Abstract
Evaluating the risk of emergence and transmission of vector‐borne diseases requires knowledge of the genetic and environmental contributions to pathogen transmission traits. Compared to the significant effort devoted to understanding the biology of malaria transmission from vertebrate hosts to mosquito vectors, the strategies that malaria parasites have evolved to maximize transmission from vectors to vertebrate hosts have been largely overlooked. While determinants of infection success within the mosquito host have recently received attention, the causes of variability for other key transmission traits of malaria, namely the duration of parasite development and its virulence within the vector, as well as its ability to alter mosquito behavior, remain largely unknown. This important gap in our knowledge needs to be bridged in order to obtain an integrative view of the ecology and evolution of malaria transmission strategies. Associations between transmission traits also need to be characterized, as they trade‐offs and constraints could have important implications for understanding the evolution of parasite transmission. Finally, theoretical studies are required to evaluate how genetic and environmental influences on parasite transmission traits can shape malaria dynamics and evolution in response to disease control.
Keywords: host–parasite interactions, malaria, mosquito, transmission
1. INTRODUCTION
Human malaria remains one of the most common causes of human mortality, accounting for nearly half a million deaths each year (WHO, 2015). Malaria is caused by Plasmodium parasites transmitted among humans by the bites of infected Anopheles mosquitoes (Box 1). More than 85% of malaria cases and 90% of malaria deaths occur in sub‐Saharan Africa, mainly among young children (WHO, 2015). Five species of the genus Plasmodium cause all human malaria infections. Of these parasites, Plasmodium falciparum causes the highest mortality and presents one of the most pressing challenges facing public health systems worldwide (White et al., 2014; WHO, 2015). Ongoing control efforts, relying mostly on antimalarial drugs and insecticide‐based interventions such as long‐lasting insecticidal nets and indoor residual spraying against mosquito vectors, have reduced malaria transmission (Bhatt et al., 2015). However, these interventions have selected for drug and insecticide resistance which could jeopardize control efforts (Huijben & Paaijmans, 2017; Sternberg & Thomas, 2017).
Box 1. Malaria life cycle and the contrasting amount of knowledge on transmission traits variability within‐vertebrate hosts versus within‐mosquito vectors.
1.
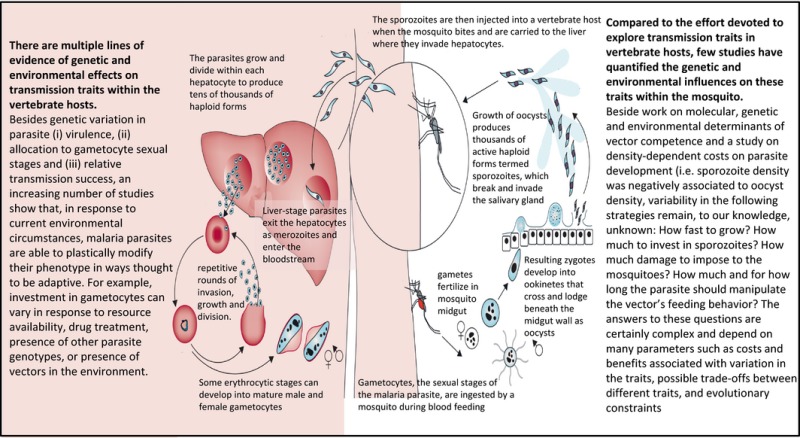
Despite the public health importance of these pathogens, many fundamental aspects of transmission remain unexplored. In particular, the sources of variation in traits that predict transmission from vectors to vertebrate hosts have been largely overlooked (Box 1). Like any vector‐borne parasite, malaria parasites must exploit patchy resources, encountering different environments with varying resources and selective forces as they make their way between the human host and insect vector. Parasite transmission traits can thus be influenced by multiple interacting factors including the direct influence of parasite genetic characteristics, the within‐vertebrate or within‐vector environment (vertebrate/vector genotype, immune responses, resource availability, presence of co‐infecting parasites, age, etc.), and the indirect influence of the external environment (temperature, humidity, host's predators, competitors, etc.). In recent years, a great deal of effort has been invested in studying transmission traits of malaria parasites in their vertebrate host (Cameron, Reece, Drew, Haydon, & Yates, 2013; Greischar, Mideo, Read, & Bjornstad, 2016; Neal & Schall, 2014; Reece, Ramiro, & Nussey, 2009). As we would predict, studies have shown that both genetic and environmental factors are important in determining parasite transmission from vertebrate hosts to mosquitoes. Like any other phenotypic trait, transmission traits can respond to environmental changes either plastically or evolutionarily (Box 2). For example, work using rodent malaria models suggests that parasite genotype can predict virulence and transmission success (De Roode et al., 2005). Furthermore, studies have shown that the investment of malaria parasites in gametocyte transmission stages can vary in response to environmental conditions, such as the presence of drugs, the availability of resources, the host immune response, coinfection with different strains, and the presence of vectors (Cornet, Nicot, Rivero, & Gandon, 2014; Mideo & Reece, 2012; Pollitt et al., 2011). While some of these responses may illustrate cases of passive susceptibility to environmental changes, others are likely examples of adaptive plasticity (Box 2). For example, Plasmodium chabaudi can detect the presence of unrelated conspecifics and adjust the proportion of male and female gametes in a way that supports sex ratio theory (Reece, Drew, & Gardner, 2008). This research demonstrates that unicellular parasites can evolve finely tuned mechanisms to detect information about their within‐host environment and plastically adjust some of their transmission traits.
Box 2. Genetically fixed responses and (adaptive vs. nonadaptive) phenotypic plasticity.
1.
Like any other organism trait, changes in parasite phenotypic traits can occur through two nonmutually exclusive processes: genetically fixed responses and/or phenotypic plasticity (Pigliucci, 2005). First, there may be genetic variation underlying transmission traits, and natural selection will favor the genetic variants which produce the phenotypes most fitted to the current conditions. This is the classic evolutionary response whereby some genetic variants can spread through the population over generations. Genetic variation is the raw material for evolution; therefore, characterizing genetic variability in transmission traits is key to understanding how control interventions can drive evolutionary changes in the parasite. As one hypothetical example, reduced vector longevity following insecticide exposure might select individuals with shorter EIP in the parasite population.
Second, a given parasite genotype may be able to produce different phenotypes in response to different environmental conditions, that is, phenotypic plasticity. In contrast to genetic changes over generations, modifications in phenotypic traits through plasticity can occur within a generation. Many examples of phenotypic plasticity are clearly adaptive such as some immune responses, antipredator defenses, and diapauses allowing individuals to adjust to environmental variation in real time (Whitman & Agrawal, 2009). In this case, organisms possess mechanisms to detect cues that predict environmental changes and induce adaptive plasticity. Such plasticity does not necessarily involve changes in gene frequencies in the parasite population and can provide a more rapid response to unpredictably changing environments. Using the above hypothetical example, parasites could detect cues associated with imminent death of their vectors (e.g., directly through the presence of insecticides or indirectly through modifications of vector physiology) and adaptively accelerate their sporogonic development to achieve transmission prior to vector death.
In contrast to adaptive plasticity, other environmentally induced changes in phenotype may illustrate mere susceptibilities to environmental stresses with no adaptive value (Ghalambor, McKay, Carroll, & Reznick, 2007). In this case, the phenotypic changes can arise from a “passive” disruption of physiological processes and do not require any mechanisms for how cues are detected. For example, a longer EIP in mosquitoes exposed to insecticides and hence with reduced potential for transmission compared to mosquitoes with greater longevity would indicate that environmental variation (here a reduction in mosquito longevity) does influence this trait, but this would also be intuitively interpreted as a case of phenotypic plasticity with maladaptive value. However, it is often difficult to conclude whether or not altered phenotypes are adaptive or nonadaptive (Pigliucci, 2005).
In any case, determining the extent to which parasite transmission traits are genetically fixed or plastic will help predict the consequences of control interventions on parasite evolution. Experimental designs with some form of genetic structure (clones, family lines) and environmental treatments are extremely powerful for studying genetic effects and phenotypic plasticity (Whitman & Agrawal, 2009). Measuring transmission trait (EIP, virulence, manipulation, infection level) variation among different genetic backgrounds or environmental conditions will help to quantify the relative importance of phenotypic plasticity and genetic variation. The statistical measure of variation is variance, which quantifies the deviation of values around a mean. The variance of a phenotypic trait can be partitioned as follows:
V P = V G + V E + V G×E + V error
where V P = Total phenotypic variance for a trait;V G = Genetic variance (proportion of phenotypic variation attributable to genes);V E = Environmental variance (proportion of variation caused by the environment);V G×E = Genotype × Environment interaction (genetic variation for phenotypic plasticity);V error = Unexplained variance, including developmental noise.
Quantifying phenotypic variation across different parasite clones or mosquito genotypes in controlled conditions will minimize environmental variance, and the phenotypic variance will be close to the genetic variance. Similarly, randomly assigning mosquito genotypes infected with single parasite clones (monoclonal infections) to different environmental treatments will lead to a robust estimate of phenotypic plasticity (Whitman & Agrawal, 2009).
