Abstract
An increased prevalence of the inflammatory bowel diseases, ulcerative colitis and Crohn’s disease, was found amongst residents in a livestock dense area. We hypothesised that exposure to livestock farms might be a substantial environmental factor that contributes to the development of these diseases and that in the lead up to inflammatory bowel diseases potential risk factors can be identified. This study aimed to investigate the contribution of livestock exposure to the development of these diseases and the clinical events prior to the diagnosis. Electronic health records from 2006–2013 of general practices were used. The study population consisted of patients with a new diagnosis of inflammatory bowel diseases resident in areas with a high (n = 141) or lower (n = 109) livestock density. Patients with low back pain (n = 10,469) were used as controls. For those in a livestock dense area, distance to livestock farms was determined. Associations between morbidities and drug prescriptions in the reporting year and three years previous to the diagnosis, and the residential proximity to livestock exposure were investigated with multivariable logistic regression analyses. Acute and chronic morbidity of the gastrointestinal tract and associated drug prescriptions were predictive for the development of inflammatory bowel diseases. In addition, a positive association was found between infections and living within 500 meter of poultry farms and the development of inflammatory bowel diseases [OR: 3.3 (1.1–9.9)]. Nonetheless, overall livestock exposure contributed little to the development of these diseases. These results suggest that exposure to livestock farms on its own contributes minimal to the development of inflammatory bowel diseases. Nonetheless, having infections appeared to be a risk factor for neighbouring residents of poultry farms. More research is warranted to explain the increased prevalence of inflammatory bowel diseases amongst residents in areas with a high density of livestock.
Introduction
Ulcerative colitis and Crohn’s disease are chronic inflammatory diseases of the gastrointestinal tract (GI-tract) and are known as inflammatory bowel diseases (IBD). Ulcerative colitis is characterised by inflammation of the mucosal layer of the colon and rectum. In contrast, Crohn’s disease is characterised by transmural inflammation causing strictures and fistulas, and may affect the entire GI-tract. Clinically, ulcerative colitis often manifests with abdominal pain and diarrhoea associated with blood, while Crohn’s disease often manifests with abdominal pain and constipation. However, in practice the distinction between these two diseases can be a challenge, resulting in diagnostic delays for months in adults up to several years in children [1,2]. Although ulcerative colitis and Crohn’s disease share similar features, such as a relapsing-remitting course, and although both affect young people, these diseases are considered to have different aetiological mechanisms [3]. Unfortunately, these mechanisms are still poorly understood. Nevertheless, it is presumed that the cause of IBD is multifactorial, including genetic mutations as well as lifestyle-related and environmental factors such as smoking habits and infections, respectively [4,5]. Interestingly, Heederik and Yzermans (2011) showed a higher prevalence rate of IBD in neighbouring residents of livestock farms in a rural area with a high density of these farms, as compared with those in areas with a much lower density of those farms [6]. This difference was confirmed in ongoing analyses investigating a longer period of time. This suggests that exposure to large-scale livestock farming, as an environmental factor, may contribute to the development of IBD, possibly by repeatedly stimulating mucosal tissue triggering an immune response [7]. On the contrary, others have shown that growing up on a farm is inversely associated with the development of juvenile IBD or IBD later in life [8–10]. Whether this also applies for neighbouring residents remains to be investigated.
As the diagnostic process of IBD can be challenging, longitudinal studies investigating the clinical events preceding this diagnosis are warranted. In one such study, Pimentel and colleagues showed that the clinical threshold of IBD-related symptoms were present on average seven years before diagnosis [11]. Whether the nature of symptoms preceding the diagnosis of IBD differs in light of exposure to livestock farms has not yet been investigated. We hypothesise that people exposed to a high density of livestock experience more often gastro-intestinal symptoms and appeal more often to the general practitioner. As every citizen in the Netherlands is obligatorily enrolled in the list of just one general practice, electronic health records of those practices can be used to investigate the medical history preceding the diagnosis of IBD. By combining this information with exposure estimates, we performed an observational study with a high quality of longitudinal registration of health records, including morbidity and drug prescriptions.
This study was designed to investigate whether livestock exposure contributes to the development of IBD and whether potential risk factors can be identified. To this end, we investigated clinical events preceding the diagnosis of IBD using health records in the reporting year and three previous years to the diagnosis of these diseases. These records were gathered for residents in a rural area with a high density of livestock farms and residents in rural areas with a much lower density of those farms, and findings compared to those gathered in a control group.
Materials and methods
Research design
A case-control study was performed using electronic health records (EHRs) of general practitioners (GPs) in the Netherlands. These records include morbidity and drug prescriptions at the patient level. Clinical events in the reporting year and three previous years to the diagnosis were investigated for patients with IBD, the cases, in comparison with patients with low back pain with or without radiating pain, the controls. Patients with low back pain were included as controls since these patients constitute typical general practice patients who also contact and visit the GP regularly, but their symptom patterns and associated healthcare utilisation are theoretically unrelated to IBD, as well as livestock exposure [12]. In addition, an area with a high density of livestock farms, so called exposed area, was compared with areas with a lower density of livestock farms, altogether referred to as the reference area. The exact specification of the exposed and reference area has been previously published [13,14]. Briefly, the exposed area included the Eastern part of the province Noord-Brabant and North-Western part of the province Limburg and is an area with the highest density of livestock farms in the Netherlands. The reference area included rural areas with mainly crop farming in the provinces of Noord-Holland, Zuid-Holland, Gelderland, Groningen, Overijssel and Utrecht. In both areas, cities with more than 30.000 residents were excluded to reduce the influence of urban air pollution.
Ethical aspects
Research was performed in accordance with the Dutch Medical Treatment Act and Personal Data Protection Act. The protocol of the VGO study was approved by the Medical Ethical Committee of the University Medical Centre Utrecht. Privacy was ensured by using a Trusted Third Party (IVZ, Houten, The Netherlands), keeping medical information and address records separated at all times.
Data availability statement
In consultation with the Medical Ethical Committee that approved the study protocol, data from the VGO study are not publicly available due to privacy protection of participants. The study's privacy regulations stated that only co-authors and researchers from NIVEL, IRAS, and RIVM (consortium partners) have access to the study database and researchers who are not part of the consortium partners will not have access to the study database since sharing an anonymized and de-identified dataset would still contain Electronic Health Records and the personal data of participants, which could potentially lead to the identification of subjects. Researchers may reach a data transfer and privacy agreement to access the data, which will include agreement on co-authorship with the consortium members who were responsible for designing the study and collecting the data. Researchers may contact the data access committee through Remco Coppen, PhD, LLM (r.coppen@nivel.nl) or non-personal departmental email (NIVEL: zorgregistraties@nivel.nl; IRAS secretary: Seeoh903@uu.nl), or contact Christos Baliatsas, PhD (c.baliatsas@nivel.nl), Lidwien Smit, PhD (l.a.smit@uu.nl) or Joris Yzermans, PhD (j.ijzermans@nivel.nl).
