Abstract
Background
Meconium-stained amniotic fluid (MSAF) represents the passage of fetal colonic content into the amniotic cavity. Meconium aspiration syndrome (MAS) is a complication that occurs in a subset of infants with MSAF. Secreted phospholipase A2 (sPLA2) is detected in meconium and is implicated in the development of MAS. The purpose of this study was to determine if sPLA2 concentrations are increased in the amniotic fluid of women in spontaneous labor at term with MSAF.
Materials and methods
This was a cross-sectional study of patients in spontaneous term labor who underwent amniocentesis (n = 101). The patients were divided into two study groups: (1) MSAF (n = 61) and (2) clear fluid (n = 40). The presence of bacteria and endotoxin as well as interleukin-6 (IL-6) and sPLA2 concentrations in the amniotic fluid were determined. Statistical analyses were performed to test for normality and bivariate analysis. The Spearman correlation coefficient was used to study the relationship between sPLA2 and IL-6 concentrations in the amniotic fluid.
Results
Patients with MSAF have a higher median sPLA2 concentration (ng/mL) in amniotic fluid than those with clear fluid [1.7 (0.98–2.89) versus 0.3 (0–0.6), p < 0.001]. Among patients with MSAF, those with either microbial invasion of the amniotic cavity (MIAC, defined as presence of bacteria in the amniotic cavity), or bacterial endotoxin had a significantly higher median sPLA2 concentration (ng/mL) in amniotic fluid than those without MIAC or endotoxin [2.4 (1.7–6.0) versus 1.7 (1.3–2.5), p <0.05]. There was a positive correlation between sPLA2 and IL-6 concentrations in the amniotic fluid (Spearman Rho=0.3, p <0.05).
Conclusion
MSAF that contains bacteria or endotoxin has a higher concentration of sPLA2, and this may contribute to induce lung inflammation when meconium is aspirated before birth.
Keywords: Acute phase protein reactant, interleukin-6, intra-amniotic inflammation/infection, prostaglandins, sPLA2
Introduction
Meconium-stained amniotic fluid (MSAF) represents the passage of fetal colonic content into the amniotic cavity [1–13]. MSAF is a risk factor for maternal infection-related complications (e.g. chorioamnionitis [8,14–20], puerperal endomyometritis [16,17,20,21]), neonatal sepsis [3,22–25], cerebral palsy [26–29], hypoxic-ischemic encephalopathy [3,30–33], meconium aspiration syndrome (MAS) [3,6,8,12,13,34–54], and fetal death [55–57].
MAS occurs in a subset of infants born to mothers with MSAF [3,6,8,12,13,34–54]. However, why some infants with MSAF develop MAS, and others do not, remains an open question [6,38,41–43,45,51]. Meconium-induced lung injury has been attributed to mechanical obstruction [51,52,58–60], chemical injury [58,61–66], pulmonary cell apoptosis [35,36,60,65,67–70] and an inflammatory response [35,59,67,71–87]. A series of experimental and clinical studies have made a strong case for a role of secreted phospholipase A2 (sPLA2) in MAS [67,88–95]. This enzyme can exert deleterious effects by eliciting inflammation [92,93,96–112] and inactivating lung surfactant [89,90,113–115]. The purpose of this study was to determine if sPLA2 concentration is increased in the amniotic fluid of women in spontaneous labor at term with MSAF.
Materials and methods
Study design and population
A cross-sectional study was conducted which included patients at term with MSAF (n = 61) and clear amniotic fluid (n = 40, controls). Inclusion and exclusion criteria for the study population were similar to a previous report [116]. All women provided written informed consent before collection of the amniotic fluid samples. The collection and utilization of the samples was approved by the Human Investigation Committee of the participating institutions and the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/ DHHS). The clinical definitions, sample collection, microbiological studies, detection of endotoxin, and statistical analysis have been described in a previous report [116]. sPLA2 immunoassay was performed according to the methods defined by Stoner et al. [117,118].
Results
Among women with spontaneous labor at term, 60.4% (61/101) had MSAF and 39.6% (40/101) had clear amniotic fluid. The median maternal age was significantly higher in patients with MSAF than in those with clear fluid (p = 0.03). Otherwise, the clinical characteristics of the two study groups were similar (p >0.05).
Microorganisms in the AF were identified in 16.4% (10/61) of patients in the MSAF group and in 5% (2/40) of those with clear fluid (p < 0.05). The most common microorganisms were Gram-negative rods (n = 6), followed by Ureaplasma urealyticum (n = 2), Gram-positive rods (n = 2) and Mycoplasma hominis (n = 1). One patient’s amniotic fluid had both a Gram-positive rod and M. hominis. Two patients with clear amniotic fluid had positive cultures for bacteria (U. urealyticum).
The Limulus amebocyte lysate (LAL) assay for bacterial endotoxin in the amniotic fluid was positive in 32.8% (20/61) of patients with MSAF, but in only 2.5% (1/40) of those with clear amniotic fluid (p < 0.001). After heat treatment to eliminate the effect of trypsin [119], the frequency of a positive LAL assay was still significantly higher in the MSAF group compared to those with clear amniotic fluid, even after heat treatment [19.7% (12/61) versus 2.5% (1/40); p <0.05].
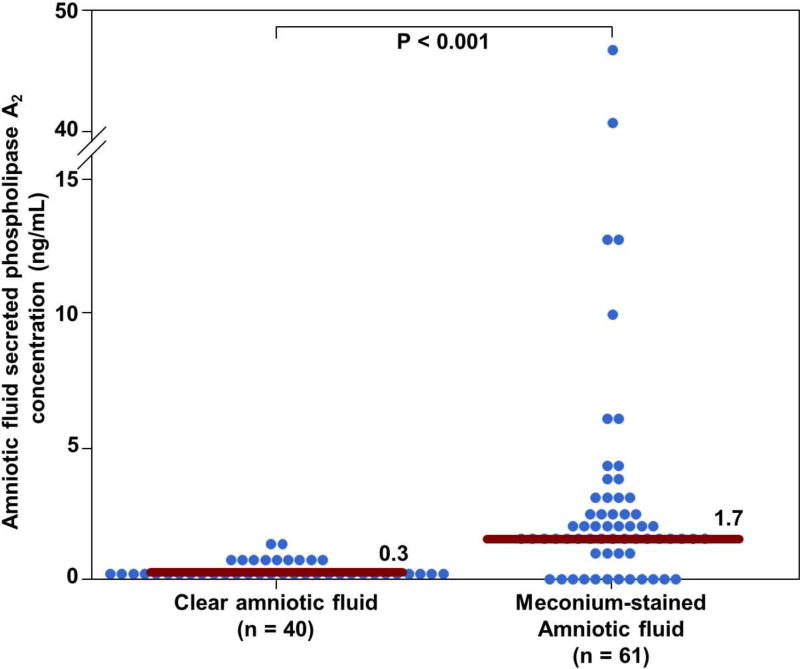
Patients with MSAF had a significantly higher median amniotic fluid sPLA2 concentration (ng/mL) than those with clear amniotic fluid [1.7 (0.98–2.89) versus 0.3 (0–0.6); p < 0.001] (Figure 1). Moreover, in the MSAF group, those with endotoxin or microorganisms (defined by LAL or amniotic fluid Gram stain or positive amniotic fluid culture) had a significantly higher median amniotic fluid sPLA2 concentration (ng/mL) than those with the absence of endotoxin or microorganisms [2.4 (1.7–6.9) versus 1.7 (1.3–2.5); p = 0.049] (Figure 2). Amniotic fluid sPLA2 concentration had a significant positive correlation with amniotic fluid IL-6 concentration (Spearman Rho =0.3, p =0.045).
Figure 1.
Amniotic fluid secreted phospholipase A2 concentrations (sPLA2) in women at term with clear amniotic fluid and MSAF. Patients with MSAF had a significantly higher median amniotic fluid secreted phospholipase A2 concentration (ng/mL) than those with clear amniotic fluid [1.7 (1–2.9) versus 0.3 (0–0.6); p < 0.001].
Figure 2.
Amniotic fluid secreted phospholipase A2 concentration (sPLA2) among women with MSAF at term with presence and absence of endotoxin or microorganisms. Patients with MSAF and intra-amniotic inflammation/infection at term had a significantly higher median secreted phospholipase A2 concentration (ng/mL) than those without intra-amniotic inflammation/infection [2.4 (1.7–6.99) versus 1.7 (1.3–2.5); p = 0.049].
Discussion
Principal findings of the study
(1) Patients with MSAF in spontaneous labor at term had a higher median sPLA2 concentration in amniotic fluid than those with clear amniotic fluid; (2) among patients with MSAF, women with either microbial invasion of the amniotic cavity (MIAC; defined as a positive amniotic fluid culture for microorganisms) or the presence of endotoxin in the amniotic cavity had a higher median sPLA2 concentration in the amniotic fluid than those without MIAC or bacterial endotoxin; and (3) there was a positive correlation between amniotic fluid sPLA2 and amniotic fluid IL-6 concentration. Since sPLA2 is an acute-phase reactant protein induced by IL-6, this observation suggests that an inflammatory response is associated with an increase in sPLA2.