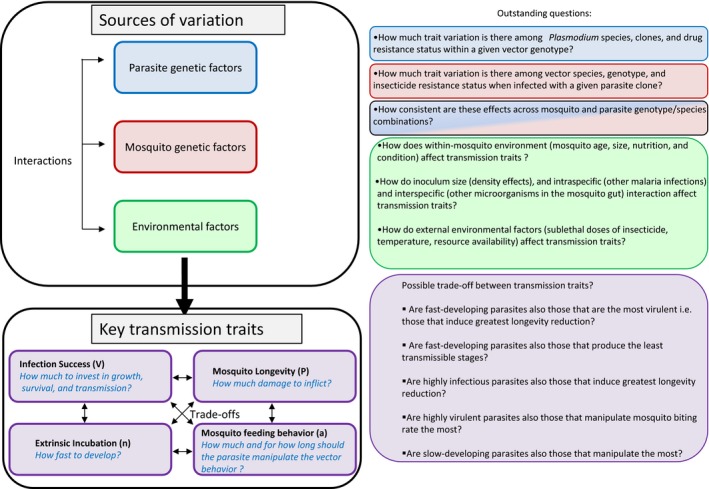
In comparison with explorations of within‐host factors that affect transmission from hosts to vectors, little work has been performed on the other half of the parasite transmission cycle: from vectors to vertebrate hosts. We propose that a complete understanding of factors that shape the evolution of transmission strategies must consider not only the within‐vertebrate host factors contributing to transmission, but also those factors within the vector (Box 1). We use vectorial capacity (C), one of the most common metrics of transmission for vector‐borne diseases, to establish a framework for investigating genetic and environmental variation in transmission traits within the mosquito vector. C is defined as the potential intensity of vertebrate‐to‐vertebrate parasite transmission by mosquito vectors and can be described by the formula:
where m is the density of vectors per vertebrate hosts, a is the vector biting rate and host preference, V is vector competence, p is the daily probability of adult vector survival, and n is the duration in days of the parasite's extrinsic incubation period (EIP; Dye, 1992). Four of these critical components of transmission—the biting rate, mosquito competence, mosquito survival, and EIP—are traits that could potentially be determined directly or indirectly by parasites (Table 1). The vectorial capacity equation predicts that parasites could enhance transmission by influencing vector physiology to increase competence (V), altering the timing and propensity of mosquito biting (a), shortening EIP (n), or by increasing vector longevity (p).
Table 1.
The critical components of malaria transmission that can either be determined directly or indirectly by parasites and how they affect our understanding of transmission
| Component of vectorial capacity | Effect of increase in component on disease transmission (everything else being equal) | Interactions or trade‐offs to consider | Key questions to address | Applications and outlook |
|---|---|---|---|---|
| Mosquito competence (V) | ↑ | Virulence transmission trade‐off could result in mosquitoes with higher competence having reduced survival | Does mosquito competence correlates with a, n and/or p? Is competence predicted by G p × G H × E? Do environmentally driven changes in competence illustrate adaptive or nonadaptive phenotypic plasticity on the part of the parasite or the vector? | Understanding the genetic basis of competence can identify targets for genetic modification‐based control strategies |
| Vector biting rate and host preference (a) | ↑ | Biting rate increases mortality risk and could reduce vector survival. Changing host preference could increase survival by reducing exposure to insecticides | How does parasite impact vector biting rate? Can malaria parasites manipulate the vertebrate host choice of their vectors? Is malaria manipulation of vector biting rate a general phenomenon among the different mosquito–parasite combinations? Is there any parasite genetic variation for manipulation? Does the intensity of manipulation vary with environmental conditions (e.g., seasonally with mosquito densities)? Does a correlate with n and/or p? | Identifying parasite–mosquito associations that exhibit altered feeding behavior during infection will help improve transmission predictions by more accurately estimating biting rates and could also provide the opportunity to selectively target infected mosquitoes for control |
| The extrinsic incubation period (n) | ↓ | Faster developing parasites might inflict higher fitness costs on mosquitoes and reduce their ability to transmit | How does EIP length respond to within‐vector and environmental conditions? Can EIP be predicted by vector or parasite genotype? What affects EIP length besides temperature? | Shorter EIPs could evolve in response to interventions if there is a genetic basis for EIP length and sufficient selection pressure. For example, insecticides that reduce vector lifespan may favor faster parasite development |
| Mosquito longevity (p) | ↑ | Longer‐lived mosquitoes may have reduced biting rates | Do parasite traits, such as EIP or virulence, covary with mosquito lifespan? How does malaria infection impact vector lifespan? How does vector lifespan affect selection on parasites with different traits? | Mosquito longevity is the most sensitive parameter in vectorial capacity, and understanding how this trait covaries with other vector and parasite traits related to transmission is crucial for better characterizing transmission in the field |
The degree to which variation in any one of these parameters affects transmission outcomes depends both on how sensitive vectorial capacity is to perturbations in a given parameter and the extent to which a given parameter can vary. Sensitivity analyses can evaluate the relative effect small changes in one parameter have on the outcome of what the model is predicting. Previous sensitivity analyses on the vectorial capacity equation have indicated that vectorial capacity is highly sensitive to adult mosquito survival (Brady et al., 2016; MacDonald, 1957; Smith & McKenzie, 2004). This has consequently led to suggestions that interventions targeting adult survival may be the most effective means of vector control, even when weighted by the relative effort of implementing an intervention (Brady et al., 2016). Using similar analyses to weight sensitivity by the capacity of a trait to vary cannot currently be conducted on key vector traits (V, a, n, and p) because variation in traits is poorly characterized. Control strategy design and transmission predictions could be improved by understanding the extent of variation in these parameters. Here, we explore each of these traits, review the extent of observed and predicted genetic and environmental variation, and discuss how variation in any one of these components of vectorial capacity impacts parasite transmission.
2. MOSQUITO COMPETENCE (V)
Mosquito competence is the ability of mosquitoes to support malaria development and transmission. It can be measured in the laboratory by exposing mosquitoes to a given dose of parasite gametocytes during blood feeding directly on an infected vertebrate host (Direct Feeding Assay (Bousema et al., 2012)), or through a membrane containing either cultured parasites (Standard Membrane Feeding Assays (van der Kolk et al., 2005)) or blood drawn from naturally infected hosts (Direct Membrane Feeding Assays (Bousema et al., 2012; Ouédraogo et al., 2013)). The measure of competence captures both parasite prevalence (the proportion of malaria‐exposed mosquitoes harboring at least one oocyst in their midgut or sporozoite in their salivary gland) and parasite intensity (the number of oocysts in the guts, or the number of sporozoites in the salivary glands of infected mosquitoes). Competence is a combined estimate of parasite infectivity (the parasite's ability to successfully establish and develop in the mosquito) and vector susceptibility to infection. It thus encompasses both mosquito resistance mechanisms used to fight the infection and parasite mechanisms used to overcome the vector's defenses.
The molecular and genetic bases of mosquito competence for malaria parasites have been well characterized for a number of mosquito–parasite associations (Aly, Vaughan, & Kappe, 2009; Beier, 1998; Bennink, Kiesow, & Pradel, 2016; Cirimotich, Dong, Garver, Sim, & Dimopoulos, 2010; Li et al., 2013; Molina‐Cruz et al., 2012; Redmond et al., 2015; Severo & Levashina, 2014; Sinden, 2016; Sinden, Alavi, & Raine, 2004). For example, different strains or families of Anopheles gambiae, the primary vector of malaria in Africa, display a wide range of susceptibility for a given parasite genotype (Blandin et al., 2009; Harris et al., 2010) and different Plasmodium isolates also vary in their infectivity to a given mosquito strain (Molina‐Cruz et al., 2012). Some studies have also demonstrated the existence of mosquito–parasite genetic interactions (Harris et al., 2012; Lambrechts, Halbert, Durand, Gouagna, & Koella, 2005; Molina‐Cruz et al., 2015). As yet, however, this large body of research has provided only limited insight into transmission dynamics in the field.
Besides mosquito and parasite genetic factors, there is a great diversity of ways in which biotic and abiotic external and within‐vector environmental factors (temperature, mosquito diet, insecticide exposure, microbial gut flora, infection history, mosquito age, etc.) can influence with mosquito competence (Alout, Djègbè, et al., 2014; Gendrin et al., 2015; Hien et al., 2016; Lefèvre, Vantaux, Dabiré, Mouline, & Cohuet, 2013; Murdock, Blanford, Luckhart, & Thomas, 2014; Murdock, Paaijmans, Cox‐foster, Read, & Thomas, 2012; Pigeault, Nicot, Gandon, & Rivero, 2015; Pollitt, Bram, Blanford, Jones, & Read, 2015; Shapiro, Murdock, Jacobs, Thomas, & Thomas, 2016; Takken et al., 2013; Vantaux, Dabiré, Cohuet, & Lefèvre, 2014). However, it is still unknown whether these environmentally driven changes in competence illustrate mere passive susceptibilities to environmental stresses (nonadaptive plasticity) or active beneficial shifts in either parasite growth and development or mosquito immune responses (parasite or vector adaptive phenotypic plasticity; Box 2).
Malaria transmission depends on the production of gametocytes that infect mosquitoes, which in turn develop in the mosquito vector to produce the transmissible stage of parasites, known as sporozoites (Box 1). Although there has been a great deal of effort to understand variation in gametocyte investment in several Plasmodium species (Bousema et al., 2008; Carter et al., 2013; Gadalla et al., 2016; McKenzie, Jeffery, & Collins, 2002; Neal & Schall, 2014; Box 1), it remains controversial as to whether or not the parasite is able to modulate its growth, survival, and sporozoite production within the mosquito vector. Similar to parasite stages within the vertebrate host, stages within the mosquito experience variation in their environment. Factors that may influence the parasite's within‐vector environment include vector age, resource availability, and presence of competitors. Whether the parasite is able to actively detect these variations and adjust its development through adaptive phenotypic plasticity remains enigmatic. In particular, it is still unclear whether intermediate “optimum” parasite densities exist for maximizing vector‐to‐vertebrate transmission. Parasite numbers during sporogonic development exhibit marked fluctuations, with the gametocyte to ookinete transition, the ookinete to oocyst transition, and the salivary gland invasion by sporozoites representing three major bottlenecks (reviewed in (Vaughan, 2007), see also Box 1). Studies using the Plasmodium berghei—Anopheles stephensi experimental system found that these developmental transitions experienced negative density dependence, possibly due to resources and space limitation and/or to an elevated mosquito immune response (Pollitt et al., 2013; Sinden et al., 2007). In addition, high‐density P. berghei infections can cause significant lifespan reduction in An. stephensi (Dawes, Churcher, Zhuang, Sinden, & Basanez, 2009; Pollitt et al., 2013). Together, the observations that high‐density infections limit both parasite development and vector survival support the possible existence of a selective pressure for parasites to modulate growth and reproduction within the vector to maintain densities at which transmission is maximized (Pollitt et al., 2013).
