Abstract
Phentermine is the most widely prescribed obesity medication in adults, yet studies of its use in the pediatric population are limited. We conducted a retrospective chart review of adolescents with obesity treated in a pediatric weight management clinic to examine the weight loss effectiveness of phentermine added to standard of care lifestyle modification therapy (SOC) versus SOC alone. All patients receiving phentermine plus SOC (n=25) were matched with a comparison group receiving only SOC (n=274). Differences at 1, 3, and 6 months were evaluated using generalized estimated equations adjusting for age, sex, and baseline body mass index (BMI) and robust variance standard error estimates for confidence intervals and P-values. Phentermine use was associated with a greater percent change in BMI at 1 month (−1.6% [95% CI (−2.6%, −0.6%); p=0.001), 3 months (−2.9% [95% CI (−4.5%, −1.4%); p<0.001]) and 6 months (−4.1% [95% CI (−7.1%, −1.0%); p=0.009]) compared to SOC alone, with no differences in systolic or diastolic blood pressure between groups. Heart rate was higher at all time-points in the phentermine plus SOC compared to SOC only group. These data suggest that short-term use of phentermine added to SOC may enhance weight loss in adolescents with obesity in the clinical setting.
Introduction
Pharmacotherapy as an adjunct to lifestyle modification therapy is indicated for youth with obesity complicated by co-morbid conditions or severe obesity (body mass index (BMI) ≥120% of the 95th percentile or ≥35 kg/m2).1, 2 However, the pharmacologic options for the treatment of pediatric obesity are limited.1, 3
Phentermine, a norepinephrine reuptake inhibitor, was originally approved in 1959 by the United States Food and Drug Administration as an appetite-suppressing drug for the treatment of obesity in adults. Since that time, it has seen widespread use and is currently the most commonly prescribed medication for the treatment of obesity in adults4, 5 Given phentermine’s long track record without major safety concerns and its similarity to other stimulants which are widely prescribed for attention-deficit/hyperactivity disorder in children, phentermine may prove to be a viable pharmacological treatment option for pediatric obesity.
To date, the only studies examining phentermine for obesity in the pediatric population are from the 1960’s.6, 7 These studies, which were small in sample size (n = 24 - 30) and had no control or comparison groups, offer little in terms of clinical relevance for today’s youth with obesity. As such, data on the clinical effectiveness of phentermine would provide valuable information guiding decision-making about the need for subsequent clinical trials evaluating this agent, especially because phentermine is sometimes prescribed “off-label” for the treatment of adolescent obesity.4 Therefore, we conducted a retrospective chart review of pediatric weight management clinic patients who were treated with phentermine in addition to standard of care (SOC) lifestyle modification therapy versus those receiving SOC only. We further examined changes in heart rate and blood pressure that occurred over time.
Methods
This was a retrospective chart review of patients treated at the University of Minnesota Masonic Children’s Hospital Pediatric Weight Management Clinic between July 3, 2011 and May 28, 2016. All patients were treated with SOC lifestyle modification therapy which included nutrition and exercise counseling supported by behavioral modification strategies. The SOC was delivered by a multidisciplinary team including a registered dietician, physical therapist, pediatric psychologist, and pediatrician who specializes in childhood obesity. Patients were seen approximately every four weeks, though some more frequently and some less frequently, depending on their need. Patients in the phentermine plus SOC group were included if they were additionally prescribed phentermine at a dose of 15 mg/day for weight loss and had at least one follow-up visit. Patients were excluded if they were taking any other medication with weight altering properties (either gain or loss) and those who underwent bariatric surgery. Patients were not excluded from either group based upon comorbidities. Weight, height, heart rate, and blood pressure data were abstracted from the medical records. This study was approved by the University of Minnesota Institutional Review Board.
