Abstract
Purpose of review
Stroma is a broad term referring to the connective tissue matrix in which other cells reside. It is composed of diverse cell types with functions such as extracellular matrix maintenance, blood and lymph vessel development, and effector cell recruitment. The tissue microenvironment is determined by the molecular characteristics and relative abundances of different stromal cells such as fibroblasts, endothelial cells, pericytes, and mesenchymal precursor cells. Stromal cell phenotypic heterogeneity is due to embryonic developmental lineage, stages of differentiation to other cell types, and activation states. The interaction of the immune system with the stroma is critical to wound healing, cancer, and a wide range of inflammatory diseases. Here we review recent studies of rheumatic diseases that use functional genomics and single-cell technologies to identify and characterize stromal cell types associated with pathogenesis.
Recent findings
High dimensional strategies using mRNA sequencing, mass cytometry, and fluorescence activated cell-sorting with fresh primary tissue samples are already producing detailed views of what is happening in diseased tissue in rheumatoid arthritis, inflammatory bowel disease, and cancer. Fibroblasts positive for CD90 (THY1) are enriched in the synovium of rheumatoid arthritis patients, and also in prostate cancer tumors. Single cell RNA-seq offers potential for even more discoveries about the stroma in the near future.
Summary
Stromal cells form the microenvironment of inflamed and diseased tissues. Functional genomics is producing an increasingly detailed view of subsets of stromal cells with pathogenic functions in rheumatic diseases and cancer. Future genomics studies will discover disease mechanisms by perturbing molecular pathways with chemokines and therapies known to affect patient outcomes. Functional genomics studies with large sample sizes of patient tissues will identify patient subsets with different diseases or treatment responses.
Keywords: Functional genomics, stromal cells, fibroblasts, microenvironment, rheumatic disease, inflammation
Introduction
The stroma is the background of every tissue in the body, and plays a critical role in supporting the normal epithelium, forming the general architecture of the tissue, and modulating the local microenvironment. While many types of cells constitute the stroma in varying proportions across tissues, fibroblasts are the major constituent of stroma in all tissues. One of the first demonstrations of functional fibroblast heterogeneity was done 40 years ago, showing three distinct phenotypes of fibroblast response to prostaglandin [1]. Many studies since then have shown how fibroblasts in different systems support tissue homeostasis and shape immune responses [2].
Fibroblasts are central to tissue biology because they organize the extracellular matrix in which other cells are embedded, and they communicate with these cells [2]. Since cell functions are dependent on the context in which they are embedded, in vitro studies may fail to capture physiologically relevant context essential to tissue functions. A major impediment to discovery is the lack of well-defined cell surface markers that distinguish different functional types of fibroblasts. One marker, the GPI-anchored protein Thy-1 (CD90), has been studied in many tissues. Thy-1 is differentially expressed on different types of fibroblasts in the spleen [3], lung [4,5], female reproductive system [6,7], ocular orbit [8], liver [9], and prostate cancer tumors [10].
Functional genomics offers a promising path forward to understand the diversity of fibroblast functions in health and disease. By assessing global measures of all genes, it has the potential to find new factors important for production and degradation of extracellular matrix, development of blood and lymph vessels, wound healing, and communication with leukocytes during inflammation.
Currently, most genomic studies have effectively averaged signals in bulk high dimensional assays across millions of cells with diverse phenotypes, which complicates interpretations about the roles of specific cell types. In other words, differences in cell composition cannot be distinguished from differences in gene regulation at the whole tissue level. More recently, studies using low-input genomics on subpopulations sorted by cell surface markers and studies using single-cell genomics demonstrated that the average signals can be explained by independent contributions from different proportions of functionally distinct single cells. Further, single-cell technologies are directly addressing the challenge of discovering cell surface markers that distinguish functional subsets of cells relevant to tissue biology and disease pathology.