General practices
Health records from GPs participating in the Netherlands Institute for Health Services Research Primary Care Database (NIVEL PCD) between 1 January 2006 and 31 December 2013 were used. The practices are representative of the Dutch GP population with respect to age, gender, region and urbanisation [15]. Furthermore, the NIVEL PCD has only small changes in the composition of practices between years. More information about the NIVEL Primary Care Database can be found at https://www.nivel.nl/en/node/4814. Following previous approaches [13], a number of a-priori inclusion criteria was used in terms of data completeness. More specifically, data on morbidity should be available for at least 65% of the contacts and for 46 or more weeks per year. Regarding the prescription data, drug prescriptions should have been registered in more than 85% of prescription records and for 46 or more weeks per year. As a result, 16 practices in the exposed area and 15 practices in the reference area were included from a total of 32 and 24, respectively.
Patient selection
Following the selection of practices, incident patients with IBD and low back pain were identified according to their first registration of IBD or low back pain in their EHR. GPs registered the reason of contact, including diseases and presented symptoms, according to the International Classification of Primary Care (ICPC) [16]. In this classification, symptoms and diseases are given an unique code according to organ or system, as well as general/unspecified, psychological problems or social problems. The code consists of one letter followed by two numbers. The letter refers to the organ or system, the numbers 1–29 to symptoms and the numbers 70–99 to diseases. Incident IBD patients were identified by the ICPC-code D94, whereas incident patients with low back pain were identified by the ICPC-codes L03 and L86, without and with radiating pain, respectively. Patients with IBD and low back pain were excluded if EHRs were unavailable in the three years prior to their diagnosis. In addition, patients with low back pain with a registration of IBD were also excluded. Finally, when investigating potential associations between the development of IBD and variables of livestock exposure in the exposed area, presumed farmers were excluded as we were only interested in neighbouring residents. These were identified as patients living within 50 meters of livestock farm stables as shown by others [13,17].
Morbidity and drug prescriptions
Health records in the reporting year and three previous years to the diagnosis were investigated for all patients using the ICPC-classification for morbidity and the Anatomical Therapeutical Chemical (ATC)-classification for drug prescriptions. In the ATC-classification, drug prescriptions are classified “according to the organ or system on which they act, and their therapeutic, pharmacological and chemical properties” [18]. In order to utilise a step-wise approach, both classifications were separately used to group morbidity or drug prescriptions in the reporting year and three previous years to the diagnosis into clusters and categories. Whereas the clustering of relevant ICPC-codes was adapted from Van Gils-Van Rooij et al. [19], clustering of the drug prescriptions was performed according to the guidelines for ATC classification and Defined Daily Dose assignment [18]. Morbidity included amongst others symptoms of the gastro-intestinal tract including diarrhoea and chronic diseases concerning the GI-tract but also autoimmune diseases and infections. Drug prescriptions were categorised for example in antidiarrheal and intestinal anti-inflammatory/anti-infective agents, corticosteroids and laxatives. Details of these clusters and categories, including the ICPC- and ATC-codes, are provided in S1 Appendix and S2 Appendix.
Exposure to livestock farms
Detailed information on livestock farms was used to investigate the influence of livestock exposure to the development of IBD. The provinces of Noord-Brabant and Limburg provide mandatory licenses and therefore retain information among others on the number of livestock farms and type of animals, including pigs, poultry and cattle. As previously described, the distance between the patients’ residences in the exposed area and livestock farms was calculated by a geographic information system using the residential addresses and area codes (ArcGis 10.1; Esri, Redlands, CA, USA) [13,17,20]. Contrary to patients in the exposed area, computing individual exposure estimates for those in the reference area was not possible as we did not have information on their residential addresses. Subsequently, the following variables of exposure were determined in the exposed area: distance to nearest livestock farm, less than 500m versus more than 500m, the number of livestock farms within 500m and the presence of farm animals within 500m, all used as binary variables. When investigating the presence of farm animals (e.g. pigs) within 500m, the reference group comprised patients with no pigs or any other farm animals within 500m. Information on livestock exposure was used from 2012 [20].
Data analysis
Differences in total healthcare utilisation for cases and controls between the exposed and reference area, including mean number of presented morbidity and drug prescriptions from all years together, was tested using the Mann-Whitney U test. Differences in gender and age at diagnosis between those areas as well as between cases and controls were tested using the Student’s t test.
In order to investigate potential associations between incidence of IBD and morbidity and drug prescriptions in the reporting year and three previous years to the diagnosis and to investigate whether the area of residence modifies these associations, logistic regression analyses were performed. Inflammatory bowel disease—‘1’ was IBD, ‘0’ was low back pain—was used as the dependent variable and the presence of the morbidity and drug prescription clusters were used as independent variables. Separate analyses were performed for each independent variable, and results were corrected for gender and age at diagnosis. Results were stratified for the area, and p-values for the multiplicative interactions (area × morbidity, area × drug prescriptions and area × exposure variable) are shown.
To investigate the influence of livestock exposure as an environmental factor to the development of IBD, logistic regression analysis was performed. Development of IBD was the dependent variable and livestock exposure the independent variable. Again, correction was performed for the potential confounders gender and age at diagnosis. Expanding on this analysis, the influence of livestock exposure variables, such as type of livestock, as effect modifiers was investigated in the exposed area. Results were stratified for the exposure variable, and p-values for multiplicative interactions (reference no animals) were determined from combined models.
Finally, given the hierarchical structure of the data, analyses were repeated on the basis of multilevel logistic regression to verify consistency of the results. Multilevel analyses showed similar results and are therefore not presented. Stata (version 13.1, StataCorp LP) was used to perform all analyses.
Results
Characteristics of the study population
Details of the study population are shown in Fig 1 and Table 1. In total, 250 patients with IBD and 10,469 patients with low back pain were selected (Fig 1). Of the 250 cases, 141 (56%) lived in the exposed area. Of the controls, 6,127 (59%) lived in the exposed area. Details with respect to estimated exposure to livestock farms were available for 135 cases and 5,790 controls. Controls did not differ significantly in age and gender from cases. Overall distribution of gender and age for cases was not statistically different between both areas. In contrast, controls in the exposed area were on average older than those in the reference area (p = 0.001, Table 1). Concerning the total healthcare utilisation in the reporting year and three previous years to the diagnosis, controls in the exposed area presented on average less often with morbidity and had less drug prescriptions than those in the reference area (p = 0.001 and p < 0.01, respectively). For cases, no significant differences in total healthcare utilisation between the areas were observed. Furthermore, 47% of the cases and 55% of the controls lived at a distance of 500m or less from the nearest livestock farm. Most of these patients lived within 500m of cattle (32% of cases and 43% of controls). Finally, almost 2% of cases and 4% of controls did not receive any drug prescriptions during the three-year period prior to diagnosis (data not shown).
Fig 1. Selection of study population.
