Abstract
Over the past few decades, the large, international, randomized controlled trials of anticoagulant therapies for patients with sepsis have not yielded any improvement in mortality rates. However, in Japan, anticoagulant therapies are administered for sepsis patients with disseminated intravascular coagulation (DIC), but not for sepsis patients without DIC. Furthermore, epidemiological data regarding sepsis in Japan are scarce. Therefore, a nationwide multicenter retrospective observational study, the Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study, was undertaken. The JSEPTIC DIC study enrolled 42 intensive care units and included 3,195 patients with sepsis. The results of the JSEPTIC DIC study indicated the following: (i) anticoagulant therapy may be effective in sepsis‐induced DIC patients at high risk for death, (ii) recombinant human soluble thrombomodulin administration and antithrombin supplementation are associated with survival benefits in patients with sepsis‐induced DIC.
Keywords: coagulopathy, sepsis/multiple organ failure
Introduction
In cases of sepsis and septic shock, the dysregulation of systemic coagulation and fibrinolytic systems frequently leads to disseminated intravascular coagulation (DIC).1, 2, 3 Moreover, DIC often induces multiple organ failure, which is associated with a high mortality rate, owing to the development of microthrombi that cause tissue hypoperfusion.1, 2, 3
Over the past few decades, although some anticoagulant agents, such as antithrombin (AT), tissue factor pathway inhibitor, and activated protein C, have been investigated for use in patients by international large randomized controlled trials (RCTs), no remarkable effect on the mortality rate has been reported.4, 5, 6, 7 However, the post‐hoc subgroup analyses of those RCTs indicated that the anticoagulant therapies resulted in improved mortality rates in patients with sepsis‐induced DIC.8, 9 In Japan, anticoagulant therapy has been approved for use as adjunctive therapy for patients with sepsis‐induced DIC and is now widely applied in clinical settings. However, this approach is not practiced in cases of sepsis without DIC. A recent meta‐analysis of RCTs reported that the survival benefit of anticoagulant therapy was observed only in sepsis patients with DIC, but not in those without DIC.10 Furthermore, although many epidemiological studies for sepsis have been carried out,11, 12, 13, 14, 15 epidemiological data regarding sepsis in Japan is limited.16, 17
Therefore, we undertook a nationwide multicenter retrospective observational study, named the Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study, which reported the effects of anticoagulant therapy on sepsis in real world clinical settings.18, 19, 20, 21 Herein, we present a summary of the JSEPTIC DIC study.
Basic information regarding the JSEPTIC DIC study
The JSEPTIC DIC study involved 42 intensive care units (ICUs) from 40 institutions throughout Japan. The ICU characteristics are presented in Table 1. The JSEPTIC DIC study included 3,195 consecutive adult patients with severe sepsis or septic shock, which was defined on the basis of the International Sepsis Definitions Conference criteria,22 diagnosed between January 2011 and December 2013. The JSEPTIC DIC study excluded patients who were <16 years old or patients who developed severe sepsis or septic shock following ICU admission.
Table 1.
Characteristics of the intensive care units (ICUs) that participated in the Japan Septic Disseminated Intravascular Coagulation study
| n = 42 | |
|---|---|
| Hospital characteristics | |
| University hospital | 22 (52.4) |
| Other | 20 (47) |
| ICU characteristics | |
| General ICU | 24 (57.1) |
| Emergency ICU | 18 (42.9) |
| ICU policy | |
| Closed policy | 17 (40.5) |
| Open policy | 18 (42.9) |
| Other | 7 (16.7) |
| Number of beds | 11 (8–15) |
Data are presented as median (interquartile range) or n (%).
Characteristics, treatments, and outcomes of patients with sepsis treated in the ICU
The JSEPTIC DIC study included 1,916 men (60%) and 1,279 women (40%). The mean age was 70 ± 15 years. The mean Acute Physiology and Chronic Health Evaluation II score was 23 ± 9. The median Sequential Organ Failure Assessment (SOFA) score was 9 (interquartile range, 6–12). The primary infection sites are presented in Table 2. The in‐hospital mortality rate was 33%. These characteristics of the septic patients treated in the ICU were almost the same as that previously reported in Japanese published works.16, 17
Table 2.
