Abstract
Purpose
Chemohormonal therapy with docetaxel and androgen deprivation therapy (ADT+D) for metastatic hormone-sensitive prostate cancer improves overall survival as compared with androgen deprivation therapy (ADT) alone. We compared the quality of life (QOL) between patients with metastatic hormone-sensitive prostate cancer who were treated with ADT+D and those who were treated with ADT alone.
Methods
Men were randomly assigned to ADT+ D (six cycles) or to ADT alone. QOL was assessed by Functional Assessment of Cancer Therapy-Prostate (FACT-P), FACT-Taxane, Functional Assessment of Chronic Illness Therapy-Fatigue, and the Brief Pain Inventory at baseline and at 3, 6, 9, and 12 months. The Wilcoxon signed rank test was used to examine changes over time. Mixed-effect models compared the QOL between arms at each time point.
Results
Seven hundred ninety men were randomly assigned (ADT+D [n = 397] and ADT[ n = 393]) and completed FACT-P (90% at baseline, 86% at 3 months, 83% at 6 months, 78% at 9 months, and 77% at 12 months). ADT+D patients reported a statistically significant decline in FACT-P at 3 months (P < .001) but FACT-P did not differ significantly between baseline and 12 months (P = .38). ADT+D FACT-P scores were significantly lower at 3 months (P = .02) but significantly higher at 12 months (P = .04) when compared with ADT FACT-P scores. Differences did not exceed the minimal clinically important difference at any time point. ADT+D patients reported significantly lower Functional Assessment of Chronic Illness Therapy-Fatigue scores at 3 months than did ADT patients (P < .001). Over time, both arms reported significantly poorer FACT-Taxane scores (P < .001) when compared with baseline. Brief Pain Inventory scores were similar between arms.
Conclusion
Although ADT+D was associated with statistically worse QOL at 3 months, QOL was better at 12 months for ADT+D patients than for ADT patients. Both arms reported a similar minimally changed QOL over time, suggesting that ADT+D is not associated with a greater long-term negative impact on QOL.
INTRODUCTION
Prostate cancer (PCa) is the second most common cancer diagnosed in men worldwide, and is the third leading cause of cancer death among American men.1,2 Two landmark studies have demonstrated prolonged survival for men with metastatic hormone-sensitive prostate cancer (mHSPC) treated with chemohormonal therapy.3,4 A prospectively defined subgroup analysis in E3805 with long-term follow-up further demonstrated that the survival benefit associated with treatment was driven by men with high-volume mHSPC.5 Chemohormonal therapy has since become a standard option for the treatment of mHSPC with adequate performance status, particularly among men with high-volume disease.6
Quality of life (QOL) is of paramount importance for men with metastatic PCa because the disease is incurable and it commonly afflicts elderly men with multiple comorbid illnesses or a borderline performance status. In the PCa population, docetaxel has an adverse event profile, with up to a 51% risk of grade 3 to 5 adverse events including neutropenic fever, peripheral neuropathy, and fluid retention.3,4,7 It has been hypothesized that QOL in this population is the product of the alleviation of disease-related symptoms and the introduction of treatment-related symptoms.8 Understanding QOL as it relates to particular therapies is critical to inform patient-provider treatment decisions.
To more clearly understand the burden of treatment, E3805 evaluated the change in QOL over 12 months to describe QOL outcomes associated with chemohormonal therapy in men with mHSPC.
METHODS
Study Oversight
The primary objective of E3805 was to assess whether chemohormonal therapy was associated with prolonged survival as compared with androgen deprivation therapy (ADT) alone in men with mHSPC.3 A patient-reported QOL assessment was performed as a secondary outcome of E3805. As detailed in a previous publication, E3805 was designed and coordinated by the Eastern Cooperative Oncology Group (ECOG; now ECOG-ACRIN) and was approved by local institutional review boards at participating institutions.3 Sanofi provided docetaxel (before progression when receiving ADT) and a grant to ECOG-ACRIN. The company did not participate in the design of the study, data collection or analysis, or manuscript preparation.
Patients
Participants were enrolled by ECOG-ACRIN, Southwest Oncology Group, Alliance for Clinical Trials in Oncology, and NRG Oncology, and the study was supported by the Clinical Trials Support Unit.3 Eligible men had an ECOG performance status of ≤ 2 without significant organ dysfunction, a pathologic diagnosis of prostate adenocarcinoma, and radiographic evidence of metastatic disease. Exposure to previous ADT for ≤ 24 months was permissible if > 12 months had passed since exposure. Men without progression when receiving ADT for mHSPC were eligible if they had started ADT within 120 days. All participants provided written informed consent.
Treatment Plan, Random Assignment, and Stratification
Men were randomly assigned to receive treatment with chemohormonal therapy (docetaxel 75 mg/m2 once every 3 weeks for six cycles plus ADT; ADT+D) or with ADT alone.3 Patients were stratified by the extent of metastatic burden (high volume, defined as the presence of four or more bone metastases with one or more outside of the vertebral bodies and pelvis or visceral metastases; and low volume).3 Dose modifications to ADT were not allowed, and dose modifications for docetaxel were limited. Dose modification details are in the Data Supplement.
Data Collection
QOL surveys were administered at baseline and at 3, 6, 9, and 12 months. When necessary, staff provided neutral assistance for survey completion and prompted patients to complete incomplete items. If the patient refused, it was indicated on the questionnaire. If a patient missed a scheduled appointment, the questionnaire was completed by telephone on the appointed date or at the rescheduled appointment.