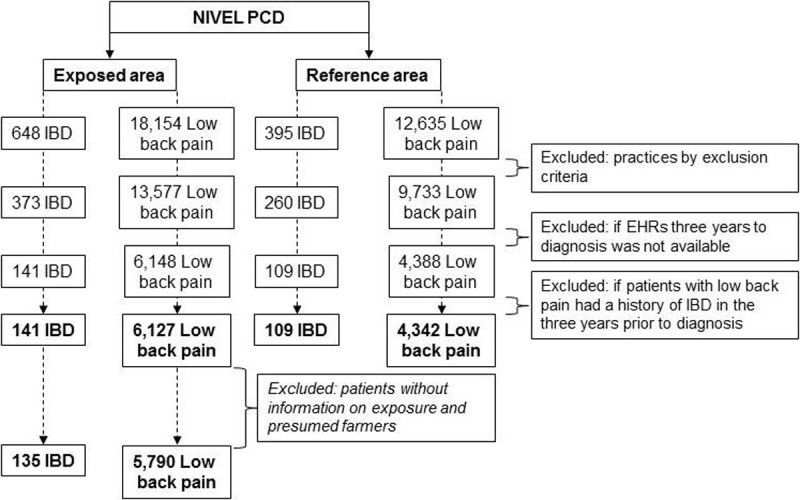
Patients without information on estimated exposure and presumed farmers were excluded only for logistic regression analyses with livestock exposure variables. EHRs, Electronic health records; IBD, Inflammatory bowel disease; NIVEL PCD, Netherlands Institute for Health Services Research Primary Care Database.
Table 1. Characteristics of the study population.
| Characteristic | Exposed area | Reference area | ||
|---|---|---|---|---|
| Inflammatory bowel disease | Low back pain | Inflammatory bowel disease | Low back pain | |
| Patients, n | 141 | 6,127 | 109 | 4,342 |
| Gender (female) | 54% | 51% | 48% | 52% |
| Age, in years, mean (±SD) | 50 (±17) | 51 (±18)*** | 49 (±20) | 50 (±19) |
| 3–24, n (%) | 11 (8%) | 651 (11%) | 20 (18%) | 494 (11%) |
| 25–44, n (%) | 41 (29%) | 1,406 (23%) | 19 (17%) | 1,126 (26%) |
| 45–64, n (%) | 58 (41%) | 2,522 (41%) | 41 (38%) | 1,720 (40%) |
| ≥65–104, n (%) | 31 (22%) | 1,548 (25%)*** | 29 (27%) | 1,002 (23%) |
| Healthcare utilisation | ||||
| Total number of presented morbidity with GP in the reporting year and three previous years to the diagnosis, mean (±SD) | 31 (±32) | 29 (±31)*** | 38 (±42) | 34 (±39) |
| Total number of drug prescriptions in the reporting year and three previous years to the diagnosis, mean (±SD) | 43 (±65) | 32 (±57)** | 46 (±78) | 34 (±55) |
| Exposure to livestock | ||||
| Patients, n | 135 | 5,790 | N/A | N/A |
| Distance to nearest livestock farm, n (%) | N/A | N/A | ||
| ≤500m | 64 (47%) | 3,165 (55%) | ||
| >500m | 71 (53%) | 2,625 (45%) | ||
| Number of livestock farms ≤500m, n (%) |
||||
| 0 | 72 (53%) | 2,663 (46%) | ||
| 1–2 | 32 (24%) | 1,810 (31%) | ||
| 3 > 13 | 30 (22%) | 1,314 (23%) | ||
| Presence of farm animals ≤500m (yes or no), n (%) |
||||
| Pigs | 33 (24%) | 1,508 (26%) | ||
| Poultry | 22 (16%) | 642 (11%) | ||
| Cattle | 43 (32%) | 2,465 (43%) | ||
| Others (goats, sheep and minks) |
25 (19%) | 657 (11%) | ||
** p-value < 0.01
*** p-value = 0.001, ref are patients in the reference area
Association between morbidity in the reporting year and three previous years to the diagnosis and incident IBD
In general, morbidity in the reporting year and three previous years to the diagnosis was positively associated with the development of IBD (Table 2). Irrespective of the area, experiencing acute somatic symptoms of the GI-tract was positively associated with the development of IBD [OR: 3.0 (95%CI: 1.6–5.7) in the exposed area and OR: 4.7 (95%CI: 2.1–10) in the reference area]. This was likewise for patients with chronic/long-term diseases of the GI-tract [OR: 1.9 (95%CI: 1.1–3.2) and OR: 3.4 (95%CI: 2.0–5.8), respectively, Table 2]. A history of autoimmune diseases in the reporting year and three previous years to the diagnosis was positively associated with the development of IBD, but only in the exposed area [OR: 3.1 (95%CI: 1.7–5.7)]. Nonetheless, this association was not statistically different between the areas (p for interaction = 0.25). In contrast, residents with infections of the GI-tract in the reference area were more at risk for development of IBD than those in the exposed area (p for interaction = 0.01, Table 2).
Table 2. Association between incidence of IBD and morbidity (diseases and presented symptoms) in the reporting year and three previous years to the diagnosis.
| Morbidity (yes or no) | Exposed area | Reference area | P-value for interactionc | ||
|---|---|---|---|---|---|
| n (%)a | OR (95% CI)b | n (%)a | OR (95% CI)b | ||
| Total acute somatic symptoms/MUPS | 130 (92%) | 1.4 (0.8–2.7) | 102 (94%) | 2.1 (0.95–4.5) | 0.53 |
| Acute somatic symptoms: symptoms of the GI-tract | 85 (60%) | 3.0 (1.6–5.7)** | 67 (61%) | 4.7 (2.1–10)** | 0.58 |
| Total infections | 88 (62%) | 1.1 (0.8–1.6) | 80 (73%) | 1.7 (1.1–2.6)* | 0.16 |
| Infections of the eye | 10 (7%) | 1.3 (0.6–2.5) | 11 (10%) | 2.1 (1.0–4.2)* | 0.35 |
| Infections of the skin | 39 (28%) | 1.3 (0.8–1.9) | 30 (28%) | 1.6 (0.97–2.7) | 0.51 |
| Infections of the airways | 48 (34%) | 1.2 (0.8–1.8) | 43 (39%) | 1.7 (1.1–2.8)* | 0.26 |
| Infections of the GI-tract | 8 (6%) | 1.6 (0.7–3.3) | 17 (16%) | 6.3 (3.3–12)** | 0.01 |
| Infections of the urinary tract | 19 (13%) | 1.1 (0.6–2.0) | 15 (14%) | 1.4 (0.7–2.8) | 0.82 |
| Total chronic/long-term diseases | 96 (68%) | 1.3 (0.9–1.9) | 74 (68%) | 1.3 (0.9–2.1) | 0.99 |
| Chronic/long-term diseases of the GI-tract | 29 (21%) | 1.9 (1.1–3.2)* | 33 (30%) | 3.4 (2.0–5.8)*** | 0.23 |
| Chronic/long-term diseases of the cardiovascular system | 21 (15%) | 1.3 (0.7–2.7) | 25 (23%) | 1.0 (0.5–2.0) | 0.61 |
| Chronic/long-term diseases of the airways | 26 (18%) | 1.5 (0.9–2.5) | 19 (17%) | 1.2 (0.7–2.3) | 0.80 |
| Chronic/long-term diseases of the skin | 26 (18%) | 1.2 (0.7–1.9) | 22 (20%) | 1.2 (0.7–2.2) | 0.61 |
| Chronic/long-term diseases: autoimmune diseases | 24 (17%) | 3.1 (1.7–5.7)*** | 11 (10%) | 0.9 (0.4–2.2) | 0.25 |
| Total neoplasms | 22 (16%) | 0.8 (0.5–1.3) | 20 (18%) | 1.2 (0.7–1.9) | 0.39 |
| Neoplasms: cancer | 7 (5%) | 0.9 (0.4–1.9) | 7 (6%) | 1.3 (0.6–2.8) | 0.50 |
| Acute psychological and social disorders | 36 (26%) | 1.0 (0.7–1.4) | 31 (28%) | 1.0 (0.7–1.5) | 1.00 |
| Acute psychological and social problems | 23 (16%) | 1.0 (0.7–1.6) | 18 (17%) | 1.0 (0.6–1.6) | 0.76 |