Primary infection site responsible for the sepsis as reported by intensive care units participating in the Japan Septic Disseminated Intravascular Coagulation study
| Total n = 3,195 | |
|---|---|
| Abdomen | 1,032 (32) |
| Lung/thorax | 827 (26) |
| Urinary tract | 509 (16) |
| Bone/soft tissue | 374 (12) |
| Cardiovascular system | 68 (2) |
| Central nervous system | 63 (2) |
| Catheter‐related | 44 (1) |
| Other | 60 (2) |
| Unknown | 218 (7) |
Data are expressed as n (%).
Optimal targets of anticoagulant therapy in sepsis
We previously reported that anticoagulant therapy is significantly associated with lower in‐hospital mortality in sepsis patients with DIC, but not in sepsis patients without DIC (Fig. 1).19 We found that the associations between anticoagulant therapy and lower in‐hospital mortality were not significant, regardless of the DIC diagnosis criteria applied. We also evaluated the relationship between the effects of anticoagulant therapy and disease severity. A significant association between anticoagulant therapy and lower in‐hospital mortality was observed in high‐risk sepsis patients (SOFA score 13–17) but not in low‐risk to moderate‐risk sepsis patients (SOFA score ≤12; Fig. 2). These results indicate that anticoagulant therapies may be effective in sepsis‐induced DIC patients at high risk of death.
Figure 1.
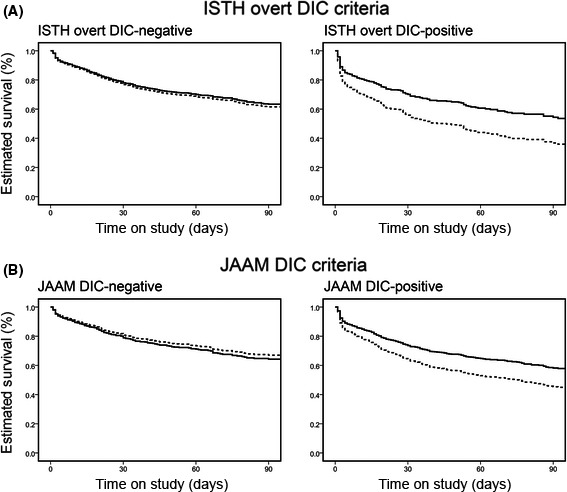
Adjusted estimated survival curves in patients with or without disseminated intravascular coagulation (DIC). A, DIC was diagnosed on the basis of the International Society on Thrombosis and Haemostasis (ISTH) criteria for overt DIC. Significant associations between anticoagulant therapy and lower in‐hospital mortality rates were observed only in patients with DIC (adjusted hazard ratio [HR], 0.609; 95% confidence interval [CI], 0.456–0.814; P = 0.001), whereas mortality rates in patients without DIC were not different regardless of anticoagulant therapy (adjusted HR, 0.941; 95% CI, 0.773–1.145; P = 0.543). B, DIC was diagnosed on the basis of the Japanese Association for Acute Medicine (JAAM) DIC criteria. Significant associations between anticoagulant therapy and lower in‐hospital mortality rates were observed only in patients with DIC (adjusted HR, 0.685; 95% CI, 0.559–0.839; P < 0.001), whereas mortality rates in patients without DIC were not different regardless of anticoagulant therapy (adjusted HR, 1.104; 95% CI, 0.839–1.453; P = 0.478). Dotted line, patients in the control group; solid line, patients in the anticoagulant group. Cited as Figure 3 in our previous report.19
Figure 2.