Measures
Primary outcome: Overall QOL by Functional Assessment of Cancer Therapy-Prostate.
Functional Assessment of Cancer Therapy-Prostate (FACT-P; version 4) is a patient-reported measure of QOL that contains 39 items distributed over five subscales: Physical (seven items), Social or Familial (seven items), Emotional (six items), and Functional (seven items) well-being, and additional concerns related to the Prostate Cancer Scale (12 items).9 Higher scores indicate better QOL. Clinically meaningful change on the FACT-P total score was considered a change of 6 to 10 points.10
Secondary outcomes: Treatment and disease-related QOL.
The Trial Outcome Index (TOI) is a measure of physical and functional well-being calculated by summing the Physical and Functional Well-being subscales and the Prostate Cancer subscale.9 FACT-P subscales have been used in clinical trials of QOL in patients with advanced PCa to characterize specific domains.11-13
The FACT-Taxane (FACT-T) Scale includes 16 items associated with adverse effects of taxane treatment, and it has excellent internal consistency, reliability, validity, and responsiveness to change.14 Higher scores indicate better QOL. A clinically meaningful difference in FACT-Taxane was a change of ≥ 1 standard error of measurement.14 The Fatigue subscale of the FACIT-F includes13 items that assess the physical experience of fatigue.15 It exhibits strong convergent and discriminant validity, internal consistency, test-retest reliability, and sensitivity to group differences in performance status.15 Higher scores indicate better QOL. A minimal important difference (MID) for FACIT-F was considered a change of ≥ 3 points.16 The Brief Pain Inventory (BPI) Short Form includes 11 questions that assess pain intensity and interference with daily functioning.17,18 The reliability and validity of the BPI are well established.17,18 Higher scores indicate greater pain. MID was a change of ≥ 2 points for pain intensity and ≥ 0.5 of the standard deviation for pain interference.19,20
In addition, we assessed QOL by PCa disease burden by comparing FACT-P total scores and subscales between treatment arms by disease burden.
Scoring Methods
FACT-P, FACT-T, and FACIT-F scores were calculated by summing all subscale items after reversing negatively stated items. If items were missing, subscale scores were prorated (we multiplied the sum of item scores by the number of total subscale items, then divided by the number of items answered) only if ≥ 50% of items in the subscale were answered. The FACT-P total score was considered valid only if the FACT-P was ≥ 80% complete. The BPI includes four items assessing pain intensity that were averaged to determine the average pain score. The remaining seven pain interference questions were averaged to obtain the average interference score. Score calculation required ≥ 50% subscale completion. The response rate was calculated by dividing the number of patients answering any part of FACT-P by the number of patients due for an assessment. Patients who died, refused follow-up, or were lost to follow-up were not included in the denominator for the response rate.
Statistical Methods
We use descriptive statistics to characterize the patients at study entry and the distribution of QOL scores. Fisher’s exact test was used to compare response rates (compliance) between arms at each time point.21 Differences in the distribution of QOL scores between arms and changes in QOL scores from baseline to follow-up were evaluated using the Wilcoxon rank sum test and the Wilcoxon signed rank test, respectively.22 Mixed-effect models with random intercepts for each individual were used to evaluate the differences in FACT-P total scores and TOI between the two arms over time.23 Several baseline factors, including age (≤ 59 years v 60 to 69 years v ≥ 70 years), extent of disease (high volume v low volume), local therapy (yes v no), ECOG performance scale (PS) (0 v 1 or 2), baseline physical well-being (≤ 20 v 20 < physical well-being (PWB) ≤ 25 v > 25), and baseline pain score (0 or 1 v 2 or 3 v ≥ 4) were included in the model and were considered to be fixed effects. The assessment time point was considered a categorical variable, and the interaction between treatment arm and assessment time was included in the model. QOL data reflect all available data as of August 2015. All tests were two sided, and P values < .05 were considered significant.