An important assumption of this hypothesis is that there must be a positive relationship between sporozoite burden in the salivary glands and infection of the vertebrate host, something that has long been disputed (Beier, Davis, Vaughan, Noden, & Beier, 1991; Beier et al., 1992; Ponnudurai, Lensen, Vangemert, Bolmer, & Meuwissen, 1991; Sinden, 2016). A recent study using rodent parasites provides strong support for this relationship by showing that mosquitoes with higher numbers of sporozoites in salivary glands are indeed more likely to transmit malaria (Churcher et al., 2017).
It has also been proposed that self‐restriction strategies based on programmed cell death may reduce the mosquito immune response, competition for resources, and/or increase vector survival, hence increasing parasite transmission probability (Al‐Olayan, Williams, & Hurd, 2002; Lüder, Campos‐Salinas, Gonzalez‐Rey, & van Zandbergen, 2010; Pollitt, Colegrave, Khan, Sajid, & Reece, 2010). However, suicide of some parasites may be beneficial only if this increases transmission of closely related individuals (i.e., increased indirect fitness) such as in monoclonal infection (Ameisen et al., 1995; Nedelcu, Driscoll, Durand, Herron, & Rashidi, 2011; Reece, Pollitt, Colegrave, & Gardner, 2011). This possible strategy has been supported by a number of observations showing that zygote and ookinete stages can indeed undergo apoptosis‐like processes (Ali, Al‐Olayan, Lewis, Matthews, & Hurd, 2010; Al‐Olayan et al., 2002; Arambage, Grant, Pardo, Ranford‐Cartwright, & Hurd, 2009; Pollitt et al., 2010). Further investigations are required to determine the extent to which the occurrence and intensity of parasite apoptosis depend on parasite / mosquito genotype and on the density and relatedness of co‐infecting parasites.
3. VECTOR BITING RATE AND HOST PREFERENCE (a): PARASITE MANIPULATION OF THE VECTOR'S FEEDING BEHAVIOR
The vectorial capacity equation predicts that, when ready to be transmitted from either vertebrate to vector or vector to vertebrate, malaria parasites able to increase the vector's biting rate on suitable vertebrate hosts species would increase their probability of transmission (Dobson, 1988). This “right bite at the right time” requirement of malaria transmission represents an extremely risky point in the parasite life cycle. Although evidence show that malaria parasites can enhance mosquito’s feeding rate (Anderson, Koella, & Hurd, 1999; Cator, Lynch, Read, & Thomas, 2012; Cator, Lynch, Thomas, & Read, 2014; Cator et al., 2013, 2015; Hurd, 2003; Koella, Rieu, & Paul, 2002; Koella, Sørensen, & Anderson, 1998; Lefèvre & Thomas, 2008; Smallegange et al., 2013; Wekesa, Copeland, & Mwangi, 1992), many questions remain about the extent of such changes in natural vector–parasite combinations and the robustness of the phenomena across environmental conditions (Cornet, Nicot, Rivero, & Gandon, 2013; Vantaux et al., 2015) and whether malaria parasites can manipulate mosquito host choice in ways that enhance parasite transmission toward suitable hosts and/or reduce mosquito attraction to unsuitable hosts (i.e., specific manipulation) (Nguyen et al., 2017).
There is a reason to think that both parasite and host genetics should be selected upon to shape these phenotypes. The altered patterns in feeding behavior observed in malaria‐infected mosquitoes have been empirically demonstrated to have negative impacts on mosquito fitness (Anderson, Knols, & Koella, 2000; Ohm et al., 2016). This suggests that there is selection for both the parasite to alter mosquito behavior and the vector to resist being manipulated (Daoust et al., 2015). Historically, there has been a large emphasis on identifying specific parasite traits that in isolation lead to altered mosquito behavioral phenotypes. Recent work suggests that some components of manipulation may relate to the mosquito's own immune response (Cator et al., 2013, 2015) and that the transmission phenotype observed is likely dependent on the genotype and condition of the vector, as well as the parasite (Cator et al., 2015). How these phenotypes can vary with the environment (e.g., mosquito age or vector density) is unknown and is critical for our understanding of how they affect transmission.
4. THE EXTRINSIC INCUBATION PERIOD (n)
Natural selection will theoretically favor a developmental schedule for each parasite stage which maximizes transmission between successive hosts (Poulin, 2007). Once in the insect vector, a major challenge facing the parasite is to reach its infective stage before the insect takes its last blood meal. The extrinsic incubation period (EIP) is the duration of the parasite's development within the mosquito that starts with the ingestion of infective malaria parasites, gametocytes, in a blood meal and ends with the sporozoite invasion of the salivary glands when the mosquito becomes infectious (Box 1). For many mosquito–Plasmodium associations, this period is as long as the insect vector's average lifespan (Charlwood et al., 1997; Killeen, Mckenzie, Foy, Peter, & Beier, 2000). Plasmodium falciparum, for example, has an extremely variable EIP, but generally ranges from 10 to 14 days in high‐transmission settings (WHO, 1975). The question of why this period is so long relative to the vector lifespan has been discussed elsewhere (Cohuet, Harris, Robert, & Fontenille, 2010; Koella, 1999; Ohm et al., 2016; Paul, Ariey, & Robert, 2003).
Both mosquito and parasite are ectothermic, and the impact of temperature on the rate of sporogonic development has long been recognized (Boyd, 1949; Detinova, 1963; Murdock, Paaijmans, Cox‐foster, Read, & Thomas, 2012). In general, warming temperatures speed up parasite development, although above a certain threshold (30°C in P. falciparum), this can reduce infection level (Noden, Kent, & Beier, 1995; Okech et al., 2004). Evidence that EIP can vary in response to other environmental factors is limited. Plasmodium falciparum EIP can be modified by the quantity of food received by An. stephensi larvae (Shapiro et al., 2016) or by the source of plant sugar taken by adult Anopheles coluzzii (Hien et al., 2016). Of particular interest would be to test the extent to which malaria parasites can plastically speed up their EIP when their transmission potential is compromised by the imminent death of their vector. Such condition‐dependent developmental strategies, described in other parasite species (Donnell & Hunter, 2009; Poulin, 2007) and in blood‐stage malaria parasites (Mideo & Reece, 2012), deserve consideration in infected mosquitoes. Besides mosquito age, other environmental factors, including exposure to insecticides (Viana, Hughes, Matthiopoulos, Ranson, & Ferguson, 2016) or presence of other parasite species/genotypes (Blanford et al., 2005; Lorenz & Koella, 2012), are associated with mosquito survival and could induce an adaptive plastic shift in parasite EIP. Similar to within‐vector conditions, the extent to which parasite and/or mosquito genetic variation can influence EIP merits exploration.
At the interspecific level, some studies suggest that parasites may adapt to vector lifespan, as demonstrated by Plasmodium species with shorter EIPs associating with shorter lived vectors, such as Plasmodium mexicanum that is vectored by short‐lived sandflies. Only about 2% of sandflies capable of transmitting P. mexicanum live long enough to take a second blood meal (Fialho & Schall, 1995). Compared to other Plasmodium species, P. mexicanum has a rapid development time that ensures transmission despite the vector's high mortality, which is likely an evolved response.
At the intraspecific level, there has been no study on the influence of parasite and/or mosquito genetics on EIP duration. A recent study investigating the evolutionary potential of dengue virus EIP in Aedes aegypti demonstrated that genetic variation among a range of mosquito genetic lines can modulate the length of EIP (Ye et al., 2016). Because vectorial capacity is highly sensitive to changes in EIP, it becomes urgent to investigate the evolutionary potential of EIP in malaria parasites using family‐based breeding (Ye et al., 2016) and/or experimental evolution design (Nidelet, Koella, & Kaltz, 2009).
5. MOSQUITO LONGEVITY (p) AND OTHER DAMAGES INFLICTED TO THE MOSQUITO
Whether malaria parasites cause fitness costs to their mosquito hosts has received much attention and has long been disputed (Ferguson & Read, 2002b; Hurd, 2009; Vézilier, Nicot, Gandon, & Rivero, 2012). Given the traumatic nature of the sporogonic development (ookinetes and sporozoites perforate the midgut and salivary gland, respectively, Box 1), some degree of virulence (i.e., parasite‐induced fitness cost) might be expected. Malaria infection has been found to increase susceptibility to harmful bacterial and viral infections (Maier, Becker‐Feldman, & Seitz, 1987; Rodrigues, Brayner, Alves, Dixit, & Barillas‐Mury, 2010; Vaughan & Turell, 1996), decrease host energetic reserves (Hurd, Hogg, & Renshaw, 1995; Liu, Dong, Huang, Rasgon, & Agre, 2013; Mack, Samuels, & Vanderberg, 1979a,b), increase sugar intake requirements (Zhao et al., 2012), and decrease flight performance (Schiefer, Ward, & Eldridge, 1977). Furthermore, mounting an immune response to the parasites alone is costly (Ahmed & Hurd, 2006; Blandin, Marois, & Levashina, 2008; Cirimotich et al., 2010). Finally, increased mosquito biting rate induced by sporozoites (see above) can increase feeding‐associated mosquito mortality in the field (Anderson et al., 2000). Together, these mechanisms could negatively impact mosquito longevity and fecundity.