Fibroblasts mediate inflammation in chronic inflammatory diseases
The role of stromal cells in orchestrating local inflammatory response is becoming increasingly appreciated. Studies are suggesting that the diversity of stromal cells across anatomical sites may contribute to location-specific disease development [11–13]. There are consistent patterns of anatomical distribution for diseases like RA, IBD, psoriasis, ankylosing spondylitis, and various types of cancers. For example, ankylosing spondylitis often affects lower extremities or the spine, while RA affects small distal joints of the hands and feet [11]. These anatomical patterns suggest a few possibilities: site-specific local cell types, local responses to systemic signals, or environmental factors like mechanical stress that affect cells locally [11]. One recent study shows how expression of developmental HOX genes can influence the TNF induced activation of inflammatory molecular pathways in knee synovial fibroblasts [12].
The synovium is a thin membrane composed mostly of fibroblasts and macrophages that surrounds the joint capsule and contains the synovial fluid. In RA, it is the critical site of chronic inflammation [14]. A healthy synovium has a 1 to 2 cell thick lining layer and a sublining layer with blood vessels, lymph vessels, fibroblasts, collagen fibers, nerve fibers, and few leukocytes [15]. In RA, the inflamed synovium has a hyperplastic lining layer, dramatic expansion of blood vessels in the sublining, and dense infiltration by inflammatory leukocytes [15]. While all of the cells play some role in chronic inflammation, synovial fibroblasts orchestrate joint destruction through production of proteinases that degrade the cartilage and cytokines that activate bone resorbing osteoclasts [16].
Autoimmune diseases, such as rheumatoid arthritis, progress from an initiation phase of generating autoantibodies to an effector phase of continuous feedback between stromal cells and leukocytes [17,18]. In RA, a vicious cycle of inflammation is fueled by fibroblasts in the synovium producing chemokines that recruit leukocytes to damaged tissue, where these partners then continuously stimulate each other to maintain chronic inflammation [19,20]. A recent study are shows that there is a similar feedback loop between leukocytes and stromal cells t in IBD. In IBD fibroblasts are activated by TNF and other chemokines, causing them to produce IL6 and other chemokines to activate leukocytes [21]. In contrast to the cartilage destruction in RA, the fibroblasts of the gastrointestinal tract are responsible for excessive extracellular matrix deposition, causing fibrosis [22].
As a result of the feedback loops involving communication between stromal cells and leukocytes, fibroblasts undergo long-lasting epigenetic changes, become resistant to apoptosis, and maintain a proliferative, destructive, invasive, and migratory phenotype [23–25]. Cultured synovial fibroblasts from inflamed joints of RA patients, but not from healthy joints, express major histocompatibility complex (MHC) class II molecules in response to IFN-gamma [26]. This is consistent with a similar demonstration that lung fibroblasts within the human lung express HLA-DR and costimulatory molecules, modulating the CD4 T cell response to pathogens during infection [27]. Hence, many synovial and lung fibroblasts are actually antigen presenting cells: they are able to extract and present antigens to CD4+ T cells [26–28].
Exploring stromal cell biology with functional genomics
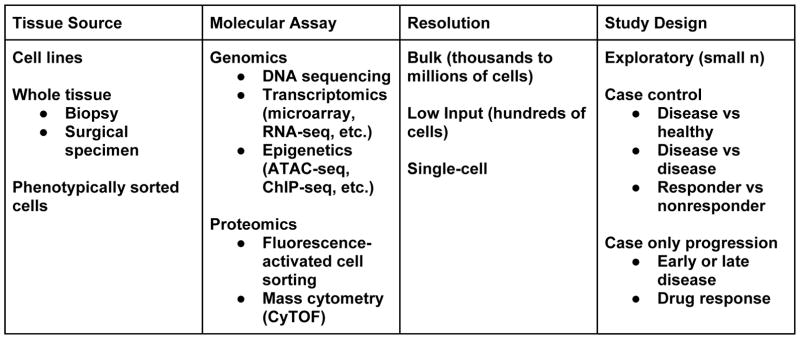
Functional genomics, or discovery of biological insights through genome-wide assays, has revolutionized the way in which we understand cell biology and tissue physiology. While there are several approaches to apply genomics to a target cell type, here we summarize some strategies and glimpse the future of stromal cell genomics (Figure 1).
Figure 1. Functional genomics in stromal cells.