RESULTS
Primary End Point: FACT-P
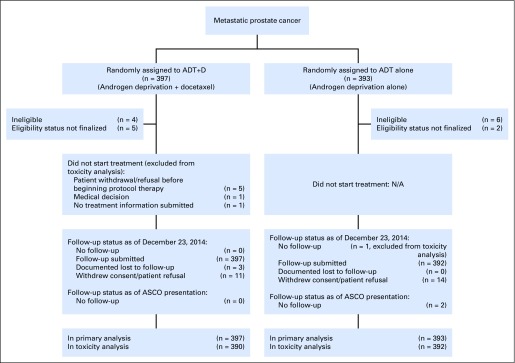
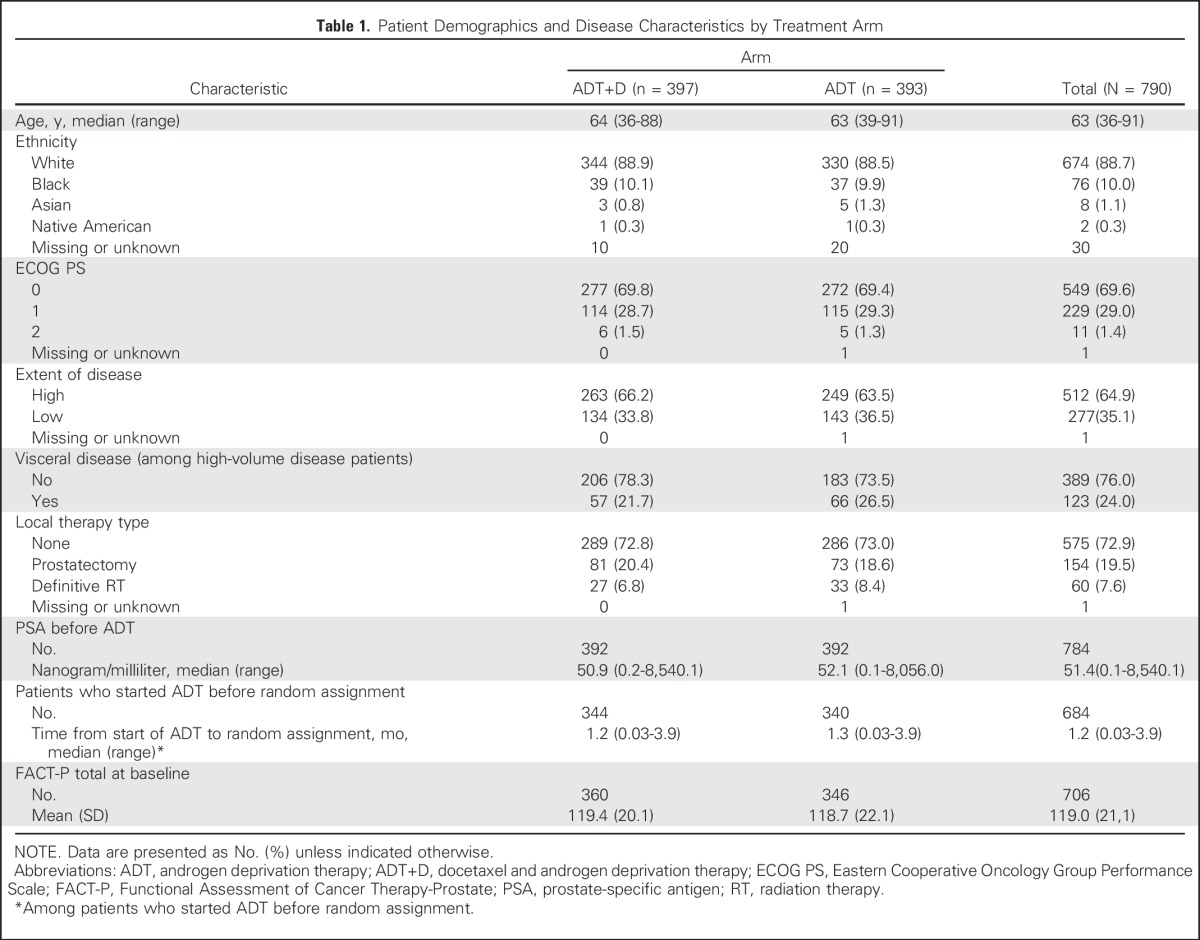
Between July 2006 and December 2012, 790 men enrolled in E3805 and were randomly assigned to ADT+D (n = 397) or to ADT alone (n = 393).3 Baseline characteristics were balanced between groups and have been described previously (Table 1).3 The results of the survival analysis of the study were released after a planned interim analysis in October 2013 due to meeting prespecified criteria for significance.
Table 1.
Patient Demographics and Disease Characteristics by Treatment Arm
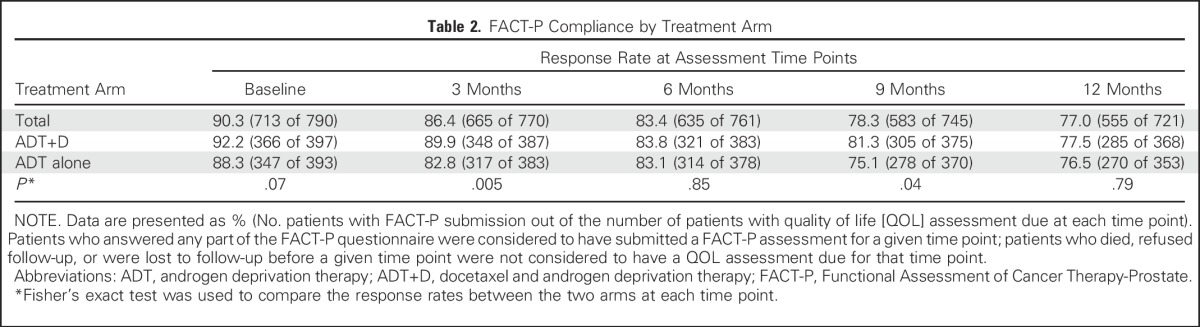
FACT-P surveys were completed by 90%, 86%, 83%, 78%, and 77% of participants at baseline and at 3, 6, 9, and 12 months, respectively (Table 2). Missing data rates were low, and there were similar response rates at all time points other than at 3 and 9 months, when there was a small but significantly better response rate for the ADT+D arm (Table 2). Reasons for missing data are cataloged in Appendix Table A1 (online only). A sensitivity analysis was performed using joint modeling of QOL and survival data to adjust for nonignorable missing data, and the conclusions were unchanged (Appendix Table A2, online only).
Table 2.