Although both mosquito and parasite could gain fitness benefits from longer vector survival, overall, there seem to be negative effects of infection on mosquito longevity, especially in specific conditions reflecting what occurs in nature, such as nutritional, predation, insecticide, or hydric stress (Aboagye‐Antwi et al., 2010; Alout, Yameogo, et al., 2014; Ferguson, Mackinnon, Chan, & Read, 2003; Lalubin, Delédevant, Glaizot, & Christe, 2014; Roux et al., 2015; Sangare et al., 2014). Furthermore, studies showed that mosquito mortality is influenced by parasite density with heavily infected mosquitoes exhibiting reduced lifespan compared to lightly infected individuals (Dawes et al., 2009; Ferguson et al., 2003; Klein, Harrison, Grove, Dixon, & Andre, 1986; Pollitt et al., 2013). Theory suggests that the optimal level of parasite virulence on mosquito longevity should be stage‐dependent. The parasite should first exhibit a low level of virulence during parasite development to prevent the death of both partners. Once the development is completed and sporozoites are in the salivary glands, parasite genotypes able to increase the biting rate of their mosquito vector could be favored (Koella, 1999; Schwartz & Koella, 2001). Consistent with these predictions, some studies reported greater survivorship in infected than in uninfected mosquitoes during the oocyst infection phase and the opposite when sporozoites have reached maturity (Anderson et al., 2000; Lyimo & Koella, 1992; Roux et al., 2015). Whether the increased survivorship observed in infected individuals during oocyst growth resulted from an active manipulation of the parasite or reflects a compensatory response of the mosquitoes to energy depletion remains unknown. Investigating the importance of parasite genetic variability and interactions with mosquito strain would also deserve consideration. In the P. chabaudi—An. stephensi model, there is evidence that different parasite genotypes vary in their effects on mosquito survival and fecundity (Ferguson & Read, 2002a; Ferguson et al., 2003). There also is evidence that some mosquito strains can suffer higher cost of infection by a given parasite genotype than others (Vézilier et al., 2012). Future studies are required to test whether Plasmodium genotype by mosquito genotype interactions impact mosquito longevity and fecundity.
6. POSSIBLE ASSOCIATIONS AND TRADE‐OFFS AMONG TRANSMISSION TRAITS
It is important to remember that it is the emergent properties of a given set of competence, biting rate, EIP, and survival values that determine transmission and that these parameters do not operate in isolation (Figure 1). For example, Plasmodium may modify resource allocation of their insect vectors in a way that changes the optimum trade‐off between reproduction and longevity, which, in turn, could favor either longer or similar vector survivorship than uninfected counterparts (Hurd, 2001, 2003, 2009). In a study using avian malaria and allowing mosquitoes to lay their eggs, infected mosquitoes were less fecund but lived longer than uninfected counterparts (Vézilier et al., 2012). This emphasizes the need to concomitantly quantify mosquito longevity and fecundity, which is rarely performed in studies on mosquito–parasite interactions. Finally, there is, to our knowledge, no study that investigated the effect of malaria infection on both mosquito longevity and fecundity over multiple gonotrophic cycles.
Figure 1.
How genetic and environmental factors contribute to variability in extrinsic incubation period, parasite manipulation, infection success, and mosquito longevity and fecundity, remain to be discovered
Beside the existing links between mosquito infection, fecundity, and longevity, an intriguing possibility is that EIP, the parasite's ability to manipulate mosquito biting rate, and mosquito survival are also correlated. For example, reduced longevity in infected mosquitoes or long parasite development duration will limit the time period for parasite transmission, but this could be compensated by increased mosquito biting rate (Koella, 1999, 2005). In turn, increased biting rate can also increase the probability of mosquito mortality (Anderson et al., 2000). Similarly, the reduction in transmission opportunities due to long parasite development duration could be compensated by increased mosquito lifespan. In other words, fast‐developing parasites might also be those that induce high level of virulence in their mosquito hosts. A recent study using dengue virus‐infected A. aegypti revealed that mosquito family lines allowing fast EIP were also those that died faster supporting the existence of a genetic trade‐off between mosquito lifespan and EIP (Ye et al., 2016). To explore these trade‐offs, future work should concomitantly quantify multiple mosquito traits.
7. KEY STEPS TO APPLIED VALUE
Understanding how transmission traits of malaria parasites are shaped by the mosquitoes that vector them can inform our approach to disease control. Frontline vector‐borne disease prevention tools such as insecticide‐treated bednets and indoor residual spraying rely on reducing mosquito contact rates with human hosts and reducing vector survival. Reduced vector survival has the benefits of decreasing mosquito abundance, the number of bites a mosquito can take over the course of its lifetime, and the probability that mosquitoes survive past the parasite's development time (Bhatt et al., 2015; Brady et al., 2016; Smith & McKenzie, 2004). These effects likely shape the selective environment for parasites within the vector. Whether parasites can respond to interventions by evolving shorter EIPs or other heritable extended phenotypes that lengthen mosquito survival or change vector behavior merit further investigation.
Human interventions often have evolutionary consequences. For example, it is well known that the use of fast‐kill insecticides selects for rapid insecticide resistance, but the evolutionary and epidemiological impact of evolved resistance traits in vectors on transmission traits of parasites is still not well understood (Alout, Djègbè, et al., 2014; Alout, Yameogo, et al., 2014; Rivero, Vezilier, Weill, Read, & Gandon, 2010). More work evaluating the consequences of insecticide‐resistant mosquitoes on parasite transmission traits will help determine how changing vector traits can influence traits of their co‐evolved parasites. In addition to physiological resistance, how mosquito behavioral resistance in response to LLINs and IRS affects parasite transmission traits is unclear. For example, some studies show that Anopheles mosquitoes can shift their host‐feeding behavior from night‐biting to day‐biting following bed net introduction (Moiroux et al., 2012). As diel rhythm shapes mosquito immune responses (Murdock et al., 2013; Rund, Hou, Ward, Collins, & Duffield, 2011; Rund, O'Donnell, Gentile, & Reece, 2016), day‐biting may also alter parasite infection prevalence and intensity. Finally, for interventions not yet deployed, such as late‐life‐acting insecticides or genetically modified mosquitoes, differences in the within‐vector environment parasites experience will also provide potentially different selective forces. Whether parasites can evolve or plastically change transmission traits in response to these interventions needs to be evaluated if we are to responsibly deploy these technologies and prepare for possible evolutionary responses.
While all of these interventions are primarily aimed at and assessed by measuring vector traits, they may have important consequences for parasite evolution. Central to understanding how variation in parasite traits will ultimately influence our approach to control is quantifying how the transmission traits identified in the vectorial capacity equation vary by vector and parasite genotypes, and the plasticity of these traits in the face of selection. Any characterization of these effects should include an estimation of trait heritability across parasite generations, within‐host environments, and external environments. Central to this will be measuring the responses of multiple traits to the within‐vector environment to determine how trade‐offs between them may constrain evolution and dictate parasite transmission.
Finally, because findings on unnatural mosquito–Plasmodium associations do not always reflect natural interactions (Aguilar, Dong, Warr, & Dimopoulos, 2005; Boëte, 2005; Cohuet et al., 2006; Dong et al., 2006; Tripet, Aboagye‐Antwi, & Hurd, 2008; Vantaux et al., 2015), it will be essential to follow‐up the discoveries in laboratory model systems such as P. berghei—An. stephensi or An. gambiae with laboratory and field studies on natural parasite–mosquito combinations.
