Abstract
Background
Controlled ovarian stimulation in assisted reproduction technology (ART) may alters endometrial receptivity by an advancement of endometrial development. Recently, technical improvements in vitrification make deferred frozen-thawed embryo transfer (Def-ET) a feasible alternative to fresh embryo transfer (ET). In endometriosis-related infertility the eutopic endometrium is abnormal and its functional alterations are seen as likely to alter the quality of endometrial receptivity. One question in the endometriosis ART-management is to know whether Def-ET could restore optimal receptivity in endometriosis-affected women leading to increase in pregnancy rates.
Objective
To compare cumulative ART-outcomes between fresh versus Def-ET in endometriosis-infertile women.
Materials and methods
This matched cohort study compared def-ET strategy to fresh ET strategy between 01/10/2012 and 31/12/2014. One hundred and thirty-five endometriosis-affected women with a scheduled def-ET cycle and 424 endometriosis-affected women with a scheduled fresh ET cycle were eligible for matching. Matching criteria were: age, number of prior ART cycles, and endometriosis phenotype. Statistical analyses were conducted using univariable and multivariable logistic regression models.
Results
135 in the fresh ET group and 135 in the def-ET group were included in the analysis. The cumulative clinical pregnancy rate was significantly increased in the def-ET group compared to the fresh ET group [58 (43%) vs. 40 (29.6%), p = 0.047]. The cumulative ongoing pregnancy rate was 34.8% (n = 47) and 17.8% (n = 24) respectively in the Def-ET and the fresh-ET groups (p = 0.005). After multivariable conditional logistic regression analysis, Def-ET was associated with a significant increase in the cumulative ongoing pregnancy rate as compared to fresh ET (OR = 1.76, CI95% 1.06–2.92, p = 0.028).
Conclusion
Def-ET in endometriosis-affected women was associated with significantly higher cumulative ongoing pregnancy rates. Our preliminary results suggest that Def-ET for endometriosis-affected women is an attractive option that could increase their ART success rates. Future studies, with a randomized design, should be conducted to further confirm those results.
Introduction
Endometriosis is a gynecologic disorder defined by the presence of endometrial-like tissue outside the uterine cavity [1]. Approximately 5–10% of reproductive-age women are affected by endometriosis, and at least one third of them are infertile [2]. While there is a fair amount of evidence demonstrating that endometriosis has a negative impact on fertility, the pathogenesis remains unclear. Current findings suggest several pathways may be involved, including inflammatory changes in peritoneal fluid resulting in altered sperm-oocyte interaction, reduced functional ovarian tissue, and hampered endometrial receptivity due to changes in the eutopic endometrium [2].
Differences in the eutopic endometrium between women with and without endometriosis is now well established [3–5]. Functional changes are believed to largely account for alteration in the quality of endometrial receptivity. Indeed, pro-inflammatory mediators, as well as candidate genes related to implantation failure and infertility, were found to be deregulated in endometrial tissues of women with endometriosis as compared to endometrial tissues from disease-free women [6,7].
Assisted reproductive technology (ART) is a reliable therapeutic option for managing endometriosis-related infertility [8]. ART protocols comprise a controlled ovarian stimulation (COS) to obtain oocytes for fertilization, which is usually followed by a fresh embryo transfer (ET). A growing concern in recent years is that COS, which generates a high level of sex-steroids, may alter endometrial receptivity by enhancing endometrial development, which could in turn contribute to a lower chance of becoming pregnant [9–11]. Technical improvements in vitrification have recently led to the emergence of a new strategy: the “freeze–all approach” [11,12]. This deferred frozen-thawed embryo transfer (Def-ET), initially developed to prevent ovarian hyper-stimulation syndrome in high-risk women [13], is now being applied to a wider population in order to improve endometrial implantation [14–17]. It consists of cryopreservation of the entire embryo-cohort, allowing the embryo transfer to be carried out separately from the COS in a subsequent cycle.
As endometriosis is an estrogen-dependent disease, it is reasonable to speculate that a high level of steroids induced by COS could interfere with endometrial receptivity: modification of gene expression profiles observed in women with endometriosis could be exacerbated by COS, leading to an inhospitable environment during the implantation process. Hence, COS could exacerbate the endometriosis-related reduction in the probability of implantation.
Thus, one of the main questions is whether deferring embryo transfers could restore optimal receptivity in endometriosis-affected women, thereby leading to an increase in pregnancy rates.
We hence undertook this matched cohort study to compare ART outcomes between fresh ET versus Def-ET in endometriosis-infertile women.
Materials and methods
Study design
We conducted a cohort study including all In Vitro Fertilization / Intra Cytoplasmic Sperm Injection (IVF/ICSI) cycles for endometriosis infertility between 01/10/2012 and 31/12/2014 in the ART unit at the university-based reproductive medicine center of our institution. The study cohort was matched in terms of patient age, the number of prior ART cycles (the number of prior ART cycle is defined as the number of prior COS leading to at least one embryo transfer with no pregnancy obtained [18]) and endometriosis phenotypes (superficial peritoneal endometriosis (SUP), ovarian endometrioma (OMA), or deeply infiltrating endometriosis (DIE) [19]. Two groups were compared: (i) a study group comprised of “exposed” women who received a Def-ET for the first transfer attempt and (ii) an “unexposed” control group comprised of women who received fresh ET for the first transfer attempt. For both groups, supernumerary embryos were frozen and transferred if pregnancy was not obtained first. All data were fully anonymized before use. Data collection and utilisation were approved by the National Data Protection Authority (Commission Nationale de l’Informatique et des Libertés, CNIL n° 1988293 v 0).
Patient cohort and the matching procedure
For both groups the inclusion criteria for this cohort study were the following: women with endometriosis-related infertility, requirement of ART (IVF or ICSI), age ≤ 43 years, and having one or more embryo(s) available for transfer. Exclusion criteria were: women without endometriosis, vitrified oocyte procedures, no embryo obtained or transferred, and patients already included in another ART research protocol.