We a priori defined the following time-points: baseline was the initial visit at which phentermine was first prescribed (or SOC was initiated for controls), 1-month time-point was any visit occurring between 24-days – 5 weeks, 3-month time-point was any visit occurring between 11-15 weeks, and 6-month time-point was any visit occurring between 23-26 weeks. The SOC only group was selected using an identical range of baseline age (11.9 – 17.7 years) and BMI (31-58 kg/m2) to that of the phentermine plus SOC group in an effort to establish a well-matched comparison group. Patients were allowed to contribute data to both groups if they first started on SOC and then later additionally initiated phentermine treatment while still receiving SOC. Differences between phentermine plus SOC and SOC only groups were evaluated using generalized estimating equations adjusting for age, sex, and baseline BMI with robust variance estimation for confidence intervals and P-values and to account for the correlated nature of some patients included in both groups. For missing values within date ranges due to inconsistent follow-up visits, imputed values were used from interpolating the most recent measurement prior and the closest measurement after a designated time point range. All analyses were conducted in R v3.2.3.8
Results
We identified 25 patients (mean age 16.1±1.3 years; mean BMI 41.2±6.9 kg/m2) who were prescribed phentermine plus SOC and a comparison group of 274 patients (mean age 14.9±1.6 years; mean BMI 38.1±5.5 kg/m2) receiving only SOC (Table 1). Figure 1 displays change in percent BMI over time. At 1-month we observed a significant reduction in weight (−1.4kg [95% CI (−2.5kg, −0.4kg); p=0.008]), absolute BMI (−0.6kg/m2 [95% CI (−1.0kg/m2, −0.2kg/m2); p=0.002]), and percent change in BMI (−1.6% [95% CI (−2.6%, −0.6%); p=0.001]) in the phentermine plus SOC group compared to the SOC group. At 3-months we observed significant reduction in weight (−2.6kg [95% CI (−4.4kg, −0.7kg); p=0.006]), absolute BMI (−1.1kg/m2 [95% CI (−1.8kg/m2, −0.5kg/m2); p<0.001]), and percent change in BMI (−2.9% [95% CI (−4.5%, −1.4%); p<0.001]) in phentermine plus SOC compared to SOC. At 6-months we observed significant reduction in weight (−3.2kg [95% CI (−6.0kg, −0.5kg); p=0.020]), absolute BMI (−1.6kg/m2 [95% CI (−2.8kg/m2, −0.4kg/m2); p=0.011]), and percent change in BMI (−4.1% [95% CI (−7.1%, −1.0%); p=0.009]) in phentermine plus SOC compared to SOC. At 3-months, 40% (8 out of 20) of the phentermine plus SOC group achieved ≥ 5% BMI reduction compared to 8.8% (15 out of 171) in the SOC group; at 6-months, these proportions increase to 63.6% (7 out of 11) of the phentermine plus SOC group compared to 20.8% (20 out of 96) in the SOC group. No statistically significant differences between groups were observed for systolic or diastolic blood pressure. At 3-months a statistically significant elevation in heart rate was observed in the phentermine plus SOC group compared to the SOC group. This effect on heart rate was not statistically significant at 1-month or 6-months.
Table 1.
Characteristics of sample and changes in weight status, BMI, blood pressure, and heart rate over time.