ACKNOWLEDGEMENTS
We thank the NIH and the BBSRC for sponsoring the “Vector Behavior in Transmission Ecology” Research Coordination Network (VectorBiTE RCN) and the RCN steering committee for organizing the 2016 meeting in Florida. We thank Pierre Echaubard, Philip Birget, and Sarah Reece for their valuable comments that improved the manuscript. TL is funded by ANR grant 16‐CE35‐0007. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Lefevre T, Ohm J, Dabiré KR, et al. Transmission traits of malaria parasites within the mosquito: Genetic variation, phenotypic plasticity, and consequences for control. Evol Appl. 2018;11:456–469. https://doi.org/10.1111/eva.12571
REFERENCES
- Aboagye‐Antwi, F. , Guindo, A. , Traore, A. S. , Hurd, H. , Coulibaly, M. , Traore, S. , & Tripet, F. (2010). Hydric stress‐dependent effects of Plasmodium falciparum infection on the survival of wild‐caught Anopheles gambiae female mosquitoes. Malaria Journal, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar, R. , Dong, Y. , Warr, E. , & Dimopoulos, G. (2005). Anopheles infection responses; laboratory models versus field malaria transmission systems. Acta Tropica, 95(3), 285–291. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16011828 [DOI] [PubMed] [Google Scholar]
- Ahmed, A. M. , & Hurd, H. (2006). Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes and Infection, 8(2), 308–315. https://doi.org/10.1016/j.micinf.2005.06.026 [DOI] [PubMed] [Google Scholar]
- Ali, M. , Al‐Olayan, E. M. , Lewis, S. , Matthews, H. , & Hurd, H. (2010). Naturally occurring triggers that induce apoptosis‐like programmed cell death in Plasmodium berghei ookinetes. PLoS ONE, 5(9), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Olayan, E. M. , Williams, G. T. , & Hurd, H. (2002). Apoptosis in the malaria protozoan, Plasmodium berghei: A possible mechanism for limiting intensity of infection in the mosquito. International Journal for Parasitology, 32(9), 1133–1143. https://doi.org/10.1016/S0020-7519(02)00087-5 [DOI] [PubMed] [Google Scholar]
- Alout, H. , Djègbè, I. , Chandre, F. , Djogbénou, L. S. , Dabiré, R. K. , Corbel, V. , & Cohuet, A. (2014). Insecticide exposure impacts vector‐parasite interactions in insecticide‐resistant malaria vectors. Proceedings of the Royal Society B: Biological Sciences, 281(1786), 20140389 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24850924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alout, H. , Yameogo, B. , Djogbénou, L. S. , Chandre, F. , Dabiré, R. K. , Corbel, V. , & Cohuet, A. (2014). Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. The Journal of Infectious Diseases, 210(9), 1464–1470. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24829465 [DOI] [PubMed] [Google Scholar]
- Aly, A. S. I. , Vaughan, A. M. , & Kappe, S. H. I. (2009). Malaria parasite development in the mosquito and infection of the mammalian host. Annual Review of Microbiology, 63, 195–221. https://doi.org/10.1146/annurev.micro.091208.073403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameisen, J. C. , Idziorek, T. , Billautmulot, O. , Loyens, M. , Tissier, J. P. , Potentier, A. , & Ouaissi, A. (1995). Apoptosis in a unicellular Eukaryote (Trypanosoma‐cruzi) – Implications for the evolutionary origin and role of programmed cell‐death in the control of cell‐proliferation, differentiation and survival. Cell Death and Differentiation, 2(4), 285–300. [PubMed] [Google Scholar]
- Anderson, R. A. , Knols, B. G. , & Koella, J. C. (2000). Plasmodium falciparum sporozoites increase feeding‐associated mortality of their mosquito hosts Anopheles gambiae s.l . Parasitology, 120, 329–333. https://doi.org/10.1017/S0031182099005570 [DOI] [PubMed] [Google Scholar]
- Anderson, R. A. , Koella, J. C. , & Hurd, H. (1999). The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proceedings of the Royal Society of London B: Biological Sciences, 266(1430), 1729–1733. https://doi.org/10.1098/rspb.1999.0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arambage, S. C. , Grant, K. M. , Pardo, I. , Ranford‐Cartwright, L. , & Hurd, H. (2009). Malaria ookinetes exhibit multiple markers for apoptosis‐like programmed cell death in vitro. Parasites & Vectors, 2(1), 32 Retrieved from http://www.parasitesandvectors.com/content/2/1/32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier, J. C. (1998). Malaria parasite development in mosquitoes. Annual Review of Entomology, 43, 519–543. https://doi.org/10.1146/annurev.ento.43.1.519 [DOI] [PubMed] [Google Scholar]
- Beier, J. C. , Beier, M. S. , Vaughan, J. A. , Pumpuni, C. B. , Davis, J. R. , & Noden, B. H. (1992). Sporozoite transmission by Anopheles freeborni and Anopheles gambiae experimentally infected with Plasmoduim falciparum . Journal of the American Mosquito Control Association, 8, 404–408. [PubMed] [Google Scholar]
- Beier, J. C. , Davis, J. R. , Vaughan, J. A. , Noden, B. H. , & Beier, M. S. (1991). Quantitation of Plasmodium falciparum sporozoites transmitted in vitro by experimentally infected Anopheles gambiae and Anopheles stephensi . American Journal of Tropical Medicine and Hygiene, 44, 564–570. https://doi.org/10.4269/ajtmh.1991.44.564 [DOI] [PubMed] [Google Scholar]
- Bennink, S. , Kiesow, M. J. , & Pradel, G. (2016). Malaria parasite development in the mosquito midgut. Cellular Microbiology, 18, 905–918. Retrieved from https://doi.org/doi.wiley.com/10.1111/cmi.12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt, S. , Weiss, D. J. , Cameron, E. , Bisanzio, D. , Mappin, B. , Dalrymple, U. , … Gething, P. W. (2015). The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature, 526, 207–211. https://doi.org/10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin, S. , Marois, E. , & Levashina, E. A. (2008). Antimalarial responses in Anopheles gambiae: From a complement‐like protein to a complement‐like pathway. Cell Host & Microbe, 3, 364–374. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18541213 [DOI] [PubMed] [Google Scholar]
- Blandin, S. , Wang‐Sattler, R. , Lamacchia, M. , Gagneur, J. , Lycett, G. , Ning, Y. , … Steinmetz, L. M. (2009). Dissecting the genetic basis of resistance to malaria parasites in Anopheles gambiae . Science, 326(5949), 147–150. https://doi.org/10.1126/science.1175241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford, S. , Chan, B. H. K. , Jenkins, N. , Sim, D. , Turner, R. J. , Read, A. F. , & Thomas, M. B. (2005). Fungal pathogen reduces potential for malaria transmission. Science, 308(5728), 1638–1641. https://doi.org/10.1126/science.1108423 [DOI] [PubMed] [Google Scholar]
- Boëte, C. (2005). Malaria parasites in mosquitoes: Laboratory models, evolutionary temptation and the real world. Trends in Parasitology, 21(10), 445–447. https://doi.org/10.1016/j.pt.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Bousema, T. , Dinglasan, R. R. , Morlais, I. , Gouagna, L. C. , van Warmerdam, T. , Awono‐Ambene, P. H. , … Churcher, T. S. (2012). Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE, 7, e42821 https://doi.org/10.1371/journal.pone.0042821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema, J. T. , Drakeley, C. J. , Mens, P. F. , Arens, T. , Houben, R. , Omar, S. A. , … Sauerwein, R. W. (2008). Increased Plasmodium falciparum gametocyte production in mixed infections with P. malariae . American Journal of Tropical Medicine and Hygiene, 78(3), 442–448. [PubMed] [Google Scholar]
- Boyd, M. F. (1949). Epidemiology: Factors related to the definitive host. Malariology, 1, 608–697. [Google Scholar]
- Brady, O. J. , Godfray, H. C. J. , Tatem, A. J. , Gething, P. W. , Cohen, J. M. , Ellis McKenzie, F. , … Smith, D. L. (2016). Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Transactions of the Royal Society of Tropical Medicine and Hygiene, 110(2), 107–117. https://doi.org/10.1093/trstmh/trv113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, A. , Reece, S. E. , Drew, D. R. , Haydon, D. T. , & Yates, A. J. (2013). Plasticity in transmission strategies of the malaria parasite, Plasmodium chabaudi: Environmental and genetic effects. Evolutionary Applications, 6(2), 365–376. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3586624&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, L. M. , Kafsack, B. F. C. , Llinás, M. , Mideo, N. , Pollitt, L. C. , & Reece, S. E. (2013). Stress and sex in malaria parasites: Why does commitment vary? Evolution, Medicine, and Public Health, 2013, 135–147. Retrieved from http://emph.oxfordjournals.org/content/2013/1/135.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator, L. , George, J. , Blanford, S. , Murdock, C. C. , Baker, T. C. , Read, A. F. , & Thomas, M. B. (2013). “Manipulation” without the parasite: Altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proceedings of the Royal Society B: Biological Sciences, 280(1763), 20130711 https://doi.org/10.1098/rspb.2013.0711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator, L. , Lynch, P. , Read, A. , & Thomas, M. (2012). Do malaria parasites manipulate mosquitoes? Trends in Parasitology, 28(11), 467–470. https://doi.org/10.1016/j.pt.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator, L. , Lynch, P. a. , Thomas, M. B. , & Read, A. F. (2014). Alterations in mosquito behaviour by malaria parasites: Potential impact on force of infection. Malaria Journal, 13(1), 164 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24885783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator, L. , Pietri, J. E. , Murdock, C. C. , Ohm, J. R. , Lewis, E. E. , Read, A. F. , … Thomas, M. B. (2015). Immune response and insulin signalling alter mosquito feeding behaviour to enhance malaria transmission potential. Scientific Reports, 5(July), 11947 Retrieved from http://www.nature.com/srep/2015/150708/srep11947/full/srep11947.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood, J. D. , Smith, T. , Billingsley, P. F. , Takken, W. , Lyimo, E. O. K. , & Meuwissen, J. H. E. T. (1997). Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bulletin of Entomological Research, 87(5), 445–453. https://doi.org/10.1017/s0007485300041304 [Google Scholar]