| Anxiety and depression | 13 (9%) | 1.6 (0.9–2.9) | 11 (10%) | 1.7 (0.9–3.3) | 0.89 |
| Lifestyle: weight and diet | 7 (5%) | 0.6 (0.3–1.4) | 9 (8%) | 0.8 (0.4–1.7) | 0.62 |
GI-tract, Gastrointestinal tract; IBD, Inflammatory bowel disease; MUPS, Medically unexplained physical symptoms
* p-value < 0.5
** p-value < 0.01
*** p-value < 0.001
a Number and percentage of patients with IBD with respective morbidity in the reporting year and three previous years to the diagnosis
b Adjusted for age at diagnosis and gender, ref: neighbouring residents without respective morbidity
c P-value for interaction: area × morbidity
Association between drug prescriptions in the reporting year and three previous years to the diagnosis and incident IBD
In general, drug prescriptions were positively associated with the development of IBD (Table 3). In particular, one or more prescriptions for antidiarrheal and intestinal anti-inflammatory/anti-infective agents in the reporting year and three previous years to the diagnosis was positively associated with the development of IBD [OR: 33 (95%CI: 22–48) in the exposed area and OR: 40 (95%CI: 26–62) in the reference area]. Laxatives were also positively associated with the development of IBD in both areas (Table 3). Furthermore, the positive association between drugs for functional GI-disorders and nasal preparations and the development of IBD for residents in the reference area were the only two associations that were significantly different compared with residents in the exposed area (p < 0.05 for interaction).
Table 3. Association between incidence of IBD and drug prescriptions in the reporting year and three previous years to the diagnosisa.
| Drug prescriptions (yes or no) | Exposed area | Reference area | P-value for interactiondd |
||
|---|---|---|---|---|---|
| n (%)b | OR (95% CI)c | n (%)b | OR (95% CI)c | ||
| Drugs for functional GI-disorders | 10 (7%) | 1.2 (0.6–2.3) | 19 (17%) | 2.9 (1.7–4.8)** | 0.046 |
| Laxatives | 59 (42%) | 4.6 (3.3–6.6)** | 48 (44%) | 5.4 (3.6–8.1)** | 0.65 |
| Antidiarrheal, intestinal anti-inflammatory/anti-infective agents | 66 (47%) | 33 (22–48)** | 54 (50%) | 40 (26–62)** | 0.53 |
| Symptoms and diseases of alimentary tract and metabolism | 64 (45%) | 1.4 (0.99–2.1) | 44 (40%) | 1.1 (0.7–1.7) | 0.42 |
| Symptoms and diseases of the blood and blood forming organs | 23 (16%) | 3.0 (1.9–4.8)** | 11 (10%) | 2.0 (1.1–3.8)* | 0.29 |
| Cardiovascular diseases | 56 (40%) | 1.2 (0.8–1.8) | 48 (44%) | 1.5 (0.9–2.3) | 0.56 |
| Antifungals for dermatological use | 28 (20%) | 1.4 (0.9–2.2) | 15 (14%) | 1.0 (0.6–1.7) | 0.29 |
| Symptoms and diseases of the skin | 64 (45%) | 1.4 (0.99–2.0) | 39 (36%) | 1.1 (0.8–1.7) | 0.39 |
| Symptoms and diseases of the genito-urinary tract and reproduction | 47 (33%) | 1.5 (1.0–2.2)* | 27 (25%) | 1.1 (0.7–1.8) | 0.21 |
| Symptoms and diseases of the endocrine glands | 32 (23%) | 1.8 (1.2–2.7)* | 21 (19%) | 1.3 (0.8–2.2) | 0.37 |
| Antibacterial drugs for systemic use | 74 (52%) | 1.3 (0.9–1.9) | 59 (54%) | 1.3 (0.9–1.9) | 0.76 |
| Antiviral medication | 29 (21%) | 1.3 (0.9–2.0) | 37 (34%) | 1.8 (1.2–2.8)* | 0.38 |
| Immunosuppressant drugs excluding corticosteroids | 11 (8%) | 8.4 (4.3–17)* | 10 (9%) | 16 (7.5–34)** | 0.21 |
| Anti-inflammatory and anti-rheumatic drugs excluding corticosteroids | 57 (40%) | 0.5 (0.4–0.8)* | 47 (43%) | 0.5 (0.4–0.8)** | 0.85 |
| Symptoms and diseases of the musculoskeletal system | 15 (11%) | 2.5 (1.4–4.5)* | 11 (10%) | 2.1 (1.1–4.2)* | 0.73 |
| Analgesic drugs | 28 (20%) | 1.0 (0.6–1.5) | 17 (16%) | 0.7 (0.4–1.1) | 0.27 |
| Antidepressants (in combination with psycholeptics) | 15 (11%) | 1.2 (0.7–2.1) | 17 (16%) | 1.6 (0.95–2.8) | 0.55 |
| Symptoms and diseases of the nervous system | 30 (21%) | 1.1 (0.7–1.6) | 28 (26%) | 1.3 (0.8–2.0) | 0.63 |
| Drugs for obstructive airway diseases, excluding corticosteroids | 26 (18%) | 1.2 (0.8–1.8) | 18 (17%) | 1.2 (0.7–2.0) | 0.94 |
| Cough and cold drugs | 19 (13%) | 1.0 (0.6–1.6) | 17 (16%) | 1.0 (0.6–1.7) | 0.97 |
| Nasal preparations and antihistamines for systemic use | 35 (25%) | 0.9 (0.6–1.3) | 43 (39%) | 1.7 (1.2–2.6)* | 0.03 |
| Symptoms and diseases of the sensory organs | 43 (30%) | 1.2 (0.8–1.7) | 25 (23%) | 0.9 (0.6–1.4) | 0.30 |
GI-disorders, Gastrointestinal disorders; IBD, Inflammatory bowel disease
* p-value < 0.5
** p-value < 0.01
a Results are shown if drugs were prescribed for at least 10 patients with IBD
b Number and percentage of patients with IBD with respective drug prescription in the reporting year and three previous years to the diagnosis
c Adjusted for age at diagnosis and gender, ref: neighbouring residents without respective drug prescription
d P-value for interaction: area × drug prescription
Influence of livestock exposure on the development of IBD
Within the exposed area, an indication was found for an inverse association between the presence of livestock farms within 500m of the patients’ residences and the development of IBD. However, it did not reach conventional levels of statistical significance [OR: 0.7 (95%CI: 0.5–1.05), not in table]. The association with the number of livestock farms within 500m was in the same direction, with an odds ratio of 0.98 (95%CI: 0.9–1.1) per farm compared with residents who did not live within 500m of livestock farms in this area.