All studies involve careful considerations of the costs and benefits for selection of a tissue source, molecular assay, desired resolution, and study design. Critically, effective application of high dimensional molecular assays requires effective strategies to disaggregate whole tissue sources without disturbing biological signal.
Three main approaches are used to apply functional genomics to understand stromal cells in rheumatic disease. First, whole tissue transcriptomics by microarray, RNA-seq, or DNA methylation is used to contrast cases and controls. This type of analysis highlights molecular markers of disease such as cytokines that are elevated in the disease state, or cell surface markers of cell types that are differentially abundant. Second, transcriptomics of purified cell populations by fluorescence activated cell sorting (FACS) after tissue dissociation or laser-dissection reveals the distinct genomic signals underlying the average signals seen in bulk tissues, helping to focus on a smaller set of cell types that are most relevant for disease pathogenesis. Third, the latest single cell RNA-seq techniques can be performed on dissociated tissue samples to provide a completely unbiased view of thousands of cells in patient samples. This overcomes the need for cell surface markers to sort different cell types, and can give a holistic perspective of how abundances and functions of different cell types relate to each other in patient tissues.
To date, many functional genomics studies have been done using cultured cell lines. The high abundance of cellular material from a culture enables a wide range of investigational strategies. However, cell lines are outside the context of the whole tissue, and they are altered by the process of growing in vitro. In many instances, studies contrast a small number (<10) of cases and controls, sometimes including perturbations by therapies or gene knockdowns. A major advantage of cultured cell lines derived from patient tissues is that they can be expanded to provide enough RNA, DNA, and other cellular material necessary for most genomics assays. In a study with differential gene expression analysis of microarrays comparing macrophages and fibroblasts from synovial samples from RA and OA, authors found that both cell types are producing inflammatory cytokines in RA [29]. POSTN and TWIST1 were identified as key regulators of fibroblast invasiveness in RA, and subsequent siRNA experiments confirmed this finding [29]. Microarray analysis with OA and RA fibroblasts revealed constitutive upregulation of the TGFB pathway in RA fibroblasts that resulted in greater expression of MMP11 [30]. Genome-wide methylation microarrays and RNA-seq data shows differential methylation and expression of developmental HOX genes between fibroblasts taken from hips and knees from RA and OA individuals [13]. In knee synovial fibroblasts, silencing the HOTAIR lncRNA in the HOX cluster resulted in greater expression of constitutive and TNF-induced collagenase MMP1 [12]. This suggests that the HOX genes not only control stromal cell phenotypes during embryonic development, but also the immunoregulatory phenotypes during disease pathogenesis.
Another common approach is to apply functional genomics to whole tissue samples. Arguably, functional genomics assays with tissue samples are a promising path toward understanding disease mechanisms that may be specific to subsets of cells in vivo, and how these mechanisms vary between individuals. However, fresh human derived tissue samples are a more limited resource, and must be used judiciously. For RA, these samples are often derived from joint arthroplasty samples or biopsy samples. In many instances obtaining whole tissue has to be considered in the clinical context of the elective surgical intervention that enabled collection of the tissue. One exciting opportunity for querying tissue samples is the use of research synovial biopsies [31]. While tissue quantities are limited with biopsies, they enable querying tissues under prescribed clinical conditions, and are possible in a trial setting. Transcriptomics by microarrays enabled definition of a rule set for discriminating rheumatoid arthritis from osteoarthritis and healthy controls [32]. Microarray analysis of RA, OA, and normal donors found that RA patients can be divided into two subsets with high or low expression of PRG4 in the synovium, and low PRG4 expression is associated with more aggressive stage of disease [33]. Pathogenic fibroblast destruction of cartilage, as well as recruitment and retainment of leukocytes is amplified by hematopoietically derived cytokines like TNF and interleukin 6 (IL6). Transcriptomics studies with synovial biopsy tissues revealed that treatment with tocilizumab, methotrexate, adalimumab, and rituximab reduces the mRNA levels of IL6 and many other chemokines and T cell activation genes after 12 weeks of therapy [34].