What are phospholipases A2?
Phospholipase A2 (PLA2) is a family of enzymes that hydrolyze the ester bond at the sn-2 position of phospholipids to generate arachidonic acid and lysophospholipids, which are precursors of eicosanoids and other lipid mediators (leukotrienes and prostaglandins) [110,112,120–135]. These enzymes are broadly classified into two groups: (1) intracellular or cytosolic PLA2 (cPLA2) and (2) extracellular or secreted PLA2 (sPLA2) [112,128]. PLA2 participates in the production of prostaglandins, which are major mediators of the onset of spontaneous labor at term [136–164], as well as preterm labor [151,159,161,163,165–168]. cPLA2 is an intracellular enzyme, while sPLA2 (in particular, group IIA isoform) is an acute phase reactant protein released in response to tissue damage and infection [169–171]. IL-6 can induce the expression of group II sPLA2 from hepatic cells in culture [172]. The properties and functions of cPLA2 and sPLA2 have been reviewed [112,124,128,130,132–134]. Recently, sPLA2 has been implicated in the pathophysiology of meconium-induced lung injury (see below).
More than 10 isoforms of sPLA2 have been described (e.g. groups I, II, III, V, etc.) [110,112,130,132–134]. Individual sPLA2 enzymes act on both cellular membrane phospholipids and non-cellular phospholipids (e.g. surfactant and lipoproteins) including foreign phospholipids (e.g. bacterial membranes and dietary phospholipids) [133]. The functions of sPLA2 depend on: (1) specific sPLA2 isoform; (2) specific target phospholipid or membrane; (3) lipid mediators produced by enzymatic activity; (4) the mechanisms responsible for the activation of sPLA2; and (5) the specific circumstances and site at which a particular sPLA2 isoform is present [133]. For example, the group I sPLA2 isoform is produced in the pancreas, and its primary function is the catalytic cleavage of dietary lipids [173]. The group II sPLA2 isoform is largely expressed and stored in inflammatory cells including neutrophils [174], eosinophils [175,176], T-lymphocytes [177,178], monocytes [179,180], macrophages [181], mast cells [182] and platelets [183]. This particular isoform (group II sPLA2) is detected in high concentrations in biological fluids in the context of inflammation (e.g. synovial fluid in rheumatoid arthritis [105,184–188], bronchoalveolar lavage (BAL) in patients with acute respiratory distress syndrome (ARDS) [100,114], and serum/plasma of patients with septic shock [189], Crohn’s disease [190], ulcerative colitis [191], acute pancreatitis [192–194], and rheumatoid arthritis [195].
The group II sPLA2 isoform has potent antimicrobial activity [112,171,196–207]. Elsbach et al. purified sPLA2 from polymorphonuclear leukocytes of rabbits, and reported that sPLA2 was bactericidal against Escherichia coli and Salmonella typhimurium, acting in concert with a ‘‘bactericidal/permeability increasing protein’’ [196]. Subsequently, Weinrauch et al. extracted group II sPLA2 from sterile peritoneal fluid of rabbits, and demonstrated that it had potent antimicrobial activity against Staphylococcus aureus [198,208]. Similarly, group II sPLA2 isolated from the plasma of baboons after a challenge with E. coli has potent bactericidal properties against S. aureus and Streptococcus pyogenes [198,203]. Such activity can be blocked by a monoclonal antibody against the enzyme [198]. Other investigators have shown antimicrobial activity against Listeria monocytogenes [197,209] and Bacillus anthracis [203,204]. sPLA2 may also participate in host defense against viruses [210–212] and parasites [213]. The presence of high sPLA2 concentrations in biological fluids (e.g. tears [214,215], semen [216], intestinal lumen [197,217,218], inflammatory exudates [105,184–188], bronchoalveolar lavage [100,114], and serum [189,219]) of both animals and humans with bacterial infections has been interpreted as indicating that sPLA2 is part of the host defense against microbial invasion [112,171].
Group II sPLA2 can induce activation of human neutrophils [101,104], exocytosis in human lung macrophages [102], neutrophils [104], eosinophils [176], and degranulation of mast cells [99]. Triggiani et al. reported that sPLA2 [group I sPLA2 from cobra venom and group II (recombinant synovial fluid) sPLA2] can increase the expression of IL-6 mRNA and the rate of secretion of IL-6 from human lung macrophages, as well as the release of β-glucuronidase (a cytosolic enzyme used as a surrogate marker for cellular exocytosis) [102]. Groups I and II sPLA2 generate an intracellular response that activates both exocytosis and cytokine gene expression in macrophages [92,102,111]. Other investigators have reported that different isoforms of sPLA2 (group IA, IB, IIA, V and X) induce the production of cytokines (e.g. IL-6, TNFα and IL-10) and chemokines [e.g. monocyte chemotactic protein-1(MCP-1)/chemokine (C-C motif) ligand 2 (CCL2), macrophage inflammatory protein-1 (MIP-1α)/CCL3 and MIP1-β/CCL4] from inflammatory cells such as monocytes [105], neutrophils [108] and eosinophils [176]. These observations collectively suggest that sPLA2 has an important role in inflammation. The catalytic action of sPLA2, cleaving membrane phospholipids to generate eicosanoid precursors (arachidonic acid, leukotrienes and prostaglandins), has been implicated in the generation of an inflammatory state [112,114,170,220].
PLA2 have been localized in lysosomes of chorioamnion [221], decidua [222,223] and amniotic fluid [224,225]. Moreover, its activity in fetal membranes was increased before the onset of labor [221]. Group II sPLA2 mRNA expression and immunoreactivity has been demonstrated in amnion, choriodecidua and placenta [226–228]. Rice et al. concluded that this isoenzyme is a major contributor of the net tissue sPLA2 activity in the human placenta and may contribute to the production of prostaglandins during labor [227]. The expression of this enzyme is increased in placentas of women in labor [228].
Phospholipase A2 in meconium and meconium-induced lung injury
sPLA2 has been reported in meconium [67,88,90,93]. The administration of meconium into the trachea of neonatal pigs results in severe histologic lung inflammation, increased apoptosis, and increased lung sPLA2 activity (measured by the concentration of arachidonic acid following incubation of lung homogenates with 1,2-dipalmitoylphosphatidylcholine (DPPC), a substrate that is specific to sPLA2) [67].
sPLA2 activity has been detected in meconium (determined by measuring DPPC metabolites in suspensions of this material before and after mixing with the substrate) [90]. Enzymatic activity is attributed to sPLA2 (rather than other phospholipases), and has been demonstrated by the formation of lysophosphatidylcholine after samples had been heat-treated (sPLA2 is heat-stable – other lipolytic enzymes are heat-sensitive). sPLA2 extracted from meconium inhibits surfactant activity in vitro [90].
sPLA2 activity in lung tissues can be induced by meconium and bile acids [115]. sPLA2 is locally produced in lung tissue and contributes to the total PLA2 activity during MAS [53,93]. Collectively, the evidence suggests that: (1) meconium contains sPLA2 activity; (2) the lungs of neonates affected with MAS contain higher amounts of sPLA2; (3) cPLA2 was not detected in meconium or alveolar fluid; and (4) there is a correlation between sPLA2 activity and TNF-α concentrations in bronchoalveolar lavage [53,67,90,92,93,115].
Phospholipase A2 in amniotic fluid with MSAF and microbial invasion of the amniotic cavity
The findings reported herein suggest that the concentration of sPLA2 is higher in MSAF than in clear amniotic fluid among patients in labor at term. After exclusion of samples with MSAF with either bacteria or endotoxin, the difference between clear amniotic fluid and MSAF disappeared. Moreover, sPLA2 concentrations in amniotic fluid correlated positively with IL-6 concentrations. These observations suggest that the elevation in sPLA2 can be attributed to the consequences of MIAC or the resulting inflammatory process.
Our findings and interpretation are consistent with those reported by Koyama et al., indicating that sPLA2 activity (measured by high-performance liquid chromatography) and group II sPLA2 concentration in amniotic fluid were higher in patients with preterm labor (with or without chorioamnionitis) than in preterm controls (i.e. pregnant women without labor who underwent amniocentesis for chromosomal studies between 17–30 weeks of gestation) [229].
We recently reported that the frequency of MIAC and bacterial endotoxin in amniotic fluid is higher among women in spontaneous labor at term with MSAF than in those with clear amniotic fluid [116]. We proposed that microorganisms or microbial products, such as endotoxin, present in amniotic fluid can be swallowed by the fetus, resulting in increased fetal peristalsis and intrauterine passage of meconium. Aspiration of meconium with microorganisms and inflammatory mediators during fetal life could predispose to MAS. Since sPLA2 has been proposed to be a major mediator of lung injury in MAS, our findings suggest that the meconium of patients with MIAC or endotoxin contains higher concentrations of sPLA2. Although exposure to sPLA2 may begin during fetal life, aspirated meconium and microbial products contained in such meconium, as well as inflammatory mediators, may induce further production of sPLA2 and other inflammatory mediators by the lung that may eventually lead to lung injury and respiratory insufficiency observed in MAS.
