Abstract
Deficits in cognitive functions that rely on the integrity of the frontal and temporal lobes are characteristic of normative human aging. Due to similar aging phenotypes and homologous cortical organization between nonhuman primates and humans, several species of macaque monkeys are used as models to explore brain senescence. These macaque species are typically regarded as equivalent models of cognitive aging, yet no direct comparisons have been made to support this assumption. Here we used adult and aged rhesus and bonnet macaques (Macaca mulatta and Macaca radiata) to characterize the effect of age on acquisition and retention of information across delays in a battery of behavioral tasks that rely on prefrontal cortex and medial temporal lobe networks. The cognitive functions that were tested include visuospatial short-term memory, object recognition memory, and object-reward association memory. In general, bonnet macaques at all ages outperformed rhesus macaques on tasks thought to rely primarily on the prefrontal cortex, and were more resilient to age-related deficits in these behaviors. On the other hand, both species were comparably impaired by age on tasks thought to preferentially engage the medial temporal lobe. Together, these results suggest that rhesus and bonnet macaques are not equivalent models of cognitive aging and highlight the value of cross-species comparisons. These observations should enable improved design and interpretation of future experiments aimed at understanding changes in cognition across the lifespan.
Keywords: Object recognition memory, visuospatial short-term memory, object-reward association memory, bonnet macaques, rhesus macaques, WGTA
1. INTRODUCTION
Frontal and temporal lobe-dependent cognitive functions, including spatial working memory, associative memory, and other executive processes, decline across the human lifespan even in the absence of neurodegenerative diseases [1–4]. Nonhuman primates, such as macaque monkeys, offer several advantages over other animal models in studying healthy cognitive aging. For example, humans and macaques share numerous homologous brain structures and common cytoarchitecture in the prefrontal cortex and temporal lobes, differing mainly in the sizes and laminar thicknesses of the regions [5–8]. Additionally, similar or identical behavioral tests can be used to assess multiple mental operations in both humans and macaques across their lifespans, allowing for relatively straightforward inter-species comparisons [9,10]. Importantly, while senescent rhesus macaques do develop some of the pathological markers associated with Alzheimer’s disease, the accumulation is minor, never progressing to meet criteria for disease diagnosis, which makes them particularly suitable for modeling the normal cognitive aging process [11,12]. Similar pathological studies have not been carried out on bonnet macaques. Together, these characteristics make macaque models useful for studying the molecular, cellular, and systems-level biological correlates of normative age-related changes in executive function and memory [13].
A variety of macaque species have contributed to our knowledge of cognitive aging, with the rhesus macaque (Macaca mulatta) being the most commonly used nonhuman primate model. Several other macaque species have also been used to make significant contributions to cognitive aging research, including the bonnet macaque (M. radiata [14,15]), crab-eating or long-tailed macaque (M. fascicularis [16,17]), pig-tailed macaque (M. nemestrina [17,18]), and Japanese macaque (M. fuscata, [19,20]). These different species of macaque have generally been treated as comparable models of brain senescence in the nonhuman primate literature, although no direct inter-species comparisons currently exist to support this assumption [21]. This is due in great part to the relatively large sample sizes necessary to draw meaningful conclusions. Such comparisons in rodents have revealed important disparities between species and even between strains of the same species at multiple levels of analysis [22–28]. In general, this has led the field to treat different rodents as distinct animal models. Revealing inter-species similarities and differences in learning, memory, and executive function across macaque lifespans will be a critical consideration in future experiments designed to use these animals to investigate different aspects of human cognitive aging.
The present experiment compares the performance of adult and aged bonnet and rhesus macaques on a behavioral test battery that includes tasks that have been used extensively to characterize cognitive aging in monkeys and humans [29–42]. Specifically, macaques each performed a delayed response task, a delayed nonmatching-to-sample task, and an object discrimination task. These tasks access visuospatial working memory, nonspatial object recognition memory, and object-reward association memory, respectively, across different delay periods. Importantly, the acquisition and performance of the tasks across delays depend on different regions of the frontal and parietal cortices, as well as the medial temporal lobe [10], allowing some inferences to be made with respect to the neural origin of species and age differences in behavioral outcome.
2. MATERIALS AND METHODS
2.1. Subjects
Thirty-five rhesus macaques of Indian origin were studied in these experiments (Table 1). Monkeys were separated into two age groups based on their ages at the completion of behavioral testing using 20 years of age as the cutoff. The adult group included 15 rhesus monkeys (mean 10.4 years, range 7.2–14.1 years, 3 female, 12 male), and the aged group included 20 rhesus monkeys (mean 24.5 years, range 20.6–29.5 years, 8 female, 12 male). A factor of three can be used to estimate the equivalent human age. Thus the adult group ranged in approximate human ages from 22 to 42 years old, and the aged group ranged from 62 to 89 approximate human years [43]. All rhesus macaques were born, reared by mothers, and tested at the California National Primate Research Center at the University of California, at Davis (Davis, CA). Subjects were opposite-sex pair-housed, and pregnancies were prevented by performing vasectomies on males.
Table 1.
Total number of macaques of each species and age group that participated in delayed response (DR), delayed nonmatching-to-sample (DNMS), and object discrimination (OD) tasks. Numbers of females are included in parentheses.
| Species | Age group | DR | DNMS | OD |
|---|---|---|---|---|
| Bonnet | Adult | 6 (6) | 6 (6) | 6 (6) |
| Aged | 7 (7) | 7 (7) | 7 (7) | |
| Rhesus | Adult | 15 (3) | 15 (3) | 14 (3) |
| Aged | 19 (6) | 20 (7) | 12 (4) |
In addition, 13 female bonnet macaques (Macaca radiata) were tested in this study, and were also separated into two groups with the same 20-year old age cutoff (Table 1). This resulted in two groups consisting of six adult (mean 11.4 years, range 11.0–12.2 years) and 7 aged (mean 25.3 years, range 21.3–30.9 years) bonnet macaques. Thus, the adult bonnet macaque group ranged in age from approximately 33 to 36 human years, and the aged bonnet macaques ranged from roughly 76 to 93 human equivalent years [43]. The bonnet macaques were born and reared by mothers in a naturalistic environment at the State University of New York (SUNY), Downstate, prior to being relocated to the University of Arizona (Tucson, AZ), where they were pair-housed and tested.
Macaques at both the Davis, CA and Tucson, AZ facilities were housed in temperature- and humidity-controlled environments on a 12-hour light/dark cycle. All animals in this study were in good health as documented by semiannual health exams by each facility’s on-site veterinary staff. Eye exams were included in regular health testing to ensure that no vision issues interfered with task performance. Diets consisted of monkey chow and fresh food supplements, with ad libitum access to water. Both colonies had comparable enrichment in the form of toys, treats, and videos, for example. Macaques underwent behavioral shaping to learn to be transported between vivaria and testing rooms in specialized transportation boxes. All animals were behaviorally naïve prior to these experiments. With few exceptions, each animal completed all three behavioral tasks (Table 1). The tasks were administered sequentially in the following order: delayed response (DR), delayed nonmatching-to-sample (DNMS), and then object discrimination (OD). Importantly, both colonies were overseen by the same PI and the behavioral protocols were identical for both colonies. Procedures were conducted in accordance with National Institutes of Health guidelines and the protocols were approved by the Institutional Animal Care and Use Committees at the California National Primate Research Center at UC Davis and the University of Arizona.