There are three phenotypes for endometriotic lesions: superficial peritoneal endometriosis (SUP), ovarian endometrioma (OMA), or deeply infiltrating endometriosis (DIE). In our referral center, all endometriosis infertile women underwent an appropriate pre-ART imaging work-up in order to obtain a clear diagnosis and staging of the endometriosis. For DIE and OMA phenotypes, diagnosis and staging of endometriosis was based on previously published imaging criteria using transvaginal ultrasound (TVUS) [20–22], magnetic resonance imaging (MRI) [23–26], or transrectal ultrasonography [27]. Additionally, for patients with a history of prior surgery, the diagnosis was also confirmed by histological proof of endometriosis. Patients were classified as SUP in the following cases: no OMA nor DIE lesions at the pre-ART imaging work-up and previously histologically proven superficial peritoneal endometriosis. Since these phenotypes are frequently associated, patients were assigned to the group corresponding to the most severe lesion according to a previous published classification [28,29], ordered from the least to the most severe, i.e. SUP, OMA, and then DIE.
The decision about whether to defer the embryo transfer or to perform a fresh embryo transfer for the first attempt was based on a joint decision by the patient and the doctor. The information process was conducted according to the specific elements of Braddock and colleagues [30] required for completeness of informed decision making. During the appointment, the practitioner fully involves the patient in the strategy to choose. After having informed the women of her role in decision making, the doctor clearly explains the nature of the decision and the 2 choices are proposed: The fresh embryo strategy or the deferred embryo strategy. Pros and cons of each strategy are carefully discussed during the appointment, especially the results of extended embryo culture, vitrification and survival rates. After assessment of the patient’s understanding, a joint decision by the patient and the doctor is made between the 2 strategies.
Matching criteria were the patients’ age, the number of prior ART cycles, and the endometriosis phenotypes (SUP, OMA, or DIE). Blind matching to the results was performed. Each Def-ET was matched to one fresh ET. Matching was performed by staff members who were cognizant of the matching criteria but who were otherwise blinded to the results. Matched records were used only once.
Ovarian stimulation
Women were monitored and managed according to our institutional clinical protocols as reported previously [18]. Thus, all patients were synchronized using timed administration of an oral contraceptive (OC) containing 0.03 mg of ethynil estradiol (EE) and 0.15 mg of levonorgestrel (LNG) (Minidril, Pfizer Holding, Paris, France) [31]. Various COS protocols were used according to our institutional clinical protocols, with 150–450 IU/day of recombinant Follicle-stimulating hormone (FSH) (Puregon-MSD, Courbevoie, France; Gonal-F, Merck, Lyon, france) and urinary FSH (hMG, Menopur, Ferring Pharmaceuticals, Gentilly, France): (i) a GnRH antagonist protocol, (ii) a long agonist protocol, and (iii) a short agonist protocol [32]. Gonadotropin doses and the type of COS protocol were determined according to the individual patient’s characteristics. As previously described in the literature, antagonist protocol is preferentially used in the Def-ET strategy [12,33]. Final oocyte maturation was triggered when ≥ 3 ovarian follicles of ≥ 17 mm were visible by ultrasound: (i) for the Def-ET group, final oocyte maturation was achieved using either a single injection of 0.2 mg of GnRH agonist (Triptoreline, Decapeptyl, Ipsen, Boulogne Billancourt, France), or by 250 μg of recombinant hCG (rhCG, Ovitrelle, Serono, Lyon, France), according to the COS protocol; (ii) for the fresh ET group, final oocyte maturation was achieved using rhCG triggering. Oocyte retrieval was performed 35–36h later by transvaginal aspiration under ultrasound guidance.
Oocyte insemination
Semen samples were collected by masturbation after a sexual abstinence period ranging from 2 to 5 days. Conventional IVF or ICSI were performed according to the sperm parameters [34]. Fertilization was assessed by the presence of two pronuclei (2PN) and two polar bodies at 17-18h following oocyte insemination or injection.
Embryo culture, cryopreservations and thawing
Concerning prolonged cultures, embryos were transferred into a 50 μl droplet of one-step Global culture medium (LifeGlobal, USA) and cultured until day 5 or 6 at 37°C in an atmosphere of 5% CO2, 5% O2 and 90% N2. The culture medium was changed on day 3. Embryo morphology was evaluated on the morning of day 5 and 6. Blastocysts were scored according to the grading system of Gardner and Schoolcraft [35] and considered eligible for cryopreservation on day 5 or 6 if qualifying as full (B3) or expanded (B4-5) blastocysts with a type A-C inner cell mass and/or a type A-C trophectoderm. Blastocyst that did not meet these criteria on day 5 were kept in culture and re-examined on day 6. Blastocysts with a type “C” inner cell mass (ICM) and a type “C” trophectoderm were not cryopreserved regardless of their degree of expansion and the day of observation (days 5–6).
The precise vitrification and thawing protocol in our unit has been reported in detail previously [18]. Briefly, embryo vitrification was performed using closed Cryo-Bio-System vitrification (CBS-VIT) High Security (HS) straws in combination with DMSO-EG-S as the cryoprotectants (Irvine Scientific Freeze Kit). For thawing, the Irvine Scientific Thaw Kit was used. Zygotes were warmed the day prior to the embryo transfer and kept in culture for 24 hours in the same culture medium (50 μl droplet of one-step Global culture medium (LifeGlobal, USA) at 37°C in an atmosphere of 5% CO2, 5% O2, and 90% N2). On day 2, the embryos were morphologically assessed according to the criteria published in the Istanbul Consensus workshop on embryo assessment guide [36]. One or two best quality embryos were chosen for transfer. Supernumerary embryos, if any, were maintained in extended culture, and vitrified if they reached the blastocyst stage. Blastocysts were warmed on the day of transfer. When the warmed blastocyst had < 50% intact cells, an additional blastocyst was warmed if available. If the blastocyst was > 50% intact, expansion and re-expansion were assessed 2–3 hours later.
Endometrial preparation and embryo transfer
In the fresh ET group, all of the women began progesterone treatment (200 mg vaginal capsule t.i.d, Utrogestan, Besins International, Montrouge, France) the day of the oocyte retrieval, and estradiol (E2) was delivered transdermally (0.2 mg/day, through two Vivelledot 100 systems simultaneously, Novartis Pharma SA, Rueil malmaison, France) or orally (8 mg/day, Provames, Sanofi Aventis, Paris, France) 48h after the ET. Day-2 embryos were assessed morphologically, and the one or two embryos deemed to be the most suitable were selected for transfer. In the Def-ET group, all of the women received progesterone (one 200 mg vaginal capsule, daily) for 10 days in order to assure proper occurrence of menses. Embryo transfers were scheduled 4–5 weeks later. For this, women received an estradiol (E2) priming regimen that was delivered transdermally (0.2 mg/day) or orally (8 mg/day). Patients were examined again 2–3 weeks after menses in order to assess endometrial thickness and to determine progesterone levels. When conditions were appropriate (e.g. endometrium thickness ≥ 7mm and progesterone < 1.5 ng/ml), vaginal progesterone treatment was initiated at a dose of 200 mg t.i.d. Day-2 cleaved-stage embryos were transferred on the 4th day of progesterone exposure. Blastocysts were transferred immediately on the 5th day of progesterone exposure. The best quality embryos were chosen for transfer. The women who became pregnant by these procedures continued with the same dose of progesterone and E2 treatment until 12 weeks of gestation.