Conclusion
Term meconium-stained amniotic fluid that contains bacteria or endotoxin has a higher concentration of secreted phospholipase A2, and this may contribute to induce lung inflammation when meconium is aspirated before birth.
Acknowledgments
Funding: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C. The research was also supported by a grant from the Walter Scott Foundation for Medical Research.
Footnotes
Declaration of interest: The authors report no conflicts of interest.
References
- 1.Woods JR, Glantz JC. Significance of amniotic fluid meconium. In: Creasy RK, Resnik R, editors. Maternal fetal medicine principles and practices. 3. Philadelphia: W.B.: Saunders Company; 1994. pp. 413–22. [Google Scholar]
- 2.Ross MG. Meconium aspiration syndrome – more than intrapartum meconium. N Engl J Med. 2005;353:946–8. doi: 10.1056/NEJMe058149. [DOI] [PubMed] [Google Scholar]
- 3.Ahanya SN, Lakshmanan J, Morgan BL, et al. Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv. 2005;60:45–56. doi: 10.1097/01.ogx.0000149659.89530.c2. quiz 73–44. [DOI] [PubMed] [Google Scholar]
- 4.Lakshmanan J, Ross MG. Mechanism(s) of in utero meconium passage. J Perinatol. 2008;28:S8–13. doi: 10.1038/jp.2008.144. [DOI] [PubMed] [Google Scholar]
- 5.Caughey AB, Musci TJ. Complications of term pregnancies beyond 37 weeks of gestation. Obstet Gynecol. 2004;103:57–62. doi: 10.1097/01.AOG.0000109216.24211.D4. [DOI] [PubMed] [Google Scholar]
- 6.Bhat R, Vidyasagar D. Delivery room management of meconiumstained infant. Clin Perinatol. 2012;39:817–31. doi: 10.1016/j.clp.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Kariniemi V, Harrela M. Significance of meconium staining of the amniotic fluid. J Perinat Med. 1990;18:345–9. doi: 10.1515/jpme.1990.18.5.345. [DOI] [PubMed] [Google Scholar]
- 8.Maymon E, Chaim W, Furman B, et al. Meconium stained amniotic fluid in very low risk pregnancies at term gestation. Eur J Obstet Gynecol Reprod Biol. 1998;80:169–73. doi: 10.1016/s0301-2115(98)00122-5. [DOI] [PubMed] [Google Scholar]
- 9.Abramovici H, Brandes JM, Fuchs K, et al. Meconium during delivery: a sign of compensated fetal distress. Am J Obstet Gynecol. 1974;118:251–5. doi: 10.1016/0002-9378(74)90556-0. [DOI] [PubMed] [Google Scholar]
- 10.Fujikura T, Klionsky B. The significance of meconium staining. Am J Obstet Gynecol. 1975;121:45–50. doi: 10.1016/0002-9378(75)90973-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee KA, Mi Lee S, Jin Yang H, et al. The frequency of meconiumstained amniotic fluid increases as a function of the duration of labor. J Matern Fetal Neonatal Med. 2011;24:880–5. doi: 10.3109/14767058.2010.531329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheiner E, Hadar A, Shoham-Vardi I, et al. The effect of meconium on perinatal outcome: a prospective analysis. J Matern Fetal Neonatal Med. 2002;11:54–9. doi: 10.1080/jmf.11.1.54.59. [DOI] [PubMed] [Google Scholar]
- 13.Oyelese Y, Culin A, Ananth CV, et al. Meconium-stained amniotic fluid across gestation and neonatal acid-base status. Obstet Gynecol. 2006;108:345–9. doi: 10.1097/01.AOG.0000226853.85609.8d. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Hanaoka S, Mazor M, et al. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1991;164:859–62. doi: 10.1016/0002-9378(91)90529-z. [DOI] [PubMed] [Google Scholar]
- 15.Mazor M, Furman B, Wiznitzer A, et al. Maternal and perinatal outcome of patients with preterm labor and meconium-stained amniotic fluid. Obstet Gynecol. 1995;86:830–3. doi: 10.1016/0029-7844(95)00265-S. [DOI] [PubMed] [Google Scholar]
- 16.Markovitch O, Mazor M, Shoham-Vardi I, et al. Meconium stained amniotic fluid is associated with maternal infectious morbidity in pre term delivery. Acta Obstet Gynecol Scand. 1993;72:538–42. doi: 10.3109/00016349309058159. [DOI] [PubMed] [Google Scholar]
- 17.Piper JM, Newton ER, Berkus MD, et al. Meconium: a marker for peripartum infection. Obstet Gynecol. 1998;91:741–5. doi: 10.1016/s0029-7844(98)00048-9. [DOI] [PubMed] [Google Scholar]
- 18.Chapman S, Duff P. Incidence of chorioamnionitis in patients with meconium-stained amniotic fluid. Infect Dis Obstet Gynecol. 1995;2:210–12. doi: 10.1155/S1064744995000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usta IM, Sibai BM, Mercer BM, et al. Use of maternal plasma level of zinc-coproporphyrin in the prediction of intrauterine passage of meconium: a pilot study. J Matern Fetal Med. 2000;9:201–3. doi: 10.1002/1520-6661(200007/08)9:4<201::AID-MFM2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Tran SH, Caughey AB, Musci TJ. Meconium-stained amniotic fluid is associated with puerperal infections. Am J Obstet Gynecol. 2003;189:746–50. doi: 10.1067/s0002-9378(03)00767-1. [DOI] [PubMed] [Google Scholar]
- 21.Josephson A. An epidemiologic study of postcesarean infection. Am J Infect Control. 1984;12:19–25. doi: 10.1016/0196-6553(84)90068-3. [DOI] [PubMed] [Google Scholar]
- 22.Berkus MD, Langer O, Samueloff A, et al. Meconium-stained amniotic fluid: increased risk for adverse neonatal outcome. Obstet Gynecol. 1994;84:115–20. [PubMed] [Google Scholar]
- 23.Escobar GJ, Li DK, Armstrong MA, et al. Neonatal sepsis workups in infants 4/¼2000 grams at birth: A population-based study. Pediatrics. 2000;106:256–63. doi: 10.1542/peds.106.2.256. [DOI] [PubMed] [Google Scholar]
- 24.Kayange N, Kamugisha E, Mwizamholya DL, et al. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. 2010;10:39. doi: 10.1186/1471-2431-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrag SJ, Cutland CL, Zell ER, et al. Risk factors for neonatal sepsis and perinatal death among infants enrolled in the prevention of perinatal sepsis trial, Soweto, South Africa. Pediatr Infect Dis J. 2012;31:821–26. doi: 10.1097/INF.0b013e31825c4b5a. [DOI] [PubMed] [Google Scholar]
- 26.Altshuler G, Arizawa M, Molnar-Nadasdy G. Meconium-induced umbilical cord vascular necrosis and ulceration: a potential link between the placenta and poor pregnancy outcome. Obstet Gynecol. 1992;79:760–6. [PubMed] [Google Scholar]
- 27.Spinillo A, Fazzi E, Capuzzo E, et al. Meconium-stained amniotic fluid and risk for cerebral palsy in preterm infants. Obstet Gynecol. 1997;90:519–23. doi: 10.1016/s0029-7844(97)00308-6. [DOI] [PubMed] [Google Scholar]
- 28.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452–7. doi: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 29.McIntyre S, Taitz D, Keogh J, et al. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol. 2013;55:499–508. doi: 10.1111/dmcn.12017. [DOI] [PubMed] [Google Scholar]
- 30.Andres RL, Saade G, Gilstrap LC, et al. Association between umbilical blood gas parameters and neonatal morbidity and death in neonates with pathologic fetal acidemia. Am J Obstet Gynecol. 1999;181:867–71. doi: 10.1016/s0002-9378(99)70316-9. [DOI] [PubMed] [Google Scholar]
- 31.Ellis M, Manandhar N, Manandhar DS, et al. Risk factors for neonatal encephalopathy in Kathmandu, Nepal, a developing country: unmatched case-control study. BMJ. 2000;320:1229–36. doi: 10.1136/bmj.320.7244.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes BC, Cooley S, Donnelly J, et al. The placenta in infants >36 weeks gestation with neonatal encephalopathy: a case control study. Arch Dis Child Fetal Neonatal Ed. 2013;98:F233–9. doi: 10.1136/archdischild-2012-301992. [DOI] [PubMed] [Google Scholar]
- 33.Hayes BC, McGarvey C, Mulvany S, et al. A case-control study of hypoxic-ischemic encephalopathy in newborn infants at >36 weeks gestation. Am J Obstet Gynecol. 2013;209:29.e1–29.e19. doi: 10.1016/j.ajog.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Vidyasagar D, Harris V, Pildes RS. Assisted ventilation in infants with meconium aspiration syndrome. Pediatrics. 1975;56:208–13. [PubMed] [Google Scholar]
- 35.Vidyasagar D, Lukkarinen H, Kaapa P, et al. Inflammatory response and apoptosis in newborn lungs after meconium aspiration. Biotechnol Prog. 2005;21:192–7. doi: 10.1021/bp0497886. [DOI] [PubMed] [Google Scholar]