2.2. Behavioral experiments
2.2.1. Testing apparatus
A modified Wisconsin General Testing Apparatus (WGTA; [44]) was used for behavioral testing. Briefly, the WGTA consisted of an animal compartment, one side of which was made of spaced vertical bars that separated the macaque from a tray with three equally-spaced wells. Stimuli and rewards in these wells could be reached by the animals through the bars. Two vertical separators could be lowered across the bars to block access to the wells: a clear acrylic glass door blocked physical interaction with stimuli, while a wooden guillotine door blocked both visualization of and interaction with the stimuli. Finally, a one-way mirror allowed the experimenter to monitor each animal’s behavior without detection.
2.2.2. Delayed response
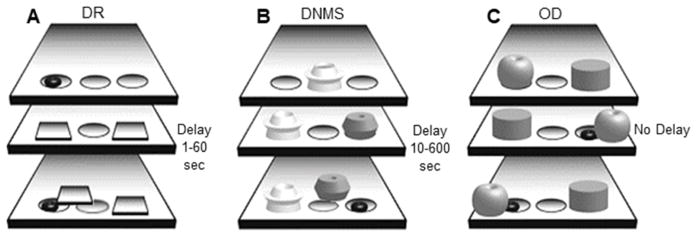
The delayed response task (DR, Figure 1A) is a standard test of visuospatial working memory [30]. In short, one of the two lateral wells in the WGTA was baited with a solid food reward. The macaque observed the baiting through the acrylic glass door. Bait location was pseudorandomized between trials so that the left and right wells were correct for an equal number of trials. Both lateral wells were then covered with identical opaque plaques so that the animal had to remember the bait location, and then the wooden door was lowered to impose a delay period. After the delay, both doors were raised and the macaque could displace only one plaque from a well. If the macaque chose the correct well, they uncovered the reward. The incorrect well was not baited. A one second delay was used during training. Learning criterion was ≥ 90% correct across 90 consecutive trials (3 days of 30 trials/day). Once criterion had been reached in the training phase, the full task began with sequentially increasing delays of 5, 10, 15, 30, and 60 seconds. Three days of testing with 30 trials/day and 20 second inter-trial intervals were conducted at each delay, and then the delay was extended regardless of performance levels.
Fig. 1.
2.2.3. Delayed nonmatching-to-sample
The delayed nonmatching-to-sample task (DNMS, Figure 1B) is used to assess nonspatial recognition memory [45]. In brief, a sample object was presented over the baited center well in the WGTA. The macaque displaced the sample object and retrieved the food reward, making certain that the sample object was observed. Following a delay period imposed by lowering the opaque door, the sample object and a novel object were presented over the two lateral wells. Only the well beneath the novel object was baited, and displacing the novel object was always the correct response. The location of the novel object was pseudorandomized to balance the number of correct left- and right-well choices. Object stimuli were plastic toys of similar size (~8 cm3). Objects were unique across trials so that no object repeated or introduced bias. Training trials had a 10 second delay. Learning criterion was ≥ 90% correct across 100 consecutive trials (5 days of 20 trials/day). After reaching criterion, 5 days of testing were completed at sequentially increasing delays. Twenty trials were completed per day at each delay of 15, 30, 60, and 120 seconds. Five trials were completed per day of testing at a 600 second delay, as is standard in the macaque-DNMS literature. A 30 second interval was imposed between trials. Animals progressed sequentially through increasing delays regardless of performance levels.
2.2.4. Object discrimination
The object discrimination task (OD, Figure 1C) is a test of object-reward association memory [39,42]. During training, macaques were presented with a pair of distinct objects over the lateral wells of the WGTA. Object stimuli were visually distinct; they did not share overlapping features. One of the two objects was consistently baited, although the locations of the correct and incorrect objects were pseudorandomized so that the rewarded object was on the left and right for an equal number of trials. For each object pair, the animal was allowed two 30 trial training sessions, administered on back-to-back days. The monkeys were given a 48 hour rest period before the probe session, which was also 30 trials long. This protocol was repeated sequentially for 4 distinct object pairs. Inter-trial intervals for all OD sessions were 15 seconds. Estimated learning trials were extracted using the state-space model described in Smith et al., 2004 [46]. Briefly, this analysis outputs the estimated trial at which each monkey learned each object pair above chance level. Each of the 4 object pairs were analyzed independently and then averaged to yield a single value per animal. For consistency we refer to this value as the OD trials to criterion measure, but note that it is not derived the same way as the DR and DNMS tasks since no predefined performance threshold was imposed on the animals.
2.3. Data analysis
2.3.1. Task acquisition (trials to criterion)
The measurement of task acquisition for each subject and for all three tasks was the number of trials to reach criterion. To assess effects of age and species in these data, we fitted a separate linear mixed model for each task with terms for age, species, and age-species interaction. The trials to criterion for subject i, TTCi, is
| (1) |
Agei and Speciesi were indicators for age (adult = −1, aged = +1) and species (rhesus = −1, bonnet = +1), respectively. Calculations were performed using linear mixed models in MATLAB (The Mathworks, Natick, MA). Posthoc tests were performed by computing the p-value of an F-test for a contrast matrix H, under the null hypothesis H*beta = 0, where beta is the fixed-effects vector. The same linear mixed models were employed to assess errors to criterion for the DR and DNMS tasks for most monkeys, with the exception of 6 aged rhesus macaques (Appendix A).
The potential for response bias to one side was quantified using an open source signal detection theory calculator (ComputerPsych LLC), which can be used to measure response bias in yes-no discrimination experiments [47]. A response table of left and right responses versus left- and right-correct conditions was employed. The model output two probability density functions that reflected the likelihood of observing two different outcomes; a correct-left response or a correct-right response. Additionally, a response bias measure was extrapolated from the model that reflects the distance in standard deviations from the center of the two distributions to the crossover point of the two distributions. This measure was extracted for all learning sessions from all animals, then averaged by animal. Threshold values of +/−0.5 standard deviations [47] were used to determine whether monkeys showed significant response biases.
2.3.2. Task performance across delays (percent correct)
For the DR and DNMS tasks, task performance was measured with the percentage of correct trials for each subject at 5 delays. The model for percentage correct in subject i at delay k, PCi,k, was
| (2) |
Posthoc tests were performed for DR and DNMS tasks across delays as described in section 2.3.1. For the DR and DNMS tasks, we also tested whether the percent correct performance of each species and age group differed from 50% chance performance at the longest delays (60 and 600 seconds, respectively) by performing t-tests that computed the probability that the values were different from a normal distribution with mean = 0.5 and unknown variance.
For the OD task, percent correct on the probe day was measured 4 times for each subject (once per object pair, with two exceptions where there was missing data for one object pair). The model for percent correct, PCi,k, for subject i and measure k = 1, 2, 3, 4, was
| (3) |
where Agei and Speciesi were as defined above. As for the DR and DNMS tasks, the final term was a random effect that allowed each subject to have a unique intercept term and accounted for the repeated measures. For all results, confidence intervals (CIs) are the lower and upper 95% bounds.