Data analysis and statistics
The general characteristics of the patients in both groups were recorded prospectively, prior to the COS. The following data were collected: age (in years); height (in meters); weight (in kilograms), body mass index (BMI, calculated as weight (kg)/[height (m)] 2); the number of prior ART cycles; the duration of their infertility; ovarian reserve (day 3 FSH, Luteinizing hormone (LH), estradiol; antral follicle count (AFC)); AMH levels; associated factors of infertility (e.g. a male factor, tubal factor, and associated adenomyosis). Associated adenomyosis was diagnosed using imaging criteria based on TVUS and MRI [37].
Clinical pregnancy rates (cPR) were determined by ultrasonographic documentation of at least one fetus with a heart beat at 6–7 weeks of gestation [38]. The ongoing pregnancy rate (oPR) was defined as the sonographic detection of one or more intrauterine fetuses with a positive heartbeat at 12 weeks of gestation [39]. Live birth rate (LBR) was defined as delivery of any viable infant at 22 weeks or more of gestation [38].
Cumulative cPR, oPR and LBR were the proportion of women that had at least one clinical pregnancy, ongoing pregnancy and live birth, respectively, whether from the first transfer attempt or subsequent transfers of frozen–thawed supernumerary embryos [15] after the oocyte retrieval. Once a woman obtained a pregnancy from IVF/ICSI she did not contribute any more to the cumulative rates [40].
The main ART outcome measure was cumulative cumulative oPR. Secondary outcomes were Cumulative cPR, cumulative LBR and cPR, oPR and LBR after the first ET. All of the data were entered into a digital database and analyzed using SPSS software (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered to be statistically significant. Given the matched design of our groups (fresh and Def-ET) we used matched-pair statistics for the univariate statistical analysis. Dichotomous variables were compared by the McNemar test, and continuous variables were assessed by paired t-test, as appropriate. For subgroup analysis of non-matched groups we used the unpaired t-test for continuous variables, and the Pearson’s χ2 test or the Fisher’s exact test for qualitative variables. Then, a second analysis was performed to identify risk factors of cumulative ongoing pregnancy with a conditional logistic regression analysis. Potential confounding factors found to be statistically significant at the threshold of p ≤ 0.10 after an univariable analysis and variables with clinical relevance were included in the conditional multiple logistic regression model.
The model built to retain variables into the final model was processed according to scientific knowledge and clinical relevance of variables.
Results
Study population
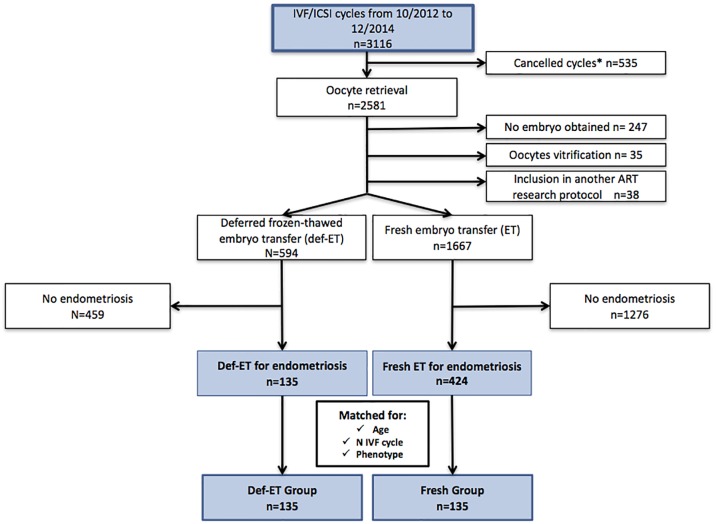
The process of our cohort selection is detailed in Fig 1. From the initial cohort of 3 116 ART procedures scheduled in our reproductive medicine unit between the 1st of October 2012 and the end of December 2014, 535 cycles were cancelled before oocyte retrieval. Three hundred and twenty were excluded from the study for the following reasons: no embryo obtained (n = 247), oocyte vitrification procedure (n = 35), and inclusion in another ART research protocol (n = 38). For the remaining 2 261 cycles, we excluded women without endometriosis [459 women who received a Def-ET and 1 276 women who received a fresh ET] from the study. Overall, 135 Def-ET cycles and 424 fresh ET cycles performed for endometriotic women were eligible for matching. Of these, 135 pairs of fresh and deferred cycles were matched for age, ART ranking, and the endometriosis phenotype. The endometriosis phenotypes were as follows: SUP, 27 patients (10.00%); OMA, 66 patients (24.44%); and DIE, 177 patients (65.56%). One hundred and two (37.78%) DIE patients exhibited an associated OMA.
Fig 1. Patient inclusion flowchart.
IVF/ICSI, in vitro fertilization / intra cytoplasmic sperm injection; Def-ET: Deferred frozen-thawed embryo transfer—*Cancelled cycles: Poor response—Personal or medical (e.g. non-gynecological) reasons.
The patient characteristics are presented in Table 1. A prior history of surgery for endometriosis was significantly more frequent in the fresh ET group [100 (74.07%) versus 43 (31.85%), p < 0.001]. Moreover, the distribution of the type of stimulation protocol was different between the two groups. For the fresh group, the long agonist protocol [67 (49.63%) versus 16 (11.85%) for the Def-ET group] and the short agonist protocol [41 (30.37%) versus 9 (6.67%) for the Def-ET group] were more often performed than in the Def-ET group. Therefore, the total dose of injected gonadotropin was significantly higher for the fresh group (3 064.1 ± 1 210.3 versus 2 498.5 ± 809.9, p < 0.001). Conversely, age, the number of prior ART procedures, BMI, the duration of the infertility, gravidity and parity, associated infertility factors, ovarian reserve parameters, and the endometriosis phenotypes, were not significantly different between the two groups (Table 1 and S1 Table).