| Baseline | Change at 1 Month | Change at 3 Months | Change at 6 Months | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Phentermine + SOC | SOC | Treatment Effect* (95% CI) |
p-value | Treatment Effect* (95% CI) |
p-value | Treatment Effect* (95% CI) |
p-value | |
| n | 25 | 274 | P+SOC (25) SOC (274) | P+SOC (20) SOC (171) | P+SOC (11) SOC (96) | |||
| Sex (male) | 6 (24.0%) | 109 (39.8%) | – | – | – | – | – | – |
| Age at Visit (years) | 16.1 (1.33) | 14.9 (1.63) | – | – | – | – | – | – |
| BMI Percentile (%) | 99.9 (0.6) | 100.0 (0.4) | – | – | – | – | – | – |
| -Class 1 Obesity | 6 (24.0%) | 39 (14.2%) | – | – | – | – | – | – |
| -Class 2 Obesity | 4 (16.0%) | 123 (44.9%) | – | – | – | – | – | – |
| -Class 3 Obesity | 15 (60.0%) | 112 (40.9%) | – | – | – | – | – | – |
| Height (cm) | 166 (8.98) | 166 (9.55) | 0.18 (−0.05,0.42) | 0.127 | 0.32 (−0.17,0.80) | 0.204 | 0.67 (−0.15,1.49) | 0.109 |
| Weight (kg) | 114 (22.2) | 106 (21.0) | −1.41 (−2.45,−0.36) | 0.008 | −2.56 (−4.39,−0.73) | 0.006 | −3.23 (−5.95,−0.52) | 0.020 |
| BMI (kg/m2) | 41.2 (6.9) | 38.1 (5.5) | −0.62 (−1.00,−0.24) | 0.002 | −1.14 (−1.76,−0.51) | <0.001 | −1.57 (−2.78,−0.36) | 0.011 |
| - % change | – | – | −1.61 (−2.59,−0.64) | 0.001 | −2.94 (−4.51,−1.37) | <0.001 | −4.05 (−7.06,−1.03) | 0.009 |
| BMI Z-score | 2.44 (0.31) | 2.44 (0.27) | −0.03 (−0.05,−0.01) | 0.016 | −0.06 (−0.10,−0.01) | 0.010 | −0.09 (−0.16,−0.02) | 0.019 |
| Percent of the 95th Percentile | 144 (23.9) | 139 (20.0) | −1.99 (−3.38,−0.60) | 0.007 | −4.09 (−6.13,−2.05) | <0.001 | −5.71 (−9.86,−1.56) | 0.010 |
| Systolic BP (mmHg) | 120 (9.28) | 122 (12.4) | 0.24 (−2.8,3.28) | 0.878 | 1.44 (−3.03,5.91) | 0.528 | −1.18 (−7.58,5.22) | 0.717 |
| Diastolic BP (mmHg) | 70.5 (7.65) | 70.7 (10.3) | −0.74 (−3.82,2.34) | 0.638 | 1.53 (−2.90,5.96) | 0.498 | 4.12 (−3.3,11.54) | 0.276 |
| Heart Rate (bpm) | 81.6 (12.3) | 82.2 (13.2) | 3.82 (−0.17,7.80) | 0.060 | 8.53 (1.59,15.47) | 0.016 | 6.92 (−2.1,15.95) | 0.133 |
Treatment effect is change Phentermine + SOC with SOC subtracted. Analyses are further adjusted for age, gender, and baseline BMI. Data are presented as mean (SD) or n (%). Abbreviations (in order of appearance): SOC = Standard of care; P+SOC = Phentermine + Standard of care; BMI = Body mass index; Class 1 obesity = 95th percentile to <20% of 95th percentile; Class 2 obesity = ≥20% of the 95th percentile and <40% of the 95th percentile; Class 3 obesity = ≥40% of the 95th percentile ; BP = Blood Pressure
Figure 1.
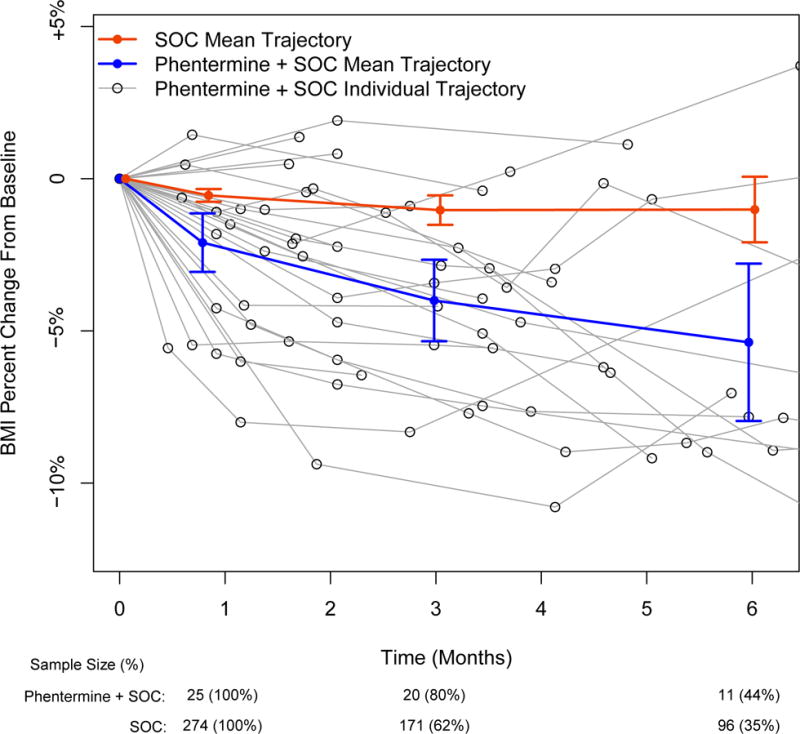
BMI percent change from baseline for phentermine plus standard of care (SOC; Blue) versus SOC (Red). Individual data for the phentermine plus SOC are also shown (Black). Mean data are unadjusted means and standard deviations at 1 month, 3 months, and 6 months.