3. RESULTS
3.1. Delayed response
3.1.1. Task acquisition
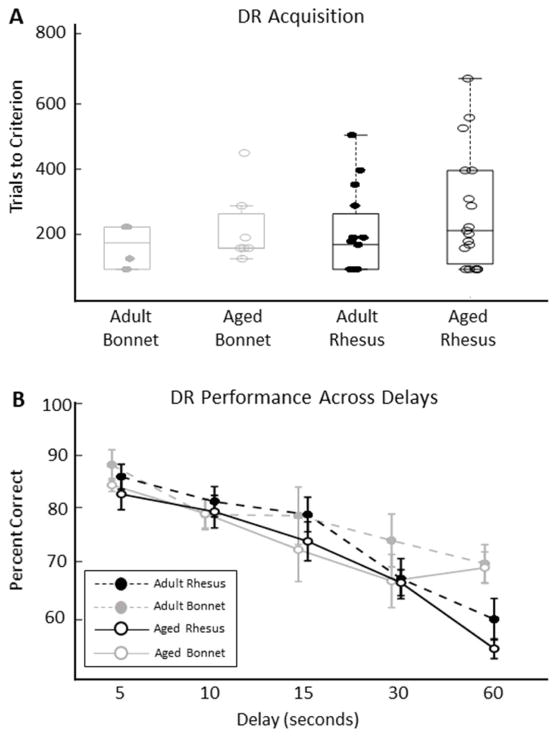
Adult and aged bonnet and rhesus macaques were trained on the delayed response (DR) test of visuospatial working memory to a criterion of 90% correct over 3 consecutive days of 30 trials per day. No left- or right-side response bias was observed in any monkey’s behavior on this task (bias measures all < 0.5; see methods). The number of trials to reach criterion did not differ significantly between age groups or species, and there was no age-by-species interaction (Figure 2A, Table 2; LMM, all p values > 0.180). Qualitatively equivalent results were found when errors to criterion were assessed instead of trials to criterion. No significant age or species effects or interactions were observed (Appendix A, Figure A1; LMM, all p values > 0.158).
Fig. 2.
Table 2.
Descriptive statistics for delayed response (DR), delayed nonmatching-to-sample (DNMS), and object discrimination (OD) tasks. Mean and standard deviation (std) for each species and age group are provided for learning data (trials to criterion) and performance data across delays (percent correct). NA indicates that delay in seconds is not applicable to the OD task.
| Task | Data type | Delay (seconds) | Statistic | Adult Bonnet | Aged Bonnet | Adult Rhesus | Aged Rhesus |
|---|---|---|---|---|---|---|---|
| DR | Trials to criterion | 1 | mean | 155 | 205.71 | 184 | 248.33 |
| std | 61.24 | 105.96 | 121.82 | 165.54 | |||
| Percent correct | 5 | mean | 87.78 | 84.31 | 85.73 | 83.77 | |
| std | 6.53 | 3 | 8.41 | 11 | |||
| 10 | mean | 79.17 | 79.34 | 81.41 | 80.09 | ||
| std | 6.43 | 6.74 | 10.2 | 12.54 | |||
| 15 | mean | 78.97 | 73.09 | 79.18 | 75.45 | ||
| std | 12.26 | 14.72 | 11.66 | 14.61 | |||
| 30 | mean | 74.67 | 67.63 | 68.03 | 67.01 | ||
| std | 11.24 | 12.24 | 13.47 | 9.97 | |||
| 60 | mean | 70.65 | 69.94 | 60.99 | 54.72 | ||
| std | 8.08 | 7.23 | 13.96 | 5.69 | |||
| DNMS | Trials to criterion | 10 | mean | 313.33 | 571.43 | 241.43 | 1048.42 |
| std | 234.83 | 385.55 | 126.54 | 409.9 | |||
| Percent correct | 15 | mean | 86.67 | 87.14 | 92.53 | 88.26 | |
| std | 5.54 | 4.56 | 3.2 | 3.5 | |||
| 30 | mean | 89.67 | 86.14 | 88.51 | 85.51 | ||
| std | 2.66 | 4.26 | 6.03 | 4.07 | |||
| 60 | mean | 86.33 | 85.83 | 86.7 | 82.78 | ||
| std | 3.27 | 5.05 | 5.76 | 5.95 | |||
| 120 | mean | 77.33 | 83.71 | 83.39 | 78.83 | ||
| std | 7.71 | 2.98 | 6.89 | 5.41 | |||
| 600 | mean | 71.83 | 60.29 | 76.13 | 65.43 | ||
| std | 26.2 | 31.73 | 10.76 | 8.06 | |||
| OD | Trials to criterion | NA | mean | 5.96 | 7.35 | 9.32 | 13.48 |
| std | 2.09 | 1.58 | 4.94 | 4.85 | |||
| Percent correct | NA – 48 hours later | mean | 92.19 | 88.09 | 90.57 | 85.98 | |
| std | 3.2 | 2.61 | 7.02 | 4.72 |
3.1.2. Task performance across delays
After reaching criterion, adult and aged bonnet and rhesus macaques performed the DR task at sequentially increasing delays from 5 seconds to 1 minute. Because all macaques had reached criterion, any differences in performance across delays can be attributed to the effect of the memory load on each species and age group. As expected, there was a significant effect of delay on the percent of correct trials out of 90 trials at each delay (Figure 2B, Tables 2–3; LMM, β3 = −0.360, CIs =[−0.415, −0.306], t(222) = −13.03, p < 1e−6). All species and age groups retained performance significantly greater than 50% chance at the longest delay (Bonferroni corrected α/4 = 0.0125; all p values < 0.0087). Although there was no significant age effect (Figure 2B; LMM, β1 = −1.28, CIs = [−4.30, 1.75], t(222) = −0.831, p = 0.407) or species effect (Figure 2B; LMM, β2 = −1.45, CIs =[−4.48, 1.58], t(222)= −0.944, p = 0.346), there was a significant species-by-delay interaction (Figure 2B, Tables 2–3; LMM, β6 = 0.121, CIs = [0.0668, 0.176], t(222)= 4.38, p = 0.0000182) indicating that performance was impaired by increasing delays for both species, but rhesus macaques were especially impaired at longer delays compared to bonnets.
Table 3.
Summary of significant age, species, and delay effects and interactions for initial acquisition and performance across delays of each behavioral task.
| DR | DNMS | OD | |
|---|---|---|---|
| Spatial short-term memory | Object recognition memory | Object-reward association memory | |
|
| |||
| Acquisition | - No significant effects |
Age*species - Age |
- Species - Age |
| Trials to reach criterion | Aged macaques were slower than adults, and aged rhesus macaques were especially impaired | Rhesus macaques were slower than bonnet macaques and aged macaques were slower than adult macaques | |
|
| |||
| Performance across delays |
- Species*delay - Delay |
- Age*delay - Delay |
- Age |
| Percent correct | Rhesus macaques scored worse than did bonnet macaques, especially at longer delays | Aged macaques scored worse than did adults, especially at longer delays | Aged macaques scored worse than did adults |
Non-significant parameter estimates were β0 = 83.75, β4 = −0.713, β5 = −0.0119 and β7 = 0.0243. Substitution in Equation 2 indicates that the slope of rhesus and bonnet macaque groups are given by (β3 − β6) = −0.48 and (β3 + β6) = −0.24, corresponding to a drop of 0.48 and 0.24 percentage points per second of delay, respectively. Posthoc comparisons indicate that the groups are significantly different at a delay of 60 seconds (F(222) = 11.6, p = 0.000766), approach significance at delay of 30 seconds (F(222) = 2.45, p = 0.119) and are not significant at delays of 5, 10 and 15 seconds.