Table 1. Baseline characteristics in matched fresh and deferred frozen-thawed embryo transfer groups.
| Fresh-ET group (n = 135) | Def-ET group (n = 135) | |
|---|---|---|
| Patient’s Age at COS (years) (mean ± SD) | 34.3 ± 3.9 | 34.3 ± 4.1a |
| Number of prior ART cycles (mean ± SD) | 1.9 ± 1.0 | 2.0 ± 1.1a |
| Duration of the infertility (years) (mean ± SD) | 4.4 ± 2.3 | 4.7 ± 2.7 |
| BMI (kg/m2) (mean ± SD) | 22.3 ± 3.3 | 22.8 ± 3.6 |
| Gravidity—n (%) | ||
| 0 | 101 (74.8) | 112 (83.0) |
| ≥1 | 34 (25.2) | 23 (17.0) |
| Parity—n (%)) | ||
| 0 | 123 (91.1) | 125 (92.6) |
| ≥1 | 12 (8.9) | 10 (7.4) |
| Associated male infertility—n (%) | 26 (19.3) | 19 (14.1) |
| Associated tubal factor—n (%) | 12 (8.9) | 12 (8.9) |
| Associated adenomyosis—n (%) | 56 (41.5) | 57 (42.2) |
| Prior endometriosis surgery—n (%) | 100 (74.07) | 43 (31.85) |
| Patient’s ovarian reserve—n (%) | ||
| AMH ≤ 1.5ng/ml | 34 (25.19) | 23 (17.04) |
| AFC ≤ 10 | 63 (46.67) | 49 (36.30) |
| Endometriosis phenotypes n (%) | ||
| SUP | 12 (8.89) | 15 (11.11)a |
| OMA | 34 (25.19) | 32 (23.70) |
| DIE | 89 (65.93) | 88 (65.19) |
| Stimulation protocol n (%) | ||
| Long agonist protocol | 67 (49.63) | 16 (11.85)b |
| Antagonist Protocol | 27 (20.00) | 110 (81.48)b |
| Short agonist protocol | 41 (30.37) | 9 (6.67)b |
| Total dose of injected gonadotropin (IU) (mean ± SD) | 3064.1 ± 1210.3 | 2498.5 ± 809.9c |
Fresh-ET, Fresh embryo transfer; Def-ET, Deferred embryo transfer; ART, Assisted reproductive technology; COS, controlled ovarian stimulation; BMI, Body mass index; FSH, Follicle-stimulating hormone; LH, Luteinizing hormone; AFC, Antral follicle count; AMH, Anti mullerian hormone; SUP, superficial peritoneal endometriosis; OMA, endometriomas; DIE, Deep infiltrating endometriosis; GnRH, Gonadotropin-releasing hormone
a p value not provided because groups were matched for age, number of prior ART cycles and endometriosis phenotypes
b Same superscript letter in a row indicate a statistically significant difference (P < 0.05) using a McNemar test.
c Same superscript letter in a row indicate a statistically significant difference (P < 0.05) using a paired t test.
IVF-ICSI outcomes
Table 2 depicts IVF/ICSI outcomes in matched fresh and Def-ET groups after univariable analysis. This non-adjusted analysis found a lower number of oocytes retrieved in the fresh group (7.4 ± 4.3 versus 9.9 ± 7.0 in the Def-ET group, p < 0.001) (Table 2) and the cumulative cPR [40 (29.6%) versus 58 (43.0%), p = 0.047], the cumulative oPR [24 (17.8%) vs. 47 (34.8%), p = 0.005] and the cumulative LBR [21 (15.6%) vs. 41 (29.6%), p = 0.012] were significantly higher in Def-ET group as compared to fresh ET group. Conversely, the miscarriage rate was significantly higher in the fresh ET group. The multiple pregnancy rates were not significantly different between the two groups (Table 2).
Table 2. IVF/ICSI-characteristics and outcomes in matched fresh and deferred frozen embryo transfer groups.
| Fresh-ET group (n = 135) | Def-ET group (n = 135) | p-value | |
|---|---|---|---|
| Number of oocytes retrieved (mean ± SD) | 7.4 ± 4.3 | 9.9 ± 7.0 | 0.001 pt |
| Total number of embryos transferred | 278 | 224 | NA |
| Mean No. of embryos transferred (mean ± SD) | 2.1 ± 0.9 | 1.7 ± 0.9 | <0.001 pt |
| Total number of embryo transfers | 155 | 170 | NA |
| Mean No. of transfers (mean ± SD) | 1.2 ± 0.4 | 1.3 ± 0.7 | 0.132 pt |
| Cumulative clinical pregnancy rate—(n,%) | 40 (29.6) | 58 (43.0) | 0.047 mn |
| Miscarriage—(n,%) | 16/40 (40.0) | 11/58 (19.0) | 0.022 k |
| Multiple pregnancy—(n,%) | 7/40 (17.5) | 5/58 (8.6) | 0.220 k |
| Cumulative ongoing pregnancy—(n,%) | 24 (17.8) | 47 (34.8) | 0.005 mn |
| Cumulative live birth rate a—(n,%) | 21 (15.6) | 41 (29.6) | 0.012 mn |
IVF/ICSI, in vitro fertilization / intra cytoplasmic sperm injection; Fresh-ET, Fresh embryo transfer; Def-ET, Deferred frozen- thawed embryo transfer; NA, non applicable
pt, Paired t-test;
mn, McNemar test;
k Pearson’s chi-square test.
a 2 and 5 women were lost to follow up in Fresh and Def-ET group respectively
IVF-ICSI outcomes after the first-ET were depicted in S2 Table. Ongoing PR [42 (31.1%) versus 24 (17.8%), p = 0.003] and LBR [39 (28.9%) versus 21 (15.6%), p = 0.025] after the first-ET were significantly higher in Def-ET group as compared to fresh ET group (S2 Table). The miscarriage rate was significantly higher in the fresh ET group.
Risk factors for cumulative ongoing pregnancy
Univariable analysis comparing patients who became pregnant and those who did not is presented in Table 3. A prior history of surgery for endometriosis (OR = 0.35; 95% CI = 0.16–0.78; p = 0.010) and having a Def-ET versus a Fresh ET (OR = 1.94; 95% CI = 1.19–3.18; p = 0.008) were statistically significantly associated with cumulative ongoing pregnancy.
