Abstract
Background
Statins have been known to increase the risk of incident type 2 diabetes mellitus (DM); however, other factors, especially hypertension, are also associated with DM development.
Objective
We investigated whether statin use increases the risk of DM and further analyzed whether the relation between statin use and incident DM differs according to the presence of hypertension and gender.
Methods
From a nationwide health-screening cohort, 40,164 participants with total cholesterol levels ≥eve mg/dL and without pre-diagnosed DM, cardiovascular disease, or cancer, who underwent a series of regular health check-ups, were enrolled. Statin users were defined as participants who were prescribed statins more than twice during 6 months.
Results
There were 17,798 statin non-users and 22,366 statin users. During 7.66±3.21 years of follow-up, incident DM developed in 5.68% of statin non-users and 7.64% of statin users. Among the entire study population, statin use was associated with new-onset DM after adjusting for clinical risk factors. In sub-analysis according to hypertension, statin use significantly increased the risk of incident DM only in normotensive patients [hazard ratio (HR) 1.31, 95% confidence interval (CI) 1.09 to 1.58, p = 0.004], and not in hypertensive patients (p>0.05). Furthermore, continuous statin use was strongly associated with new-onset DM in women, regardless of hypertension presence (all p<0.05). However, in men, statin was associated with new-onset DM only in normotensive males (HR 1.61, 95% CI 1.35 to 1.92, p<0.001) and not in hypertensive males (p>0.05).
Conclusions
Statin use increased the risk of new-onset DM only in normotensive patients and hypertensive women, suggesting that these groups should be more carefully monitored for the development of DM during the course of follow-up.
Introduction
The impact of statins on the development of type 2 diabetes mellitus (DM) has been studied in both randomized controlled trials and meta-analyses [1–5]. Based on the results, the risk of new-onset DM has been added to labels of statins by the US Food and Drug Administration [6]. However, because DM itself is often associated with dyslipidemia, hypertension, obesity, and elevated glucose levels, it remains controversial whether only patients who are already at high risk for developing DM are susceptible to statin-induced DM or patients who are at low risk for type 2 DM also have a risk for statin-induced DM. Therefore, all these confounding factors should be considered to determine whether a sex difference exists in susceptibility to the development of new-onset DM associated with statin use.
Especially, it has been shown that hypertensive patients without DM have an increased risk of developing diabetes and a high prevalence of insulin resistance, when compared with the risk and prevalence in normotensive subjects [7,8]. Furthermore, antihypertensive therapy, including both beta-blockers and diuretics, has been shown to increase the risk of new-onset DM [9,10]. As a result, recent guidelines do not recommend thiazides or beta-blockers in patients with hypertension who are at high risk of developing diabetes [11]. Therefore, it is required to consider the presence of hypertension separately when assessing the impact of statins on the development of type 2 DM.
Therefore, in the present study, we first investigated whether a relation exists between statin use and incident type 2 DM in a large cohort of a relatively healthy population undergoing national health screening. Furthermore, we investigated whether the effect of statins on incident DM is dependent on the presence of hypertension and evaluated sex differences.
Materials and methods
Study population
In the Republic of Korea, the National Health Insurance System (NHIS) is compulsory for all citizens, and it is important in the management and maintenance of health service use. The NHIS operates a biennial health-screening program at the national level, including a general health examination for all its participants over the age of 40 years.
In 2016, data from the NHIS-National Health-screening Cohort (NHIS-HEALS), representing approximately 10% of the total source population, were released. The cohort consisted of 514,795 randomly sampled individuals who had started their biennial NHIS health screening between years 2002 and 2003. Each individual in the cohort had received follow-up until either the end of study period (2013) or the time of health service disqualification due to death or emigration, and had their health screening every two years. The NHIS-HEALS contains eligibility and demographic information regarding health insurance and medical aid beneficiaries, histories of medical treatment or disease, details of medical bills, and drug prescriptions.