Influence of livestock exposure on the association between morbidity in the reporting year and three previous years to the diagnosis and incident IBD
Positive and statistically significant associations between infections in the reporting year and three previous years to the diagnosis and the development of IBD were only found among those living within 500m of poultry farms, in particular for infections of the airways (p = 0.006 for interaction, Table 4). Furthermore, no positive associations were found between morbidity and the development of IBD for neighbouring residents living within 500m of cattle (data not shown). Finally, the associations between drug prescriptions and the development of IBD as shown in Table 3 were not modified by the exposure variables and are therefore not presented.
Table 4. Association in incident IBD patients between morbidity in the reporting year and three previous years to the diagnosis and farm animals ≤500m.
| Morbidity (yes or no) | Farm animals ≤500m (yes or no) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No animals | All animals | Pigs | Poultry | ||||||||
| n (%)a | OR (95% CI)b | n (%)a | OR (95% CI)b | P-value for interactionc |
n (%)a | OR (95% CI)b | P-value for interactionc |
n (%)a | OR (95% CI)b | P-value for interactionc |
|
| Total acute somatic symptoms/MUPS | 70 (52%) | 3.6 (0.9–15) | 55 (41%) | 1.1 (0.5–2.4) | 0.19 | 31 (23%) | 2.3 (0.5–9.8) | 0.55 | 21 (16%) | 2.8 (0.4–21) | 0.85 |
| Acute somatic symptoms: symptoms of the GI-tract | 44 (33%) | 6.5 (1.6–27)* | 25 (19%) | 2.6 (1.1–6.1)* | 0.34 | 19 (14%) | 4.9 (1.1–22)* | 0.67 | 17 (13%) | 7.1 (0.9–56) | 0.97 |
| Total infections | 39 (29%) | 0.7 (0.5–1.2) | 43 (32%) | 1.6 (0.9–2.7) | 0.06 | 22 (16%) | 1.3 (0.6–2.6) | 0.26 | 18 (13%) | 3.3 (1.1–9.9)* | 0.02 |
| Infections of the eye | 4 (3%) | 0.7 (0.3–2.1) | 6 (4%) | 2.2 (0.8–5.7) | 0.16 | 3 (2%) | 1.7 (0.4–6.5) | 0.36 | 3 (2%) | 6.0 (1.2–29)* | 0.047 |
| Infections of the skin | 16 (12%) | 0.8 (0.4–1.5) | 20 (15%) | 1.9 (0.99–3.6) | 0.07 | 9 (7%) | 1.4 (0.6–3.3) | 0.35 | 8 (6%) | 4.2 (1.2–15)* | 0.02 |
| Infections of the airways | 20 (15%) | 0.7 (0.4–1.3) | 23 (17%) | 1.7 (0.9–3.1) | 0.07 | 12 (9%) | 1.4 (0.6–3.2) | 0.24 | 12 (9%) | 4.5 (1.4–15)* | 0.006 |
| Infections of the GI-tract | 4 (3%) | 1.1 (0.4–3.2) | 4 (3%) | 2.4 (0.8–7.3) | 0.33 | 3 (2%) | 2.7 (0.7–10) | 0.33 | 1 (1%) | 3.5 (0.4–34) | 0.39 |
| Infections of the urinary tract | 13 (10%) | 1.3 (0.6–2.6) | 5 (4%) | 1.0 (0.4–3.0) | 0.56 | 3 (2%) | 1.2 (0.3–5.0) | 0.62 | 2 (1%) | 3.1 (0.4–24) | 0.63 |
| Total chronic/long-term diseases | 52 (39%) | 1.2 (0.7–2.2) | 39 (29%) | 1.3 (0.8–2.3) | 0.39 | 19 (14%) | 1.2 (0.6–2.5) | 0.24 | 14 (10%) | 1.2 (0.5–3.1) | 0.70 |
| Chronic/long-term diseases of the GI-tract | 14 (10%) | 1.4 (0.7–3.1) | 14 (10%) | 2.5 (1.2–5.1)* | 0.96 | 8 (6%) | 3.3 (1.3–8.5)* | 0.99 | 4 (3%) | 1.8 (0.5–6.5) | 0.75 |
| Chronic/long-term diseases of the cardiovascular system | 14 (10%) | 1.1 (0.4–2.7) | 6 (4%) | 1.4 (0.5–4.3) | 0.19 | 5 (4%) | 1.9 (0.5–6.9) | 0.51 | 4 (3%) | 1.7 (0.4–8.1) | 0.89 |
| Chronic/long-term diseases of the airways | 12 (9%) | 1.3 (0.6–2.9) | 12 (9%) | 1.5 (0.7–3.1) | 0.97 | 4 (3%) | 0.8 (0.3–2.6) | 0.39 | 4 (3%) | 1.1 (0.3–3.9) | 0.93 |
| Chronic/long-term diseases of the skin | 16 (12%) | 1.3 (0.6–2.6) | 8 (6%) | 0.9 (0.4–2.0) | 0.22 | 3 (2%) | 0.6 (0.2–2.3) | 0.14 | 2 (1%) | 0.5 (0.1–2.7) | 0.28 |
| Chronic/long-term diseases: autoimmune diseases | 14 (10%) | 3.2 (1.4–7.1)** | 8 (6%) | 2.6 (1.0–6.7)* | 0.38 | 4 (3%) | 2.8 (0.8–9.9) | 0.37 | 2 (1%) | 1.1 (0.2–6.7) | 0.42 |
| Total neoplasms | 13 (10%) | 0.99 (0.5–1.8) | 9 (7%) | 0.8 (0.4–1.6) | 0.46 | 6 (4%) | 1.1 (0.4–2.7) | 0.94 | 2 (1%) | 0.5 (0.1–2.0) | 0.33 |
| Neoplasms: cancer | 3 (2%) | 0.6 (0.2–2.0) | 4 (3%) | 1.5 (0.5–4.5) | 0.49 | 3 (2%) | 3.3 (0.9–12) | 0.27 | 0 (0%) | - | - |
| Acute psychological and social disorders | 24 (18%) | 1.2 (0.7–1.9) | 12 (9%) | 0.8 (0.4–1.6) | 0.29 | 5 (4%) | 0.6 (0.2–1.7) | 0.20 | 4 (3%) | 0.8 (0.3–2.4) | 0.45 |
| Acute psychological and social problems | 15 (11%) | 1.2 (0.7–2.2) | 8 (6%) | 1.0 (0.5–2.1) | 0.49 | 1 (1%) | 0.2 (0.03–1.6) | 0.09 | 3 (2%) | 1.1 (0.3–3.8) | 0.69 |
| Anxiety and depression | 10 (7%) | 2.3 (1.1–4.6)* | 3 (2%) | 1.0 (0.3–3.3) | 0.18 | 2 (1%) | 1.2 (0.3–5.2) | 0.37 | 1 (1%) | 1.1 (0.1–8.3) | 0.48 |
| Lifestyle: weight and diet | 5 (4%) | 0.8 (0.3–1.9) | 2 (1%) | 0.5 (0.1–2.0) | 0.40 | 1 (1%) | 0.6 (0.1–4.9) | 0.55 | 1 (1%) | 0.6 (0.1–4.7) | 0.75 |
GI-tract, Gastrointestinal tract; IBD, Inflammatory bowel disease; MUPS, Medically unexplained physical symptoms
* p-value < 0.5
** p-value < 0.01
a Number and percentage of patients with IBD with respective morbidity in the reporting year and three previous years to the diagnosis
b Adjusted for age at diagnosis and gender, ref: neighbouring residents without respective morbidity
c P-value for interaction: exposure variable × morbidity
Discussion
The present study was designed to investigate whether livestock exposure contributes to the development of IBD and whether potential risk factors can be identified. Therefore the clinical events over a three-year period prior to established IBD diagnosis were explored. Morbidity of the GI-tract was confirmed to be highly associated with the development of IBD. In addition, having infections appeared to be a risk factor, but only in neighbouring residents living in the vicinity of poultry farms. Nonetheless, livestock exposure, as an environmental factor, contributed little to the development of IBD. These results support the hypothesis that residents in livestock dense areas generally are protected for the development of inflammatory diseases.