The power of whole tissue transcriptomic profiling was recently revealed in a study of a cohort of patients with Crohn’s disease (CD) and ulcerative colitis (UC); in that study West et al found a TNFi-resistant pathway specific to gut-resident stromal cells [21]. Analysis of public transcriptomics data showed that oncostatin M (OSM) is more highly expressed in biopsy tissues from IBD patients. The authors found that OSM is mostly derived from hematopoietic cells, and stromal fibroblasts have highest expression of the OSM receptor (OSMR) in the gut. These PDPN+ fibroblasts are highly abundant in inflamed tissue from Crohn’s disease (CD) and ulcerative colitis (UC) patients, and they produce inflammatory chemokines in response to TNF and OSM stimulation. This study highlights the novel system of leukocyte-stromal communication behind the chronic inflammation in IBD.
The OSM result may translate to human therapies. Notably, OSM has phase 1 and 2 trials for RA showing that humanized anti-OSM monoclonal antibodies are well tolerated, and OSMR-deficient mice are healthy and viable. Large scale functional genomics studies with biopsies can help to identify different subsets of patients. In a large scale study with 210 Crohn’s disease patients and 35 healthy controls, CD patients were clearly distinguished from healthy controls by biopsy gene expression of 29 genes enriched with genetic risk variants [35]. Further, the 29 gene signal was also able to identify patients more likely to progress to complicated disease.
Opportunities to advance fibroblast biology with single-cell genomics
Transcriptomic profiling of single cells is a powerful technique to reveal cellular and molecular heterogeneity in tissues with complex and dynamic cellular compositions. These technologies are being applied at scale in efforts such as the human single-cell atlas, with the goal of providing a reference map of all common types of cells in the human body [36]. These types of large scale studies will be valuable to all researchers. The ability to visualize molecular pathways at a single cell level can transform our understanding of cell identity and function. Single cell RNA-seq overcomes the need for cell surface markers to sort different cell types, and can give a holistic perspective of how abundances and functions of different cell types relate to each other in patient tissues.
Recent advances in immunoprofiling and transcriptomic assay technologies has created an opportunity to assay sorted stromal cells directly from tissues [37]. Advances in sequencing library construction have lowered the requirements for genomics assays to 10s or 100s of cells per sample [38].
Discoveries from single-cell genomics will include new cell types specific to anatomical sites or disease states, dysregulations of molecular pathways exclusive to particular cell types, and genetic effects on those pathways [39]. One recent study demonstrates that genetic effects on gene expression can be masked when assaying total peripheral blood mononuclear cells (PBMCs). This single-cell eQTL analysis shows that the same variant influences expression of TSPAN13 in CD4 T cells, but not in dendritic cells or other cell types [40].
Investigators have begun applying single cell technologies to query tissues in pathological conditions. Critical to successful application of single cell technologies include preserving cell viability and effective dissociation of tissue into single cell suspension. Recent advances in droplet-based platforms for single cell RNA-seq have enabled analysis of large number of single cells from a single donor [41,42].
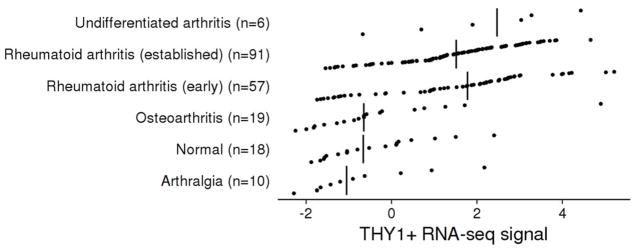
Two independent studies have identified Thy-1 positive and negative fibroblasts in the synovium [41,43]. By applying cell sorting, low-input RNA-seq, and single-cell RNA-seq to synovial biopsies, they identified different functional subsets of fibroblasts [43]. Notably, the Thy-1 positive fibroblasts were found to be significantly overabundant in the synovial tissue of rheumatoid arthritis (RA) relative to osteoarthritis [43]. We checked if we could identify overabundance of Thy-1 positive fibroblasts in a recent study of synovial biopsies [44], and found that RA has higher relative expression of Thy-1 positive fibroblast genes than Thy-1 negative fibroblast genes (Figure 2). Expression of Thy-1 influences multiple aspects of fibroblast biology such as propensity to differentiate into adipocytes [45], and this protein is also involved in neuronal development and oncogenesis [5].