FACT-P Compliance by Treatment Arm
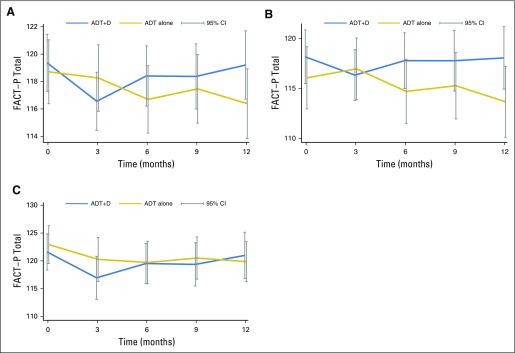
For the primary end point, there was a statistically significant decrease in the mean FACT-P score between baseline and 3 months with ADT+D (−2.7 points, P < .001), but no significant change in mean FACT-P score between baseline and 3 months with ADT (−1.1 points, P = .4; Fig 1A). There was no significant difference in mean FACT-P score between baseline and 12 months with ADT+D (−0.7 points, P = .38), but the mean FACT-P score significantly decreased between baseline and 12 months with ADT (−4.2 points, P < .001; Appendix Table A3, online only). None of these changes met the criteria for a clinically meaningful change. A sensitivity analysis of the 25% of men reporting the greatest decline in QOL between baseline and 3 months was performed, and similar FACT-P score changes were seen.
Fig 1.
Comparison of Functional Assessment of Cancer Therapy-Prostate (FACT-P) scores over time between the docetaxel and androgen deprivation therapy (ADT+D) and androgen deprivation therapy (ADT) arms, including (A) FACT-P scores for the entire population; (B) FACT-P scores for high-volume patients; and (C) FACT-P scores for low-volume patients. Clinically meaningful change on the FACT-P total score was considered a change of 6 to10 points.
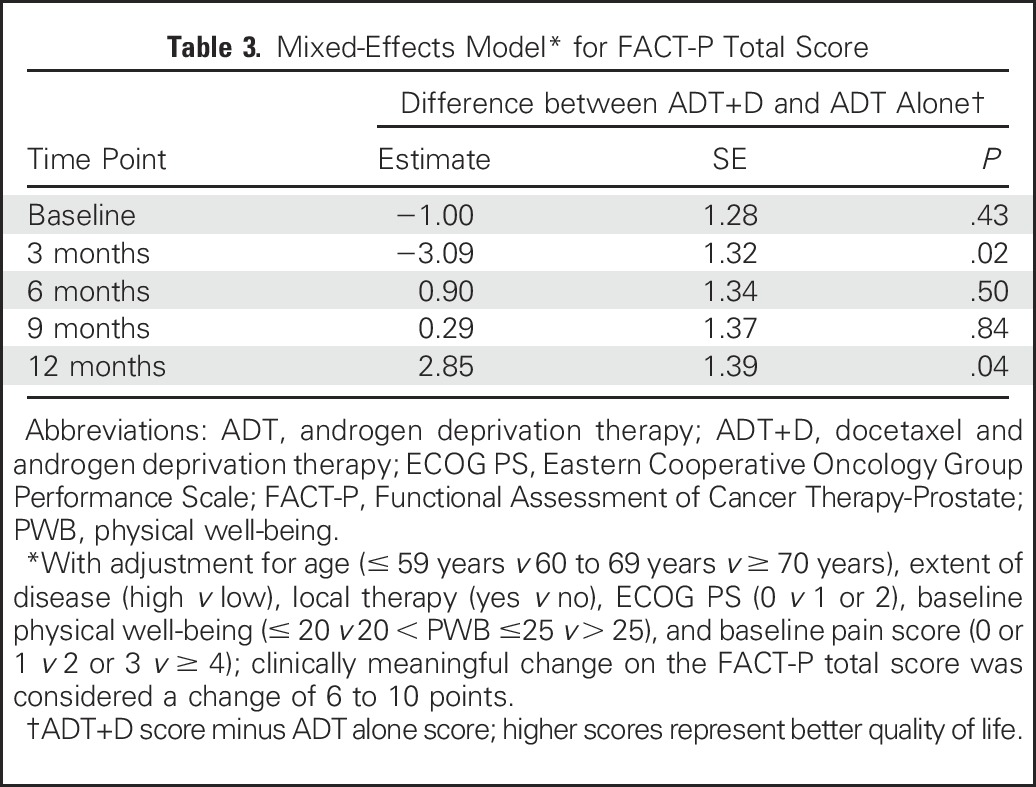
A direct comparison of mean FACT-P total scores between treatment arms at 3 months (during chemotherapy) showed no significant difference (116.6 v 118.3, P = .07; Appendix Table A4, online only). In a mixed-effects model adjusted as described in Patients and Methods, the ADT+D group was associated with a significantly poorer adjusted mean FACT-P score at 3 months when compared with the ADT group (−3.09, P = .02; Table 3). Conversely, at 12 months, ADT+D was associated with a higher mean adjusted FACT-P score when compared with that of ADT (2.85, P = .04; Table 3). Differences between arms did not meet the criteria for a clinically meaningful difference at any time point.
Table 3.
Mixed-Effects Model* for FACT-P Total Score
Secondary End Points
FACT-P subscales.
We evaluated the TOI and FACT-P subscales to determine the effect of treatment and disease on specific domains of QOL (Appendix Table A4). At 3 months, mean TOI and the physical and functional well-being subscales were significantly lower with ADT+D than with ADT alone (TOI, 74.9 v 77.6, P = .003), but they were similar at all other time points. In a mixed-effects model adjusted as described in Patients and Methods, ADT+D was associated with significantly lower TOI scores than was ADT at 3 months only (−3.44, P < .001; Appendix Table A5, online only).
FACT-Taxane.