- Churcher, T. S. , Sinden, R. E. , Edwards, N. J. , Poulton, I. , Rampling, T. W. , Brock, P. M. , … Blagborough, A. M. (2017). Probability of transmission of malaria from mosquito to human is regulated by mosquito parasite density in naïve and vaccinated hosts. PLoS Pathogens, 13(1), e1006108 https://doi.org/10.1371/journal.ppat.1006108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich, C. M. , Dong, Y. , Garver, L. S. , Sim, S. , & Dimopoulos, G. (2010). Mosquito immune defenses against Plasmodium infection. Developmental and Comparative Immunology, 34(4), 387–395. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20026176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohuet, A. , Harris, C. , Robert, V. , & Fontenille, D. (2010). Evolutionary forces on Anopheles: What makes a malaria vector? Trends in Parasitology, 26(3), 130–136. https://doi.org/10.1016/j.pt.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Cohuet, A. , Osta, M. A. , Morlais, I. , Awono‐Ambene, P. H. , Michel, K. , Simard, F. , … Kafatos, F. C. (2006). Anopheles and Plasmodium: From laboratory models to natural systems in the field. EMBO Reports, 7(12), 1285–1289. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1794687&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, F. H. , Sakai, R. K. , Vernick, K. D. , Paskewitz, S. , Seeley, D. C. , Miller, L. H. , … Gwadz, R. W. (1986). Genetic selection of a Plasmodium‐refractory strain of the malaria vector Anopheles gambiae . Science, 234(4776), 607–610. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3532325 [DOI] [PubMed] [Google Scholar]
- Cornet, S. , Nicot, A. , Rivero, A. , & Gandon, S. (2013). Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malaria Journal, 12(1), 179 https://doi.org/10.1186/1475-2875-12-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet, S. , Nicot, A. , Rivero, A. , & Gandon, S. (2014). Evolution of plastic transmission strategies in avian malaria. PLoS Pathogens, 10(9), e1004308 https://doi.org/10.1371/journal.ppat.1004308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoust, S. P. , King, K. C. , Brodeur, J. , Roitberg, B. D. , Roche, B. , & Thomas, F. (2015). Making the best of a bad situation: Host partial resistance and bypass of behavioral manipulation by parasites? Trends in Parasitology, 31, 413–418. https://doi.org/10.1016/j.pt.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Dawes, E. J. , Churcher, T. S. , Zhuang, S. , Sinden, R. E. , & Basanez, M. G. (2009). Anopheles mortality is both age‐ and Plasmodium‐density dependent: Implications for malaria transmission. Malaria Journal, 8, 228 https://doi.org/10.1186/1475-2875-8-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roode, J. C. , Pansini, R. , Cheesman, S. J. , Helinski, M. E. H. , Huijben, S. , Wargo, A. R. , … Read, A. F. (2005). Virulence and competitive ability in genetically diverse malaria infections. Proceedings of the National Academy of Sciences of the United States of America, 102(21), 7624–7628. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1140419&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detinova, T. S. (1963). Age‐grouping methods in Diptera of medical importance. Bulletin of the World Health Organization, 47. [PubMed]
- Dobson, A. P. (1988). The population biology of parasite‐induced changes in host behavior. The Quarterly Review of Biology, 63(2), 139–165. https://doi.org/10.1086/415837 [DOI] [PubMed] [Google Scholar]
- Dong, Y. , Aguilar, R. , Xi, Z. , Warr, E. , Mongin, E. , & Dimopoulos, G. (2006). Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathogens, 2(6), 13 Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1475661&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnell, D. M. , & Hunter, M. S. (2002). Developmental rates of two congeneric parasitoids, Encarsia formosa and E. pergandiella (Hymenoptera: Aphelinidae), utilizing different egg provisioning strategies. Journal of Insect Physiology, 48, 487–493. https://doi.org/10.1016/s0022-1910(02)00070-7 [DOI] [PubMed] [Google Scholar]
- Dye, C. (1992). The analysis of parasite transmission by bloodsucking insects. Annual Review of Entomology, 37, 1–19. https://doi.org/10.1146/annurev.en.37.010192.000245 [DOI] [PubMed] [Google Scholar]
- Ferguson, H. M. , Mackinnon, M. J. , Chan, B. H. , & Read, A. F. (2003). Mosquito mortality and the evolution of malaria virulence. Evolution, 57(12), 2792–2804. https://doi.org/10.1554/03-211 [DOI] [PubMed] [Google Scholar]
- Ferguson, H. M. , & Read, A. F. (2002a). Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proceedings of the Royal Society B: Biological Sciences, 269(1497), 1217–1224. https://doi.org/10.1098/rspb.2002.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, H. M. , & Read, A. F. (2002b). Why is the effect of malaria parasites on mosquito survival still unresolved? Trends in Parasitology, 18(6), 256–261. Retrieved from http://www.sciencedirect.com/science/article/pii/S147149220202281X [DOI] [PubMed] [Google Scholar]
- Fialho, R. F. , & Schall, J. J. (1995). Thermal ecology of a malarial parasite and its insect vector: Consequences for the parasite’ s transmission success. Journal of Animal Ecology, 64(5), 553–562. https://doi.org/10.2307/5799 [Google Scholar]
- Gadalla, A. A. H. , Schneider, P. , Churcher, T. S. , Nassir, E. , Abdel‐Muhsin, A. M. A. , Ranford‐Cartwright, L. C. , … Babiker, H. A. (2016). Associations between season and gametocyte dynamics in chronic Plasmodium falciparum infections. PLoS ONE, 11(11), e0166699 https://doi.org/10.1371/journal.pone.0166699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrin, M. , Rodgers, F. H. , Yerbanga, R. S. , Ouédraogo, J. B. , Basáñez, M.‐G. , Cohuet, A. , & Christophides, G. K. (2015). Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nature Communications, 6, 5921 Retrieved from http://www.nature.com/doifinder/10.1038/ncomms6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor, C. K. , McKay, J. K. , Carroll, S. P. , & Reznick, D. N. (2007). Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology, 21, 394–407. https://doi.org/10.1111/j.1365-2435.2007.01283.x [Google Scholar]
- Greischar, M. A. , Mideo, N. , Read, A. F. , & Bjornstad, O. N. (2016). Quantifying transmission investment in malaria parasites. Plos Computational Biology, 12, e1004718 https://doi.org/10.1371/journal.pcbi.1004718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, C. , Lambrechts, L. , Rousset, F. , Abate, L. , Nsango, S. E. , Fontenille, D. , … Cohuet, A. (2010). Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum . PLoS Pathogens, 6(9), e1001112 https://doi.org/10.1371/journal.ppat.1001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, C. , Morlais, I. , Churcher, T. S. , Awono‐Ambene, P. , Gouagna, L. C. , Dabire, R. K. , … Cohuet, A. (2012). Plasmodium falciparum produce lower infection intensities in local versus foreign Anopheles gambiae populations. PLoS ONE, 7(1), e30849 https://doi.org/10.1371/journal.pone.0030849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien, D. F. , Dabiré, K. R. , Roche, B. , Diabaté, A. , Yerbanga, S. R. , Cohuet, A. , … Lefevre, T. (2016). Plant‐mediated effects on mosquito capacity to transmit human malaria. PLoS Pathogens, 12(8), e1005773 https://doi.org/10.5061/dryad.9s690.funding [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijben, S. , & Paaijmans, K. P. (2017). Putting evolution in elimination: Winning our ongoing battle with evolving malaria mosquitoes and parasites. Evolutionary Applications, 1–16. https://doi.org/10.1111/eva.12530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd, H. (2001). Host fecundity reduction: A strategy for damage limitation? Trends in Parasitology, 17, 363–368. https://doi.org/10.1016/S1471-4922(01)01927-4 [DOI] [PubMed] [Google Scholar]
- Hurd, H. (2003). Manipulation of medically important insect vectors by their parasites. Annual Review of Entomology, 48(1), 141–161. https://doi.org/10.1146/annurev.ento.48.091801.112722 [DOI] [PubMed] [Google Scholar]
- Hurd, H. (2009). Evolutionary drivers of parasite‐induced changes in insect life‐history traits: From theory to underlying mechanisms In Webster J. P. (Ed.), Advances in parasitology (Vol. 68, pp. 85–110). Waltham, MA: Academic Press; Retrieved from http://www.sciencedirect.com/science/article/pii/S0065308X08006040 [DOI] [PubMed] [Google Scholar]
- Hurd, H. , Hogg, J. C. , & Renshaw, M. (1995). Interactions between bloodfeeding, fecundity and infection in mosquitoes. Parasitology Today, 11, 411–416. https://doi.org/10.1016/0169-4758(95)80021-2 [Google Scholar]
- Killeen, G. F. , Mckenzie, R. E. , Foy, B. D. , Peter, C. S. , & Beier, J. C. (2000). A simplified model for predicting malaria entomologic inoculation to control. American Journal of Tropical Medicine and Hygiene, 62(5), 535–544. https://doi.org/10.4269/ajtmh.2000.62.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, T. A. , Harrison, B. A. , Grove, J. S. , Dixon, S. V. , & Andre, R. G. (1986). Correlation of survival rates of Anopheles dirus A (Diptera, Culicidae) with different infection densities of Plasmodium cynomolgi . Bulletin of the World Health Organization, 64(6), 901–907. [PMC free article] [PubMed] [Google Scholar]
- Koella, J. C. (1999). An evolutionary view of the interactions between anopheline mosquitoes and malaria parasites. Microbes and Infection, 1(4), 303–308. Retrieved from http://www.sciencedirect.com/science/article/B6VPN-3WV4XBW-5/2/5ed9a710445551cc9c7b1026815bd7ea [DOI] [PubMed] [Google Scholar]
- Koella, J. C. (2005). Malaria as a manipulator. Behavioural Processes, 68(3), 271–273. https://doi.org/10.1016/j.beproc.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Koella, J. C. , Rieu, L. , & Paul, R. E. L. (2002). Stage‐specific manipulation of a mosquito's host‐seeking behavior by the malaria parasite Plasmodium gallinaceum . Behavioral Ecology, 13(6), 816–820. https://doi.org/10.1093/beheco/13.6.816 [Google Scholar]
- Koella, J. C. , Sørensen, F. L. , & Anderson, R. A. (1998). The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae . Proceedings of the Royal Society B: Biological Sciences, 265(1398), 763–768. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1689045&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk, M. , de Vlas, S. J. , Saul, A. , van de Vegte‐Bolmer, M. , Eling, W. M. , & Sauerwein, R. W. (2005). Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission‐reducing activity using empirical data. Parasitology, 130, 13–22. https://doi.org/10.1017/S0031182005008826 [DOI] [PubMed] [Google Scholar]