Figure 2. THY1+ synovial fibroblast gene expression in RA.
First, we selected 4 genes from single-cell RNA-seq data [43] that are highly expressed in sublining CD34-THY1+ fibroblasts (THY1, POSTN, TGFBI, CCL2) and 4 genes highly expressed in lining CD34-THY1− fibroblasts (CLIC5, TSPAN7, SLC2A12, SELP). Next, we took the synovial biopsy RNA-seq expression data from a recent study (GSE89408), computed the average of each gene set, and plotted the ratio of the gene sets. Vertical lines are medians. Early RA is significantly different than OA (Wilcox rank sum test) (P = 2.0e–4), and established RA is also significantly different than OA (P = 1.8e–5). This result is consistent with the idea that synovial biopsy RNA-seq signal is explained by an overabundance of the THY1+ fibroblast population in RA patients relative to OA.
Despite these exciting initial applications, little is known about the complexity of stromal populations, and single cell genomics offers a powerful way to capture the heterogeneity and compare gene expression of membrane proteins, transcriptional regulators, and signaling pathways that might reveal phenotypic and functional subsets of fibroblasts. Building on the foundation of the human single-cell atlas, new studies will focus on relevant tissues from patients. In the coming years, each new study of single cells from chronic inflammatory diseases will give deeper insights about the commonalities and differences between them. For example, the Accelerating Medicines Partnership (AMP) is a consortium where one focus is generating large scale datasets that include single cell genomics from disease affected tissues in RA and SLE patients. These datasets will show how cell type abundances and functions change over the course of disease and in response to therapy. We envision many future studies will take advantage of the new clarity provided by single-cell technologies.
Conclusion
In the past few years, oncologists have moved from general chemotherapy to personalized treatment based on genetic measurements. Treatment of rheumatic disease can follow a similar path as ongoing and future studies pave the way forward [46]. For example, measuring OSM gene expression in IBD patients may promise to predict successful response to anti-TNF therapy [21]. As synovial biopsy tissue acquisition becomes more common, researchers will have more opportunities to use functional genomics technologies to uncover disease relevant molecular phenotypes. Researchers will assay transcriptomics, DNA methylation, chromatin accessibility, histone modifications, and other molecular signals to find associations with disease activity and response to therapies. Armed with this information, biopsy data may be then used to predict optimal treatments for patients. The advancement of molecular phenotyping will help to deliver on the promise of precision medicine, where therapies are adjusted for maximum benefit to each individual patient.
Functional genomics has the potential to reveal common and differential sets of molecular pathways across multiple anatomical sites and diseases. Stromal biology has thus far been mostly focused on single systems like cancer associated fibroblasts from prostate cancer, gut fibroblasts from IBD patients, or synovial fibroblasts from inflamed joints. Many pathological perturbations of stromal cells are likely to be different across diseases. However, careful analysis of the genome-wide measurements from functional genomics techniques can reveal the commonalities in molecular phenotypes across those diseases. For example, the cytokine gene expression profiles of prostate cancer associated fibroblasts are similar to synovial fibroblasts from rheumatoid arthritis joints as well as fibroblasts from the gut.
We envision that synovial biopsy samples will be treated in vitro to study responses to known and novel therapies. We expect that hematopoietic and stromal cells from treatment responders might behave differently than cells from nonresponders, and future studies will find the molecular basis for these differences. These findings will give unprecedented resolution about pathogenic functions in the synovium, and will suggest new diagnostic assays and therapeutic targets that might be addressed by existing or future therapies [15].
Key Points.
The potential to define disease related cell types in stroma is rapidly expanding with emerging single-cell technologies such as single-cell RNA-seq and mass cytometry.
CD90 (THY1) positive synovial fibroblasts are significantly overabundant in rheumatoid arthritis, indicative of major phenotypic changes in the rheumatoid synovium.
Functional genomics can shed light on the interaction of the stroma with the immune system.