3.2. Delayed nonmatching-to-sample
3.2.1. Task acquisition
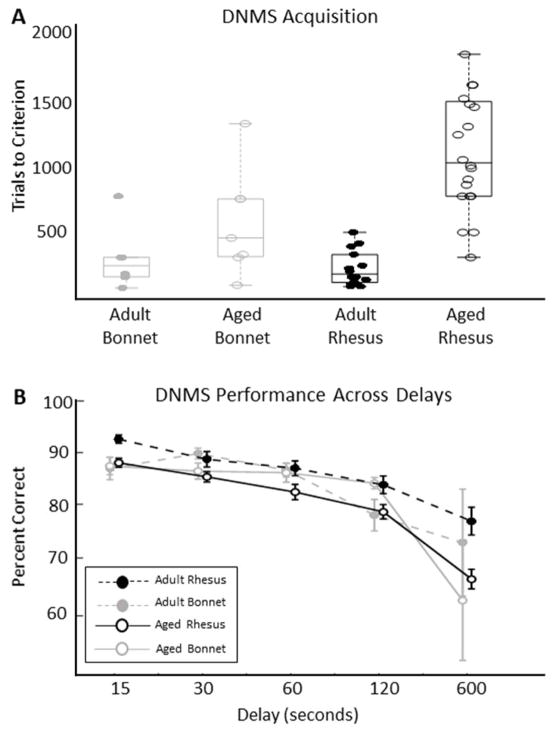
On the delayed nonmatching-to-sample (DNMS) task, the number of trials to reach criterion of 90% correct over 5 days of 20 trials per day differed between age, with older macaques requiring more trials to reach criterion (Figure 3A, Tables 2–3; LMM, β0 = 544, CIs = [441, 646], t(42) = 10.68, p < 1e−6; β1 = 266, CIs = [164, 369], t(42) = 5.23, p = 0.00001). Although rhesus macaques had a higher average number of trials to reach criterion than did bonnet macaques, the effect of species only approached significance (Figure 3A; LMM, β2 = −101.3, CIs = [−204.0, 1.47], t(42) = −1.99, p = 0.0532). Moreover, there was a significant interaction between age and species (Figure 3A, Tables 2–3; LMM, β3 = −137, CIs = [−240, −34.5], t(42)= −2.70, p = 0.0100). No left- or right-side response biases were observed in any monkey’s behavior on this task (bias measures all < 0.5; see methods).
Fig. 3.
Posthoc tests indicate a significant difference between aged rhesus and aged bonnet macaques on trials to criterion (F(42) = 12.2, p = 0.0012), but not between adult rhesus and adult bonnet macaques (F(42)= 0.227, p = 0.636). These results indicate that the species effect was driven by the aged rhesus macaques that took a markedly higher number of trials to learn the DNMS task.
An assessment of errors to criterion shows similar effects (Appendix A, Figure A2). Importantly, the age-by-species interaction was replicated (Figure A2; LMM, β3 = −40.08, CIs = [−67.3, −12.9], t(36) = −2.99, p = 0.005), as was the age effect (Figure A2; LMM, β1 = 71.02, CIs = [43.8, −98.2], t(36) = −5.30, p = 0.009e−6). However, there was a significant effect of species for errors to criterion that was not observed in the trials to criterion data (Figure A2; LMM, β2 = −32.99, CIs = [−60.2, −5.8], t(36) = −2.46, p = 0.0188).
3.2.2. Task performance across delays
The DNMS task was completed at increasing delays from 5 seconds to 10 minutes. As expected, the percent correct trials measure was affected significantly by the delay length (Figure 3B, Tables 2–3; LMM, β3 = −0.0327, CIs = [−0.0382, −0.0272], t(227) = −11.8, p < 0.00001), with monkeys performing fewer correct trials at longer delays. At the longest delay (10 minutes) adult and aged bonnet macaque performance dropped to chance level (Bonferroni corrected α/4 = 0.0125; adult and aged bonnet macaques’ p values > 0.09), while the rhesus monkey’s performance approached, but never reached, chance performance (Bonferroni corrected α/4 = 0.0125; rhesus’ p values < 1e−7). There was no significant age effect (Figure 3B; LMM, β1 = −0.427, CIs = [−2.12, 1.27], t(227) = −0.495, p = 0.621) or species effect on the percent correct DNMS trials (Figure 3B, Table 3; LMM, β2 = −0.0563, CIs = [−1.754, 1.64], t(227) = −0.0654, df = 227, p = 0.948,). Importantly, there was an age-by-delay interaction (Figure 3B, Tables 2–3; LMM, β5 = −0.00807, CIs = [−0.0135, −0.00261], t(227) = −2.91, p = 0.00393) suggesting that at longer delays, aged animals were especially impaired on the DNMS task, regardless of species.
Non-significant parameter estimates were β0 = 87.54, β4 = 1.20, β6 = −0.00363, and β7 = −0.00191. Posthoc tests for age differences at each delay indicate that age differences are not large. Specifically, only at delay of 600 seconds does the between-age-group difference approach significance (F(227) = 3.881, p = 0.050).
3.3. Object discrimination
3.3.1. Task acquisition
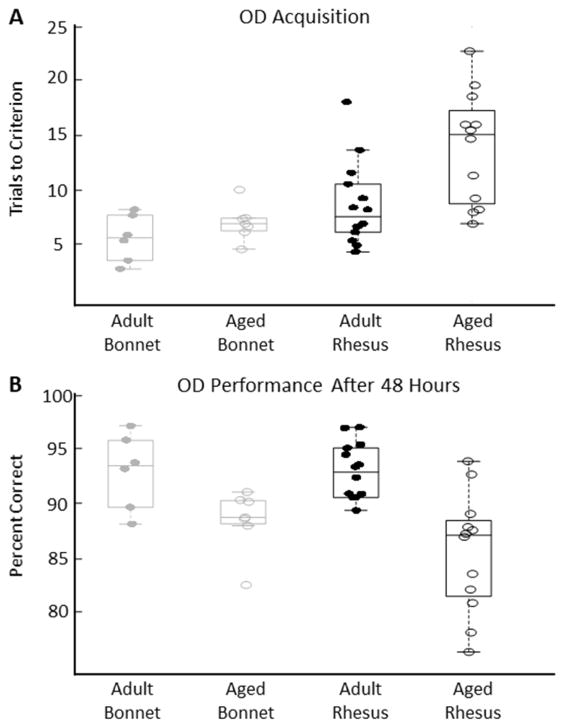
Both age and species had a significant effect on the estimated number of trials it took to perform above chance on the object discrimination task (OD, Figure 4A, Tables 2–3; LMM; β0 = 8.885, CIs = [7.76, 10.0], t(33) = 16.1, p = 16.073; Age: β1 = 1.69, CIs = [0.568, 2.82], t(33) = 3.06, p = 0.0043; Species: β2 = −2.17, CIs = [−3.30, −1.05], t(33) = −3.93, p = 0.00041). There was no age-by-species interaction (Figure 4A; LMM, β3 = −0.979, CIs = [−2.10, 0.146], t(33) = −1.77, p = 0.086).