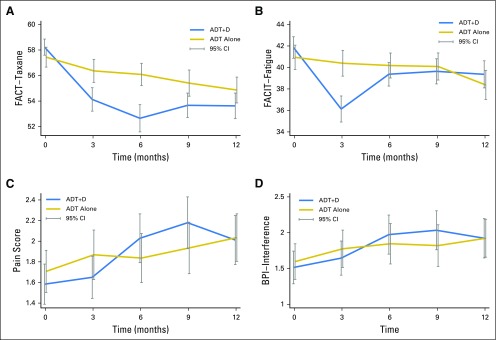
We used the FACT-Taxane to assess the treatment effect within and between arms over time. FACT-Taxane scores were significantly lower for ADT+D than for ADT at all time points after baseline (Appendix Table A6, online only). FACT-Taxane scores declined significantly from baseline in both treatment arms at all time points (Fig 2A). No differences between groups or changes over time met the criteria for MID.
Fig 2.
Treatment-associated and disease-associated symptoms over time between the docetaxel and androgen deprivation therapy (ADT+D) and androgen deprivation therapy (ADT) arms, including(A) the Functional Assessment of Cancer Therapy-Taxane (FACT-Taxane) subscale; (B) the FACIT-Fatigue subscale; (C) the Brief Pain Inventory (BPI) pain subscale; and (D) the BPI interference subscale.
FACIT-Fatigue.
Mean scores for the FACIT-Fatigue were similar between arms at baseline and at all subsequent time points, with the exception of 3 months (36.1 v 40.4 for the ADT+D and ADT arms at 3 months, respectively; P < .001; Appendix Table A6; Fig 2B). At 3 months, both the change in FACIT-Fatigue score from baseline with ADT+D and the difference between arms met the criteria for MID.
BPI.
There was no significant difference between arms in BPI pain intensity or interference scores at any time point (Figs 2C and 2D). Pain increased slightly over time in both groups but did not meet the minimal clinically important difference at any time point (Appendix Table A6).
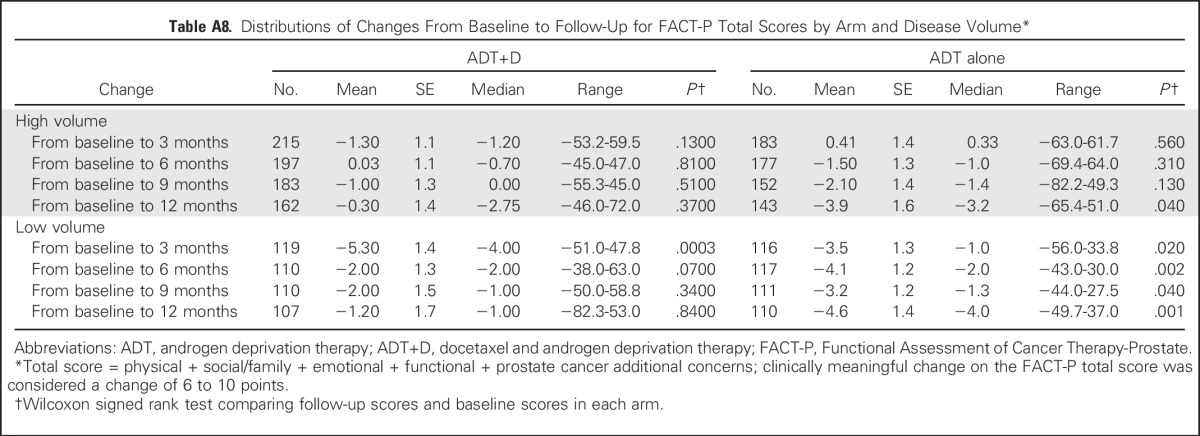
High- Versus Low-Volume Disease
We assessed overall QOL between arms and over time by comparing FACT-P scores by disease burden (Figs 1B and 1C). There was no significant difference in mean FACT-P scores between treatment arms within the high- or low-volume groups (Appendix Table A7, online only). Among high-volume patients, there was no significant change in mean FACT-P score between baseline and any time point with ADT+D (Appendix Table A8, online only). Among high-volume patients receiving ADT alone, the mean FACT-P score at 12 months was significantly lower than at baseline (−3.9, P = .04; Appendix Table A8). Among ADT+D low-volume patients, the mean FACT-P score decreased significantly between baseline and 3 months (−5.3, P < .001), but scores did not differ from baseline at 6, 9, or 12 months (Appendix Table A8). Among ADT low-volume patients, the mean FACT-P score was significantly lower than at baseline at all time points (Appendix Table A8).
DISCUSSION
We assessed QOL over time in men treated with ADT+D or ADT alone to assess the benefit-to-harm ratio of chemohormonal therapy for mHSPC. Our data suggest that overall QOL declines for men in the ADT+D arm at 3 months, but improves to nearly baseline levels by 12 months. As we might expect, ADT+D was associated with poorer physical and functional QOL at 3 months, presumably because of treatment effects, but differences between the groups resolved by 6 months. There was a gradual decline in QOL over time in the ADT-alone arm, consistent with findings in other metastatic PCa populations on effective hormonal therapy.24 Treatment burden as reflected by the FACT-Taxane demonstrated significantly poorer scores among men treated with ADT+D than with ADT alone at all time points after baseline. Fatigue was significantly greater in the ADT+D arm when compared with ADT only at 3 months, and pain intensity and interference were similar between arms at all time points. Although the identified differences were significantly different statistically, changes in QOL did not meet the criteria for clinically meaningful change by any measure.