Acknowledgments
None
Financial support and sponsorship
This work is supported in part by funding from the Ruth L. Kirschstein National Research Service Award (F31AR070582) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K.S.); the Rheumatology Research Foundation Scientist Development Award (K.W); the National Institutes of Health (UH2AR067677, 1U01HG009088, and 1R01AR063759), and the Doris Duke Charitable Foundation Grant #2013097 (S.R.).
Footnotes
Conflicts of interest
None
References
- 1*.Ko SD, Page RC, Narayanan AS. Fibroblast heterogeneity and prostaglandin regulation of subpopulations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3429–32. doi: 10.1073/pnas.74.8.3429. This article is one of the first to demonstrate fibroblast subsets that are functionally distinct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Sorrell JM, Caplan AI. Fibroblasts—a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. doi: 10.1016/S1937-6448(09)76004-6. Fibroblast diversity and significance of interactions with other cell types, especially in the skin. [DOI] [PubMed] [Google Scholar]
- 3.Borrello MA, Phipps RP. Differential Thy-1 expression by splenic fibroblasts defines functionally distinct subsets. Cell Immunol. 1996 Nov 1;173(2):198–206. doi: 10.1006/cimm.1996.0268. [DOI] [PubMed] [Google Scholar]
- 4.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994 Sep;72(3):283–92. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 5.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006 Jun;20(8):1045–54. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 6.Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP. Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(−) human female reproductive tract fibroblasts. Am J Pathol. 2001 Sep;159(3):925–35. doi: 10.1016/S0002-9440(10)61768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malmström E, Sennström M, Holmberg A, Frielingsdorf H, Eklund E, Malmström L, et al. The importance of fibroblasts in remodelling of the human uterine cervix during pregnancy and parturition. Mol Hum Reprod. 2007 May;13(5):333–41. doi: 10.1093/molehr/gal117. [DOI] [PubMed] [Google Scholar]
- 8.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002 Feb;32(2):477–85. doi: 10.1002/1521-4141(200202)32:2<477::AID-IMMU477>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Dudas J, Mansuroglu T, Batusic D, Saile B, Ramadori G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007;329(3):503–14. doi: 10.1007/s00441-007-0437-z. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009 Jun 15;69(9):991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Ospelt C, Frank-Bertoncelj M. Why location matters - site-specific factors in rheumatic diseases. Nat Rev Rheumatol. 2017 Jul;13(7):433–42. doi: 10.1038/nrrheum.2017.96. This review gives a lucid perspective of the relation between embryonic development and anatomical disease patterning. [DOI] [PubMed] [Google Scholar]
- 12*.Frank-Bertoncelj M, Trenkmann M, Klein K, Karouzakis E, Rehrauer H, Bratus A, et al. Epigenetically-driven anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. Nat Commun. 2017 Mar 23;8:14852. doi: 10.1038/ncomms14852. One of the first demonstrations that HOX expression in fibroblasts is directly related to inflammatory response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai R, Hammaker D, Boyle DL, Morgan R, Walsh AM, Fan S, et al. Joint-specific DNA methylation and transcriptome signatures in rheumatoid arthritis identify distinct pathogenic processes. Nat Commun. 2016 Jun 10;7:11849. doi: 10.1038/ncomms11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223(1):252–70. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 15.van de Sande MG, Baeten DL. Immunopathology of synovitis: from histology to molecular pathways. Rheumatology. 2016 Apr;55(4):599–606. doi: 10.1093/rheumatology/kev330. [DOI] [PubMed] [Google Scholar]
- 16.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013 Jan;9(1):24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005 Dec;1(2):102–10. doi: 10.1038/ncprheum0047. [DOI] [PubMed] [Google Scholar]
- 18.Sabat R, Wolk K. Psoriasis. John Wiley & Sons, Ltd; 2014. Pathogenesis of psoriasis; pp. 28–48. [Google Scholar]