From the entire database, statin users were identified as patients who were prescribed statins more than twice during 6 months (n = 137,784), and statin nonusers were identified as patients who were not prescribed statins between 2002 and 2013 (n = 330,805). Among these 468,589 patients, we excluded those who met the following criteria: 1) treatment with statins before the first examination (n = 39,039), 2) treatment with statins after the diagnosis of DM (n = 17,978), 3) only one examination (n = 53,786), 4) an elevated fasting glucose level at the baseline examination (n = 13,564), 5) a low total cholesterol level (<240 mg/dL) at the baseline examination (n = 297,815), 6) previous history of DM (10th revision of the International Classification of Diseases [ICD-10] code E11 with prescription history of diabetic drugs), cardiac disease (ICD-10 code I21, I22, or I23), stroke (ICD-10 code I63), malignancy (ICD-10 codes C00–C97) (n = 4,875), and 7) previous history of impaired fasting glucose or gestational DM (ICD-10 codes E10–14, O24, or R73) (n = 1,367). The final dataset included 40,164 patients (17,798 statin non-user and 22,366 statin users). The date of enrollment was set as the date on which statin was first prescribed.
This study was approved by the institutional review board of Yonsei University College of Medicine. Informed consent was not obtained from each participant owing to the characteristics of the data obtained from the NHIS and the fact that the data were fully de-identified and anonymized.
Clinical variables
Laboratory tests, including fasting glucose and total cholesterol tests, were performed after overnight fasting at each examination visit. Systolic and diastolic blood pressure, body weight, height, and waist circumference were measured at every visit. Body mass index (BMI) was calculated using the documented body weight and height. Detailed histories of alcohol consumption and smoking status were obtained via a questionnaire.
The medical history of the participants was assessed using a combination of the following: clinical and pharmaceutical codes of ICD-10, list of previously prescribed medicines, and past medical information. Prescription of well-known diabetogenic drugs other than statins (beta-blockers and hydrochlorothiazide) was identified and adjusted [9,10,12].
Study endpoint
The primary endpoint was the development of new-onset DM, identified according to the newly entered ICD-10 codes E11–14, with prescription of diabetic drugs.
Statistical analysis
Variables are reported as percentages or means ± standard deviations (SDs) for normally distributed variables, and medians (with 25–75% ranges) for non-normally distributed variables, as necessary. The chi-square test was used for nominal variables. Development rate of new-onset DM was computed by time-dependent Cox regression. Hazard ratios (HR) were calculated to model the effects of statins on development of DM in univariate and multivariate analyses. Variables in multivariate model were age, sex, body mass index, blood pressure, fasting blood glucose level, total cholesterol level, history of smoking and drinking, exercise, family history of type 2 DM, duration of statin treatment, and history of taking beta-blocker or hydrochlorothiazide. A p-value <0.05 was considered statistically significant. All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical and laboratory results at baseline and last follow-up
Overall, 40,164 patients (mean age, 55.1±9.0 years; 47.0% male) were enrolled from the NHIS-HEALS. The mean total follow-up duration was 7.7±3.2 years (Table 1). Among the entire study population, 12,527 (31.2%) patients were diagnosed with hypertension at baseline. There were 17,798 statin non-users (44.3%; mean age, 52.2±9.4 years; 59.9% male) and 22,366 statin users (55.7%; mean age, 57.4±8.0 years; 36.8% male). At the time of enrollment, the mean total cholesterol level of the entire study population was 264.8±25.9 mg/dL and the mean fasting blood glucose level was 94.0±12.5 mg/dL.
Table 1. Baseline clinical characteristics and laboratory results.