- 36.Vidyasagar D, Zagariya A. Studies of meconium-induced lung injury: inflammatory cytokine expression and apoptosis. J Perinatol. 2008;28:S102–7. doi: 10.1038/jp.2008.153. [DOI] [PubMed] [Google Scholar]
- 37.Katz VL, Bowes WA., Jr Meconium aspiration syndrome: reflectionson a murky subject. Am J Obstet Gynecol. 1992;166:171–83. doi: 10.1016/0002-9378(92)91856-6. [DOI] [PubMed] [Google Scholar]
- 38.Cleary GM, Wiswell TE. Meconium-stained amniotic fluid and the meconium aspiration syndrome. An update. Pediatr Clin North Am. 1998;45:511–29. doi: 10.1016/s0031-3955(05)70025-0. [DOI] [PubMed] [Google Scholar]
- 39.Blackwell SC, Carreno CA, Hassan SS, et al. Meconium staining and meconium aspiration syndrome. Is there seasonal variation? Fetal Diagn Ther. 2001;16:208–10. doi: 10.1159/000053911. [DOI] [PubMed] [Google Scholar]
- 40.Ziadeh SM, Sunna E. Obstetric and perinatal outcome of pregnancies with term labour and meconium-stained amniotic fluid. Arch Gynecol Obstet. 2000;264:84–7. doi: 10.1007/s004040000088. [DOI] [PubMed] [Google Scholar]
- 41.Blackwell SC, Moldenhauer J, Hassan SS, et al. Meconium aspiration syndrome in term neonates with normal acid-base status at delivery: is it different? Am J Obstet Gynecol. 2001;184:1422–5. doi: 10.1067/mob.2001.115120. discussion 1425–6. [DOI] [PubMed] [Google Scholar]
- 42.Manganaro R, Mami C, Palmara A, et al. Incidence of meconium aspiration syndrome in term meconium-stained babies managed at birth with selective tracheal intubation. J Perinat Med. 2001;29:465–8. doi: 10.1515/JPM.2001.065. [DOI] [PubMed] [Google Scholar]
- 43.Sedaghatian MR, Othman L, Hossain MM, et al. Risk of meconium-stained amniotic fluid in different ethnic groups. J Perinatol. 2000;20:257–61. doi: 10.1038/sj.jp.7200367. [DOI] [PubMed] [Google Scholar]
- 44.Srinivasan HB, Vidyasagar D. Meconium aspiration syndrome: current concepts and management. Compr Ther. 1999;25:82–9. doi: 10.1007/BF02889600. [DOI] [PubMed] [Google Scholar]
- 45.Dargaville PA, Copnell B. The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics. 2006;117:1712–21. doi: 10.1542/peds.2005-2215. [DOI] [PubMed] [Google Scholar]
- 46.Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004;364:597–602. doi: 10.1016/S0140-6736(04)16852-9. [DOI] [PubMed] [Google Scholar]
- 47.Fraser WD, Hofmeyr J, Lede R, et al. Amnioinfusion for the prevention of the meconium aspiration syndrome. N Engl J Med. 2005;353:909–17. doi: 10.1056/NEJMoa050223. [DOI] [PubMed] [Google Scholar]
- 48.Becker S, Solomayer E, Dogan C, et al. Meconium-stained amniotic fluid – perinatal outcome and obstetrical management in a low-risk suburban population. Eur J Obstet Gynecol Reprod Biol. 2007;132:46–50. doi: 10.1016/j.ejogrb.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 49.Fanaroff AA. Meconium aspiration syndrome: historical aspects. J Perinatol. 2008;28:S3–7. doi: 10.1038/jp.2008.162. [DOI] [PubMed] [Google Scholar]
- 50.de Beaufort AJ. Early human development at the perinatal interface: meconium stained amniotic fluid (MSAF) and meconium aspiration syndrome (MAS) Early Hum Dev. 2009;85:605. doi: 10.1016/j.earlhumdev.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 51.van Ierland Y, de Beaufort AJ. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Hum Dev. 2009;85:617–20. doi: 10.1016/j.earlhumdev.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Martin GI, Vidyasagar D. Introduction: proceedings of the first international conference for meconium aspiration syndrome and meconium-induced lung injury. J Perinatol. 2008;28:S1–2. doi: 10.1038/jp.2008.176. [DOI] [PubMed] [Google Scholar]
- 53.De Luca D, Minucci A, Tripodi D, et al. Role of distinct phospholipases A2 and their modulators in meconium aspiration syndrome in human neonates. Intensive Care Med. 2011;37:1158–65. doi: 10.1007/s00134-011-2243-z. [DOI] [PubMed] [Google Scholar]
- 54.Uhing MR, Bhat R, Philobos M, et al. Value of amnioinfusion in reducing meconium aspiration syndrome. Am J Perinatol. 1993;10:43–5. doi: 10.1055/s-2007-994699. [DOI] [PubMed] [Google Scholar]
- 55.Mandelbaum B. Gestational meconium in the high-risk pregnancy. Obstet Gynecol. 1973;42:87–92. [PubMed] [Google Scholar]
- 56.Ohana O, Holcberg G, Sergienko R, et al. Risk factors for intrauterine fetal death (1988–2009) J Matern Fetal Neonatal Med. 2011;24:1079–83. doi: 10.3109/14767058.2010.545918. [DOI] [PubMed] [Google Scholar]
- 57.Brailovschi Y, Sheiner E, Wiznitzer A, et al. Risk factors for intrapartum fetal death and trends over the years. Arch Gynecol Obstet. 2012;285:323–9. doi: 10.1007/s00404-011-1969-8. [DOI] [PubMed] [Google Scholar]
- 58.Tyler DC, Murphy J, Cheney FW. Mechanical and chemical damage to lung tissue caused by meconium aspiration. Pediatrics. 1978;62:454–9. [PubMed] [Google Scholar]
- 59.Kisala JM, Ayala A, Stephan RN, et al. A model of pulmonary atelectasis in rats: activation of alveolar macrophage and cytokine release. Am J Physiol. 1993;264:R610–14. doi: 10.1152/ajpregu.1993.264.3.R610. [DOI] [PubMed] [Google Scholar]
- 60.Zagariya A, Bhat R, Uhal B, et al. Cell death and lung cell histology in meconium aspirated newborn rabbit lung. Eur J Pediatr. 2000;159:819–26. doi: 10.1007/s004310000581. [DOI] [PubMed] [Google Scholar]
- 61.Clark DA, Nieman GF, Thompson JE, et al. Surfactant displacement by meconium free fatty acids: an alternative explanation for atelectasis in meconium aspiration syndrome. J Pediatr. 1987;110:765–70. doi: 10.1016/s0022-3476(87)80021-5. [DOI] [PubMed] [Google Scholar]
- 62.Moses D, Holm BA, Spitale P, et al. Inhibition of pulmonary surfactant function by meconium. Am J Obstet Gynecol. 1991;164:477–81. doi: 10.1016/s0002-9378(11)80003-7. [DOI] [PubMed] [Google Scholar]
- 63.Sun B, Curstedt T, Robertson B. Surfactant inhibition in experimental meconium aspiration. Acta Paediatr. 1993;82:182–9. doi: 10.1111/j.1651-2227.1993.tb12635.x. [DOI] [PubMed] [Google Scholar]
- 64.Sun B, Herting E, Curstedt T, et al. Exogenous surfactant improves lung compliance and oxygenation in adult rats with meconium aspiration. J Appl Physiol. 1994;77:1961–71. doi: 10.1152/jappl.1994.77.4.1961. [DOI] [PubMed] [Google Scholar]
- 65.Zagariya A, Bhat R, Navale S, et al. Inhibition of meconiuminduced cytokine expression and cell apoptosis by pretreatment with captopril. Pediatrics. 2006;117:1722–7. doi: 10.1542/peds.2005-0274. [DOI] [PubMed] [Google Scholar]
- 66.El Shahed AI, Dargaville P, Ohlsson A, et al. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database Syst Rev. 2007:CD002054. doi: 10.1002/14651858.CD002054.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Holopainen R, Aho H, Laine J, et al. Human meconium has high phospholipase A2 activity and induces cellular injury and apoptosis in piglet lungs. Pediatr Res. 1999;46:626–32. doi: 10.1203/00006450-199911000-00022. [DOI] [PubMed] [Google Scholar]
- 68.Lukkarinen H, Laine J, Lehtonen J, et al. Angiotensin II receptor blockade inhibits pneumocyte apoptosis in experimental meconium aspiration. Pediatr Res. 2004;55:326–33. doi: 10.1203/01.PDR.0000100901.88697.66. [DOI] [PubMed] [Google Scholar]
- 69.Rosenfeld CR, Zagariya AM, Liu XT, et al. Meconium increases type 1 angiotensin II receptor expression and alveolar cell death. Pediatr Res. 2008;63:251–6. doi: 10.1203/PDR.0b013e318163a2b8. [DOI] [PubMed] [Google Scholar]
- 70.Zagariya A, Sierzputovska M, Navale S, et al. Role of meconium and hypoxia in meconium aspiration-induced lung injury in neonatal rabbits. Mediators Inflamm. 2010;2010:204831. doi: 10.1155/2010/204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopez A, Bildfell R. Pulmonary inflammation associated with aspirated meconium and epithelial cells in calves. Vet Pathol. 1992;29:104–11. doi: 10.1177/030098589202900202. [DOI] [PubMed] [Google Scholar]
- 72.Clark P, Duff P. Inhibition of neutrophil oxidative burst and phagocytosis by meconium. Am J Obstet Gynecol. 1995;173:1301–5. doi: 10.1016/0002-9378(95)91375-0. [DOI] [PubMed] [Google Scholar]
- 73.de Beaufort AJ, Pelikan DM, Elferink JG, et al. Effect of interleukin 8 in meconium on in-vitro neutrophil chemotaxis. Lancet. 1998;352:102–5. doi: 10.1016/S0140-6736(98)85013-7. [DOI] [PubMed] [Google Scholar]