Conclusion
To our knowledge this is the first study to evaluate the weight loss effectiveness of phentermine as an adjunctive treatment to SOC lifestyle modification therapy among adolescents with obesity in the setting of a pediatric weight management clinic. Compared to SOC alone, phentermine added to SOC resulted in statistically significant weight loss at 1-month, 3-months, and 6-months among adolescents with obesity. At 3- and 6-months, a higher proportion of patients on phentermine achieved a clinically meaningful weight loss of ≥ 5% BMI reduction. Furthermore, no statistically significant or clinically meaningful differences between groups were observed in blood pressure, although heart rate was higher in the phentermine plus SOC group at all time-points.
Our observation of weight loss in adolescents who were prescribed phentermine is consistent with the limited data available in adults with obesity.9, 10 However, it is important to note that the degree of weight loss observed in our study was less than that reported in adults in terms of both mean weight loss and proportion of those achieving 5% and 10% weight loss goals. This may be due to a number of factors including dosing differences (15 mg/d versus 30 mg/d) and the higher baseline BMIs observed in our population. The mean percent BMI reduction observed at 3-months (−2.90%) and 6-months (−4.10%) is similar to what has been observed in adolescents with severe obesity in randomized controlled trials of other pharmacological agents such as exenatide (glucagon-like peptide-1 receptor agonist)11, 12 and greater than that observed with either metformin or orlistat.13, 14,15 However, it is important to note that the present study is observational in nature and may be biased, either positively or negatively, when compared to the results of randomized controlled trials.
Our findings of no adverse changes in either systolic or diastolic blood pressure are in agreement with studies of adults.9, 10 Hendricks and colleagues, in 269 adults with obesity taking phentermine (15 – 37.5 mg/d) in addition to lifestyle modification therapy, observed significant decreases in systolic and diastolic blood pressure at 26 and 52 weeks along with significant weight loss. Interestingly, heart rate was not appreciably different across time points which is in contrast to our data showing an elevation at all time-points, although only statistically significant at 3 months. This may be due to a number of factors including differences in sample size, cardiac cycle difference in youth versus adults,16 and our utilization of a pediatric population with a higher relative body mass for age. Nevertheless, the potential risk of elevated heart rate should be monitored closely by clinicians and followed in any future clinical trials and may have potential implications if phentermine is used for long-term treatment. Other safety considerations when using phentermine, a class of amphetamines, include addiction potential and withdrawal after long-term use. However, limited data in adults suggests that phentermine is non-addictive and produces no withdrawal-like symptoms.17 Due to the retrospective nature of the study we were unable to account for changes that may have occurred in diet, appetite, eating behaviors, physical activity, or medication adherence. Additionally, the small proportion of males included in this study and potential variability of data collection should be noted as limitations.
In conclusion, results from this retrospective chart review suggests that phentermine, when added to SOC lifestyle modification therapy, produces significantly greater weight loss at 1-month, 3-months, and 6-months compared to SOC alone in adolescents with obesity. These data support the further investigation of phentermine as a monotherapy or in combination with other medications as a treatment option for pediatric obesity. Future clinical trials should include additional endpoints such as changes in cardiometabolic risk factors, quality of life, and non-invasive vascular measurements, as well as investigate potential mechanisms of action and predictors of response.