When using the findings from this study in clinical decisions, individual patient characteristics, specific comorbidities, performance status, and other psychosocial factors that may contribute to a patient’s tolerance of treatment and treatment experience should be considered. This patient population represents a slightly younger age than the average age of men with PCa (median age of 63 years in the E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer [CHAARTED] v median age of 66 years in men with PCa), and a majority have high-volume metastatic disease (64.9% overall) and an ECOG performance status of 0 (nearly 70%) or 1 (approximately 29%).2 The findings may be less applicable to older, frailer men and should be considered in concert with the updated analysis that fails to find a survival advantage from chemohormonal therapy in men with low-volume disease.5 Clinicians should also consider QOL data from treatment with ADT, abiraterone acetate, and prednisone until progression in the mHSPC setting.26,27 Abiraterone acetate and chemohormonal therapy may confer similar survival benefits in this patient population, and considerations of differences in the adverse effect profile of long-term abiraterone acetate and prednisone are likely to be distinct from those associated with six cycles of docetaxel and ongoing ADT.28
Although patient-reported outcomes are commonly incorporated into PCa clinical trials for localized disease, they have been less consistently included in studies of advanced PCa.29-31 A challenge for the field has been a lack of consistency across studies in the QOL instruments included in trials of advanced PCa, making comparisons of different treatment approaches less than straightforward. Treatment trials in men with metastatic PCa have not consistently demonstrated a significant change in global measures of QOL.8,32 To address this, we included an analysis of FACT-P subscales and additional QOL instruments and compared QOL between and within arms, to provide a more nuanced description of QOL than could be provided by relying on a single instrument alone.
To our knowledge, this prospective, randomized study is the first QOL comparison between chemohormonal therapy and ADT alone, and the first longitudinal analysis of these treatments. As clinicians determine whether to guide decisions by overall survival data in CHAARTED, LATITUDE, and STAMPEDE, by CHAARTED subgroup analysis data for high- versus low-volume mHSPC, or by QOL data from these studies, this QOL analysis will enable them to consider the QOL effects of these treatments in the shared decision-making process.3-5,26,27,33 This study is limited in that the analysis cannot definitively attribute detriments in QOL to treatment or disease progression with the included instruments, although FACT-T changes are likely more specific to treatment than to disease progression. However, to the best of our knowledge, it is the most comprehensive study of the QOL to date in this patient population. The longitudinal data demonstrating an improvement in overall QOL at 12 months in the chemohormonal arm suggests that the physical and functional deficits present at the 3-month time point are reversible short-term deficits. Because participants were not blinded to treatment, it is also possible that the knowledge of having received an additional treatment (chemotherapy) might have affected their perspective and QOL reports, although this was likely reduced by the comprehensive assessment of diverse aspects of QOL (physical, functional, prostate cancer [PCa] specific, and so forth). In addition, we report QOL differences that were statistically significant as reported by patients, but we cannot define within this study how clinically meaningful they were to patients. Separate work has defined clinically meaningful change in the QOL measures used in various clinical settings.10,14,16,19,20 Clinically meaningful differences can be calculated by multiple methods, including anchoring to patient-reported global rating scales or objective physician-designated clinical measures (eg, performance status), or on the basis of standard deviation within a cohort.34,35 Although the differences in QOL in this study did not meet previously defined MID levels, these changes and differences were rigorously collected from patients during treatment and are a valid patient-reported contribution to consider when choosing treatment of mHSPC. Finally, we acknowledge that this study does not report on late effects from treatment that may be important in clinical decision making.
This analysis compares the QOL of men receiving ADT+D and ADT alone, assesses QOL over time with multiple instruments, and evaluates QOL by disease burden. Men treated with ADT+D had poorer QOL outcomes than did men treated with ADT alone at 3 months while undergoing chemotherapy. However, by 12 months, ADT+D patients reported statistically superior overall QOL outcomes by FACT-P. These findings must be considered in the context of the population assessed and in the context of a simultaneously reported subgroup analysis that failed to find an improvement in overall survival in men with low-volume mHSPC treated with ADT+D. Clinicians treating men with mHSPC who are fit enough to receive chemotherapy should consider that ADT+D is associated with both stable to improved QOL at 12 months and greater longevity than ADT alone.
Appendix
Descriptive statistics were used to characterize reasons for Functional Assessment of Cancer Therapy-Prostate (FACT-P) noncompliance and the distribution of quality of life (QOL) scores. Differences in the distribution of QOL scores between arms and changes in QOL scores from baseline to follow-up were evaluated using the Wilcoxon rank sum test and the Wilcoxon signed rank test, respectively.22 Distribution of FACT-P total scores and changes from baseline were also presented by volume of disease and among the 25% of patients with the greatest alterations at 3 months. Mixed-effect models with random intercepts for each individual were used to evaluate the differences in Trial Outcome Index score between the two arms over time.23 Several baseline factors, including age (≤ 59 years v 60 to 69 years v ≥ 70 years), extent of disease (high volume v low volume), local therapy (yes v no), Eastern Cooperative Oncology Group PS (0 v 1 or 2), baseline physical well-being (≤ 20 v 20 < PWB ≤ 25 v > 25) and baseline pain score (0 or 1 v 2 or 3 v ≥ 4) were included in the model and were considered to be fixed effects. Assessment time point was considered a categorical variable, and the interaction between treatment arm and assessment time was included in the model. A joint model (Schluchter MD: Methods for the analysis of informatively censored longitudinal data. Stat Med 11(14-15):1861-1870, 1992) of longitudinal FACT-P measurements and survival data was performed to explore the trend of QOL changes over time. The mean slope of FACT-P scores over time was estimated for each arm, and bootstrap standard errors were computed. QOL data reflect all available data as of August 2015. All tests were two sided, and P values < .05 were considered significant.