- 74.Kytola J, Uotila P, Kaapa P. Meconium stimulates cyclooxygenase-2 expression in rat lungs. Prostaglandins Leukot Essent Fatty Acids. 1999;60:107–10. doi: 10.1054/plef.1998.0015. [DOI] [PubMed] [Google Scholar]
- 75.Tamura DY, Moore EE, Partrick DA, et al. Acute hypoxemia in humans enhances the neutrophil inflammatory response. Shock. 2002;17:269–73. doi: 10.1097/00024382-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 76.de Beaufort AJ, Bakker AC, van Tol MJ, et al. Meconium is a source of pro-inflammatory substances and can induce cytokine production in cultured A549 epithelial cells. Pediatr Res. 2003;54:491–5. doi: 10.1203/01.PDR.0000082017.97479.39. [DOI] [PubMed] [Google Scholar]
- 77.Kytola J, Kaapa P, Uotila P. Meconium aspiration stimulates cyclooxygenase-2 and nitric oxide synthase-2 expression in rat lungs. Pediatr Res. 2003;53:731–6. doi: 10.1203/01.PDR.0000059222.68800.1B. [DOI] [PubMed] [Google Scholar]
- 78.Lindenskov PH, Castellheim A, Aamodt G, et al. Complement activation reflects severity of meconium aspiration syndrome in newborn pigs. Pediatr Res. 2004;56:810–17. doi: 10.1203/01.PDR.0000141983.32466.2A. [DOI] [PubMed] [Google Scholar]
- 79.Castellheim A, Lindenskov PH, Pharo A, et al. Meconium is a potent activator of complement in human serum and in piglets. Pediatr Res. 2004;55:310–18. doi: 10.1203/01.PDR.0000100902.76021.8E. [DOI] [PubMed] [Google Scholar]
- 80.Zagariya A, Bhat R, Chari G, et al. Apoptosis of airway epithelial cells in response to meconium. Life Sci. 2005;76:1849–58. doi: 10.1016/j.lfs.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 81.Castellheim A, Lindenskov PH, Pharo A, et al. Meconium aspiration syndrome induces complement-associated systemic inflammatory response in newborn piglets. Scand J Immunol. 2005;61:217–25. doi: 10.1111/j.1365-3083.2005.01532.x. [DOI] [PubMed] [Google Scholar]
- 82.Mollnes TE, Castellheim A, Lindenskov PH, et al. The role of complement in meconium aspiration syndrome. J Perinatol. 2008;28:S116–19. doi: 10.1038/jp.2008.148. [DOI] [PubMed] [Google Scholar]
- 83.Castellheim A, Pharo A, Fung M, et al. Complement C5a is a key mediator of meconium-induced neutrophil activation. Pediatr Res. 2005;57:242–7. doi: 10.1203/01.PDR.0000150725.78971.30. [DOI] [PubMed] [Google Scholar]
- 84.Okazaki K, Kondo M, Kato M, et al. Serum cytokine and chemokine profiles in neonates with meconium aspiration syndrome. Pediatrics. 2008;121:e748–53. doi: 10.1542/peds.2007-1697. [DOI] [PubMed] [Google Scholar]
- 85.Salvesen B, Fung M, Saugstad OD, et al. Role of complement and CD14 in meconium-induced cytokine formation. Pediatrics. 2008;121:e496–505. doi: 10.1542/peds.2007-0878. [DOI] [PubMed] [Google Scholar]
- 86.Cayabyab RG, Kwong K, Jones C, et al. Lung inflammation and pulmonary function in infants with meconium aspiration syndrome. Pediatr Pulmonol. 2007;42:898–905. doi: 10.1002/ppul.20675. [DOI] [PubMed] [Google Scholar]
- 87.Salvesen B, Nielsen EW, Harboe M, et al. Mechanisms of complement activation and effects of C1-inhibitor on the meconium-induced inflammatory reaction in human cord blood. Mol Immunol. 2009;46:688–94. doi: 10.1016/j.molimm.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Pulkkinen MO, Eskola J, Kleimola V, et al. Pancreatic and catalytic phospholipase A2 in relation to pregnancy, labor and fetal outcome. Gynecol Obstet Invest. 1990;29:104–7. doi: 10.1159/000293312. [DOI] [PubMed] [Google Scholar]
- 89.Hite RD, Seeds MC, Jacinto RB, et al. Hydrolysis of surfactant associated phosphatidylcholine by mammalian secretory phospholipases A2. Am J Physiol. 1998;275:L740–7. doi: 10.1152/ajplung.1998.275.4.L740. [DOI] [PubMed] [Google Scholar]
- 90.Schrama AJ, de Beaufort AJ, Sukul YR, et al. Phospholipase A2 is present in meconium and inhibits the activity of pulmonary surfactant: an in vitro study. Acta Paediatr. 2001;90:412–16. [PubMed] [Google Scholar]
- 91.Kaapa P. Meconium aspiration syndrome: a role for phospholipase A2 in the pathogenesis? Acta Paediatr. 2001;90:365–7. [PubMed] [Google Scholar]
- 92.Granata F, Petraroli A, Boilard E, et al. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J Immunol. 2005;174:464–74. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
- 93.Sippola T, Aho H, Peuravuori H, et al. Pancreatic phospholipase A2 contributes to lung injury in experimental meconium aspiration. Pediatr Res. 2006;59:641–5. doi: 10.1203/01.pdr.0000214685.31232.6a. [DOI] [PubMed] [Google Scholar]
- 94.Kaapa P, Soukka H. Phospholipase A2 in meconium-induced lung injury. J Perinatol. 2008;28:S120–2. doi: 10.1038/jp.2008.147. [DOI] [PubMed] [Google Scholar]
- 95.De Luca D, Capoluongo E, Rigo V. Secretory phospholipase A2 pathway in various types of lung injury in neonates and infants:a multicentre translational study. BMC Pediatr. 2011;11:101. doi: 10.1186/1471-2431-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pruzanski W, Vadas P, Fornasier V. Inflammatory effect of intradermal administration of soluble phospholipase A2 in rabbits. J Invest Dermatol. 1986;86:380–3. doi: 10.1111/1523-1747.ep12285639. [DOI] [PubMed] [Google Scholar]
- 97.Niewoehner DE, Rice K, Duane P, et al. Induction of alveolar epithelial injury by phospholipase A2. J Appl Physiol. 1989;66:261–7. doi: 10.1152/jappl.1989.66.1.261. [DOI] [PubMed] [Google Scholar]
- 98.Tocker JE, Durham SK, Welton AF, et al. Phospholipase A2-induced pulmonary and hemodynamic responses in the guinea pig. Effects of enzyme inhibitors and mediators antagonists. Am Rev Respir Dis. 1990;142:1193–9. doi: 10.1164/ajrccm/142.5.1193. [DOI] [PubMed] [Google Scholar]
- 99.Murakami M, Hara N, Kudo I, et al. Triggering of degranulation in mast cells by exogenous type II phospholipase A2. J Immunol. 1993;151:5675–84. [PubMed] [Google Scholar]
- 100.Kim DK, Fukuda T, Thompson BT, et al. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am J Physiol. 1995;269:L109–18. doi: 10.1152/ajplung.1995.269.1.L109. [DOI] [PubMed] [Google Scholar]
- 101.Takasaki J, Kawauchi Y, Yasunaga T, et al. Human type II phospholipase A2-induced Mac-1 expression on human neutrophils. J Leukoc Biol. 1996;60:174–80. doi: 10.1002/jlb.60.2.174. [DOI] [PubMed] [Google Scholar]
- 102.Triggiani M, Granata F, Oriente A, et al. Secretory phospholipases A2 induce beta-glucuronidase release and IL-6 production from human lung macrophages. J Immunol. 2000;164:4908–15. doi: 10.4049/jimmunol.164.9.4908. [DOI] [PubMed] [Google Scholar]
- 103.Fonteh AN, Marion CR, Barham BJ, et al. Enhancement of mast cell survival: a novel function of some secretory phospholipase A(2) isotypes. J Immunol. 2001;167:4161–71. doi: 10.4049/jimmunol.167.8.4161. [DOI] [PubMed] [Google Scholar]
- 104.Silliman CC, Moore EE, Zallen G, et al. Presence of the M-type sPLA(2) receptor on neutrophils and its role in elastase release and adhesion. Am J Physiol Cell Physiol. 2002;283:C1102–13. doi: 10.1152/ajpcell.00608.2001. [DOI] [PubMed] [Google Scholar]
- 105.Triggiani M, Granata F, Oriente A, et al. Secretory phospholipases A2 induce cytokine release from blood and synovial fluid monocytes. Eur J Immunol. 2002;32:67–76. doi: 10.1002/1521-4141(200201)32:1<67::AID-IMMU67>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 106.Fuentes L, Hernandez M, Fernandez-Aviles FJ, et al. Cooperation between secretory phospholipase A2 and TNF-receptor superfamily signaling: implications for the inflammatory response in atherogenesis. Circ Res. 2002;91:681–8. doi: 10.1161/01.res.0000038341.34243.64. [DOI] [PubMed] [Google Scholar]
- 107.Beck G, Yard BA, Schulte J, et al. Secreted phospholipases A2 induce the expression of chemokines in microvascular endothelium. Biochem Biophys Res Commun. 2003;300:731–7. doi: 10.1016/s0006-291x(02)02920-0. [DOI] [PubMed] [Google Scholar]
- 108.Jo EJ, Lee HY, Lee YN, et al. Group IB secretory phospholipase A2 stimulates CXC chemokine ligand 8 production via ERK and NF-kappa B in human neutrophils. J Immunol. 2004;173:6433–9. doi: 10.4049/jimmunol.173.10.6433. [DOI] [PubMed] [Google Scholar]