- 19.Ospelt C, Kyburz D, Pierer M, Seibl R, Kurowska M, Distler O, et al. Toll-like receptors in rheumatoid arthritis joint destruction mediated by two distinct pathways. Ann Rheum Dis. 2004 Nov;63(Suppl 2):ii90–1. doi: 10.1136/ard.2004.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Buckley CD. Why do leucocytes accumulate within chronically inflamed joints? Rheumatology. 2003 Dec 1;42(12):1433–44. doi: 10.1093/rheumatology/keg413. Fundamental concepts about interactions between stromal cells and leukocytes in the synovial microenvironment, focusing on chemokines and cytokines involved in the rheumatoid joint. [DOI] [PubMed] [Google Scholar]
- 21**.West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017 May;23(5):579–89. doi: 10.1038/nm.4307. Demonstration that fibroblasts are necessary for IBD, and fibroblast gene expression predicts whether patients respond to anti-TNF therapy. Also, a key example of the value of public gene expression data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016 Jan;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 23.Böhm BB, Freund I, Krause K, Kinne RW, Burkhardt H. ADAM15 adds to apoptosis resistance of synovial fibroblasts by modulating focal adhesion kinase signaling. Arthritis Rheum. 2013 Nov;65(11):2826–34. doi: 10.1002/art.38109. [DOI] [PubMed] [Google Scholar]
- 24.Whitaker JW, Shoemaker R, Boyle DL, Hillman J, Anderson D, Wang W, et al. An imprinted rheumatoid arthritis methylome signature reflects pathogenic phenotype. Genome Med. 2013 Apr 30;5(4):40. doi: 10.1186/gm444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartok B, Hammaker D, Firestein GS. Phosphoinositide 3-kinase δ regulates migration and invasion of synoviocytes in rheumatoid arthritis. J Immunol. 2014 Mar 1;192(5):2063–70. doi: 10.4049/jimmunol.1300950. [DOI] [PubMed] [Google Scholar]
- 26.Boots AM, Wimmers-Bertens AJ, Rijnders AW. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology. 1994 Jun;82(2):268–74. [PMC free article] [PubMed] [Google Scholar]
- 27.Hutton AJ, Polak ME, Spalluto CM, Wallington JC, Pickard C, Staples KJ, et al. Human Lung Fibroblasts Present Bacterial Antigens to Autologous Lung Th Cells. J Immunol. 2017 Jan 1;198(1):110–8. doi: 10.4049/jimmunol.1600602. [DOI] [PubMed] [Google Scholar]
- 28.Tran CN, Davis MJ, Tesmer LA, Endres JL, Motyl CD, Smuda C, et al. Presentation of arthritogenic peptide to antigen-specific T cells by fibroblast-like synoviocytes. Arthritis & Rheumatism. 2007;56(5):1497–506. doi: 10.1002/art.22573. [DOI] [PubMed] [Google Scholar]
- 29.You S, Yoo S-A, Choi S, Kim J-Y, Park S-J, Ji JD, et al. Identification of key regulators for the migration and invasion of rheumatoid synoviocytes through a systems approach. Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):550–5. doi: 10.1073/pnas.1311239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohlers D, Beyer A, Koczan D, Wilhelm T, Thiesen H-J, Kinne RW. Constitutive upregulation of the transforming growth factor-beta pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2007;9(3):R59. doi: 10.1186/ar2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orr C, Sousa E, Boyle DL, Buch MH, Buckley CD, Cañete JD, et al. Synovial tissue research: a state-of-the-art review. Nat Rev Rheumatol. 2017 Aug;13(8):463–75. doi: 10.1038/nrrheum.2017.115. [DOI] [PubMed] [Google Scholar]
- 32.Woetzel D, Huber R, Kupfer P, Pohlers D, Pfaff M, Driesch D, et al. Identification of rheumatoid arthritis and osteoarthritis patients by transcriptome-based rule set generation. Arthritis Res Ther. 2014 Apr 1;16(2):R84. doi: 10.1186/ar4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ungethuem U, Haeupl T, Witt H, Koczan D, Krenn V, Huber H, et al. Molecular signatures and new candidates to target the pathogenesis of rheumatoid arthritis. Physiol Genomics. 2010 Nov 29;42A(4):267–82. doi: 10.1152/physiolgenomics.00004.2010. [DOI] [PubMed] [Google Scholar]
- 34.Ducreux J, Durez P, Galant C, Nzeusseu Toukap A, Van den Eynde B, Houssiau FA, et al. Global molecular effects of tocilizumab therapy in rheumatoid arthritis synovium. Arthritis Rheumatol. 2014 Jan;66(1):15–23. doi: 10.1002/art.38202. [DOI] [PubMed] [Google Scholar]
- 35.Marigorta UM, Denson LA, Hyams JS, Mondal K, Prince J, Walters TD, et al. Transcriptional risk scores link GWAS to eQTLs and predict complications in Crohn’s disease. Nat Genet [Internet] 2017 Aug 14; doi: 10.1038/ng.3936. Available from: http://dx.doi.org/10.1038/ng.3936. [DOI] [PMC free article] [PubMed]
- 36.Regev A, Teichmann S, Lander ES, Amit I, Benoist C, Birney E, et al. The Human Cell Atlas [Internet] bioRxiv. 2017:121202. cited 2017 Aug 16. Available from: http://www.biorxiv.org/content/early/2017/05/08/121202.