Table 3. Risk factors of cumulative ongoing pregnancy: Results from the conditional univariable and multivariable logistic regression analysis.
| Parameters | No cumulative ongoing pregnancy (-) (n = 199) | Cumulative ongoing pregnancy (+) (n = 71) | OR (95% CI) | p value | Adjusted OR(95%CI) | p value |
|---|---|---|---|---|---|---|
| Duration of the infertility (years) (mean ± SD) | 4.7 ± 2.6 | 4.0 ± 2.1 | 0.90 (0.77–1.06) | 0.199 | ||
| BMI (kg/m2) (mean ± SD) | 22.7 ± 3.4 | 22.2 ± 3.5 | 0.97 (0.89–1.06) | 0.482 | ||
| Gravidity—n (%) | 0.488 | |||||
| 0 | 157 (78.9) | 56 (78.9) | 1 | |||
| ≥1 | 42 (21.1) | 15 (21.1) | 1.34 (0.59–3.06) | |||
| Parity—n (%) | 0.711 | |||||
| 0 | 184 (92.5) | 64 (90.1) | 1 | |||
| ≥1 | 15 (7.5) | 7 (9.9) | 1.23 (0.41–3.68) | |||
| Associated male infertility—n (%) | 37 (18.6) | 8 (11.3) | 0.48(0.21–1.12) | 0.089 | 0.59 (0.27–1.32) | 0.439 |
| Associated tubal factor—n (%) | 20 (10.1) | 4 (5.6) | 0.67 (0.19–2.36) | 0.530 | ||
| Associated adenomyosis—n (%) | 88 (44.2) | 25 (35.2) | 0.75 (0.36–1.54) | 0.430 | ||
| Prior endometriosis surgery—n (%) | 116 (58.3) | 27 (38.0) | 0.35 (0.16–0.78) | 0.010 | 0.66(0.39–1.12) | 0.121 |
| Patient’s ovarian reserve—n (%) | ||||||
| AMH ≤ 1.5ng/ml | 46 (23.1) | 11 (15.5) | 0.44(0.19–1.02) | 0.056 | 0.67(0.32–1.45) | 0.313 |
| AFCa ≤10 | 87 (43.7) | 25 (35.2) | 0.56(0.30–1.06) | 0.075 | ||
| Stimulation protocol—n (%) | ||||||
| GnRH antagonist | 66 (33.2) | 17 (23.9) | 1 | 1 | ||
| Long agonist protocol | 90 (45.2) | 47 (66.2) | 0.62(0.30–1.29) | 0.203 | 1.32(0.54–3.23) | 0.541 |
| Short agonist protocol | 43 (21.6) | 7 (9.9) | 0.60 (0.23–1.57) | 0.298 | 0.95 (0.32–2.85) | 0.927 |
| Total dose of injected gonadotropin (IU) (mean ± SD) | 2884.2 ± 1088.7 | 2492.4 ± 949.4 | 1.00(0.99–1.01) | 0.136 | 1.00(0.99–1.01) | 0.439 |
| Type of embryo transfer—n (%) | 0.008 | 0.028 | ||||
| Fresh-ET | 111 (55.8) | 24 (33.8) | 1 | 1 | ||
| Def-ET | 88 (44.2) | 47 (66.2) | 1.94 (1.19–3.18) | 1.76(1.06–2.92) | ||
| Number of oocytes retrieved (mean ± SD) | 8.06 ± 5.3 | 10. ± 7.1 | 1.06 (0.99–1.12) | 0.065 | 1.04 (1.01–1.07) | 0.038 |
BMI, Body mass index; AMH, Anti-mullerian hormone; AFC, Antral follicle count; GnRH, Gonadotropin-releasing hormone; Fresh-ET, Fresh embryo transfer; Def-ET, Deferred embryo transfer; n number of cases, OR Odds ratio, CI Confidence interval;
a omitted from multivariable regression model due to multicollinearity
A multivariable analysis was performed to identify risk factors of cumulative ongoing pregnancy. Potential confounding factors included in the model were: An associated male infertility, a prior history of surgery for endometriosis, AMH level (≤ 1.5ng/ml versus >1.5 ng/ml), the type of stimulation protocol, the total dose of the injected gonadotropin (IU), the type of ET (fresh versus Def-ET), and the number of oocytes retrieved. The type of ET (fresh versus Def-ET) (fresh embryo transfer, OR = 1, Def-ET, OR = 1.76; 95% CI = 1.06–2.92; p = 0.028) and the number of oocytes retrieved (OR = 1.04; 95% CI = 1.01–1.07; p = 0.038) remained independent factors associated with cumulative ongoing pregnancy, as shown in Table 3.
Discussion
Main findings
This study compared ART outcomes of endometriosis-affected women, according to the type of ET: fresh versus deferred. In our population, a significantly higher cumulative pregnancy rate was observed after a deferred ET strategy. Based on a multivariable multivariate analysis, there were two independent predictive factors for ongoing pregnancy rates after ART: the number of oocytes retrieved and the type of ET (deferred ET strategy), both of which enhanced pregnancy rates.
Strengths and limitations
1) Our study focused on the important issue of management of endometriosis-related infertility. To the best of our knowledge, this is the first study to specifically examine ART outcomes in endometriosis-affected women after a fresh versus a deferred ET strategy; 2) Given the disease heterogeneity, we selected patients with well-defined endometriosis phenotypes (SUP, OMA, or DIE) [41]. For DIE and OMA phenotypes, we only included patients whose diagnosis of endometriosis was based on stringent image-based criteria [20–22,25]; Additionally, for patients with a history of prior surgery for endometriosis, the diagnosis was also confirmed by histological proof of endometriosis. For the SUP phenotype, the diagnosis was proven histologically, and no OMA nor DIE lesions at the pre-ART imaging work-up were identified; 3) The strengths of the study are also derived from some of the specifics of the methodological design: this is a matched controlled study including a large number of endometriosis affected-patients (270 women undergoing IVF/ICSI cycles) and great care was taken to minimize the possibility of biases. As advanced age and prior ART failure could have a negative influence on ART outcomes [42,43] we selected the age of the women and the number of prior ART as matching criteria. Additionally, we decided to match based on the endometriosis phenotypes. Since the link between endometriosis phenotypes and ART outcomes is a matter of debate [8,44], it seemed relevant to have comparable groups in terms of endometriosis phenotypes. 4) In order to compare the fresh versus the deferred ET strategy, in this study we used the cumulative pregnancy rate per oocyte retrieval, as published previously [40,45]. By providing an all-inclusive success rate, this analysis is of considerable relevance for clinicians since cryopreservation has become an integral part of ART. 5) Lastly, numerous epidemiological variables were collected prospectively through face-to-face interviews before ART (in regard to surgical history, infertility data, and ovarian stimulation characteristics).