- Lalubin, F. , Delédevant, A. , Glaizot, O. , & Christe, P. (2014). Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. Journal of Animal Ecology, 83(4), 850–857. https://doi.org/10.1111/1365-2656.12190 [DOI] [PubMed] [Google Scholar]
- Lambrechts, L. , Halbert, J. , Durand, P. , Gouagna, L. C. , & Koella, J. C. (2005). Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum . Malaria Journal, 4(1), 3 https://doi.org/10.1186/1475-2875-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre, T. , & Thomas, F. (2008). Behind the scene, something else is pulling the strings: Emphasizing parasitic manipulation in vector‐borne diseases. Infection, Genetics and Evolution, 8(4), 504–519. https://doi.org/10.1016/j.meegid.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Lefèvre, T. , Vantaux, A. , Dabiré, K. R. , Mouline, K. , & Cohuet, A. (2013). Non‐genetic determinants of mosquito competence for malaria parasites. PLoS Pathogens, 9(6), e1003365 https://doi.org/10.1371/journal.ppat.1003365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Wang, X. , Zhang, G. , Githure, J. I. , Yan, G. , & James, A. A. (2013). Genome‐block expression‐assisted association studies discover malaria resistance genes in Anopheles gambiae . Proceedings of the National Academy of Sciences of the United States of America, 110(51), 20675–20680. https://doi.org/10.1073/pnas.1321024110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Dong, Y. , Huang, Y. , Rasgon, J. L. , & Agre, P. (2013). Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proceedings of the National Academy of Sciences of the United States of America, 110(43), 17504–17509. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3808592&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, L. M. , & Koella, J. C. (2012). The microsporidian parasite Vavraia culicis as a potential late life‐acting control agent of malaria. Evolutionary Applications, 4(6), 783–790. https://doi.org/10.1111/j.1752-4571.2011.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüder, C. G. , Campos‐Salinas, J. , Gonzalez‐Rey, E. , & van Zandbergen, G. (2010). Impact of protozoan cell death on parasite‐host interactions and pathogenesis. Parasites & Vectors, 3(1), 116 Retrieved from http://www.parasitesandvectors.com/content/3/1/116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyimo, E. O. , & Koella, J. C. (1992). Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum . Parasitology, 104, 233–237. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1594289 [DOI] [PubMed] [Google Scholar]
- MacDonald, G. (1957). The epidemiology and control of malaria. London, UK: Oxford University Press. [Google Scholar]
- Mack, S. R. , Samuels, S. , & Vanderberg, J. P. (1979a). Hemolymph of Anopheles stephensi from noninfected and Plasmodium berghei‐infected mosquitoes. 3. Carbohydrates. The Journal of Parasitology, 65(2), 217–221. https://doi.org/10.2307/3280149 [PubMed] [Google Scholar]
- Mack, S. R. , Samuels, S. , & Vanderberg, J. P. (1979b). Hemolymph of Anopheles stephensi from uninfected and Plasmodium berghei‐infected mosquitoes. 2. Free amino acids. The Journal of Parasitology, 65, 130–136. https://doi.org/10.2307/3280217 [PubMed] [Google Scholar]
- Maier, W. A. , Becker‐Feldman, H. , & Seitz, H. M. (1987). Pathology of malaria‐infected mosquitoes. Parasitology Today, 3, 216–218. https://doi.org/10.1016/0169-4758(87)90063-9 [DOI] [PubMed] [Google Scholar]
- McKenzie, F. E. , Jeffery, G. M. , & Collins, W. E. (2002). Plasmodium malariae infection boosts Plasmodium falciparum gametocyte production. American Journal of Tropical Medicine and Hygiene, 67(4), 411–414. https://doi.org/10.4269/ajtmh.2002.67.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mideo, N. , & Reece, S. E. (2012). Plasticity in parasite phenotypes: Evolutionary and ecological implications for disease. Future Microbiology, 7(1), 17–24. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22191443 [DOI] [PubMed] [Google Scholar]
- Moiroux, N. , Gomez, M. B. , Pennetier, C. , Elanga, E. , Djènontin, A. , Chandre, F. , … Corbel, V. (2012). Changes in anopheles funestus biting behavior following universal coverage of long‐lasting insecticidal nets in benin. Journal of Infectious Diseases, 206, 1622–1629. https://doi.org/10.1093/infdis/jis565 [DOI] [PubMed] [Google Scholar]
- Molina‐Cruz, A. , Canepa, G. E. , Kamath, N. , Pavlovic, N. V. , Mu, J. , Ramphul, U. N. , … Barillas‐Mury, C. (2015). Plasmodium evasion of mosquito immunity and global malaria transmission: The lock‐and‐key theory. Proceedings of the National Academy of Sciences of the United States of America, 112(49), 15178–15183. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4679011&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina‐Cruz, A. , DeJong, R. J. , Ortega, C. , Haile, A. , Abban, E. , Rodrigues, J. , … Barillas‐Mury, C. (2012). Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement‐like system of Anopheles gambiae mosquitoes. Proceedings of the National Academy of Sciences of the United States of America, 109(28), E1957–E1962. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22623529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C. C. , Blanford, S. , Luckhart, S. , & Thomas, M. B. (2014). Ambient temperature and dietary supplementation interact to shape mosquito vector competence for malaria. Journal of Insect Physiology, 67, 37–44. https://doi.org/10.1016/j.jinsphys.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C. C. , Moller‐Jacobs, L. L. , Thomas, M. B. , Christophides, G. , Vlachou, D. , Kafatos, F. , … Curtis, C. (2013). Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proceedings of the Royal Society B: Biological Sciences, 280, 20132030 https://doi.org/10.1098/rspb.2013.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C. C. , Paaijmans, K. P. , Cox‐foster, D. , Read, A. F. , & Thomas, M. B. (2012). Rethinking vector immunology: The role of environmental temperature in shaping resistance. Nature Reviews Microbiology, 10(December), 869–876. https://doi.org/10.1038/nrmicro2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal, A. T. , & Schall, J. J. (2014). Life history focus on a malaria parasite: Linked traits and variation among genetic clones. Evolutionary Ecology, 28(1), 89–102. https://doi.org/10.1007/s10682-013-9654-y [Google Scholar]
- Nedelcu, A. M. , Driscoll, W. W. , Durand, P. M. , Herron, M. D. , & Rashidi, A. (2011). On the paradigm of altruistic suicide in the unicellular world. Evolution, 65(1), 3–20. https://doi.org/10.1111/j.1558-5646.2010.01103.x [DOI] [PubMed] [Google Scholar]
- Nguyen, P. L. , Vantaux, A. , Hien, D. F. , Dabiré, K. R. , Yameogo, B. K. , Gouagna, L.‐C. , … Lefèvre, T. (2017). No evidence for manipulation of Anopheles gambiae, An. coluzzii and An. arabiensis host preference by Plasmodium falciparum . Scientific Reports, 7(1), 9415 https://doi.org/10.1038/s41598-017-09821-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidelet, T. , Koella, J. C. , & Kaltz, O. (2009). Effects of shortened host life span on the evolution of parasite life history and virulence in a microbial host‐parasite system. BMC Evolutionary Biology, 9, 65 Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19320981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden, B. H. , Kent, M. D. , & Beier, J. C. (1995). The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi . Parasitology, 111, 539–545. https://doi.org/10.1017/S0031182000077003 [DOI] [PubMed] [Google Scholar]
- Ohm, J. R. , Teeple, J. , Nelson, W. A. , Thomas, M. B. , Read, A. F. , & Cator, L. (2016). Fitness consequences of altered feeding behavior in immune‐challenged mosquitoes. Parasites & Vectors, 9(1), 113 Retrieved from http://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-016-1392-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okech, B. a. , Gouagna, L. C. , Kabiru, E. W. , Walczak, E. , Beier, J. C. , Yan, G. , & Githure, J. I. (2004). Resistance of early midgut stages of natural Plasmodium falciparum parasites to high temperatures in experimentally infected Anopheles gambiae (Diptera: Culicidae). The Journal of Parasitology, 90(4), 764–768. https://doi.org/10.1645/ge-135r1 [DOI] [PubMed] [Google Scholar]
- Ouédraogo, A. L. , Guelbéogo, W. M. , Cohuet, A. , Morlais, I. , King, J. G. , Gonçalves, B. P. , … Bousema, T. (2013). A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae . Malaria World Journal, 4(16), 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, R. E. L. , Ariey, F. , & Robert, V. (2003). The evolutionary ecology of Plasmodium . Ecology Letters, 6(9), 866–880. https://doi.org/10.1046/j.1461-0248.2003.00509.x [Google Scholar]
- Pigeault, R. , Nicot, A. , Gandon, S. , & Rivero, A. (2015). Mosquito age and avian malaria infection. Malaria Journal, 14(1), 383 https://doi.org/10.1186/s12936-015-0912-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci, M. (2005). Evolution of phenotypic plasticity: Where are we going now? Trends in Ecology & Evolution, 20, 481–486. https://doi.org/10.1016/j.tree.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Pollitt, L. C. , Bram, J. T. , Blanford, S. , Jones, M. J. , & Read, A. F. (2015). Existing infection facilitates establishment and density of malaria parasites in their mosquito vector. PLoS Pathogens, 11(7), 1–18. https://doi.org/10.1371/journal.ppat.1005003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt, L. C. , Churcher, T. S. , Dawes, E. J. , Khan, S. M. , Sajid, M. , Basáñez, M. G. , … Reece, S. E. (2013). Costs of crowding for the transmission of malaria parasites. Evolutionary Applications, 6(4), 617–629. https://doi.org/10.1111/eva.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt, L. C. , Colegrave, N. , Khan, S. M. , Sajid, M. , & Reece, S. E. (2010). Investigating the evolution of apoptosis in malaria parasites: The importance of ecology. Parasites & Vectors, 3(1), 105 Retrieved from http://parasitesandvectors.biomedcentral.com/articles/10.1186/1756-3305-3-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt, L. C. , Mideo, N. , Drew, D. R. , Schneider, P. , Colegrave, N. , & Reece, S. E. (2011). Competition and the evolution of reproductive restraint in malaria parasites. The American Naturalist, 177(3), 358–367. https://doi.org/10.1086/658175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnudurai, T. , Lensen, A. H. W. , Vangemert, G. J. A. , Bolmer, M. G. , & Meuwissen, J. (1991). Feeding behavior and sporozoite ejection by infected Anopheles stephensi . Transactions of the Royal Society of Tropical Medicine and Hygiene, 85(2), 175–180. https://doi.org/10.1016/0035-9203(91)90012-n [DOI] [PubMed] [Google Scholar]