| Risk factors | Total (n = 40,164) | Statin (-) (n = 17,798) | Statin (+) (n = 22,366) |
|---|---|---|---|
| Age (years) | 55.1±9.0 | 52.2±9.4 | 57.4±8.0 |
| Male gender, n (%) | 18,882 (47.0) | 10,653 (59.9) | 8,229 (36.8) |
| Follow-up duration (years) | 7.7±3.2 | 10.2±1.7 | 5.6±2.6 |
| BMI (kg/m2) | 24.5±2.8 | 24.4±2.8 | 24.6±2.8 |
| SBP (mmHg) | 128.6±17.4 | 127.4±17.6 | 129.5±17.1 |
| DBP (mmHg) | 80.5±11.3 | 80.4±11.6 | 80.5±11.0 |
| Total Cholesterol (mg/dL) | 264.8±25.9 | 258.7±23.4 | 269.7±26.8 |
| FSG (mg/dL) | 94.0±12.5 | 92.8±13.1 | 95.0±11.9 |
| Smoking, n (%) | 7,735 (19.3) | 4,916 (27.6) | 2,819 (12.6) |
| Alcohol use, n (%) | 15,822 (39.4) | 8,432 (47.4) | 7,390 (33.0) |
| Exercise, n (%) | 21,148 (52.7) | 7,694 (43.2) | 13,454 (60.2) |
| FHx of type 2 DM, n (%) | 5,504 (13.7) | 2,541 (14.3) | 2,963 (13.3) |
| Duration of statin (years) | - | - | 4.4±2.9 |
| Drug history, n (%) | 6,057 (15.1) | 819 (4.6) | 5,238 (23.4) |
| Use of beta-blocker | 2,181 (5.4) | 359 (2.0) | 1822 (8.2) |
| Use of thiazide | 3,876 (9.7) | 460 (2.6) | 3,416 (15.3) |
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FHx, family history; FSG, fasting serum glucose; SBP, systolic blood pressure
At follow up, the total cholesterol level decreased when compared to the level at baseline, regardless of statin use, although the absolute difference was greater in statin users (Δ56.0 mg/dL) than in non-users (Δ32.3 mg/dL) (S1 Table). The decreases in the total cholesterol level among both statin users and non-users were maintained when the population was divided according to the presence of hypertension. In contrast to the total cholesterol level, the fasting serum glucose level increased in both statin users and non-users.
Time-dependent multivariate Cox regression analysis for development of DM
Development of incident type 2 DM was noted in 5.7% of statin non-users (1,011 patients) and 7.6% of statin users (1,709 patients) (p = 0.045). When considering the entire study population (Table 2), statin use was associated with the development of new-onset type 2 DM (hazard ratio [HR]: 1.66, 95% confidence interval [CI]: 1.49 to 1.85, p<0.001) after adjusting for age, sex, BMI, blood pressure, fasting glucose level, total cholesterol level, smoking, drinking, exercise, family history of type 2 DM, and history of taking a beta-blocker or hydrochlorothiazide, using time-dependent Cox regression analysis.
Table 2. Time dependent multivariate Cox regression analysis for development of new-onset type 2 diabetes mellitus*.
| Risk factors | Total patients | HTN (-) | HTN (+) | |||
|---|---|---|---|---|---|---|
| (n = 40,164) | (n = 27,637) | (n = 12,827) | ||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Statin use | 1.66 (1.49, 1.85) | < .001 | 1.31 (1.09, 1.58) | 0.004 | 1.18 (0.96, 1.46) | 0.114 |
| Female | 1.24 (1.09, 1.41) | 0.001 | 1.29 (1.03, 1.60) | 0.024 | 1.10 (0.90, 1.33) | 0.361 |
| Age | 1.02 (1.01, 1.03) | < .001 | 1.01 (0.99, 1.03) | 0.217 | 1.02 (1.00, 1.03) | 0.021 |
| BMI | 1.08 (1.07, 1.10) | < .001 | 1.08 (1.06, 1.11) | < .001 | 1.06 (1.04, 1.09) | < .001 |
| SBP | 1.01 (1.00, 1.01) | 0.004 | 1.01 (1.00, 1.02) | 0.051 | 0.10 (0.99, 1.01) | 0.640 |
| DBP | 1.00 (0.99, 1.01) | 0.672 | 1.00 (0.99, 1.01) | 0.729 | 1.00 (0.99, 1.01) | 0.428 |
| FSG | 1.04 (1.04, 1.04) | < .001 | 1.04 (1.04, 1.04) | < .001 | 1.04 (1.03, 1.04) | < .001 |
| TC | 0.99 (0.99, 1.00) | < .001 | 1.00 (0.99, 1.00) | < .001 | 1.00 (0.99, 1.00) | < .001 |