- 109.Ramoner R, Putz T, Gander H, et al. Dendritic-cell activation by secretory phospholipase A2. Blood. 2005;105:3583–7. doi: 10.1182/blood-2004-08-3001. [DOI] [PubMed] [Google Scholar]
- 110.Triggiani M, Granata F, Frattini A, et al. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta. 2006;1761:1289–300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 111.Granata F, Frattini A, Loffredo S, et al. Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. Eur J Immunol. 2006;36:1938–50. doi: 10.1002/eji.200535567. [DOI] [PubMed] [Google Scholar]
- 112.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 113.Holm BA, Keicher L, Liu MY, et al. Inhibition of pulmonary surfactant function by phospholipases. J Appl Physiol. 1991;71:317–21. doi: 10.1152/jappl.1991.71.1.317. [DOI] [PubMed] [Google Scholar]
- 114.Arbibe L, Koumanov K, Vial D, et al. Generation of lysophospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J Clin Invest. 1998;102:1152–60. doi: 10.1172/JCI3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Luca D, Minucci A, Zecca E, et al. Bile acids cause secretory phospholipase A2 activity enhancement, revertible by exogenous surfactant administration. Intensive Care Med. 2009;35:321–6. doi: 10.1007/s00134-008-1321-3. [DOI] [PubMed] [Google Scholar]
- 116.Romero R, Yoon BH, Chaemsaithong P, et al. Bacteria and endotoxin in meconium-stained amniotic fluid at term: could intra-amniotic infection cause meconium passage? J Matern Fetal Neonatal Med. 2014 May;27:775–88. doi: 10.3109/14767058.2013.844124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stoner CR, Reik LM, Donohue M, et al. Human group II phospholipase A2. Characterization of monoclonal antibodies and immunochemical quantitation of the protein in synovial fluid. J Immunol Methods. 1991;145:127–36. doi: 10.1016/0022-1759(91)90318-a. [DOI] [PubMed] [Google Scholar]
- 118.Romero R, Brandt F, Sepulveda W, et al. Extracellular phospholipase A2 in term and preterm parturition; 39th Annual Meeting of the Society for Gynecologic Investigation: March 18–21, 1992: scientific program and abstracts: San Antonio Marriott Rivercenter; San Antonio, Texas. Abstract 278. [Google Scholar]
- 119.Lisowska-Myjak B, Pachecka J. Trypsin and antitrypsin activities and protein concentration in serial meconium and feces of healthy newborns. J Matern Fetal Neonatal Med. 2006;19:477–82. doi: 10.1080/14767050600746720. [DOI] [PubMed] [Google Scholar]
- 120.Verheij HM, Slotboom AJ, de Haas GH. Structure and function of phospholipase A2. Rev Physiol Biochem Pharmacol. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- 121.Dennis EA. The enzymes. New York: Academic Press; 1983. [Google Scholar]
- 122.Vadas P, Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest. 1986;55:391–404. [PubMed] [Google Scholar]
- 123.Waite M. The phospholipases. New York: Plenum Press; 1987. [Google Scholar]
- 124.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–60. [PubMed] [Google Scholar]
- 125.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 126.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A(2) and their receptors and binding proteins. Biochim Biophys Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 127.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 128.Murakami M, Kudo I. Phospholipase A2. J Biochem. 2002;131:285–92. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 129.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–59. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 130.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50:S237–42. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murakami M, Taketomi Y, Girard C, et al. Emerging roles of secreted phospholipase A2 enzymes: lessons from transgenic and knockout mice. Biochimie. 2010;92:561–82. doi: 10.1016/j.biochi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 132.Boyanovsky BB, Webb NR. Biology of secretory phospholipase A2. Cardiovasc Drugs Ther. 2009;23:61–72. doi: 10.1007/s10557-008-6134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murakami M, Taketomi Y, Sato H, et al. Secreted phospholipase A2 revisited. J Biochem. 2011;150:233–55. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- 134.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murakami M, Taketomi Y, Miki Y, et al. Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog Lipid Res. 2011;50:152–92. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 136.Wiqvist N, Bygdeman M, Green K, et al. Endogenous prostaglandins and the initiation of labor. Acta Obstet Gynecol Scand Suppl. 1974;37:7–16. doi: 10.3109/00016347409156407. [DOI] [PubMed] [Google Scholar]
- 137.Schwarz BE, Schultz FM, Macdonald PC, et al. Initiation of human parturition. III. Fetal membrane content of prostaglandin E2 and F2alpha precursor. Obstet Gynecol. 1975;46:564–8. [PubMed] [Google Scholar]
- 138.Csapo AI. Prostaglandins and the initiation of labor. Prostaglandins. 1976;12:149–64. doi: 10.1016/s0090-6980(76)80013-5. [DOI] [PubMed] [Google Scholar]
- 139.Kinoshita K, Satoh K, Sakamoto S. Prostaglandin F2alpha and E1 in plasma and amniotic fluid during human pregnancy and labor. Endocrinol Jpn. 1977;24:155–62. doi: 10.1507/endocrj1954.24.155. [DOI] [PubMed] [Google Scholar]
- 140.Liggins GC, Forster CS, Grieves SA, et al. Control of parturition in man. Biol Reprod. 1977;16:39–56. doi: 10.1095/biolreprod16.1.39. [DOI] [PubMed] [Google Scholar]
- 141.Mitchell MD, Flint AP. Progesterone withdrawal: effects on prostaglandins and parturition. Prostaglandins. 1977;14:611–14. doi: 10.1016/0090-6980(77)90279-9. [DOI] [PubMed] [Google Scholar]
- 142.Mitchell MD, Bibby JG, Hicks BR, et al. Possible role for prostacyclin in human parturition. Prostaglandins. 1978;16:931–7. doi: 10.1016/0090-6980(78)90108-9. [DOI] [PubMed] [Google Scholar]
- 143.Liggins GC. Initiation of parturition. Br Med Bull. 1979;35:145–50. doi: 10.1093/oxfordjournals.bmb.a071561. [DOI] [PubMed] [Google Scholar]
- 144.Okita JR, MacDonald PC, Johnston JM. Initiation of human parturition. Am J Obstet Gynecol. 1982;142:432–5. doi: 10.1016/s0002-9378(16)32385-7. [DOI] [PubMed] [Google Scholar]
- 145.Mitchell MD. Mechanisms of human parturition: role of prostaglandins and related compounds. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:613–15. [PubMed] [Google Scholar]
- 146.Okazaki T, Sagawa N, Ban C, et al. Regulation of prostaglandin formation during human parturition. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:617–18. [PubMed] [Google Scholar]
- 147.Jenkins DM. Prostaglandins and parturition. Lancet. 1985;2:163. doi: 10.1016/s0140-6736(85)90276-4. [DOI] [PubMed] [Google Scholar]
- 148.Romero R, Mazor M, Wu YK, et al. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids. 1989;37:183–6. doi: 10.1016/0952-3278(89)90083-5. [DOI] [PubMed] [Google Scholar]
- 149.Romero R, Wu YK, Mazor M, et al. Amniotic fluid concentration of 5-hydroxyeicosatetraenoic acid is increased in human parturition at term. Prostaglandins Leukot Essent Fatty Acids. 1989;35:81–3. doi: 10.1016/0952-3278(89)90169-5. [DOI] [PubMed] [Google Scholar]
- 150.Romero R, Wu YK, Sirtori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins. 1989;37:149–61. doi: 10.1016/0090-6980(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 151.Walsh SW. 5-Hydroxyeicosatetraenoic acid, leukotriene C4, and prostaglandin F2 alpha in amniotic fluid before and during term and preterm labor. Am J Obstet Gynecol. 1989;161:1352–60. doi: 10.1016/0002-9378(89)90696-0. [DOI] [PubMed] [Google Scholar]
- 152.Lundin-Schiller S, Mitchell MD. The role of prostaglandins in human parturition. Prostaglandins Leukot Essent Fatty Acids. 1990;39:1–10. doi: 10.1016/0952-3278(90)90164-g. [DOI] [PubMed] [Google Scholar]
- 153.Dowling DD, Romero RJ, Mitchell MD, et al. Isolation of multiple substances in amniotic fluid that regulate amnion prostaglandin E2 production: the effects of gestational age and labor. Prostaglandins Leukot Essent Fatty Acids. 1991;44:253–5. doi: 10.1016/0952-3278(91)90026-2. [DOI] [PubMed] [Google Scholar]