We investigated 141 cases in a livestock dense area and 109 cases in a reference area with less livestock farms, selected from electronic health records, as registered by GPs, of 648 and 395 patients with IBD, respectively. We found a positive association between other autoimmune diseases and the development of IBD. This positive association was only significant in the exposed area, irrespective of the presence of livestock within 500m. We considered the following autoimmune diseases: pernicious anaemia, diabetes mellitus type 1 and 2, psoriasis, multiple sclerosis and rheumatoid arthritis. For all these diseases, an association with IBD has also been found by others [21–23], which supports the hypothesis that patients with autoimmune diseases are prone to develop other autoimmune diseases [24]. However, shared aetiologies, which lie at the heart of most of these relations, may not fully explain why we only found this positive association for those in a livestock dense area. Further research is required to investigate the contribution of other environmental factors, such as particulate matter and microbial agents in this area, in more detail.
Our finding that infections, including respiratory infections, in the reporting year and three previous years to the diagnosis were positively associated with the development of IBD is in line with the literature [25–27]. This association was influenced by exposure to poultry farms. Although there is a lack of studies investigating the role of exposure to poultry in the development of IBD, associations between residential proximity to poultry and stimulation of mucosal tissue have been shown. These studies mainly focus on the respiratory system, showing increased exacerbations in patients with chronic obstructive pulmonary disease [12] and incidence of pneumonia in residents near poultry farms [13], suggesting irritation of mucosal cells by poultry-related emission. Furthermore, there have been suggestions of a possible shared pathological mechanism between respiratory diseases, such as asthma, and IBD (reviewed by Adar et al. [7]). This mechanism involves the increased recruitment of eosinophils, a specific type of innate immune cells, to the intestinal mucosa by the chemokine eotaxin-1. In patients with IBD, increased levels of this cytokine have been found in serum [28] and intestinal mucosal tissues [29,30]. In these latter two studies by Banks et al. (2003) and Coburn et al. (2013), tissue levels of eotaxin-1 correlated with disease activity. These results are in accordance with another study that showed a positive association between the number of eosinophils in the intestines of patients with IBD and disease severity [31]. Currently, a phase 2 clinical trial is being conducted with an antibody to eotaxin-1, called Bertilimumab, in patients with ulcerative colitis (ClinicalTrial.gov, NCT01671956). Altogether, these studies support the hypothesis that, besides their role in respiratory diseases, eosinophils may also play a role in the pathology of IBD. Whether their role is influenced by livestock exposure, requires further research. And as we only focussed on a three-year period before diagnosis, additional longitudinal studies should be performed to investigate whether patients may have presented with infections prior to this period.
In the current study, we confirmed some of the most typical symptoms and related manifestations that precede the diagnosis of ulcerative colitis and Crohn’s disease. For instance, patients with acute somatic symptoms of the GI-tract mainly reported abdominal pain, rectal bleeding, constipation and diarrhoea, as well as anal fissures and diverticulosis for chronic/long-term diseases of the GI-tract. The association between gastrointestinal morbidity and the development of IBD was also supported by the investigation of the drug prescriptions in the reporting year and three previous years to the diagnosis. Classic prescriptions for the treatment of these typical symptoms, such as laxatives, antidiarrheal and immunosuppressant drugs, showed a positive association with the development of IBD. This was also the case for drug prescriptions for anaemia. Being aware of false-positives, GPs might use these findings to achieve early recognition of IBD when they are presented with patients with these type of symptoms and manifestations. It is important to keep diagnostic delays to a minimum, as longer delays correlate with more severe disease and complications thereof [32]. In addition, others have shown that patients with Crohn’s disease benefit from treatment early in the disease course [33]. Nonetheless, reducing diagnostic delays is also dependent on the patients’ decision to visit the GP [32]. Identification of biomarkers and other factors involved in the development of IBD will further contribute to early recognition and management of IBD.
Our study was the first to investigate clinical events prior to the diagnosis of IBD in neighbouring residents of livestock farms, using health records from GPs in combination with estimated livestock exposure at the level of the patients’ home. Nonetheless, one source of weakness in this study is the small sample size, which could have affected the outcomes. This was mainly due to the criteria used to study the clinical events in the three years prior to diagnosis. Although the prevalence of IBD increased over the years, it should be mentioned that IBD is low-prevalent in the general population [34]. In addition, by limiting the medical history of patients for this period, potential risk factors that developed years before were therefore excluded. However, as shown by Hutfless et al., onset of Crohn’s disease can follow GI-tract infections within one year [27]. Other limiting factors are the lack of information on risk factors including smoking habits and family history, and other confounders such as the patients’ residential history and the patients’ occupation, which might be related to farms [35]. These factors and a family history of farming in neighbouring residents of livestock farms might explain why we found a negative association between development of IBD and living in an area with a high density of livestock farms [8,9]. Further research including questionnaires would be useful to take these confounders into account. In addition, whereas we had insight of individual exposure to livestock for residents in the exposed area, no information on residential addresses was available for those in the reference area. Finally, using the ICPC-code D94 did not allow us to make a distinction between ulcerative colitis and Crohn’s disease. Although these diseases have common features, they also show distinct characteristics. Therefore, future studies should aim to investigate these diseases separately. In contrast to these limitations, one of the major strengths of our study design was the selection of practices. Since every citizen in the Netherlands is obligatorily enrolled in the list of just one general practice, we could use a known denominator of the population. Furthermore, we only selected practices with a reliable quality of registration and did not base our selection on the number of patients or the location of the practice. This allowed us to use reliable data without selection bias. Another strong aspect of our study was its case-control design, including patients with low back pain as controls who showed a similar distribution of age and gender compared with the cases. Additionally, low back pain has no known relation with IBD or livestock exposure, yielding a control group that also contacts and visits the GP. Finally, other major strengths of this study were the use of detailed livestock exposure, although taken into account only a single calendar year, and the longitudinal study design. The latter strength allowed us to investigate risk factors for the development of IBD on the patient level.