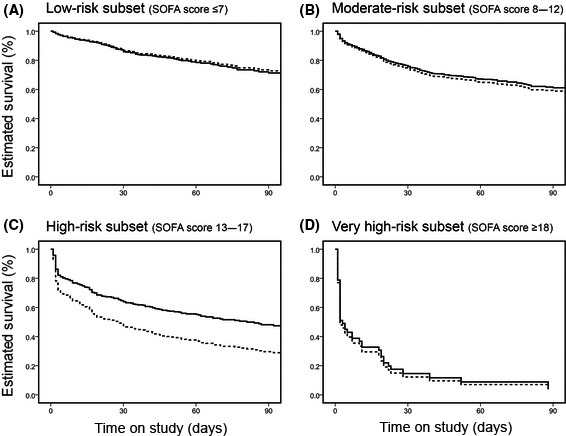
Adjusted estimated survival curves in four subsets of patients with sepsis‐induced disseminated intravascular coagulation, stratified according to baseline Sequential Organ Failure Assessment (SOFA) score. A, Low‐risk subset (SOFA score ≤7): adjusted hazard ratio [HR], 1.063; 95% confidence interval [CI], 0.716–1.580; P = 0.761. B, Moderate‐risk subset (SOFA score 8–12): adjusted HR, 0.927; 95% CI, 0.728–1.181; P = 0.540. C, High‐risk subset (SOFA score 13–17): adjusted HR 0.601; 95% CI, 0.451–0.800; P < 0.001. D, Very high‐risk subset (SOFA ≥18): adjusted HR 0.915; 95% CI, 0.418–2.003; P = 0.825. However, this analysis was not definitive because of the small sample sizes of the subsets. Dotted line, patients in the control group; solid line, patients in the anticoagulant group. Cited as Figure 4 in our previous report.19
Recombinant human soluble thrombomodulin
Recombinant human soluble thrombomodulin (rhTM), like AT, is frequently used as an anticoagulant for treating DIC.18, 23 Thrombomodulin is a receptor of thrombin and protein C on the endothelial cell surface and plays an important role in the regulation of coagulation and the innate immune system.24 Recombinant human soluble thrombomodulin was developed and approved in Japan in 2008 for treating patients with DIC.25 However, there is very limited clinical evidence supporting the use of rhTM in patients with sepsis‐induced DIC. Therefore, we evaluated the effect of rhTM treatment on sepsis‐induced DIC using a propensity score analysis for the dataset of the JSEPTIC DIC study.20
We selected 1,784 patients with sepsis‐induced DIC from the 3,195 patients in the JSEPTIC DIC study. Among these patients, 645 were given rhTM (rhTM group) and 1,139 patients were not (control group). Propensity score matching yielded 452 matched pairs, after which the characteristics and therapeutic interventions of the two groups were appropriately balanced. Lower in‐hospital all‐cause mortality was significantly associated with rhTM administration according to the three propensity score analyses (propensity score matching, inverse probability of treatment weighting, and quintile‐stratified analyses). Survival time analysis revealed a higher survival rate in the propensity score‐matched rhTM group than in the propensity score‐matched control group (hazard ratio, 0.781; 95% confidence interval, 0.624–0.977; P = 0.03) (Fig. 3).20 These results revealed that rhTM administration was associated with reduced in‐hospital mortality among patients with sepsis‐induced DIC.
Figure 3.
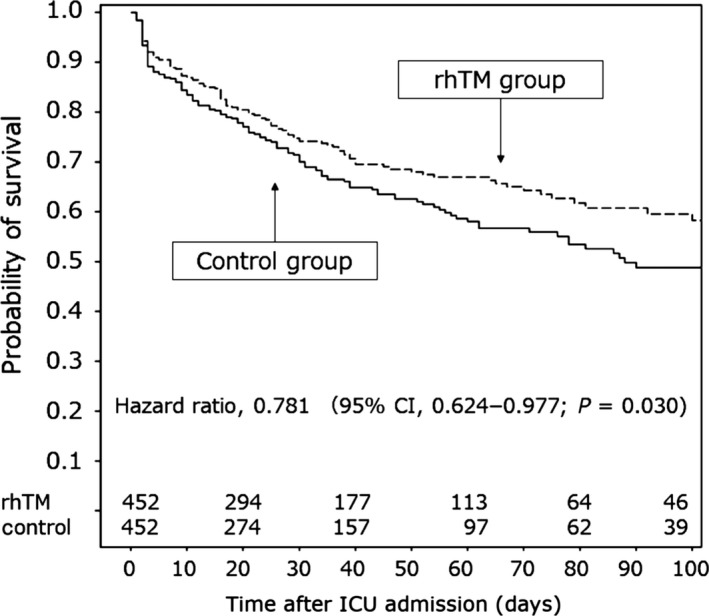
Survival time curves for patients with sepsis‐induced disseminated intravascular coagulation in propensity score‐matched groups according to treatment with recombinant human soluble thrombomodulin (rhTM group) or without (control group). The survival rate was higher in the rhTM group than in the control group. Cited as Figure 3 in our previous report.20
Antithrombin