Despite the precautions taken, our study may still be subject to certain shortcomings and/or biases. 1) This study was conducted in a referral center for endometriosis management. The women referred to our center may therefore have suffered from particularly severe forms of endometriosis (65.6% of the women in our study had a DIE phenotype). This referral bias for women with severe lesions might have influenced the ART outcomes. However, in light of the criteria for matching, the distribution of endometriosis phenotypes was identical in both groups; 2) Some differences persisted between the groups: The women in the fresh ET group had significantly more often a prior history of surgery for endometriosis, and this could potentially have an impact on the ovarian response to stimulation [46]. We are cognizant that women in the fresh group had a less efficient response to stimulation. Despite a higher mean dose of injected gonadotropins for the fresh group, the mean number of oocytes retrieved was lower compared to the deferred ET group.
In order to evaluate the possible impact of these differences on pregnancy outcomes and therefore to determine which factors are independently linked to cumulative ongoing PR, we have performed a second analysis comparing patients who became pregnant to those who did not in our study population. After a multivariable logistic regression analysis, two factors were independently associated with cumulative ongoing PR: the type of transfer (i.e. the fresh versus deferred ET strategies) and the number of oocytes retrieved. Having ‘a prior history of surgery for endometriosis’ was not found to be independently associated with cumulative ongoing pregnancy rates. 3)Finally, this analysis consists in a preliminary cohort study. We are aware that randomized controlled studies should confirmed those results. Bias inherent in the study design cannot be excluded. However, it still allows us to identify the deferred embryo strategy as a new approach for endometriosis infertile women.
Interpretation
The goal of this preliminary study was to compare the overall strategy of embryo transfer (i.e. fresh versus deferred). Given the existence of differences in regard to the number of oocytes, these results need to be interpreted with caution. We were nonetheless able to show that the deferred ET procedure could constitute a promising strategy for patients with endometriosis-related infertility. Indeed, in our model, carrying out a deferred ET rather a fresh ET was significantly linked with becoming pregnant. Our study hence provides new insight regarding ART management in endometriosis-related infertility. Deferring the ET relative to the ovarian stimulation appears to improve cumulative pregnancy rates in endometriosis-affected women undergoing IVF/ICSI.
Based on what has previously been reported the literature, it appears that endometrial alterations occur in women with endometriosis. Endometriosis induces changes in the eutopic endometrium in a natural cycle [3,4]. These endometrial-alterations have been related to the occurrence of a self-survival loop involving different pathways: inflammation, angiogenesis, and a disruption of steroidogenesis [47]. The endometrium in endometriosis-affected women could be less receptive than in women without endometriosis [2]. Even if there is currently no formal evidence for this, ovarian stimulation is widely thought to be a factor that adversely affects endometrial receptivity [11]. Our results are in accordance with the hypothesis that endometrial receptivity in endometriosis-affected women could be aggravated by ovarian stimulation.
The high rate of miscarriages (27.55%) that occurred in our study is consistent with findings in previous reports that investigated the association between endometriosis and miscarriages. As published previously, the risk of pregnancy loss in endometriosis-affected women is higher than in women without endometriosis [48–50]. Additionally, in the present study, a significantly higher rate of pregnancy loss was observed after a fresh versus a def-ET strategy (p = 0.022). These results are in accordance with a previous randomized controlled study focusing on ART outcomes in case of polycystic ovary syndrome after a fresh or a deferred ET [17].
The best practice for treating moderate to severe endometriosis-related infertility is still a matter of debate in the medical community. Nevertheless, patients with endometriosis-associated infertility exhibit good pregnancy rates after IVF/ICSI treatment compared to women with no associated endometriosis [51,52]. Some gynecologists are concerned that ART could negatively impact on endometriosis related symptoms. This concern no longer appears to be justified since recent prospective studies have shown no worsening of pain scores during IVF/ICSI procedures in endometriosis-affected women as compared to women without endometriosis [53,54]. Furthermore, the use of GnRHa triggering in association with a def-ET strategy has been recently been reported to limit pain symptoms during IVF/ICSI treatment [55]. Therefore, the def-ET strategy could be beneficial for endometriosis-affected women undergoing IVF/ICSI: firstly, by increasing cumulative pregnancy rates and, secondly, in association with a GnRHa triggering, by limiting physically painful symptoms [55].
Conclusion
In conclusion, these preliminary results suggest that deferred ET for endometriosis-affected women is an attractive option to increase ART success rates. Furthers studies, with a randomized design, should be conducted to more firmly confirm whether def-ET enhances pregnancy rates in endometriosis-affected women and to confirm these preliminary results.