| Smoking | 1.37 (1.16, 1.61) | < .001 | 1.26 (0.95, 1.66) | 0.106 | 1.32 (1.03, 1.70) | 0.028 |
| Drink | 0.73 (0.64, 0.82) | < .001 | 0.88 (0.70, 1.10) | 0.247 | 0.76 (0.62, 0.94) | 0.009 |
| Exercise | 0.89 (0.81, 0.99) | 0.02 | 0.87 (0.73, 1.04) | 0.119 | 0.88 (0.75, 1.03) | 0.099 |
| Family DM | 1.34 (1.18, 1.52) | < .001 | 1.49 (1.18, 1.89) | 0.001 | 1.28 (1.04, 1.59) | 0.022 |
| Drug | 1.04 (0.92, 1.17) | 0.526 | 0.95 (0.70, 1.31) | 0.773 | 0.93 (0.77, 1.09) | 0.362 |
BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; FHx, family history; FSG, fasting serum glucose; HR, hazard ratio; HTN, hypertension; SBP, systolic blood pressure; TC, total cholesterol
*Adjusted with age, sex, body mass index, blood pressure, fasting serum glucose, smoking, drinking, exercise, family history of type 2 DM, total cholesterol level and history of taking beta-blocker or hydrochlorothiazide
To determine the impact of hypertension on the development of DM associated with statin use, patients were divided into normotensive and hypertensive groups. Statins tended to be more commonly prescribed in hypertensive patients than in normotensive patients (84.3% vs. 42.7%, p<0.001). Statin therapy significantly increased the risk of incident type 2 DM only in normotensive patients (HR: 1.31, 95% CI: 1.09 to 1.58, p = 0.0039) and not in hypertensive patients (HR: 1.18, 95% CI: 0.96 to 1.46, p = 0.114). Notably, female gender was a risk factor for development of DM only in normotensive group.
Sex differences in the impact of hypertension on the development of incident DM with statin therapy
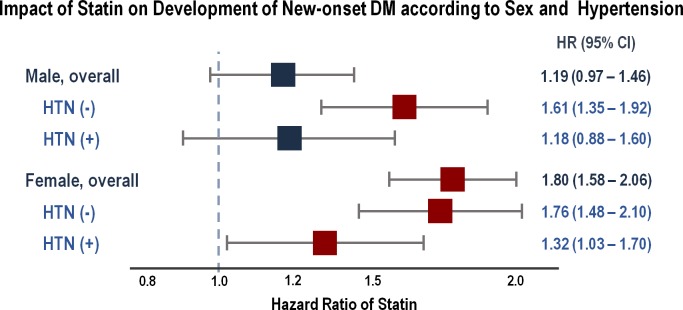
In the sub-analysis according to sex, in men, statin use was not associated with new-onset DM (HR: 1.19, 95% CI: 0.97 to 1.46, p = 0.101), even after correcting for the change in the total cholesterol level (Fig 1). In contrast, in women, statin use was significantly associated with the development of incident DM (HR: 1.80, 95% CI: 1.58 to 2.06, p<0.001).
Fig 1. Effect of statins on development of diabetes mellitus according to sex and hypertension.
CI, confidence interval; HR, hazard ratio; HTN, hypertension. *Adjusted with age, body mass index, blood pressure, fasting serum glucose, smoking, drinking, exercise, family history of type 2 DM, total cholesterol level and history of taking beta-blocker or hydrochlorothiazide.
On further dividing the patients according to the presence of hypertension, in men, statin use was not associated with DM among hypertensive patients (HR: 1.18, 95% CI: 0.88 to 1.60, p = 0.274) (Fig 1, S2 Table). However, notably, statin use significantly increased the risk of developing DM among normotensive men (HR: 1.61, 95% CI: 1.35 to 1.92, p<0.001). In contrast, the presence of hypertension did not influence the impact of statins on the development of DM in women. Statin use was associated with an increased risk of new-onset DM in both normotensive (HR: 1.76, 95% CI: 1.48 to 2.10, p<0.001) and hypertensive (HR: 1.32, 95% CI: 1.21 to 1.70, p = 0.030) women.
Discussion
The main findings of the current study using a nationwide time-series health-screening cohort are as follows: (1) statin therapy increased the risk of new-onset type 2 DM at the total population level, and (2) the risk of DM associated with statin use increased only in normotensive patients. Moreover, statin therapy did not show any impact on the development of DM in only hypertensive male patients.