Antithrombin is among the most important physiologic anticoagulants.26 However, in sepsis‐induced DIC, a decrease in AT activity is frequently observed27, 28, 29, 30 and is associated with high mortality rates.31, 32 Several RCTs to investigate the effects of high‐dose AT administration in patients with sepsis have been carried out.4, 33, 34, 35, 36 Although some RCTs indicated benefits of high‐dose AT administration in patients with sepsis,33, 34, 35 a large RCT (the KyberSept trial) failed to show any survival benefits.4 However, a subgroup analysis of the KyberSept trial indicated that AT administration significantly improved survival rates in patients with sepsis‐induced DIC.9
In Japan, a supplemental dose of AT (4,500 IU over 3 days) is widely given to patients with sepsis‐induced DIC and low AT levels in clinical settings, although high‐dose AT is not recommended as treatment for patients with sepsis‐induced DIC. Recently, two studies that used a nationwide administrative database reported that AT supplementation was beneficial in treating sepsis‐induced DIC.37, 38 However, the evidence for this practice is insufficient. Therefore, we analyzed the effect of AT supplementation in patients with sepsis‐induced DIC using propensity score analyses based on the JSEPTIC DIC study dataset.21
We selected 1,784 patients with sepsis‐induced DIC from among 3,195 patients in the JSEPTIC DIC study, similar to the approach used for the analysis of rhTM effects.20 Among these patients, 715 were given rhTM (rhTM group) and 1,069 patients were not (control group). Propensity score matching created 461 matched pairs, and the characteristics and therapeutic interventions of the two groups were appropriately balanced. The three propensity score analyses (propensity score matching, inverse probability of treatment weighting, and quintile‐stratified analyses) revealed the same tendency: lower in‐hospital all‐cause mortality was associated with AT administration. However, statistically significant differences were not clearly observed. The survival rates in the matched groups were not different.21
Some experts indicated that an analysis of the survival benefits of AT supplementation therapy over a short period, such as 28 days after ICU admission, yielded more concrete results than that conducted over a long period (the results reported in our previous study21). Therefore, we re‐analyzed the survival benefits of AT supplementation therapy over the 28‐day period after ICU admission. Survival time analysis revealed higher survival rates in the propensity score‐matched AT group than in the propensity score‐matched control group (hazard ratio, 0.717; 95% confidence interval, 0.555–0.927; P = 0.011) (Fig. 4). These results revealed that AT supplementation was associated with reduced in‐hospital mortality among patients with sepsis‐induced DIC.
Figure 4.
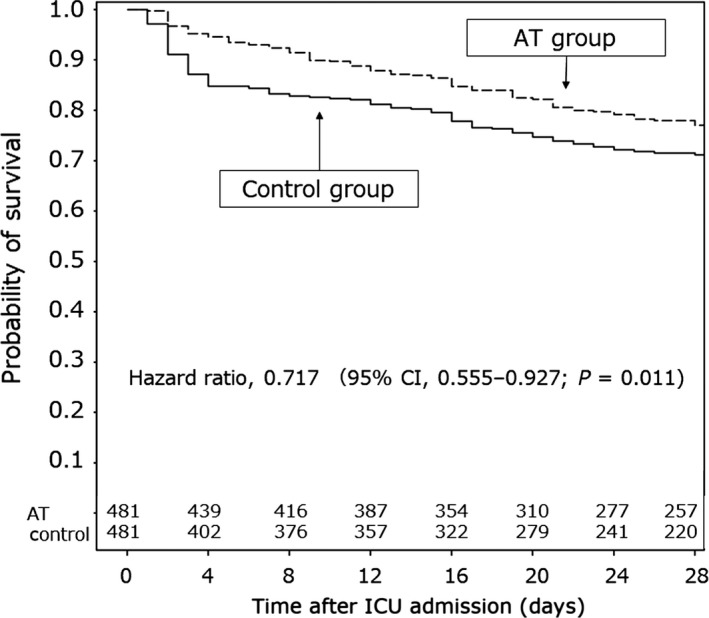
Survival time curves over the 28‐day period after admission to the intensive care unit (ICU) for patients with sepsis‐induced disseminated intravascular coagulation. Patients were allocated to groups according to treatment with antithrombin (AT group) or without (control group) in the re‐analysis. In the original analysis,21 the observation period was the duration of hospital stay (not restricted). In the re‐analysis, the observation period was restricted to a 28‐day period after ICU admission. Survival time analysis revealed that the survival rate was higher in the propensity score‐matched AT group than in the propensity score‐matched control group (hazard ratio, 0.717; 95% confidence interval, 0.555–0.927, P = 0.011).