Supporting information
(XLSX)
(DOCX)
Acknowledgments
The authors wish to thank staff members from our department for their expert assistance with data collection, and Valerie Blanchet and Julia Gonnot in particular. The authors also gratefully acknowledge Laetitia Marchand-Martin, Jessica Rousseau, and Pr Pierre Yves Ancel working in the epidemiologic research unit of INSERM UMR 1153 of our hospital for their constructive help in statistical analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was partially supported by a Finox Forward Research Grant to CC. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3: 93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 2.de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376: 730–738. doi: 10.1016/S0140-6736(10)60490-4 [DOI] [PubMed] [Google Scholar]
- 3.Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3’,5’-cyclic adenosine 5’-monophosphate inhibition of cyclin D1. Endocrinology. 2009;150: 4701–4712. doi: 10.1210/en.2009-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santulli P, Borghese B, Noël J-C, Fayt I, Anaf V, de Ziegler D, et al. Hormonal therapy deregulates prostaglandin-endoperoxidase synthase 2 (PTGS2) expression in endometriotic tissues. J Clin Endocrinol Metab. 2014;99: 881–890. doi: 10.1210/jc.2013-2950 [DOI] [PubMed] [Google Scholar]
- 5.Houshdaran S, Nezhat CR, Vo KC, Zelenko Z, Irwin JC, Giudice LC. Aberrant Endometrial DNA Methylome and Associated Gene Expression in Women with Endometriosis. Biol Reprod. 2016;95: 93 doi: 10.1095/biolreprod.116.140434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144: 2870–2881. doi: 10.1210/en.2003-0043 [DOI] [PubMed] [Google Scholar]
- 7.Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril. 2016;106: 1420–1431.e7. doi: 10.1016/j.fertnstert.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamdan M, Omar SZ, Dunselman G, Cheong Y. Influence of endometriosis on assisted reproductive technology outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2015;125: 79–88. doi: 10.1097/AOG.0000000000000592 [DOI] [PubMed] [Google Scholar]
- 9.Check JH, Choe JK, Katsoff D, Summers-Chase D, Wilson C. Controlled ovarian hyperstimulation adversely affects implantation following in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16: 416–420. doi: 10.1023/A:1020565408018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikas G, Develioglu OH, Toner JP, Jones HW. Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod Oxf Engl. 1999;14: 787–792. [DOI] [PubMed] [Google Scholar]
- 11.Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, Osianlis T, et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. 2014;20: 808–821. doi: 10.1093/humupd/dmu027 [DOI] [PubMed] [Google Scholar]
- 12.Bourdon M, Santulli P, Gayet V, Maignien C, Marcellin L, Chapron C. Deferred frozen embryo transfer: What benefits can be expected from this strategy in patients with and without endometriosis? J Endometr Pelvic Pain Disord. 2017; doi: 10.5301/jeppd.5000281 [Google Scholar]
- 13.Manzanares MA, Gómez-Palomares JL, Ricciarelli E, Hernández ER. Triggering ovulation with gonadotropin-releasing hormone agonist in in vitro fertilization patients with polycystic ovaries does not cause ovarian hyperstimulation syndrome despite very high estradiol levels. Fertil Steril. 2010;93: 1215–1219. doi: 10.1016/j.fertnstert.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 14.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96: 344–348. doi: 10.1016/j.fertnstert.2011.05.050 [DOI] [PubMed] [Google Scholar]
- 15.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Freeze-all can be a superior therapy to another fresh cycle in patients with prior fresh blastocyst implantation failure. Reprod Biomed Online. 2014;29: 286–290. doi: 10.1016/j.rbmo.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 16.Roque M, Valle M, Guimarães F, Sampaio M, Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015;103: 1190–1193. doi: 10.1016/j.fertnstert.2015.01.045 [DOI] [PubMed] [Google Scholar]
- 17.Chen Z-J, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N Engl J Med. 2016;375: 523–533. doi: 10.1056/NEJMoa1513873 [DOI] [PubMed] [Google Scholar]
- 18.Bourdon M, Santulli P, Gayet V, Maignien C, Marcellin L, Pocate-Cheriet K, et al. Assisted reproduction technique outcomes for fresh versus deferred cryopreserved day-2 embryo transfer: a retrospective matched cohort study. Reprod Biomed Online. 2016; doi: 10.1016/j.rbmo.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 19.Chapron C, Souza C, Borghese B, Lafay-Pillet M-C, Santulli P, Bijaoui G, et al. Oral contraceptives and endometriosis: the past use of oral contraceptives for treating severe primary dysmenorrhea is associated with endometriosis, especially deep infiltrating endometriosis. Hum Reprod Oxf Engl. 2011;26: 2028–2035. doi: 10.1093/humrep/der156 [DOI] [PubMed] [Google Scholar]
- 20.Abrao MS, da Gonçalves MO C, Dias JA, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod Oxf Engl. 2007;22: 3092–3097. doi: 10.1093/humrep/dem187 [DOI] [PubMed] [Google Scholar]
- 21.Piketty M, Chopin N, Dousset B, Millischer-Bellaische A-E, Roseau G, Leconte M, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod Oxf Engl. 2009;24: 602–607. doi: 10.1093/humrep/den405 [DOI] [PubMed] [Google Scholar]
- 22.Guerriero S, Ajossa S, Orozco R, Perniciano M, Jurado M, Melis GB, et al. Diagnostic accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in the recto-sigmoid: a meta-analysis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2015; [DOI] [PubMed] [Google Scholar]
- 23.Kinkel K, Frei KA, Balleyguier C, Chapron C. Diagnosis of endometriosis with imaging: a review. Eur Radiol. 2006;16: 285–298. doi: 10.1007/s00330-005-2882-y [DOI] [PubMed] [Google Scholar]
- 24.Corwin MT, Gerscovich EO, Lamba R, Wilson M, McGahan JP. Differentiation of Ovarian Endometriomas from Hemorrhagic Cysts at MR Imaging: Utility of the T2 Dark Spot Sign. Radiology. 2014;271: 126–132. doi: 10.1148/radiol.13131394 [DOI] [PubMed] [Google Scholar]
- 25.Millischer A-E, Salomon LJ, Santulli P, Borghese B, Dousset B, Chapron C. Fusion imaging for evaluation of deep infiltrating endometriosis: feasibility and preliminary results: Fusion imaging of endometriosis. Ultrasound Obstet Gynecol. 2015;46: 109–117. doi: 10.1002/uog.14712 [DOI] [PubMed] [Google Scholar]
- 26.Medeiros LR, Rosa MI, Silva BR, Reis ME, Simon CS, Dondossola ER, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291: 611–621. doi: 10.1007/s00404-014-3470-7 [DOI] [PubMed] [Google Scholar]
- 27.Chapron C, Vieira M, Chopin N, Balleyguier C, Barakat H, Dumontier I, et al. Accuracy of rectal endoscopic ultrasonography and magnetic resonance imaging in the diagnosis of rectal involvement for patients presenting with deeply infiltrating endometriosis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2004;24: 175–179. doi: 10.1002/uog.1107 [DOI] [PubMed] [Google Scholar]