- 37*.Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012 May;13(5):499–510. doi: 10.1038/ni.2262. One of the first studies to isolate stromal cell subpopulations from fresh human tissues and assay with transcriptomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goetz JJ, Trimarchi JM. Transcriptome sequencing of single cells with Smart-Seq. Nat Biotechnol. 2012 Aug;30(8):763–5. doi: 10.1038/nbt.2325. [DOI] [PubMed] [Google Scholar]
- 39.Tanay A, Regev A. Scaling single-cell genomics from phenomenology to mechanism. Nature. 2017 Jan 18;541(7637):331–8. doi: 10.1038/nature21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Wijst MGP, Brugge H, de Vries DH, Franke LH. Single-cell RNA sequencing reveals cell-type specific cis-eQTLs in peripheral blood mononuclear cells [Internet] bioRxiv. 2017:177568. cited 2017 Aug 17. Available from: http://www.biorxiv.org/content/early/2017/08/17/177568.
- 41.Stephenson W, Donlin LT, Butler A, Rozo C, Rashidfarrokhi A, Goodman SM, et al. Single-Cell RNA-Seq Of Rheumatoid Arthritis Synovial Tissue Using Low Cost Microfluidic Instrumentation [Internet] bioRxiv. 2017:140848. doi: 10.1038/s41467-017-02659-x. cited 2017 Aug 6. Available from: http://www.biorxiv.org/content/early/2017/05/22/140848. [DOI] [PMC free article] [PubMed]
- 42.Zheng C, Zheng L, Yoo J-K, Guo H, Zhang Y, Guo X, et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017 Jun 15;169(7):1342–56. e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Mizoguchi F, Slowikowski K, Marshall JL, Wei K, Rao DA, Chang SK, et al. Single Cell Transcriptomics And Flow Cytometry Reveal Disease-Associated Fibroblast Subsets In Rheumatoid Arthritis [Internet] bioRxiv. 2017:126193. cited 2017 Aug 6. Available from: http://www.biorxiv.org/content/early/2017/05/26/126193.
- 44.Guo Y, Walsh AM, Fearon U, Smith MD, Wechalekar MD, Yin X, et al. CD40L-Dependent Pathway Is Active at Various Stages of Rheumatoid Arthritis Disease Progression. J Immunol. 2017 Jun 1;198(11):4490–501. doi: 10.4049/jimmunol.1601988. [DOI] [PubMed] [Google Scholar]
- 45*.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003 Oct;163(4):1291–300. doi: 10.1016/S0002-9440(10)63488-8. Demonstration that Thy-1 negative, but not positive, fibroblasts can be differentiated into lipofibroblasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruderman EM, Mandelin AM, Perlman HR. Willie Sutton Was Right: It’s Time to Turn to the Synovium to Drive Rheumatoid Arthritis Therapy. J Rheumatol. 2016 Dec;43(12):2089–91. doi: 10.3899/jrheum.161285. [DOI] [PMC free article] [PubMed] [Google Scholar]