Fig. 4.
Parameter values indicate that being in the older age group significantly increased the number of trials to reach criterion (effect size ≈ 3.38 trials) and that bonnet macaques took fewer trials than did rhesus macaques to reach criterion (effect size ≈ −4.34 trials). No left- or right-side response bias was observed in any monkey’s behavior on this task (bias measure < 0.5; see methods), with the exception of one adult rhesus macaque for whom no corrections were made (right bias measure = 0.56).
3.3.2. Task performance after 48 hours
Two days after training on the OD task, the number of correct responses out of 30 trials was measured for each of the object pairs. There was a significant effect of age (Figure 4B, Tables 2–3; LMM: β0 = 89.63, CIs = [88.7, 90.6], t(142) = 188, p < 1e−5; β1 = −2.60, CIs = [−3.53, −1.66], t(142) = −5.46, p < 1e−7), but not of species (Figure 4B; LMM, β2 = 0.506, CIs = [−0.434, 1.45], t(142) = 1.06, p = 0.289), on the percent correct responses on the OD task. Furthermore, there was no age-by-species interaction (Figure 4B; LMM, β3 = 0.546, CIs = [−0.394, 1.49], t(142) = 1.15, p = 0.253). The model fit to age indicates that the older animals averaged about 5.2 percentage points below the younger animals.
3.4 Role of sex in learning and performance across delays
While all the bonnet macaques used in the present experiment were female, the rhesus macaque groups were composed of both males and females. Due to the small number (3) of adult female rhesus monkeys in all tasks and small number (4) of aged female rhesus in the object discrimination task, we were unable to construct detailed statistical models that included sex as a covariate, as the analyses described in section 2.3 would have had very low power. We opted to include both sexes in order to maintain a large sample size. To address the concern of sex as a potential confound to species, Appendix B contains plots of trials to criterion and percent correct data for both male and female rhesus macaques on all tasks, in addition to tables of descriptive statistics, separated by sex (Appendix B, Figures B1–B3 & Tables B1–B2). In brief, visual inspection of the plots and summary statistics suggests that although there are a few situations in which male and female rhesus macaques’ data differ, rhesus monkeys of both sexes generally learned and performed across delays similarly.
4. DISCUSSION
The aim of the present study was to compare the cognitive aging processes of two macaque species, Macaca mulatta and Macaca radiata, using a battery of tasks that rely on frontal and temporal lobe functions. The novel findings are that, irrespective of age, there are task-dependent differences between rhesus and bonnet macaques’ cognitive performance, and that age-related impairment patterns across tasks differ between species. These results emphasize the importance of understanding the nuanced differences within and between model species in order to best advance our knowledge of cognitive aging.
4.1. Inter-species differences in cognitive performance
Bonnet macaques either outperformed or performed equivalently to rhesus macaques on all tasks in the present experiment. For example, bonnet macaques showed superior performance on the delayed response task (DR) at the longest delays. Whether this discrepancy between species’ short-term spatial memory is due to differences in the neural systems responsible for these behaviors or to engagement of alternate cognitive strategies remains to be determined. Additionally, in the delayed nonmatching-to-sample task (DNMS), an age-by-species interaction showed that adult macaques required fewer trials than did aged macaques to reach the learning criterion, and that this was driven by the impaired aged rhesus macaques in particular. Similarly, bonnet macaques acquired the object discrimination task (OD) in fewer trials than did rhesus macaques. These findings are consistent with the view that the neural systems or behavioral strategies that support the acquisition of object-based rules and associations are more efficient in bonnet macaques. In contrast, performance on both the DNMS and OD tasks did not differ between species once criterion had been met. Thus, after task acquisition, the two species may either resort to the use of similar neural systems or strategies, or may achieve comparable behavioral outcomes in different ways. For example, some animals may rely on an active rehearsal strategy, while others may employ a more passive recognition strategy in the DNMS task. Previously, active cognitive control and passive memory processes have been dissociated in rhesus macaques during a delayed matching-to-sample task with varied levels of distraction during the retention phase [48]. The active approach could require more cognitive control from frontal cortical engagement since these regions are critical for modulating attention and working memory functions [49]. Additionally, the use of alternate strategies to achieve cognitive behavioral goals have been demonstrated between adult and aged rats during spatial memory tasks in which older rats relied less on place information than did younger rats [50].
4.2. Age-related differences in cognitive performance
Aging selectively impacted task acquisition and performance across delays in a task- and species-dependent manner. Older rhesus macaques required substantially more trials to acquire the DNMS task than did younger rhesus macaques, while bonnet macaques showed a relatively modest age-related impairment in learning the nonmatching rule compared to their younger counterparts.
In other components of the behavioral battery, however, both bonnet and rhesus macaques showed similar age-related impairments. On the DNMS task, for example, aged monkeys of both species performed more poorly across delays, while adult monkeys maintained consistently higher levels of performance. Similarly, bonnet and rhesus macaques were comparably impaired by age on both the acquisition and performance of the OD task.
Together, the DNMS and OD results show that, although bonnet macaques were faster to acquire the tasks, aged macaques of both species were equally susceptible to delay-induced impairments in object recognition and associative memory. These findings on the effects of age and increasing delays on behavioral outcomes are consistent with previous studies conducted in rhesus and cynomolgus monkeys [31,34,36,38,42,51, no DNMS performance age effect 30]. In the current study, no age-related impairments were observed in spatial memory in the DR task. The lack of an age effect on short-term visuospatial memory in the present experiment also finds support in previous literature [36]. However, a number of studies do suggest age impairments in rhesus macaques under certain experimental conditions [16,29–31]. Possible contributing factors to the different results include the order of the tasks and naiveté of animals prior to testing [31], smaller sample sizes that may have included particularly high- or low-performing individuals [36], animals that were feral born or born into captivity [30], and different age ranges (i.e., in [31] the aged group (21–25 years) performed significantly worse across increasing delays than did the youngest group (3–7 years), but not the adult group (10–19 years), while in the present study the adult and aged rhesus groups’ age ranges were 7–14 and 20–29 years, respectively). Furthermore, the use of different macaque species [16], different training regimes [31], and the unique cognitive demands of non-WGTA delayed spatial memory tasks [16,29], may have differentially impacted the performance of adult and aged macaques on these tasks, and thus contributed to inconsistencies in the literature.