Supplementary Material
Supplement Figure 1. Percent change in body weight (kg) from baseline for phentermine plus standard of care (SOC; Blue) versus SOC (Red). Individual data for the phentermine plus SOC are also shown (Black). Mean data are unadjusted means and standard deviations at 1 month, 3 months, and 6 months.
Acknowledgments
Funding: Dr. Ryder is supported by an individual training grant from NIH/NHLBI (F32-HL127851). Dr. Rudser and Mr. Kaizer are supported in part by the National Center for Advancing Translational Sciences/NIH (UL1TR000114). The other authors received no funding for this project.
Dr. Kelly serves as a consultant for Novo Nordisk Pharmaceuticals but does not receive personal or professional income for these activities. Dr. Kelly receives research support in the form of drug/placebo from Astra Zeneca Pharmaceuticals. Dr. Fox serves as a site principal investigator for a clinical trial sponsored by Novo Nordisk Pharmaceuticals. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Dr. Fox receives salary support for her role as a site principal investigator for Novo Nordisk Pharmaceuticals.
Footnotes
Conflict of interest: The other authors declare no conflicts of interest.
Financial Disclosure: The other authors have no financial relationships relevant to this article to disclose.
Bibliography
- 1.Kelly AS, Fox CK, Rudser KD, Gross AC, Ryder JR. Pediatric obesity pharmacotherapy: Current state of the field, review of the literature, and clinical trial considerations. Int J Obes (Lond) 2016 doi: 10.1038/ijo.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow SE. Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 3.Sherafat-Kazemzadeh R, Yanovski SZ, Yanovski JA. Pharmacotherapy for childhood obesity: present and future prospects. Int J Obes (Lond) 2013;37:1–15. doi: 10.1038/ijo.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009;17:1730–5. doi: 10.1038/oby.2009.69. [DOI] [PubMed] [Google Scholar]
- 5.Stafford RS, Radley DC. National trends in antiobesity medication use. Archives of internal medicine. 2003;163:1046–50. doi: 10.1001/archinte.163.9.1046. [DOI] [PubMed] [Google Scholar]
- 6.Lorber J. Obesity in childhood. A controlled trial of anorectic drugs. Archives of Disease in Childhood. 1966;41:309–312. doi: 10.1136/adc.41.217.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauh JL, Lipp R. Chlorphentermine as an anorexigenic agent in adolescent obesity. Report of its efficacy in a double-blind study of 30 teen-agers. Clinical pediatrics. 1968;7:138–40. doi: 10.1177/000992286800700305. [DOI] [PubMed] [Google Scholar]
- 8.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2015. [Google Scholar]
- 9.Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes, obesity & metabolism. 2010;12:876–82. doi: 10.1111/j.1463-1326.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring) 2011;19:2351–60. doi: 10.1038/oby.2011.94. [DOI] [PubMed] [Google Scholar]
- 11.Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, Fitch AK, Bomberg EM, Abuzzahab MJ. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA pediatrics. 2013;167:355–60. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM, Deering MM, Schwartz BL, Abuzzahab MJ, Gandrud LM, Moran A, Billington CJ, Schwarzenberg SJ. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring) 2012;20:364–70. doi: 10.1038/oby.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, Reynolds JC, Brady SM, Calis KA. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477–85. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–83. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 15.Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH, Osganian SV, Feldman HA. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164:116–23. doi: 10.1001/archpediatrics.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–5. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks EJ, Srisurapanont M, Schmidt SL, Haggard M, Souter S, Mitchell CL, De Marco DG, Hendricks MJ, Istratiy Y, Greenway FL. Addiction potential of phentermine prescribed during long-term treatment of obesity. Int J Obes (Lond) 2014;38:292–8. doi: 10.1038/ijo.2013.74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. Percent change in body weight (kg) from baseline for phentermine plus standard of care (SOC; Blue) versus SOC (Red). Individual data for the phentermine plus SOC are also shown (Black). Mean data are unadjusted means and standard deviations at 1 month, 3 months, and 6 months.