In conclusion, this is the first study to investigate the clinical events preceding the diagnosis of IBD in neighbouring residents of livestock farms. Although we were not able to confirm overall livestock exposure as an environmental risk factor for the development of IBD, our results suggest that residents with infections who live in the vicinity of poultry farms, have an increased risk of developing IBD. Finally, this study contributes to the body of knowledge concerning the first symptoms prior to the diagnosis of ulcerative colitis and Crohn’s disease.
Supporting information
Clusters (in bold) and categories (in italic) of morbidity according to the ICPC-classification of symptoms and diseases [16].
(DOCX)
Drug prescriptions and clusters of drug prescriptions according to the guidelines for ATC classification and Defined Daily Dose assignment 2015 [18].
(DOCX)
Acknowledgments
We would like to thank the GPs participating in this study for their cooperation. We would also like to thank Dr. Marianne Heins and Dr. Mark Nielen for their valuable contributions. Lastly, we are grateful to our colleagues at NIVEL for useful feedback on the drafts of this paper.
Data Availability
In consultation with the Medical Ethical Committee that approved the study protocol, data from the VGO study are not publicly available due to privacy protection of participants. The study's privacy regulations stated that only co-authors and researchers from NIVEL, IRAS, and RIVM (consortium partners) have access to the study database and researchers who are not part of the consortium partners will not have access to the study database since sharing an anonymized and de-identified dataset would still contain Electronic Health Records and the personal data of participants, which could potentially lead to the identification of subjects. Researchers may reach a data transfer and privacy agreement to access the data, which will include agreement on co-authorship with the consortium members who were responsible for designing the study and collecting the data. Researchers may contact the data access committee through Remco Coppen, PhD, LLM (r.coppen@nivel.nl) or non-personal departmental email (NIVEL: zorgregistraties@nivel.nl; IRAS secretary: Seeoh903@uu.nl), or contact Christos Baliatsas, PhD (c.baliatsas@nivel.nl), Lidwien Smit, PhD (l.a.smit@uu.nl) or Joris Yzermans, PhD (j.ijzermans@nivel.nl).
Funding Statement
This work was supported by the Dutch Ministry of Economic Affairs (“Ministerie van Economische Zaken”), and the Ministry of Health, Welfare and Sport (“Ministerie van Volksgezondheid, Welzijn en Sport”), as part of the VGO study (Dutch acronym for Livestock Farming and Neighbouring Residents’ Health) to CEVD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Spray C, Debelle G, Murphy M. Current diagnosis, management and morbidity in paediatric inflammatory bowel disease. Acta Paediatr. 2001;90(4):400–5. [PubMed] [Google Scholar]
- 2.Vavricka SR, Spigaglia SM, Rogler G, Pittet V, Michetti P, Felley C, et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(3):496–505. doi: 10.1002/ibd.21719 [DOI] [PubMed] [Google Scholar]
- 3.Bamias G, Nyce MR, De La Rue SA, Cominelli F. New Concepts in the Pathophysiology of Inflammatory Bowel Disease. Ann Intern Med. 2005;143(22):895–904. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg E, Järnerot G, Huitfeldt B. Smoking in Crohn’s disease: effect on localisation and clinical course. Gut. 1992;33(6):779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burisch J, Pedersen N, Cukovic-Cavka S, Turk N, Kaimakliotis I, Duricova D, et al. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe—An ECCO-EpiCom study. J Crohn’s Colitis. European Crohn’s and Colitis Organisation; 2014;8(7):607–16. [DOI] [PubMed] [Google Scholar]
- 6.Heederik D, Yzermans C. Mogelijke effecten van intensieve veehouderij op de gezondheid van omwonenden [Possible effects of concentrated animal feeding operations on residents’ health]. Utrecht/Bilthoven; 2011.
- 7.Adar T, Shteingart S, Ben Ya’acov A, Bar-Gil Shitrit A, Goldin E. From airway inflammation to inflammatory bowel disease: Eotaxin-1, a key regulator of intestinal inflammation. Clin Immunol. Elsevier Inc.; 2014;153(1):199–208. doi: 10.1016/j.clim.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 8.Timm S, Svanes C, Janson C, Sigsgaard T, Johannessen A, Gislason T, et al. Place of upbringing in early childhood as related to inflammatory bowel diseases in adulthood: A population-based cohort study in Northern Europe. Eur J Epidemiol. 2014;29(6):429–37. doi: 10.1007/s10654-014-9922-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radon K, Windstetter D, Poluda AL, Mueller B, Von Mutius E, Koletzko S. Contact With Farm Animals in Early Life and Juvenile Inflammatory Bowel Disease: A Case-Control Study. Pediatrics. 2007;120(2):354–61. doi: 10.1542/peds.2006-3624 [DOI] [PubMed] [Google Scholar]
- 10.Boneberger A, Hangl C, Schierl R, Koletzko S, Von Kries R, Kabesch M, et al. Endotoxin levels in house dust samples and juvenile inflammatory bowel disease—a case-control study. J Crohn’s Colitis. European Crohn’s and Colitis Organisation; 2011;5(6):525–30. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel M, Chang M, Chow EJ, Tabibzadeh S, Kirit-Kiriak V, Targan SR, et al. Identification of a prodromal period in Crohn’s disease but not ulcerative colitis. Am J Gastroenterol. 2000;95(12):3458–62. doi: 10.1111/j.1572-0241.2000.03361.x [DOI] [PubMed] [Google Scholar]
- 12.Van Dijk CE, Smit LAM, Hooiveld M, Zock J-P, Wouters IM, Heederik DJJ, et al. Associations between proximity to livestock farms, primary health care visits and self-reported symptoms. BMC Fam Pract. BMC Family Practice; 2016;17(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smit L, van der Sman-de Beer F, Opstal-van Winden AWJ, Hooiveld M, Beekhuizen J, Wouters IM, et al. Q fever and pneumonia in an area with a high livestock density: A large population-based study. PLoS One. 2012;7(6):e38843 doi: 10.1371/journal.pone.0038843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooiveld M, Smit LAM, van der Sman-de Beer F, Wouters IM, van Dijk CE, Spreeuwenberg P, et al. Doctor-diagnosed health problems in a region with a high density of concentrated animal feeding operations: a cross-sectional study. Environ Heal. Environmental Health; 2016;15(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prins M, Hek K, Verberne L, Nielen M, Opperhuizen G, Verheij R. Zorg voor de huisarts: jaarcijfers 2014 en trendcijfers 2010–2014 [GP care: figures 2014 and trends 2010–2014]. Utrecht; 2015.
- 16.Lamberts H, Wood M. ICPC. International Classification of Primary Care. Oxford University Press; Oxford; 1987; [Google Scholar]
- 17.Van Dijk CE, Garcia-Aymerich J, Carsin A-E, Smit LAM, Borl00E9e F, Heederik DJ, et al. Risk of exacerbations in COPD and asthma patients living in the neighbourhood of livestock farms: observational study using longitudinal data. Int J Hyg Environ Health. Elsevier GmbH.; 2016;1–10. [DOI] [PubMed] [Google Scholar]
- 18.WHOCC. Guidelines for ATC classification and DDD assignment 2015. 2014.