4.3. Potential neural bases for species and age differences
Lesion studies in adult animals have shown that the behaviors tested in the present experiment rely on the proper function of specific frontal and temporal lobe structures. This enables us to make inferences about potential sources of species- and age-related differences observed across tasks in the present experiment. In regard to the DR task, performance is severely impaired in cynomolgus monkeys after lesions of the dorsolateral prefrontal cortex (dlPFC), whereas it is more preserved when the ventromedial prefrontal cortex (vmPFC) is lesioned [52]. dlPFC is also particularly important for performance across delays in a spatial delayed alternation task, but not for a spatial task without delays [53]. Furthermore, within dlPFC, spatially-tuned neurons show persistent cellular activity during the delay period of a spatial working memory task [54]. This elevated activity during delays has been proposed to be a single-cell correlate of spatial working memory [55]. There are a number of possible explanations for the observation that the bonnet macaques had superior performance compared to rhesus macaques at longer delays on the DR task. Among them is a speculation that bonnet macaques may have more effective recurrent collateral activity that enables more reliable recall of visuospatial memory traces as delay lengths increase. This could be mediated, for example, by inter-species differences in components of the molecular cascades that instantiate rapid strengthening and weakening of PFC synapses (i.e., different HCN channel distributions, cAMP signaling, ACh levels; [56]).
With regard to the acquisition of the DNMS task, rhesus macaques with dlPFC lesions show postoperative impairments [57]. If the DNMS task is learned before dlPFC removal, however, macaques have a relatively minor postoperative impairment in reaching learning criterion [52]. The extent of PFC lesions are inconsistent across these studies, which may also contribute to their different results. In contrast to macaques with dlPFC lesions, those with vmPFC lesions fail to achieve criterion, even after 2000 learning trials, despite preoperative training [52]. Although it is unknown how vmPFC lesions impact DNMS task acquisition in the absence of preoperative training, these studies suggest that both PFC subdivisions are normally engaged in learning the DNMS task. In this study, aging compromised DNMS task acquisition more severely in rhesus than in bonnet macaques. This finding could be explained by inter-species differences in the aging of vmPFC and/or dlPFC. For example, in aged rhesus monkeys, both subdivisions have reduced α−1 and α−2 receptor binding densities and decreased grey matter volumes, compared to young adults [58,59]. These observations are correlated with slower acquisition of the DNMS task [58,59]. There is a more extensive literature on dlPFC aging than on vmPFC aging; demonstrations of age-related dlPFC changes include thin spine loss, smaller thin spine head volumes, decreased synapse density, reduced microcolumn strength, and thinning of layer I – all of which are associated with behavioral impairments in rhesus monkeys [10,36,60,61]. It remains to be determined whether any of the age-related changes observed in rhesus macaques are less pronounced in bonnet macaques, or whether aged bonnet macaques better engage compensatory neural resources to more efficiently learn the nonmatching object recognition rule.
The medial temporal lobes (MTL) also play a role in the successful execution of the cognitive tests included in this study. Thus, changes in MTL function may have mediated some of the diversity that was observed in behavioral outcomes. When the hippocampus is lesioned with ibotenic acid, or when the perirhinal cortex is removed, DNMS performance is impaired at delays of ≥ 1 minute [62,63]. Cynomolgus monkeys with combined lesions of the hippocampus and amygdala perform more poorly than do controls on the DNMS task, and are more sensitive to longer delays, even after achieving the same learning criterion [64]. In rhesus macaques, data that were originally thought to support the idea that combined hippocampus and amygdala lesions impaired DNMS performance have now been demonstrated to reflect the severe visual recognition memory deficits induced by perirhinal cortex lesions alone [65,66]. Hippocampal lesions also slow DNMS task acquisition, but not as severely as do PFC lesions [52,57,62]. These findings are consistent with the idea that successful learning of the DNMS task may rely more heavily on PFC function, while delayed object recognition memory over longer intervals preferentially engages the MTL.
With regard to object-reward association memory, cynomolgus monkeys that have previously acquired the OD task and undergo hippocampus-amygdala lesions do not retain object-reward associations after a 48-hour interval as well as do controls [64]. Furthermore, the proper function of area TE is important for visual object discrimination, on which both the DNMS and OD tasks rely [67,68]. Perirhinal cortex ablation also disrupts visual object learning, thereby impairing object discrimination [69]. The OD task itself tests not just the ability to remember objects but also associations with rewards, implicating the basal ganglia [70]. Activity in subdivisions of the striatum has been observed to synchronize with activity in the PFC during a rewarded visual category learning task in macaques, and coherent reactivation events in the hippocampus and ventral striatum are thought to contribute to place-reward associations in rats [71,72]. Dorsal striatal neurons in particular have been shown to discriminate rewarded from unrewarded visual conditioned stimuli [73]. Thus, it is possible that species- and age-related alterations in the striatum and its interactions with frontal and temporal cortex further contribute to differences in learning and remembering visual stimulus-reward associations.
Together, these studies illustrate the range of MTL and other brain processes involved in object recognition and the formation and memory of object-reward associations. A variety of age-related alterations of the MTL may account for the similar cognitive impairments observed in aged macaques on the DNMS and OD tasks, but not the DR task in the present study. These possibilities include, for example, decreased cerebral blood volume in the dentate gyrus [74], hyperexcitability of CA3 principal cells [75], and a decreased density of somatostatin-expressing inhibitory interneurons in stratum oriens of CA1 and CA3 [75], all of which are observed in behaviorally-impaired aged rhesus macaques [74,75].
4.4. Limitations of the study
This set of experiments benefits from an unusually large sample size of macaques under the same protocols for multiple cognitive tests. Data on both learning and performance of these behavioral tasks across delays in two species of two comparable age ranges also strengthens the results. Despite these advantages, conclusions about species differences in cognition and aging should be considered in light of the imbalanced overall group sample sizes and sex distributions between species. Sex has been shown to influence spatial memory in a training-dependent fashion and interact with age in rhesus macaques [76]. Although sex did not appear to systematically alter the learning and performance of rhesus macaques in the present experiments (Appendix B), larger sample sizes will be necessary to address the role it may play in species- and task-specific cognitive aging. Furthermore, the macaques of the two species included in the current study had unique upbringings and lived in different locations; the rhesus macaques were born, reared, and then cross-sex pair housed in one colony, while the bonnet macaques were born and reared at another location, then moved to a new colony where they were single-sex pair housed. Differences in enrichment procedures between the two facilities could have had differential effects on each groups’ learning and memory capacities.
4.5. Conclusions
Taken together, the results of the present study suggest that, in general, bonnet macaques execute tasks that are classically considered to be PFC-dependent more effectively than do rhesus macaques at all ages, and appear to be more resilient to the effects of age on the PFC-dependent functions probed in this battery. Both species, however, are comparably vulnerable to the effects of age on tasks traditionally thought to rely on MTL-dependent functions. Importantly, although the functions of the frontal and temporal lobes can be dissociated to some extent, bidirectional communication between these structures supports the organization, consolidation, and retrieval of memories [77–80]. For instance, crossed unilateral ablations of frontal cortex and perirhinal cortex in macaques disrupts intra-hemispheric interactions between these structures and produces marked deficits in recognition memory, even though this cognitive capacity is thought to depend mainly on MTL processes [81,82]. This suggests that multiple regional networks can participate in executing tasks like those in the current study. Thus, different behavioral strategies that macaques may have used to carry out a given task could have engaged these frontal and temporal networks to different degrees, and may in part explain some of the differences observed in behavioral outcomes. With respect to the less pronounced age effects in bonnet macaques, for example, it is possible that they more effectively invoked compensatory neural resources on PFC-dependent tasks. This idea gains support from observations in humans that under some task conditions, high-performing older individuals recruit additional networks to enhance levels of performance, while their lower-performing counterparts do not [83–86]. More specifically, a posterior-to-anterior shift of activity and increased bilateral engagement during cognitive tests in fMRI experiments are thought to serve compensatory roles [83–86]. It must be noted, however, that bonnet macaques are not exempt from age-related declines in all PFC functions, as exemplified by work showing that aged bonnet macaques are more susceptible than are young adults to interference during a recognition memory test [87].