Conclusions
The results of the JSEPTIC DIC study indicated that anticoagulant therapies, mainly rhTM and AT, were associated with survival benefits among patients with sepsis‐induced DIC in real world clinical settings.
Disclosure
Mineji Hayakawa received a grant for the basic research and a lecturer's fee from Asahi Kasei Pharma Co. The other authors have no conflict of interest.
Funding Information No funding information provided.
References
- 1. Angus DC, van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013; 369: 840–51. [DOI] [PubMed] [Google Scholar]
- 2. Hunt BJ. Bleeding and coagulopathies in critical care. N. Engl. J. Med. 2014; 370: 847–59. [DOI] [PubMed] [Google Scholar]
- 3. Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J. Intensive Care 2016; 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, et al Caring for the critically ill patient. High‐dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001; 286: 1869–78. [DOI] [PubMed] [Google Scholar]
- 5. Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, et al Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 2003; 290: 238–47. [DOI] [PubMed] [Google Scholar]
- 6. Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, et al Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N. Engl. J. Med. 2005; 353: 1332–41. [DOI] [PubMed] [Google Scholar]
- 7. Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, et al Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012; 366: 2055–64. [DOI] [PubMed] [Google Scholar]
- 8. Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson B, Brandt JT, et al Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J. Thromb. Haemost. 2004; 2: 1924–33. [DOI] [PubMed] [Google Scholar]
- 9. Kienast J, Juers M, Wiedermann CJ, Hoffmann JN, Ostermann H, Strauss R, et al Treatment effects of high‐dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J. Thromb. Haemost. 2006; 4: 90–7. [DOI] [PubMed] [Google Scholar]
- 10. Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta‐analysis of randomized controlled trials. J. Thromb. Haemost. 2016; 14: 518–30. [DOI] [PubMed] [Google Scholar]
- 11. Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, et al Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 2014; 9: e107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang CT, Tsai YJ, Tsai PR, Yu CJ, Ko WJ. Epidemiology and outcome of severe sepsis and septic shock in surgical intensive care units in Northern Taiwan. Medicine (Baltimore) 2015; 94: e2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beale R, Reinhart K, Brunkhorst FM, Dobb G, Levy M, Martin G, et al Promoting Global Research Excellence in Severe Sepsis (PROGRESS): lessons from an international sepsis registry. Infection 2009; 37: 222–32. [DOI] [PubMed] [Google Scholar]
- 14. Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit. Care Med. 2007; 35: 2538–46. [DOI] [PubMed] [Google Scholar]
- 15. Karlsson S, Varpula M, Ruokonen E, Pettila V, Parviainen I, Ala‐Kokko TI, et al Incidence, treatment, and outcome of severe sepsis in ICU‐treated adults in Finland: the Finnsepsis study. Intensive Care Med. 2007; 33: 435–43. [DOI] [PubMed] [Google Scholar]
- 16. Ogura H, Gando S, Saitoh D, Takeyama N, Kushimoto S, Fujishima S, et al Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J. Infect. Chemother. 2014; 20: 157–62. [DOI] [PubMed] [Google Scholar]
- 17. Matsuda N, Oda N, Aibiki M, Ikeda H, Imaizumi H, Endo S, et al JSICM sepsis 1st registry: management of severe sepsis and septic shock in Japan. J. Jpn. Soc. Intensive Care Med. 2007; 2013: 329–34 (Japanese). [Google Scholar]
- 18. Hayakawa M, Saito S, Uchino S, Yamakawa K, Kudo D, Iizuka Y, et al Characteristics, treatments, and outcomes of severe sepsis of 3195 ICU‐treated adult patients throughout Japan during 2011‐2013. J. Intensive Care 2016; 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamakawa K, Umemura Y, Hayakawa M, Kudo D, Sanui M, Takahashi H, et al Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit. Care 2016; 20: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayakawa M, Yamakawa K, Saito S, Uchino S, Kudo D, Iizuka Y, et al Recombinant human soluble thrombomodulin and mortality in sepsis‐induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb. Haemost. 2016; 115: 1157–66. [DOI] [PubMed] [Google Scholar]