Fig A1.
CONSORT diagram. ADT+D, docetaxel and androgen deprivation therapy; ADT, androgen deprivation therapy.
Table A1.
Reasons for FACT-P Noncompliance by Arm Over Time*
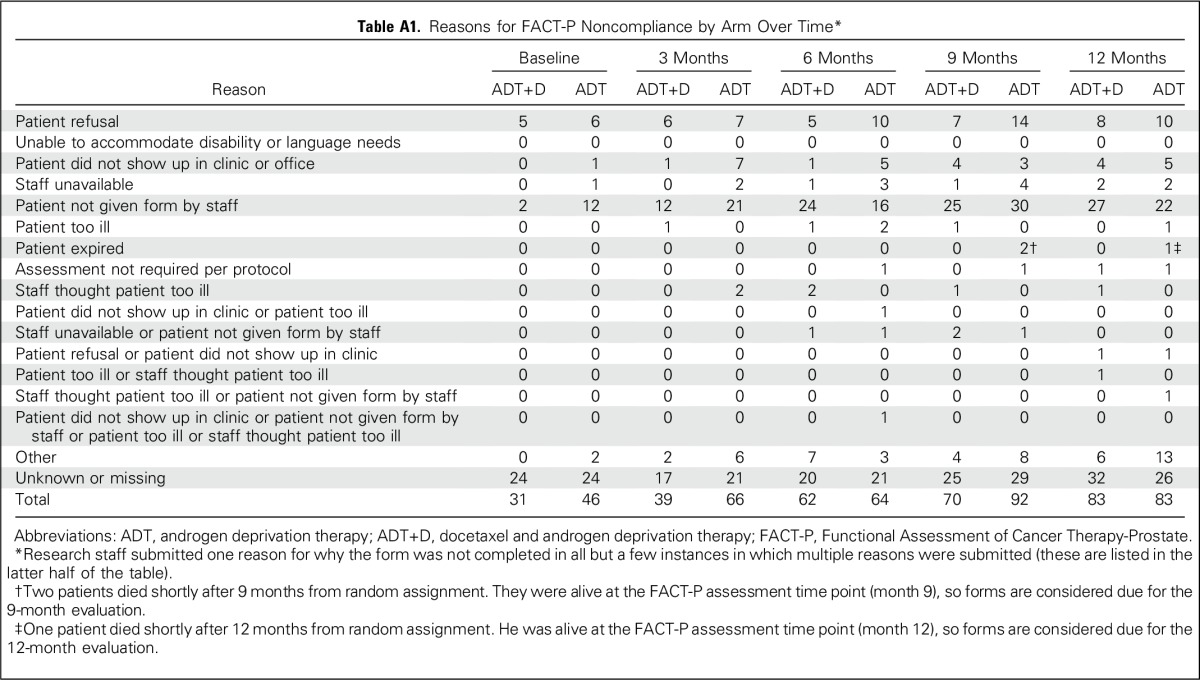
Table A2.
Estimation of Mean Slope of FACT-P Score in a Joint Model of QOL Assessments and Survival Data
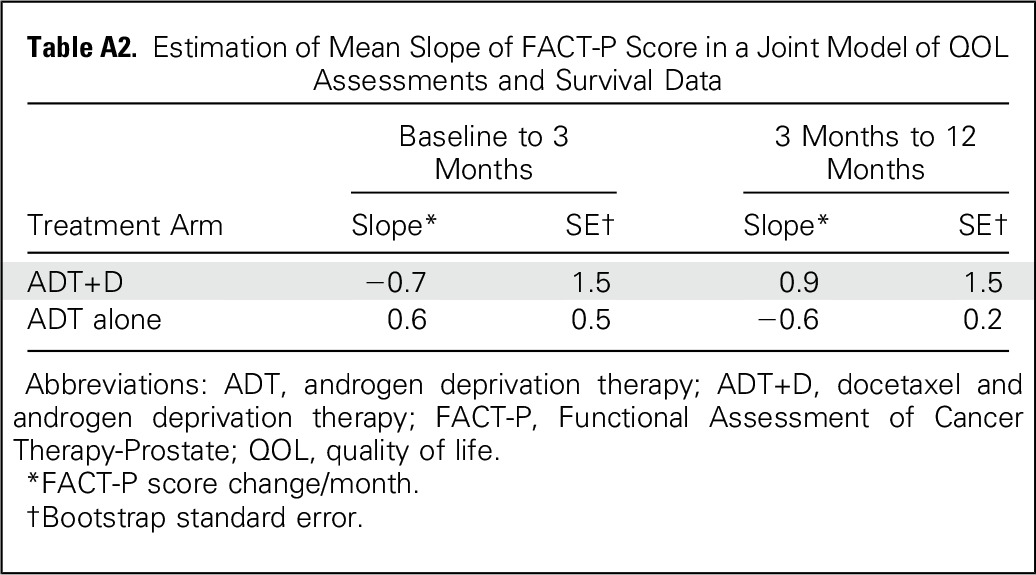
Table A3.
