–43. doi: 10.1136/neurintsurg-2012-010513. [DOI] [PubMed] [Google Scholar]
- 29.Hesselmann V, Niederstadt T, Dziewas R, et al. Reperfusion by combined thrombolysis and mechanical thrombectomy in acute stroke: effect of collateralization, mismatch, and time to and grade of recanalization on clinical and tissue outcome. AJNR Am J Neuroradiol. 2012;33:336–42. doi: 10.3174/ajnr.A2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hom J, Dankbaar JW, Soares BP, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol. 2011;32:41–8. doi: 10.3174/ajnr.A2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue M, Mlynash M, Straka M, et al. Clinical outcomes strongly associated with the degree of reperfusion achieved in target mismatch patients: pooled data from the diffusion and perfusion imaging evaluation for understanding stroke evolution studies. Stroke. 2013;44:1885–90. doi: 10.1161/STROKEAHA.111.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SJ, Son JP, Ryoo S, et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke. Ann Neurol. 2014;76:356–69. doi: 10.1002/ana.24211. [DOI] [PubMed] [Google Scholar]
- 33.Kruetzelmann A, Köhrmann M, Sobesky J, et al. Pretreatment diffusion-weighted imaging lesion volume predicts favorable outcome after intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Stroke. 2011;42:1251–4. doi: 10.1161/STROKEAHA.110.600148. [DOI] [PubMed] [Google Scholar]
- 34.Lansberg MG, Lee J, Christensen S, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Stroke. 2011;42:1608–14. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–7. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Kim YJ, Choi JW, et al. Multimodal CT: favorable outcome factors in acute middle cerebral artery stroke with large artery occlusion. Eur Neurol. 2013;69:366–74. doi: 10.1159/000350290. [DOI] [PubMed] [Google Scholar]
- 37.Ma H, Wright P, Allport L, et al. Salvage of the PWI/DWI mismatch up to 48 h from stroke onset leads to favorable clinical outcome. Int J Stroke. 2015;10:565–70. doi: 10.1111/ijs.12203. [DOI] [PubMed] [Google Scholar]
- 38.Ma L, Gao PY, Hu QM, et al. Effect of baseline magnetic resonance imaging (MRI) apparent diffusion coefficient lesion volume on functional outcome in ischemic stroke. Neurol Res. 2011;33:494–502. doi: 10.1179/016164111X13007856084124. [DOI] [PubMed] [Google Scholar]
- 39.Mishra NK, Christensen S, Wouters A, et al. Reperfusion of very low cerebral blood volume lesion predicts parenchymal hematoma after endovascular therapy. Stroke. 2015;46:1245–9. doi: 10.1161/STROKEAHA.114.008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mlynash M, Lansberg MG, De Silva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42:1270–5. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogueira RG, Haussen DC, Dehkharghani S, et al. Large volumes of critically hypoperfused penumbral tissue do not preclude good outcomes after complete endovascular reperfusion: redefining malignant profile. Stroke. 2016;47:94–8. doi: 10.1161/STROKEAHA.115.011360. [DOI] [PubMed] [Google Scholar]
- 42.Psychogios MN, Schramm P, Frölich AM, et al. Alberta Stroke Program Early CT Scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke. 2013;44:2188–93. doi: 10.1161/STROKEAHA.113.001068. [DOI] [PubMed] [Google Scholar]
- 43.Rusanen H, Saarinen JT, Sillanpää N. Collateral circulation predicts the size of the infarct core and the proportion of salvageable penumbra in hyperacute ischemic stroke patients treated with intravenous thrombolysis. Cerebrovasc Dis. 2015;40:182–90. doi: 10.1159/000439064. [DOI] [PubMed] [Google Scholar]
- 44.Saake M, Breuer L, Gölitz P, et al. Clinical/perfusion CT CBV mismatch as prognostic factor in intraarterial thrombectomy in acute anterior circulation stroke. Clin Neurol Neurosurg. 2014;121:39–45. doi: 10.1016/j.clineuro.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Seners P, Turc G, Tisserand M, et al. Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke. 2014;45:2004–9. doi: 10.1161/STROKEAHA.114.005426. [DOI] [PubMed] [Google Scholar]
- 46.Sillanpaa N, Saarinen JT, Rusanen H, et al. The clot burden score, the Boston Acute Stroke Imaging Scale, the cerebral blood volume ASPECTS, and two novel imaging parameters in the prediction of clinical outcome of ischemic stroke patients receiving intravenous thrombolytic therapy. Neuroradiology. 2012;54:663–72. doi: 10.1007/s00234-011-0954-z. [DOI] [PubMed] [Google Scholar]
- 47.Souza LCS, Payabvash S, Wang Y, et al. Admission CT perfusion is an independent predictor of hemorrhagic transformation in acute stroke with similar accuracy to DWI. Cerebrovasc Dis. 2012;33:8–15. doi: 10.1159/000331914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warach S, Al-Rawi Y, Furlan AJ, et al. Refinement of the magnetic resonance diffusion-perfusion mismatch concept for thrombolytic patient selection: insights from the desmoteplase in acute stroke trials. Stroke. 2012;43:2313–18. doi: 10.1161/STROKEAHA.111.642348. [DOI] [PubMed] [Google Scholar]
- 49.Wardlaw JM, Muir KW, Macleod MJ, et al. Clinical relevance and practical implications of trials of perfusion and angiographic imaging in patients with acute ischaemic stroke: a multicentre cohort imaging study. J Neurol Neurosurg Psychiatry. 2013;84:1001–7. doi: 10.1136/jnnp-2012-304807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu TC, Sitton C, Potter A, et al. CTP infarct core may predict poor outcome in stroke patients treated with IV t-PA. J Neurol Sci. 2014;340:165–9. doi: 10.1016/j.jns.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Yassi N, Parsons MW, Christensen S, et al. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke. 2013;44:3039–43. doi: 10.1161/STROKEAHA.113.002396. [DOI] [PubMed] [Google Scholar]
- 52.Zhu G, Michel P, Aghaebrahim A, et al. Computed tomography workup of patients suspected of acute ischemic stroke: perfusion computed tomography adds value compared with clinical evaluation, noncontrast computed tomography, and computed tomography angiogram in terms of predicting outcome. Stroke. 2013;44:1049–55. doi: 10.1161/STROKEAHA.111.674705. [DOI] [PubMed] [Google Scholar]
- 53.Zhu G, Michel P, Aghaebrahim A, et al. Prediction of recanalization trumps prediction of tissue fate: the penumbra: a dual-edged sword. Stroke. 2013;44:1014–19. doi: 10.1161/STROKEAHA.111.000229. [DOI] [PubMed] [Google Scholar]
- 54.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;313:1451–62. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 55.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 57.Keir SL, Wardlaw JM. Systematic review of diffusion and perfusion imaging in acute ischemic stroke. Stroke. 2000;31:2723–31. doi: 10.1161/01.str.31.11.2723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.