- 28.Chapron C, Lafay-Pillet M-C, Monceau E, Borghese B, Ngô C, Souza C, et al. Questioning patients about their adolescent history can identify markers associated with deep infiltrating endometriosis. Fertil Steril. 2011;95: 877–881. doi: 10.1016/j.fertnstert.2010.10.027 [DOI] [PubMed] [Google Scholar]
- 29.Ferrero S, Alessandri F, Racca A, Leone Roberti Maggiore U. Treatment of pain associated with deep endometriosis: alternatives and evidence. Fertil Steril. 2015; doi: 10.1016/j.fertnstert.2015.08.031 [DOI] [PubMed] [Google Scholar]
- 30.Braddock CH, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282: 2313–2320. [DOI] [PubMed] [Google Scholar]
- 31.de Ziegler D, Gayet V, Aubriot FX, Fauque P, Streuli I, Wolf JP, et al. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil Steril. 2010;94: 2796–2799. doi: 10.1016/j.fertnstert.2010.05.056 [DOI] [PubMed] [Google Scholar]
- 32.Santulli P, Gayet V, Fauque P, Chopin N, Dulioust E, Wolf JP, et al. HIV-positive patients undertaking ART have longer infertility histories than age-matched control subjects. Fertil Steril. 2011;95: 507–512. doi: 10.1016/j.fertnstert.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 33.Blockeel C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye H. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod Oxf Engl. 2016; doi: 10.1093/humrep/dev339 [DOI] [PubMed] [Google Scholar]
- 34.Fauque P, Léandri R, Merlet F, Juillard J-C, Epelboin S, Guibert J, et al. Pregnancy outcome and live birth after IVF and ICSI according to embryo quality. J Assist Reprod Genet. 2007;24: 159–165. doi: 10.1007/s10815-007-9115-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner DK, Lane M, Schoolcraft WB. Physiology and culture of the human blastocyst. J Reprod Immunol. 2002;55: 85–100. [DOI] [PubMed] [Google Scholar]
- 36.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod Oxf Engl. 2011;26: 1270–1283. doi: 10.1093/humrep/der037 [DOI] [PubMed] [Google Scholar]
- 37.Dueholm M, Lundorf E. Transvaginal ultrasound or MRI for diagnosis of adenomyosis. Curr Opin Obstet Gynecol. 2007;19: 505–512. doi: 10.1097/GCO.0b013e3282f1bf00 [DOI] [PubMed] [Google Scholar]
- 38.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009*. Fertil Steril. 2009;92: 1520–1524. doi: 10.1016/j.fertnstert.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 39.Brandes M, Hamilton CJCM, de Bruin JP, Nelen WLDM, Kremer JAM. The relative contribution of IVF to the total ongoing pregnancy rate in a subfertile cohort. Hum Reprod. 2010;25: 118–126. doi: 10.1093/humrep/dep341 [DOI] [PubMed] [Google Scholar]
- 40.Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod Oxf Engl. 2015;30: 2703–2707. doi: 10.1093/humrep/dev263 [DOI] [PubMed] [Google Scholar]
- 41.Chapron C, Lafay-Pillet M-C, Monceau E, Borghese B, Ngô C, Souza C, et al. Questioning patients about their adolescent history can identify markers associated with deep infiltrating endometriosis. Fertil Steril. 2011;95: 877–881. doi: 10.1016/j.fertnstert.2010.10.027 [DOI] [PubMed] [Google Scholar]
- 42.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12: 685–718. doi: 10.1093/humupd/dml034 [DOI] [PubMed] [Google Scholar]
- 43.Broekmans FJ, Verweij PJM, Eijkemans MJC, Mannaerts BMJL, Witjes H. Prognostic models for high and low ovarian responses in controlled ovarian stimulation using a GnRH antagonist protocol. Hum Reprod Oxf Engl. 2014;29: 1688–1697. doi: 10.1093/humrep/deu090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maignien C, Santulli P, Gayet V, Lafay-Pillet M-C, Korb D, Bourdon M, et al. Prognostic factors for assisted reproductive technology in women with endometriosis-related infertility. Am J Obstet Gynecol. 2016; doi: 10.1016/j.ajog.2016.11.1042 [DOI] [PubMed] [Google Scholar]
- 45.Germond M, Urner F, Chanson A, Primi M-P, Wirthner D, Senn A. What is the most relevant standard of success in assisted reproduction?: The cumulated singleton/twin delivery rates per oocyte pick-up: the CUSIDERA and CUTWIDERA. Hum Reprod Oxf Engl. 2004;19: 2442–2444. doi: 10.1093/humrep/deh501 [DOI] [PubMed] [Google Scholar]
- 46.Somigliana E, Arnoldi M, Benaglia L, Iemmello R, Nicolosi AE, Ragni G. IVF-ICSI outcome in women operated on for bilateral endometriomas. Hum Reprod Oxf Engl. 2008;23: 1526–1530. doi: 10.1093/humrep/den133 [DOI] [PubMed] [Google Scholar]
- 47.Bulun SE. Endometriosis. N Engl J Med. 2009;360: 268–279. doi: 10.1056/NEJMra0804690 [DOI] [PubMed] [Google Scholar]
- 48.Barbosa M a. P, Teixeira DM, Navarro P a. a. S, Ferriani RA, Nastri CO, Martins WP. Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2014;44: 261–278. doi: 10.1002/uog.13366 [DOI] [PubMed] [Google Scholar]
- 49.Marcellin L, Santulli P, Gogusev J, Lesaffre C, Jacques S, Chapron C, et al. Endometriosis also affects the decidua in contact with the fetal membranes during pregnancy. Hum Reprod Oxf Engl. 2015;30: 392–405. doi: 10.1093/humrep/deu321 [DOI] [PubMed] [Google Scholar]
- 50.Santulli P, Marcellin L, Menard S, Thubert T, Khoshnood B, Gayet V, et al. Increased rate of spontaneous miscarriages in endometriosis-affected women. Hum Reprod Oxf Engl. 2016;31: 1014–1023. doi: 10.1093/humrep/dew035 [DOI] [PubMed] [Google Scholar]
- 51.Opøien HK, Fedorcsak P, Omland AK, Abyholm T, Bjercke S, Ertzeid G, et al. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil Steril. 2012;97: 912–918. doi: 10.1016/j.fertnstert.2012.01.112 [DOI] [PubMed] [Google Scholar]
- 52.Hamdan M, Omar SZ, Dunselman G, Cheong Y. Influence of endometriosis on assisted reproductive technology outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2015;125: 79–88. doi: 10.1097/AOG.0000000000000592 [DOI] [PubMed] [Google Scholar]
- 53.Benaglia L, Somigliana E, Santi G, Scarduelli C, Ragni G, Fedele L. IVF and endometriosis-related symptom progression: insights from a prospective study. Hum Reprod Oxf Engl. 2011;26: 2368–2372. doi: 10.1093/humrep/der208 [DOI] [PubMed] [Google Scholar]
- 54.Santulli P, Bourdon M, Presse M, Gayet V, Marcellin L, Prunet C, et al. Endometriosis-related infertility: assisted reproductive technology has no adverse impact on pain or quality-of-life scores. Fertil Steril. 2015; doi: 10.1016/j.fertnstert.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 55.Bourdon M, Santulli P, de Ziegler D, Gayet V, Maignien C, Marcellin L, et al. Does GnRH Agonist Triggering Control Painful Symptom Scores During Assisted Reproductive Technology? A Retrospective Study. Reprod Sci Thousand Oaks Calif. 2017; 1933719116687659. doi: 10.1177/1933719116687659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.