- 154.Romero R, Baumann P, Gonzalez R, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol. 1994;171:1613–20. doi: 10.1016/0002-9378(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 155.Romero R, Gonzalez R, Baumann P, et al. Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot Essent Fatty Acids. 1994;50:97–104. doi: 10.1016/0952-3278(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 156.Neulen J, Breckwoldt M. Placental progesterone, prostaglandins and mechanisms leading to initiation of parturition in the human. Exp Clin Endocrinol. 1994;102:195–202. doi: 10.1055/s-0029-1211283. [DOI] [PubMed] [Google Scholar]
- 157.Mitchell MD, Romero RJ, Edwin SS, et al. Prostaglandins and parturition. Reprod Fertil Dev. 1995;7:623–32. doi: 10.1071/rd9950623. [DOI] [PubMed] [Google Scholar]
- 158.Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids. 1996;54:187–91. doi: 10.1016/s0952-3278(96)90015-0. [DOI] [PubMed] [Google Scholar]
- 159.Gomez R, Romero R, Edwin SS, et al. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–76. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 160.Romero R, Gotsch F, Pineles B, et al. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 161.Mitchell MD, Chang MC, Chaiworapongsa T, et al. Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J Clin Endocrinol Metab. 2005;90:4244–8. doi: 10.1210/jc.2004-2496. [DOI] [PubMed] [Google Scholar]
- 162.Lee SE, Romero R, Park IS, et al. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med. 2008;21:89–94. doi: 10.1080/14767050701830514. [DOI] [PubMed] [Google Scholar]
- 163.Kamel RM. The onset of human parturition. Arch Gynecol Obstet. 2010;281:975–82. doi: 10.1007/s00404-010-1365-9. [DOI] [PubMed] [Google Scholar]
- 164.Menon R, Fortunato SJ, Milne GL, et al. Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol. 2011;118:121–34. doi: 10.1097/AOG.0b013e3182204eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Romero R, Emamian M, Wan M, et al. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1461–7. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 166.Romero R, Wu YK, Mazor M, et al. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1988;34:141–5. doi: 10.1016/0952-3278(88)90137-8. [DOI] [PubMed] [Google Scholar]
- 167.Romero R, Wu YK, Mazor M, et al. Amniotic fluid arachidonate lipoxygenase metabolites in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1989;36:69–75. doi: 10.1016/0952-3278(89)90020-3. [DOI] [PubMed] [Google Scholar]
- 168.Hanna N, Bonifacio L, Weinberger B, et al. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol. 2006;55:19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 169.Hack CE, Wolbink GJ, Schalkwijk C, et al. A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol Today. 1997;18:111–15. doi: 10.1016/s0167-5699(97)01002-5. [DOI] [PubMed] [Google Scholar]
- 170.Uozumi N, Kume K, Nagase T, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–22. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 171.Buckland AG, Wilton DC. The antibacterial properties of secreted phospholipases A(2) Biochim Biophys Acta. 2000;1488:71–82. doi: 10.1016/s1388-1981(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 172.Crowl RM, Stoller TJ, Conroy RR, et al. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J Biol Chem. 1991;266:2647–51. [PubMed] [Google Scholar]
- 173.Slotboom AJ, van Dam-Mieras MC, Jansen EH, et al. Relationship between structure and activity of pancreatic phospholipase A2. Adv Exp Med Biol. 1978;101:137–52. doi: 10.1007/978-1-4615-9071-2_13. [DOI] [PubMed] [Google Scholar]
- 174.Rosenthal MD, Gordon MN, Buescher ES, et al. Human neutrophils store type II 14-kDa phospholipase A2 in granules and secrete active enzyme in response to soluble stimuli. Biochem Biophys Res Commun. 1995;208:650–6. doi: 10.1006/bbrc.1995.1388. [DOI] [PubMed] [Google Scholar]
- 175.Blom M, Tool AT, Wever PC, et al. Human eosinophils express, relative to other circulating leukocytes, large amounts of secretory 14-kD phospholipase A2. Blood. 1998;91:3037–43. [PubMed] [Google Scholar]
- 176.Triggiani M, Granata F, Balestrieri B, et al. Secretory phospholipases A2 activate selective functions in human eosinophils. J Immunol. 2003;170:3279–88. doi: 10.4049/jimmunol.170.6.3279. [DOI] [PubMed] [Google Scholar]
- 177.Asaoka Y, Yoshida K, Sasaki Y, et al. Possible role of mammalian secretory group II phospholipase A2 in T-lymphocyte activation: implication in propagation of inflammatory reaction. Proc Natl Acad Sci U S A. 1993;90:716–19. doi: 10.1073/pnas.90.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ho IC, Arm JP, Bingham CO, 3rd, et al. A novel group of phospholipase A2s preferentially expressed in type 2 helper T cells. J Biol Chem. 2001;276:18321–6. doi: 10.1074/jbc.M008837200. [DOI] [PubMed] [Google Scholar]
- 179.Inada M, Tojo H, Kawata S, et al. Preferential distribution of group-II-like phospholipase A2 in mononuclear phagocytic cells in rat spleen and liver. Eur J Biochem. 1991;197:323–9. doi: 10.1111/j.1432-1033.1991.tb15914.x. [DOI] [PubMed] [Google Scholar]
- 180.Sipka S, Farkas T, Gergely P, et al. Secretion of phospholipase A2 induced by interactions of human platelets with monocytes. Ann Hematol. 1994;69:307–10. doi: 10.1007/BF01696560. [DOI] [PubMed] [Google Scholar]
- 181.Hidi R, Vargaftig BB, Touqui L. Increased synthesis and secretion of a 14-kDa phospholipase A2 by guinea pig alveolar macrophages. Dissociation from arachidonic acid liberation and modulation by dexamethasone. J Immunol. 1993;151:5613–23. [PubMed] [Google Scholar]
- 182.Fonteh AN, Bass DA, Marshall LA, et al. Evidence that secretory phospholipase A2 plays a role in arachidonic acid release and eicosanoid biosynthesis by mast cells. J Immunol. 1994;152:5438–46. [PubMed] [Google Scholar]
- 183.Kramer RM, Hession C, Johansen B, et al. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989;264:5768–75. [PubMed] [Google Scholar]
- 184.Hara S, Kudo I, Chang HW, et al. Purification and characterization of extracellular phospholipase A2 from human synovial fluid in rheumatoid arthritis. J Biochem. 1989;105:395–9. doi: 10.1093/oxfordjournals.jbchem.a122675. [DOI] [PubMed] [Google Scholar]
- 185.Seilhamer JJ, Pruzanski W, Vadas P, et al. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989;264:5335–8. [PubMed] [Google Scholar]
- 186.Fierlbeck G, Rassner G, Muller C. Psoriasis induced at the injection site of recombinant interferon gamma. Results of immunohistologic investigations. Arch Dermatol. 1990;126:351–5. [PubMed] [Google Scholar]
- 187.Kortekangas P, Aro HT, Nevalainen TJ. Group II phospholipase A2 in synovial fluid and serum in acute arthritis. Scand J Rheumatol. 1994;23:68–72. doi: 10.3109/03009749409103030. [DOI] [PubMed] [Google Scholar]
- 188.Bidgood MJ, Jamal OS, Cunningham AM, et al. Type IIA secretory phospholipase A2 up-regulates cyclooxygenase-2 and amplifies cytokine-mediated prostaglandin production in human rheumatoid synoviocytes. J Immunol. 2000;165:2790–7. doi: 10.4049/jimmunol.165.5.2790. [DOI] [PubMed] [Google Scholar]
- 189.Vadas P. Elevated plasma phospholipase A2 levels: correlation with the hemodynamic and pulmonary changes in gram-negative septic shock. J Lab Clin Med. 1984;104:873–81. [PubMed] [Google Scholar]
- 190.Haapamaki MM, Gronroos JM, Nurmi H, et al. Elevated group II phospholipase A2 mass concentration in serum and colonic mucosa in Crohn’s disease. Clin Chem Lab Med. 1998;36:751–5. doi: 10.1515/CCLM.1998.133. [DOI] [PubMed] [Google Scholar]
- 191.Haapamaki MM, Gronroos JM, Nurmi H, et al. Phospholipase A2 in serum and colonic mucosa in ulcerative colitis. Scand J Clin Lab Invest. 1999;59:279–87. doi: 10.1080/00365519950185643. [DOI] [PubMed] [Google Scholar]