Because bonnet and rhesus macaques show distinct cognitive aging patterns across tasks, one or the other species may be better suited as an experimental model, depending on the aspect of cognition that is under investigation. As additional cross-species comparisons are conducted, an increasingly holistic view of the strengths and weaknesses of each model with respect to specific cognitive functions will emerge. More precise characterizations will provide a better context for interpreting existing and novel data, and for guiding future experimental designs. This will help to best advance our understanding of, and ability to ameliorate, cognitive and brain health across the lifespan for an increasingly aged human population [88,89].
HIGHLIGHTS.
Cognitive performance across age is species- and task-dependent
Species effects are only observed in prefrontal cortex-dependent tasks
Both species are similarly impaired by age on medial temporal lobe-dependent tasks
Aged bonnet macaques are less impaired on prefrontal cortex-reliant tasks
Acknowledgments
Thanks to Kojo Plange, Sara Burke, Michele Permenter, Julie Vogt, Mary Roberts, Carmel Stanko, Tracy Ojakangas Deborah Kent, Lisa Novak, and Sania Fong for macaque behavioral training, Peter Rapp for support, and Wonn Pyon and Katherine Andersh for assistance with data entry. Additional thanks to Luann Snyder, Michelle Albert, and Michelle Carroll for administrative assistance.
This work was supported by the National Institutes of Health [R01 AG003376, R01 AG050548, P51 RR000169], the McKnight Brain Research Foundation, and the University of Arizona.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 2.Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn. 2000;26:1170–1187. doi: 10.1037/0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cogn Brain Res. 2000;10:197–206. doi: 10.1016/S0926-6410(00)00029-X. [DOI] [PubMed] [Google Scholar]
- 4.Flicker C, Bartus RT, Crook TH, Ferris SH. Effects of aging and dementia upon recent visuospatial memory. Neurobiol Aging. 1984;5:275–283. doi: 10.1016/0197-4580(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 5.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 6.Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 7.David A, Pierre L. Hippocampus B. Oxford University Press; 2009. Hippocampal Neuroanatomy; pp. 37–114. [DOI] [Google Scholar]
- 8.Insausti R. Comparative anatomy of the entorhinal cortex and hippocampus in mammals. Hippocampus. 1993;3:19–26. [PubMed] [Google Scholar]
- 9.Dean RL, Bartus RT. Handb Psychopharmacol. Springer US; Boston, MA: 1988. Behavioral Models of Aging in Nonhuman Primates; pp. 325–392. [DOI] [Google Scholar]
- 10.Hara Y, Rapp PR, Morrison JH. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age (Omaha) 2012;34:1051–73. doi: 10.1007/s11357-011-9278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toledano A, Álvarez MI, López-Rodríguez AB, Toledano-Díaz A, Fernández-Verdecia CI. Does Alzheimer’s disease exist in all primates? Alzheimer pathology in non-human primates and its pathophysiological implications. Neurol (English Ed) 2012;27:354–369. doi: 10.1016/j.nrleng.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Uno H, Walker LC. The age of biosenescence and the incidence of cerebral beta-amyloidosis in aged captive rhesus monkeys. Ann N Y Acad Sci. 1993;695:232–5. doi: 10.1111/j.1749-6632.1993.tb23058.x. [DOI] [PubMed] [Google Scholar]
- 13.Hof PR, Gilissen EP, Sherwood CC, Duan H, Lee PWH, Delman BN, Naidich TP, Gannon PJ, Perl DP, Erwin JM. Comparative neuropathology of brain aging in primates. Interdiscipl Top Gerontol. 2002;31:130–154. [Google Scholar]
- 14.Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-associated deficits in pattern separation functions of the perirhinal cortex: a cross-species consensus. Behav Neurosci. 2011;125:836–47. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neill J, Fitten LJ, Siembieda DW, Ortiz F, Halgren E. Effects of guanfacine on three forms of distraction in the aging macaque. Life Sci. 2000;67:877–85. doi: 10.1016/s0024-3205(00)00681-0. [DOI] [PubMed] [Google Scholar]
- 16.Darusman HS, Call J, Sajuthi D, Schapiro SJ, Gjedde A, Kalliokoski O, Hau J. Delayed response task performance as a function of age in cynomolgus monkeys (Macaca fascicularis) Primates. 2014;55:259–267. doi: 10.1007/s10329-013-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terry AV, Buccafusco JJ, Jackson WJ, Prendergast MA, Fontana DJ, Wong EH, Bonhaus DW, Weller P, Eglen RM. Enhanced delayed matching performance in younger and older macaques administered the 5-HT4 receptor agonist, RS 17017. Psychopharmacology (Berl) 1998;135:407–15. doi: 10.1007/s002130050529. [DOI] [PubMed] [Google Scholar]
- 18.Short R, Williams DD, Bowden DM. Circulating antioxidants as determinants of the rate of biological aging in pigtailed macaques (Macaca nemestrina) Journals Gerontol. 1997;52A:B26–B38. doi: 10.1093/gerona/52A.1.B26. [DOI] [PubMed] [Google Scholar]
- 19.Kubo N, Kato A, Nakamura K. Deterioration of planning ability with age in Japanese monkeys (Macaca fuscata) J Comp Psychol. 2006;120:449–455. doi: 10.1037/0735-7036.120.4.449. [DOI] [PubMed] [Google Scholar]
- 20.Itoh K, Izumi A, Kojima S. Object discrimination learning in aged Japanese monkeys. Behav Neurosci. 2001;115:259–270. doi: 10.1037/0735-7044.115.2.259. [DOI] [PubMed] [Google Scholar]
- 21.Lacreuse A, Herndon JG. Nonhuman Primate Models of Cognitive Aging. Anim Model Hum Cogn Aging. 2009:29–59. doi: 10.1007/978-1-59745-422-3. [DOI] [Google Scholar]
- 22.Potier B, Lamour Y, Dutar P. Age-related alterations in the properties of hippocampal pyramidal neurons among rat strains. Neurobiol Aging. 1993;14:17–25. doi: 10.1016/0197-4580(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 23.Josef Van Der Staay F, Blokland A. Behavioral differences between outbred Wistar, inbred Fischer 344, Brown Norway, and hybrid Fischer 344 × Brown Norway rats. Physiol Behav. 1996;60:97–109. doi: 10.1016/0031-9384(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 24.Wyss J. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol Aging. 2000;21:671–681. doi: 10.1016/S0197-4580(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 25.Whishaw I. A comparison of rats and mice in a swimming pool place task and matching to place task: Some surprising differences. Physiol Behav. 1995;58:687–693. doi: 10.1016/0031-9384(95)00110-5. [DOI] [PubMed] [Google Scholar]
- 26.Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461–5. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- 27.Welker C, Woolsey TA. Structure of layer IV in the somatosensory neocortex of the rat: Description and comparison with the mouse. J Comp Neurol. 1974;158:437–453. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: debilitating effects on short-term memory. J Gerontol. 1978;33:858–71. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- 30.Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1969;9:3566–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991;12:99–111. doi: 10.1016/0197-4580(91)90048-O. [DOI] [PubMed] [Google Scholar]
- 32.Voytko ML, Tinkler GP. Cognitive function and its neural mechanisms in nonhuman primate models of aging, Alzheimer disease, and menopause. Front Biosci. 2004;9:1899–914. doi: 10.2741/1370. [DOI] [PubMed] [Google Scholar]
- 33.Presty SK, Bachevalier J, Walker LC, Struble RG, Price DL, Mishkin M, Cork LC. Age differences in recognition memory of the rhesus monkey (Macaca mulatta) Neurobiol Aging. 1987;8:435–40. doi: 10.1016/0197-4580(87)90038-8. [DOI] [PubMed] [Google Scholar]