- Poulin, R. (2007). The evolutionary ecology of parasites. Princeton, NJ: Princeton University Press. [Google Scholar]
- Redmond, S. N. , Eiglmeier, K. , Mitri, C. , Markianos, K. , Guelbeogo, W. M. , Gneme, A. , … Vernick, K. D. (2015). Association mapping by pooled sequencing identifies TOLL 11 as a protective factor against Plasmodium falciparum in Anopheles gambiae . BMC Genomics, 16(1), 779 https://doi.org/10.1186/s12864-015-2009-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece, S. E. , Drew, D. R. , & Gardner, A. (2008). Sex ratio adjustment and kin discrimination in malaria parasites. Nature, 453(7195), 609‐U1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece, S. E. , Pollitt, L. C. , Colegrave, N. , & Gardner, A. (2011). The meaning of death: Evolution and ecology of apoptosis in protozoan parasites. PLoS Pathogens, 7(12), 9 https://doi.org/10.1371/journal.ppat.1002320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece, S. E. , Ramiro, R. S. , & Nussey, D. H. (2009). Plastic parasites: Sophisticated strategies for survival and reproduction? Evolutionary Applications, 2(1), 11–23. https://doi.org/10.1111/j.1752-4571.2008.00060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero, A. , Vezilier, J. , Weill, M. , Read, A. F. , & Gandon, S. (2010). Insecticide control of vector‐borne diseases: When is insecticide resistance a problem? PLoS Pathogens, 6(8), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, J. , Brayner, F. A. , Alves, L. C. , Dixit, R. , & Barillas‐Mury, C. (2010). Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science, 329(5997), 1353–1355. https://doi.org/10.1126/science.1190689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, O. , Vantaux, A. , Roche, B. , Yameogo, B. , Dabiré, K. R. , Diabaté, A. , … Lefèvre, T. (2015). Evidence for carry‐over effects of predator exposure on pathogen transmission potential. Proceedings of the Royal Society B: Biological Sciences, 282, 20152430 https://doi.org/10.1098/rspb.2015.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund, S. S. C. , Hou, T. Y. , Ward, S. M. , Collins, F. H. , & Duffield, G. E. (2011). Genome‐wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae . Proceedings of the National Academy of Sciences of the United States of America, 108(32), E421–E430. https://doi.org/10.1073/pnas.1100584108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund, S. S. C. , O'Donnell, A. J. , Gentile, J. E. , & Reece, S. E. (2016). Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects, 7, 14 https://doi.org/10.3390/insects7020014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare, I. , Dabire, R. , Yameogo, B. , Da, D. F. , Michalakis, Y. , & Cohuet, A. (2014). Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii . Infection, Genetics and Evolution, 25, 57–65. https://doi.org/10.1016/j.meegid.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Schiefer, B. A. , Ward, R. A. , & Eldridge, B. F. (1977). Plasmodium cynomolgi: Effects of malaria infection on laboratory flight performance of Anopheles stephensi mosquitoes. Experimental Parasitology, 41(2), 397–404. https://doi.org/10.1016/0014-4894(77)90111-4 [DOI] [PubMed] [Google Scholar]
- Schwartz, A. , & Koella, J. C. (2001). Trade‐offs, conflicts of interest and manipulation in Plasmodium‐mosquito interactions. Trends in Parasitology, 17(4), 189–194. https://doi.org/10.1016/S1471-4922(00)01945-0 [DOI] [PubMed] [Google Scholar]
- Severo, M. S. , & Levashina, E. a. (2014). Mosquito defenses against Plasmodium parasites. Current Opinion in Insect Science, 3, 30–36. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S221457451400042X [DOI] [PubMed] [Google Scholar]
- Shapiro, L. L. M. , Murdock, C. C. , Jacobs, G. R. , Thomas, R. J. , & Thomas, M. B. (2016). Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proceedings of the Royal Society B, 283, 20160298 https://doi.org/10.1098/rspb.2016.0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden, R. E. (2016). The biology of malaria transmission In Gaur D., Chitnis C. E., & Chauhan V. S. (Eds.), Advances in malaria research (p. 600). Wiley‐Blackwell. [Google Scholar]
- Sinden, R. E. , Alavi, Y. , & Raine, J. D. (2004). Mosquito – malaria interactions : A reappraisal of the concepts of susceptibility and refractoriness. Insect Biochemistry and Molecular Biology, 34, 625–629. https://doi.org/10.1016/j.ibmb.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Sinden, R. E. , Dawes, E. J. , Alavi, Y. , Waldock, J. , Finney, O. , Mendoza, J. , … Basanez, M. G. (2007). Progression of Plasmodium berghei through Anopheles stephensi is density‐dependent. PLoS Pathogens, 3(12), 2005–2016. https://doi.org/10.1371/journal.ppat.0030195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallegange, R. C. , van Gemert, G. J. , van de Vegte‐Bolmer, M. , Gezan, S. , Takken, W. , Sauerwein, R. W. , & Logan, J. G. (2013). Malaria infected mosquitoes express enhanced attraction to human odor. PLoS ONE, 8(5), 8–10. https://doi.org/10.1371/journal.pone.0063602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. L. , & McKenzie, F. E. (2004). Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria Journal, 3(1), 13 Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=449722&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg, E. D. , & Thomas, M. B. (2017). Insights from agriculture for the management of insecticide resistance in disease vectors. Evolutionary Applications, 1–11. https://doi.org/10.1111/eva.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken, W. , Smallegange, R. C. , Vigneau, A. J. , Johnston, V. , Brown, M. , Mordue‐Luntz, A. J. , & Billingsley, P. F. (2013). Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi . Parasites & Vectors, 6(1), 345 Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4029273&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet, F. , Aboagye‐Antwi, F. , & Hurd, H. (2008). Ecological immunology of mosquito‐malaria interactions. Trends in Parasitology, 24(5), 219–227. https://doi.org/10.1016/j.pt.2008.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantaux, A. , Dabiré, K. , Cohuet, A. , & Lefèvre, T. (2014). A heavy legacy: Offspring of malaria‐infected mosquitoes show reduced disease resistance. Malaria Journal, 13(1), 442 https://doi.org/10.1186/1475-2875-13-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantaux, A. , de Sales Hien, D. F. , Yameogo, B. , Dabiré, K. R. , Thomas, F. , Cohuet, A. , & Lefèvre, T. (2015). Host‐seeking behaviors of mosquitoes experimentally infected with sympatric field isolates of the human malaria parasite Plasmodium falciparum: No evidence for host manipulation. Frontiers in Ecology and Evolution, 3(August), 1–12. Retrieved from http://journal.frontiersin.org/article/10.3389/fevo.2015.00086 [Google Scholar]
- Vaughan, J. A. (2007). Population dynamics of Plasmodium sporogony. Trends in Parasitology, 23(2), 63–70. https://doi.org/10.1016/j.pt.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Vaughan, J. A. , & Turell, M. J. (1996). Facilitation of Rift Valley fever virus transmission by Plasmodium berghei sporozoites in Anopheles stephensi mosquitoes. American Journal of Tropical Medicine and Hygiene, 55, 407–409. https://doi.org/10.4269/ajtmh.1996.55.407 [DOI] [PubMed] [Google Scholar]
- Vézilier, J. , Nicot, A. , Gandon, S. , & Rivero, A. (2012). Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proceedings of the Royal Society B: Biological Sciences, 279(1744), 4033–4041. https://doi.org/10.1098/rspb.2012.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana, M. , Hughes, A. , Matthiopoulos, J. , Ranson, H. , & Ferguson, H. M. (2016). Delayed mortality effects cut the malaria transmission potential of insecticide‐resistant mosquitoes. Proceedings of the National Academy of Sciences, 113(32), 8975–8980. https://doi.org/10.1073/pnas.1603431113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekesa, J. W. , Copeland, R. S. , & Mwangi, R. W. (1992). Effect of Plasmodium falciparum on blood feeding behavior of naturally infected Anopheles mosquitoes in western Kenya. The American Journal of Tropical Medicine and Hygiene, 47(4), 484–488. https://doi.org/10.4269/ajtmh.1992.47.484 [DOI] [PubMed] [Google Scholar]
- White, N. J. , Pukrittayakamee, S. , Hien, T. T. , Faiz, M. A. , Mokuolu, O. a. , & Dondorp, A. M. (2014). Malaria. Lancet, 383(9918), 723–735. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23953767 [DOI] [PubMed] [Google Scholar]
- Whitman, D. W. , & Agrawal, A. A. (2009). What is phenotypic plasticity and why is it important? In Whitman D. W. & Ananthakrishnan T. M. (Eds.), Phenotypic plasticity of insects: Mechanisms and consequences (pp. 1–63). Enfield, NH: Science Publishers; https://doi.org/10.1201/b10201 [Google Scholar]
- WHO (Ed.). (1975). Manual on practical entomology in malaria.
- WHO . (2015). World malaria report 2015. World Health (Vol. WHO/HTM/GM). Retrieved from http://www.who.int/malaria/publications/world_malaria_report_2013/en/%5Cnhttp://www.who.int/iris/bitstream/10665/97008/1/9789241564694_eng.pdf%5Cnhttp://www.who.int/malaria/publications/world_malaria_report_2013/wmr2013_no_profiles.pdf?ua=1
- Ye, Y. H. , Chenoweth, S. F. , Carrasco, A. M. , Allen, S. L. , Frentiu, F. D. , van den Hurk, A. F. , … McGraw, E. A. (2016). Evolutionary potential of the extrinsic incubation period of dengue virus in Aedes aegypti . Evolution, 70(11), 2459–2469. https://doi.org/10.1111/evo.13039 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. O. , Kurscheid, S. , Zhang, Y. , Liu, L. , Zhang, L. L. , Loeliger, K. , & Fikrig, E. (2012). Enhanced survival of Plasmodium‐infected mosquitoes during starvation. PLoS ONE, 7(7), 8 https://doi.org/e4055610.1371/journal.pone.0040556 [DOI] [PMC free article] [PubMed] [Google Scholar]