- 19.Van Gils-Van Rooij ESJ, Yzermans CJ, Broekman SM, Meijboom BR, Welling GP, De Bakker DH. Out-of-Hours Care Collaboration between general practitioners and hospital emergency departments in the Netherlands. J Am Board Fam Med. 2015;28(6):807–15. doi: 10.3122/jabfm.2015.06.140261 [DOI] [PubMed] [Google Scholar]
- 20.Borlée F, Yzermans C, van Dijk C, Heederik D, Smit L. Increased respiratory symptoms in COPD patients living in the vicinity of livestock farms. Eur Respir J. 2015;46(6):1605–14. doi: 10.1183/13993003.00265-2015 [DOI] [PubMed] [Google Scholar]
- 21.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–53. doi: 10.1136/gut.2009.199679 [DOI] [PubMed] [Google Scholar]
- 22.Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatology Venereol. 2009;23(5):561–5. [DOI] [PubMed] [Google Scholar]
- 23.Haapamäki J, Roine RP, Turunen U, Färkkilä MA, Arkkila PET. Increased risk for coronary heart disease, asthma, and connective tissue diseases in inflammatory bowel disease. J Crohn’s Colitis. European Crohn’s and Colitis Organisation; 2011;5(1):41–7. [DOI] [PubMed] [Google Scholar]
- 24.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76(4):561–71. doi: 10.1086/429096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brassard P, Vutcovici M, Ernst P, Patenaude V, Sewitch M, Suissa S, et al. Increased incidence of inflammatory bowel disease in Québec residents with airway diseases. Eur Respir J. 2015;45(4):962–8. doi: 10.1183/09031936.00079414 [DOI] [PubMed] [Google Scholar]
- 26.Raj AA, Birring SS, Green R, Grant A, de Caestecker J, Pavord ID. Prevalence of inflammatory bowel disease in patients with airways disease. Respir Med. 2008;102(5):780–5. doi: 10.1016/j.rmed.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 27.Hutfless S, Abramson O, Heyman MB, Bayless TM, Li D-K, Winthrop K, et al. Infections Requiring Hospitalization as Predictors of Pediatric-Onset Crohn’s Disease and Ulcerative Colitis. Gastroenterol Res Pract. Hindawi Publishing Corporation; 2015;2015:690581 doi: 10.1155/2015/690581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mir A, Minguez M, Tatay J, Pascual I, Pen A. Elevated Serum Eotaxin Levels in Patients With Inflammatory Bowel Disease. Am J Gastroenterol. 2002;97(6):1452–7. doi: 10.1111/j.1572-0241.2002.05687.x [DOI] [PubMed] [Google Scholar]
- 29.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol. 2003;199(1):28–35. doi: 10.1002/path.1245 [DOI] [PubMed] [Google Scholar]
- 30.Coburn LA, Horst SN, Chaturvedi R, Brown CT, Allaman MM, Scull BP, et al. High-throughput multi-analyte Luminex profiling implicates eotaxin-1 in ulcerative colitis. PLoS One. 2013;8(12):e82300 doi: 10.1371/journal.pone.0082300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeziorska M, Haboubi N, Schofield P, Woolley DE. Distribution and activation of eosinophils in inflammatory bowel disease using an improved immunohistochemical technique. J Pathol. 2001;194(4):484–92. [DOI] [PubMed] [Google Scholar]
- 32.Schoepfer AM, Dehlavi M-A, Fournier N, Safroneeva E, Straumann A, Pittet V, et al. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108(11):1744–53; quiz 1754. doi: 10.1038/ajg.2013.248 [DOI] [PubMed] [Google Scholar]
- 33.Kariyawasam VC, Selinger CP, Katelaris PH, Jones DB, McDonald C, Barr G, et al. Early use of thiopurines or methotrexate reduces major abdominal and perianal surgery in Crohn’s disease. Inflamm Bowel Dis. 2014;20(8):1382–90. doi: 10.1097/MIB.0000000000000119 [DOI] [PubMed] [Google Scholar]
- 34.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. Elsevier Inc.; 2012;142(1):46–54.e42. [DOI] [PubMed] [Google Scholar]
- 35.Smit L, Hooiveld M, van der Sman-de Beer F, Opstal-van Winden A, Beekhuizen J, Wouters I, et al. Air pollution from livestock farms, and asthma, allergic rhinitis and COPD among neighbouring residents. Occup Environ Med. 2014;71(2):134–40. doi: 10.1136/oemed-2013-101485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clusters (in bold) and categories (in italic) of morbidity according to the ICPC-classification of symptoms and diseases [16].
(DOCX)
Drug prescriptions and clusters of drug prescriptions according to the guidelines for ATC classification and Defined Daily Dose assignment 2015 [18].
(DOCX)
Data Availability Statement
In consultation with the Medical Ethical Committee that approved the study protocol, data from the VGO study are not publicly available due to privacy protection of participants. The study's privacy regulations stated that only co-authors and researchers from NIVEL, IRAS, and RIVM (consortium partners) have access to the study database and researchers who are not part of the consortium partners will not have access to the study database since sharing an anonymized and de-identified dataset would still contain Electronic Health Records and the personal data of participants, which could potentially lead to the identification of subjects. Researchers may reach a data transfer and privacy agreement to access the data, which will include agreement on co-authorship with the consortium members who were responsible for designing the study and collecting the data. Researchers may contact the data access committee through Remco Coppen, PhD, LLM (r.coppen@nivel.nl) or non-personal departmental email (NIVEL: zorgregistraties@nivel.nl; IRAS secretary: Seeoh903@uu.nl), or contact Christos Baliatsas, PhD (c.baliatsas@nivel.nl), Lidwien Smit, PhD (l.a.smit@uu.nl) or Joris Yzermans, PhD (j.ijzermans@nivel.nl).
In consultation with the Medical Ethical Committee that approved the study protocol, data from the VGO study are not publicly available due to privacy protection of participants. The study's privacy regulations stated that only co-authors and researchers from NIVEL, IRAS, and RIVM (consortium partners) have access to the study database and researchers who are not part of the consortium partners will not have access to the study database since sharing an anonymized and de-identified dataset would still contain Electronic Health Records and the personal data of participants, which could potentially lead to the identification of subjects. Researchers may reach a data transfer and privacy agreement to access the data, which will include agreement on co-authorship with the consortium members who were responsible for designing the study and collecting the data. Researchers may contact the data access committee through Remco Coppen, PhD, LLM (r.coppen@nivel.nl) or non-personal departmental email (NIVEL: zorgregistraties@nivel.nl; IRAS secretary: Seeoh903@uu.nl), or contact Christos Baliatsas, PhD (c.baliatsas@nivel.nl), Lidwien Smit, PhD (l.a.smit@uu.nl) or Joris Yzermans, PhD (j.ijzermans@nivel.nl).