These results are partly consistent with the findings of previous studies that demonstrated an increased risk of new-onset type 2 DM associated with statin use [1–5]. Because type 2 DM is a complex disease and multiple risk factors, including metabolic syndrome, are strongly involved in its development [13], the true pure effect of statins is difficult to acknowledge, as these diseases are often cluster together as metabolic syndrome [14]. Statin users who develop incident DM often have evidence of impaired fasting glucose levels or metabolic syndrome even before starting statin therapy [15,16]. In fact, preexisting risk factors, including baseline fasting serum glucose levels, BMI, fasting triglyceride levels, and hypertension, independently predicted new-onset DM in 3 large randomized trials involving atorvastatin [16]. The fact that statins increased the risk of DM only in normotensive patients in this study supports these previous findings, because in hypertensive patients, multiple clustering factors, other than statin use, were found to be associated with the development of DM.
Moreover, when we further divided the patients according to sex, only normotensive, not hypertensive, patients were prone to acquire DM among men. Previous studies concluded that statin use is a stronger risk factor for the development of type 2 DM in women than men [17,18]. However, it is still unclear whether these reported sex differences in the risk of DM are real or attributable to differences between women and men taking statins with respect to other risk factors for type 2 DM. The present finding of a sex difference in incident DM underscores the importance of setting treatment and monitoring strategies in women and men separately.
Despite the large amount of evidence for a higher incidence of DM with statin use, the underlying mechanisms are not yet well characterized. So far, several potential mechanisms have been suggested. Statins may directly decrease the synthesis of insulin or reduce insulin secretion [19], exacerbating insulin resistance, and/or modify insulin signaling in peripheral target tissues [20]. Another suggested mechanism states that the effect of statins associated with the reduction in cholesterol levels induces DM and not the drug itself [21]. However, in the current study, statin use was independently associated with the risk of DM even after adjusting for the change in the total cholesterol level, which suggests that the drug itself has an influence on the development of DM.
The prevalence of type 2 DM in South Korea drastically increased from 1970 to 2000, and remained relatively stable after 2000. South Korea also ranked 21st among the 30 Organization for Economic Co-operation and Development member countries in terms of adult (aged 20 to 79) diabetes prevalence, according to the International Diabetes Federation report [22,23]. This was accompanied by the increase in socioeconomic status and prevalence of adult obesity [23,24]. However, as a country with one of the highest growth rates, South Korea is expected to have the highest level of prevalence for type 2 DM by the year 2030 [23]. Furthermore, a recent study showed that the prevalence of cardiovascular disease was more than two times higher in diabetic population compared to the general Korean population [23]. These results warrant a need for planning national public health strategies, as well as identification of every possible factor that could be related to development of type 2 DM.
Furthermore, it is also important to identify patients who are at higher risk for type 2 DM in order to guide individual treatment in clinical practice, as recent modifications in the guidelines regarding management of dyslipidemia could increase the global use of statins [25]. Moreover, despite the association between statins and new-onset DM, studies investigating this relationship have equally confirmed the prevailing cardiovascular therapeutic benefit of statins in both men and women [9,15,18,26,27]. The current study found that women and normotensive men were at high risk for developing incident DM and might require more thorough monitoring during the course of follow-up.
The present study has some limitations. First, this study was conducted using observational data of South Korean population, and study subjects are East Asians. Hence, application of the data into clinical practice warrants caution, as results might differ in patients of other ethnicities. Furthermore, due to the nature of observational data, impact of the drug is inevitably susceptible to confounding factors, including indications and clinical backgrounds, which can obscure the true effect of the drug or even imply false associations between the drug and outcomes. However, it should be also acknowledged that, to date, no trials regarding statins were designed to test the effect of statins on new-onset DM, and as a result, trials using a range of methods were collected for meta-analyses. Third, potential selection bias is another inherent limitation of this study. The NHIS only recommends, but does not necessarily obligate, all health insurance subscribers to take regular health examinations. Therefore, the current cohort likely consisted of relatively healthier individuals without very serious diseases or individuals who were concerned about health. However, the data reflects a representative sample of subjects who were prospectively followed. Finally, as glycated hemoglobin was not included in the screening parameters, diagnosis of type 2 DM was made based on ICD-10 codes. However, such disease definition was frequently used in population-based studies with limited variables [22,28], while fasting glucose level and prescription history of diabetic drugs were also considered in defining patients with diabetes.