- 192.Nevalainen TJ, Gronroos JM, Kortesuo PT. Pancreatic and synovial type phospholipases A2 in serum samples from patients with severe acute pancreatitis. Gut. 1993;34:1133–6. doi: 10.1136/gut.34.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kemppainen E, Hietaranta A, Puolakkainen P, et al. Bactericidal/permeability-increasing protein and group I and II phospholipase A2 during the induction phase of human acute pancreatitis. Pancreas. 1999;18:21–7. doi: 10.1097/00006676-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 194.Miura M, Endo S, Kaku LL, et al. Plasma type II phospholipase A2 levels in patients with acute pancreatitis. Res Commun Mol Pathol Pharmacol. 2001;109:159–64. [PubMed] [Google Scholar]
- 195.Lin MK, Farewell V, Vadas P, et al. Secretory phospholipase A2 as an index of disease activity in rheumatoid arthritis. Prospective double blind study of 212 patients. J Rheumatol. 1996;23:1162–6. [PubMed] [Google Scholar]
- 196.Elsbach P, Weiss J, Franson RC, et al. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979;254:11000–9. [PubMed] [Google Scholar]
- 197.Harwig SS, Tan L, Qu XD, et al. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95:603–10. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Weinrauch Y, Abad C, Liang NS, et al. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Invest. 1998;102:633–8. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Laine VJ, Grass DS, Nevalainen TJ. Resistance of transgenic mice expressing human group II phospholipase A2 to Escherichia coli infection. Infect Immun. 2000;68:87–92. doi: 10.1128/iai.68.1.87-92.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Beers SA, Buckland AG, Koduri RS, et al. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem. 2002;277:1788–93. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 201.Koprivnjak T, Peschel A, Gelb MH, et al. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J Biol Chem. 2002;277:47636–44. doi: 10.1074/jbc.M205104200. [DOI] [PubMed] [Google Scholar]
- 202.Koduri RS, Gronroos JO, Laine VJ, et al. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A(2) J Biol Chem. 2002;277:5849–57. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 203.Gimenez AP, Wu YZ, Paya M, et al. High bactericidal efficiency of type iia phospholipase A2 against Bacillus anthracis and inhibition of its secretion by the lethal toxin. J Immunol. 2004;173:521–30. doi: 10.4049/jimmunol.173.1.521. [DOI] [PubMed] [Google Scholar]
- 204.Piris-Gimenez A, Paya M, Lambeau G, et al. In vivo protective role of human group IIa phospholipase A2 against experimental anthrax. J Immunol. 2005;175:6786–91. doi: 10.4049/jimmunol.175.10.6786. [DOI] [PubMed] [Google Scholar]
- 205.Femling JK, Nauseef WM, Weiss JP. Synergy between extracellular group IIA phospholipase A2 and phagocyte NADPH oxidase in digestion of phospholipids of Staphylococcus aureus ingested by human neutrophils. J Immunol. 2005;175:4653–61. doi: 10.4049/jimmunol.175.7.4653. [DOI] [PubMed] [Google Scholar]
- 206.Huhtinen HT, Gronroos JO, Gronroos JM, et al. Antibacterial effects of human group IIA and group XIIA phospholipase A2 against Helicobacter pylori in vitro. APMIS. 2006;114:127–30. doi: 10.1111/j.1600-0463.2006.apm_330.x. [DOI] [PubMed] [Google Scholar]
- 207.Sitkiewicz I, Stockbauer KE, Musser JM. Secreted bacterial phospholipase A2 enzymes: better living through phospholipolysis. Trends Microbiol. 2007;15:63–9. doi: 10.1016/j.tim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 208.Weinrauch Y, Elsbach P, Madsen LM, et al. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996;97:250–7. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gronroos JO, Laine VJ, Nevalainen TJ. Bactericidal group IIA phospholipase A2 in serum of patients with bacterial infections. J Infect Dis. 2002;185:1767–72. doi: 10.1086/340821. [DOI] [PubMed] [Google Scholar]
- 210.Fenard D, Lambeau G, Maurin T, et al. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol Pharmacol. 2001;60:341–7. doi: 10.1124/mol.60.2.341. [DOI] [PubMed] [Google Scholar]
- 211.Mitsuishi M, Masuda S, Kudo I, et al. Group V and X secretory phospholipase A2 prevents adenoviral infection in mammalian cells. Biochem J. 2006;393:97–106. doi: 10.1042/BJ20050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim JO, Chakrabarti BK, Guha-Niyogi A, et al. Lysis of human immunodeficiency virus type 1 by a specific secreted human phospholipase A2. J Virol. 2007;81:1444–50. doi: 10.1128/JVI.01790-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Deregnaucourt C, Schrevel J. Bee venom phospholipase A2 induces stage-specific growth arrest of the intraerythrocytic Plasmodium falciparum via modifications of human serum components. J Biol Chem. 2000;275:39973–80. doi: 10.1074/jbc.M006712200. [DOI] [PubMed] [Google Scholar]
- 214.Aho HJ, Saari KM, Kallajoki M, et al. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest Ophthalmol Vis Sci. 1996;37:1826–32. [PubMed] [Google Scholar]
- 215.Saari KM, Aho V, Paavilainen V, et al. Group II PLA(2) content of tears in normal subjects. Invest Ophthalmol Vis Sci. 2001;42:318–20. [PubMed] [Google Scholar]
- 216.Nevalainen TJ, Meri KM, Niemi M. Synovial-type (group II) phospholipase A2 human seminal plasma. Andrologia. 1993;25:355–8. doi: 10.1111/j.1439-0272.1993.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 217.Nevalainen TJ, Haapanen TJ. Distribution of pancreatic (group I) and synovial-type (group II) phospholipases A2 in human tissues. Inflammation. 1993;17:453–64. doi: 10.1007/BF00916585. [DOI] [PubMed] [Google Scholar]
- 218.Qu XD, Lloyd KC, Walsh JH, et al. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect Immun. 1996;64:5161–5. doi: 10.1128/iai.64.12.5161-5165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nevalainen TJ, Eerola LI, Rintala E, et al. Time-resolved fluoroimmunoassays of the complete set of secreted phospholipases A2 in human serum. Biochim Biophys Acta. 2005;1733:210–23. doi: 10.1016/j.bbalip.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 220.Henderson WR, Jr, Chi EY, Bollinger JG, et al. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–77. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schwarz BE, Schultz FM, MacDonald PC, et al. Initiation of human parturition. IV. Demonstration of phospholipase A2 in the lysosomes of human fetal membranes. Am J Obstet Gynecol. 1976;125:1089–92. [PubMed] [Google Scholar]
- 222.Gustavii B. The distribution within the placenta, myometrium, and decidua of 24Na-labelled hypertonic solution following intraamniotic or extra-amniotic injection. Br J Obstet Gynaecol. 1975;82:734–9. doi: 10.1111/j.1471-0528.1975.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 223.Akesson B, Gustavii B. Occurrence of phospholipase A1 and A2 in human decidua. Prostaglandins. 1975;9:667–73. doi: 10.1016/0090-6980(75)90106-9. [DOI] [PubMed] [Google Scholar]
- 224.Gebhardt DD, Beintema A, Reman FC, et al. Phospholipase-A2 in amniotic fluid. Lancet. 1978;2:1159. doi: 10.1016/s0140-6736(78)92326-7. [DOI] [PubMed] [Google Scholar]
- 225.Gebhardt DO, Beintema A, Reman FC, et al. The lipoprotein composition of amniotic fluid. Clin Chim Acta. 1979;94:93–100. doi: 10.1016/0009-8981(79)90190-6. [DOI] [PubMed] [Google Scholar]
- 226.Farrugia W, Aitken MA, van Dunne F, et al. Type II phospholipase A2 in human gestational tissues: subcellular distribution of placental immuno- and catalytic activity. Biochim Biophys Acta. 1993;1166:77–83. doi: 10.1016/0005-2760(93)90286-i. [DOI] [PubMed] [Google Scholar]
- 227.Aitken MA, Farrugia W, Wong MH, et al. Type II phospholipase A2 in human gestational tissues: extractable immuno- and enzymatic activity in fetal membranes. Biochim Biophys Acta. 1993;1170:314–20. doi: 10.1016/0005-2760(93)90016-3. [DOI] [PubMed] [Google Scholar]
- 228.Farrugia W, Rice GE, Wong MH, et al. Release of Type II phospholipase A2 immunoreactivity and phospholipase A2 enzymatic activity from human placenta. J Endocrinol. 1997;153:151–7. doi: 10.1677/joe.0.1530151. [DOI] [PubMed] [Google Scholar]
- 229.Koyama M, Ito S, Nakajima A, et al. Elevations of group II phospholipase A2 concentrations in serum and amniotic fluid in association with preterm labor. Am J Obstet Gynecol. 2000;183:1537–43. doi: 10.1067/mob.2000.107789. [DOI] [PubMed] [Google Scholar]