- 34.Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9:495–502. doi: 10.1016/S0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- 35.Shamy JLT, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27:1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WGM, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30 doi: 10.1523/jneurosci.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartus RT, Dean RL, Fleming DL. Aging in the rhesus monkey: effects on visual discrimination learning and reversal learning. J Gerontol. 1979;34:209–19. doi: 10.1093/geronj/34.2.209. [DOI] [PubMed] [Google Scholar]
- 38.Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: Spatial and object reversal learning. Neurobiol Aging. 1995;16:947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- 39.Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-associated deficits in pattern separation functions of the perirhinal cortex: a cross-species consensus. Behav Neurosci. 2011;125:836–47. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Burke SN, Ryan L, Barnes CA. Characterizing cognitive aging of recognition memory and related processes in animal models and in humans. Front Aging Neurosci. 2012;4:15. doi: 10.3389/fnagi.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104:876–884. doi: 10.1037/0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- 43.Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- 44.Harlow HF, Bromer JA. A test apparatus for monkeys. Psychol Rec. 1938;2:433–435. [Google Scholar]
- 45.Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991;12:481–486. doi: 10.1016/0197-4580(91)90077-W. [DOI] [PubMed] [Google Scholar]
- 46.Smith AC, Frank LM, Wirth S, Yanike M, Hu D, Kubota Y, Graybiel AM, Suzuki WA, Brown EN. Dynamic analysis of learning in behavioral experiments. J Neurosci. 2004;24:447–461. doi: 10.1523/jneurosci.2908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macmillan NA, Creelman CD. Response bias: Characteristics of detection theory, threshold theory, and nonparametric indexes. Psychol Bull. 1990;107:401–413. doi: 10.1037/0033-2909.107.3.401. [DOI] [Google Scholar]
- 48.Basile BM, Hampton RR. Dissociation of active working memory and passive recognition in rhesus monkeys. Cognition. 2013;126:391–396. doi: 10.1016/j.cognition.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 50.Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can J Psychol. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- 51.Walker LC, Kitt CA, Struble RG, Wagster MV, Price DL, Cork LC. The neural basis of memory decline in aged monkeys. Neurobiol Aging. 1988;9:657–666. doi: 10.1016/S0197-4580(88)80130-1. [DOI] [PubMed] [Google Scholar]
- 52.Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–61. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- 53.Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- 54.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 55.Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Arnsten AFT, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–75. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB. Impairment in delayed nonmatching to sample following lesions of dorsal prefrontal cortex. Behav Neurosci. 2012;126:772–80. doi: 10.1037/a0030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav Brain Res. 2005;160:208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Alexander GE, Chen K, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, Barnes CA. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J Neurosci. 2008;28 doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–74. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Cruz L, Roe DL, Urbanc B, Inglis A, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure correlates with cognitive decline in ventral but not dorsal area 46 of the rhesus monkey. Neuroscience. 2009;158:1509–20. doi: 10.1016/j.neuroscience.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 63.Buffalo EA, Ramus SJ, Squire LR, Zola SM. Perception and recognition memory in monkeys following lesions of area TE and perirhinal cortex. Learn Mem. 2000;7:375–82. doi: 10.1101/LM32100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- 65.Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9:4355–70. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meunier M, Bachevalier J, Mishkin M, Murray E. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13 doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem. 1999;6:572–99. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean P. Effects of inferotemporal lesions on the behavior of monkeys. Psychol Bull. 1976;83:41–71. doi: 10.1037/0033-2909.83.1.41. [DOI] [PubMed] [Google Scholar]
- 69.Buckley MJ, Gaffan D. Impairment of visual object-discrimination learning after perirhinal cortex ablation. Behav Neurosci. 1997;111:467–475. doi: 10.1037/0735-7044.111.3.467. [DOI] [PubMed] [Google Scholar]
- 70.Pennartz CMA, Ito R, Verschure PFMJ, Battaglia FP, Robbins TW. The hippocampal–striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci. 2011;34:548–559. doi: 10.1016/j.tins.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83:216–225. doi: 10.1016/j.neuron.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CMA. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White JK, Monosov IE. Neurons in the primate dorsal striatum signal the uncertainty of object–reward associations. Nat Commun. 2016;7:12735. doi: 10.1038/ncomms12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci. 2004;101:7181–6. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomé A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region- specific network dysfunction. Mol Psychiatry. 2016;21:1257–62. doi: 10.1038/mp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lacreuse A, Kim CB, Rosene DL, Killiany RJ, Moss MB, Moore TL, Chennareddi L, Herndon JG. Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta) Behav Neurosci. 2005;119:118–26. doi: 10.1037/0735-7044.119.1.118. [DOI] [PubMed] [Google Scholar]
- 77.Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–7. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- 78.Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–43. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 79.Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the Rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10:851–65. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- 80.Lavenex P, Amaral DG. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 81.Parker A, Gaffan D. Interaction of frontal and perirhinal cortices in visual object recognition memory in monkeys. Eur J Neurosci. 1998;10:3044–3057. doi: 10.1046/j.1460-9568.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 82.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bangen KJ, Kaup AR, Mirzakhanian H, Wierenga CE, Jeste DV, Eyler LT. Compensatory brain activity during encoding among older adults with better recognition memory for face-name pairs: an integrative functional, structural, and perfusion imaging study. J Int Neuropsychol Soc. 2012;18:402–13. doi: 10.1017/S1355617712000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The Posterior-Anterior Shift in Aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 86.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 87.Gray DT, Smith AC, Burke SN, Gazzaley A, Barnes CA. Attentional updating and monitoring and affective shifting are impacted independently by aging in macaque monkeys. Behav Brain Res. 2017;322:329–338. doi: 10.1016/j.bbr.2016.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leeson GW. Increasing longevity and the new demography of death. Int J Popul Res. 2014;(2014):1–7. doi: 10.1155/2014/521523. [DOI] [Google Scholar]