In conclusion, in this analysis based on a nationwide cohort, statin use was associated with an increased risk of incident DM only in normotensive patients, and only women and normotensive men were susceptible to the development of new-onset DM on statin use. The current study suggests that these patient groups should be more carefully monitored for the development of DM when taking statins.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIT), (No. 2012027176) (HJC). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. HJC had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and meta-analysis. Diabetes research and clinical practice. 2010;87: 98–107. doi: 10.1016/j.diabres.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 2.Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. The American journal of cardiology. 2013;111: 1123–1130. doi: 10.1016/j.amjcard.2012.12.037 [DOI] [PubMed] [Google Scholar]
- 3.Preiss D, Seshasai SRK, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. Jama. 2011;305: 2556–2564. doi: 10.1001/jama.2011.860 [DOI] [PubMed] [Google Scholar]
- 4.Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes care. 2009;32: 1924–1929. doi: 10.2337/dc09-0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. The Lancet. 2010;375: 735–742. [DOI] [PubMed] [Google Scholar]
- 6.Food U, Administration D. FDA Expands Advice on Statin Risks. 2015. [Google Scholar]
- 7.Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Grassi G, et al. Increased long-term risk of new-onset diabetes mellitus in white-coat and masked hypertension. Journal of hypertension. 2009;27: 1672–1678. doi: 10.1097/HJH.0b013e32832be5f9 [DOI] [PubMed] [Google Scholar]
- 8.Sowers JR, Bakris GL. Antihypertensive therapy and the risk of type 2 diabetes mellitus. New England Journal of Medicine. 2000;342: 969–970. doi: 10.1056/NEJM200003303421310 [DOI] [PubMed] [Google Scholar]
- 9.Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. The Lancet. 2002;359: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Brown M, Castaigne A, de Leeuw P, Palmer CR, Rosenthal T, et al. Outcomes with nifedipine GITS or Co-amilozide in hypertensive diabetics and nondiabetics in Intervention as a Goal in Hypertension (INSIGHT). Hypertension. 2003;41: 431–436. doi: 10.1161/01.HYP.0000057420.27692.AD [DOI] [PubMed] [Google Scholar]
- 11.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34: 2159–2219. doi: 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 12.Mason JM, Dickinson HO, Nicolson DJ, Campbell F, Ford GA, Williams B. The diabetogenic potential of thiazide-type diuretic and beta-blocker combinations in patients with hypertension. Journal of hypertension. 2005;23: 1777–1781. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. The Lancet. 2014;383: 1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112: 2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. The Lancet. 2012;380: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun C-C, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. Journal of the American College of Cardiology. 2011;57: 1535–1545. doi: 10.1016/j.jacc.2010.10.047 [DOI] [PubMed] [Google Scholar]
- 17.Culver AL, Ockene IS, Balasubramanian R, Olendzki BC, Sepavich DM, Wactawski-Wende J, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women's Health Initiative. Archives of internal medicine. 2012;172: 144–152. doi: 10.1001/archinternmed.2011.625 [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121: 1069–1077. doi: 10.1161/CIRCULATIONAHA.109.906479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sattar N, Taskinen M-R. Statins are diabetogenic–myth or reality? Atherosclerosis Supplements. 2012;13: 1–10. doi: 10.1016/j.atherosclerosissup.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Koh KK, Lim S, Sakuma I, Quon MJ. Caveats to aggressive lowering of lipids by specific statins. International journal of cardiology. 2012;154: 97–101. doi: 10.1016/j.ijcard.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 21.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA cardiology. 2016;1: 692–699. doi: 10.1001/jamacardio.2016.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo BK, Lee CH, Yang BR, Hwang SS, Choi NK. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: a National Health Insurance Database Study. PLoS One. 2014;9: e110650 doi: 10.1371/journal.pone.0110650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park Ie B, Kim J, Kim DJ, Chung CH, Oh JY, Park SW, et al. Diabetes epidemics in Korea: reappraise nationwide survey of diabetes "diabetes in Korea 2007". Diabetes Metab J. 2013;37: 233–239. doi: 10.4093/dmj.2013.37.4.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha KH, Kim DJ. Trends in the Diabetes Epidemic in Korea. Endocrinol Metab (Seoul). 2015;30: 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63: 2889–2934. doi: 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Macedo AF, Taylor FC, Casas JP, Adler A, Prieto-Merino D, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC medicine. 2014;12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trialists CT. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. The Lancet. 2015;385: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 28.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995–2005: a population-based study. Lancet. 2007;369: 750–756. doi: 10.1016/S0140-6736(07)60361-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.