- 21. Hayakawa M, Kudo D, Saito S, Uchino S, Yamakawa K, Iizuka Y, et al Antithrombin supplementation and mortality in sepsis‐induced disseminated intravascular coagulation: a multicenter retrospective observational study. Shock 2016; 46: 623–31. [DOI] [PubMed] [Google Scholar]
- 22. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 2003; 31: 1250–6. [DOI] [PubMed] [Google Scholar]
- 23. Murata A, Okamoto K, Mayumi T, Muramatsu K, Matsuda S. Recent change in treatment of disseminated intravascular coagulation in Japan: an epidemiological study based on a national administrative database. Clin. Appl. Thromb. Hemost. 2016; 22: 21–7. [DOI] [PubMed] [Google Scholar]
- 24. Weiler H. Regulation of inflammation by the protein C system. Crit. Care Med. 2010; 38: S18–25. [DOI] [PubMed] [Google Scholar]
- 25. Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al Efficacy and safety of recombinant human soluble thrombomodulin (ART‐123) in disseminated intravascular coagulation: results of a phase III, randomized, double‐blind clinical trial. J. Thromb. Haemost. 2007; 5: 31–41. [DOI] [PubMed] [Google Scholar]
- 26. Roemisch J, Gray E, Hoffmann JN, Wiedermann CJ. Antithrombin: a new look at the actions of a serine protease inhibitor. Blood Coagul. Fibrinolysis 2002; 13: 657–70. [DOI] [PubMed] [Google Scholar]
- 27. Opal SM, Kessler CM, Roemisch J, Knaub S. Antithrombin, heparin, and heparan sulfate. Crit. Care Med. 2002; 30: S325–31. [DOI] [PubMed] [Google Scholar]
- 28. Aibiki M, Fukuoka N, Umakoshi K, Ohtsubo S, Kikuchi S. Serum albumin levels anticipate antithrombin III activities before and after antithrombin III agent in critical patients with disseminated intravascular coagulation. Shock 2007; 27: 139–44. [DOI] [PubMed] [Google Scholar]
- 29. Seitz R, Wolf M, Egbring R, Havemann K. The disturbance of hemostasis in septic shock: role of neutrophil elastase and thrombin, effects of antithrombin III and plasma substitution. Eur. J. Haematol. 1989; 43: 22–8. [DOI] [PubMed] [Google Scholar]
- 30. Sie P, Letrenne E, Caranobe C, Genestal M, Cathala B, Boneu B. Factor II related antigen and antithrombin III levels as indicators of liver failure in consumption coagulopathy. Thromb. Haemost. 1982; 47: 218–20. [PubMed] [Google Scholar]
- 31. Fourrier F, Chopin C, Goudemand J, Hendrycx S, Caron C, Rime A, et al Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest 1992; 101: 816–23. [DOI] [PubMed] [Google Scholar]
- 32. Levi M, van der Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin. Thromb. Hemost. 2008; 34: 459–68. [DOI] [PubMed] [Google Scholar]
- 33. Inthorn D, Hoffmann JN, Hartl WH, Muhlbayer D, Jochum M. Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction. Shock 1997; 8: 328–34. [DOI] [PubMed] [Google Scholar]
- 34. Baudo F, Caimi TM, de Cataldo F, Ravizza A, Arlati S, Casella G, et al Antithrombin III (ATIII) replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double‐blind, randomized, multicenter study. Intensive Care Med. 1998; 24: 336–42. [DOI] [PubMed] [Google Scholar]
- 35. Eisele B, Lamy M, Thijs LG, Keinecke HO, Schuster HP, Matthias FR, et al Antithrombin III in patients with severe sepsis. A randomized, placebo‐controlled, double‐blind multicenter trial plus a meta‐analysis on all randomized, placebo‐controlled, double‐blind trials with antithrombin III in severe sepsis. Intensive Care Med. 1998; 24: 663–72. [DOI] [PubMed] [Google Scholar]
- 36. Gonano C, Sitzwohl C, Meitner E, Weinstabl C, Kettner SC. Four‐day antithrombin therapy does not seem to attenuate hypercoagulability in patients suffering from sepsis. Crit. Care 2006; 10: R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis‐associated disseminated intravascular coagulation: an observational nationwide study. J. Thromb. Haemost. 2014; 12: 1470–9. [DOI] [PubMed] [Google Scholar]
- 38. Tagami T, Matsui H, Fushimi K, Yasunaga H. Supplemental dose of antithrombin use in disseminated intravascular coagulation patients after abdominal sepsis. Thromb. Haemost. 2015; 114: 537–45. [DOI] [PubMed] [Google Scholar]