Distributions of Changes from Baseline to Follow-Up for FACT-P Total Scores*
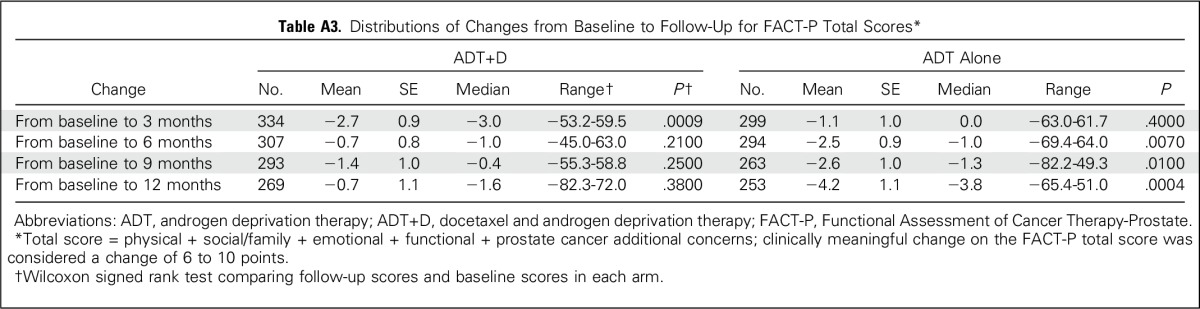
Table A4.
Distribution of FACT-P Total Scores and Subscale Scores Over Time by Arm
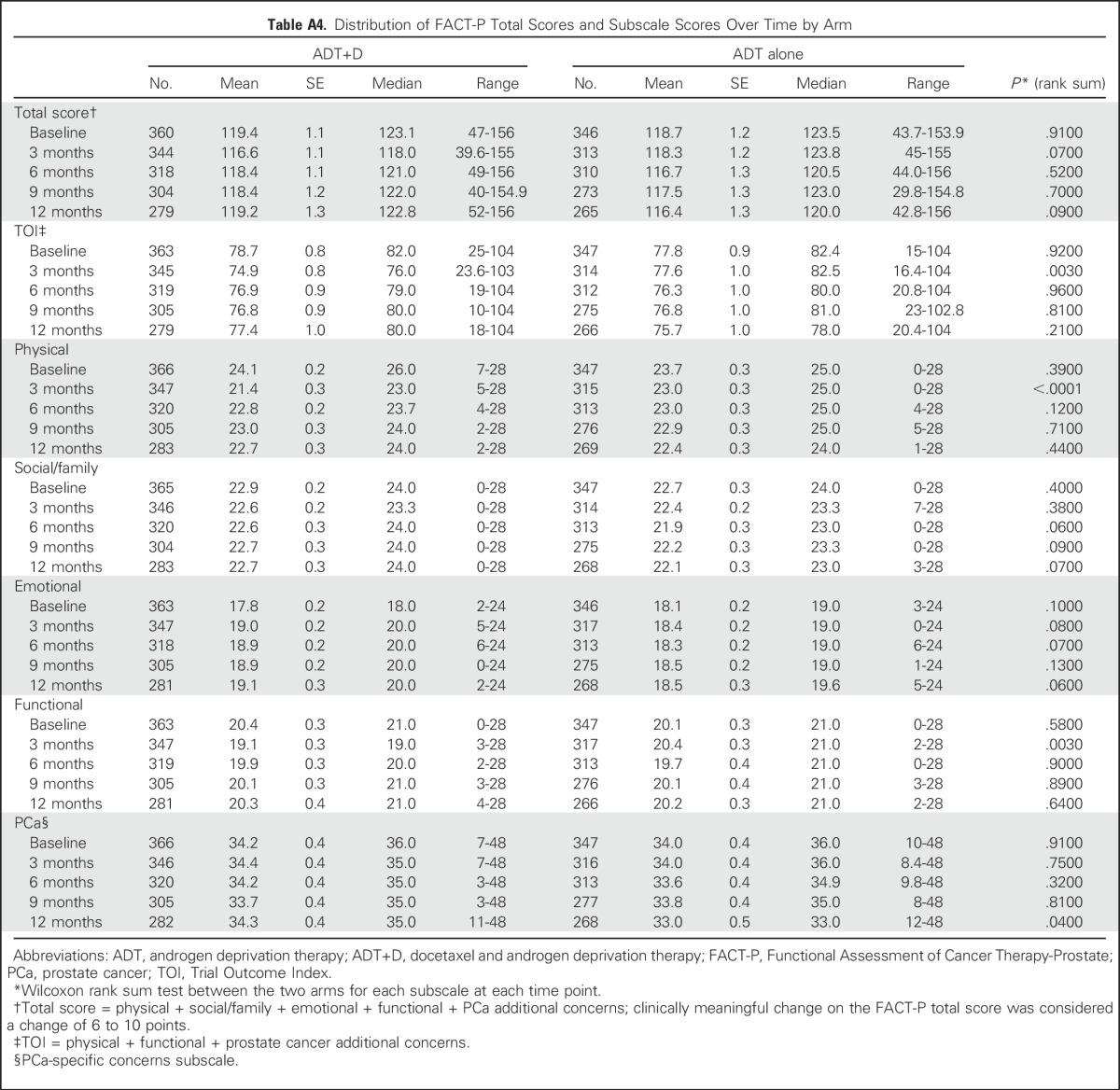
Table A5.
Mixed-Effects Model* for TOI Score
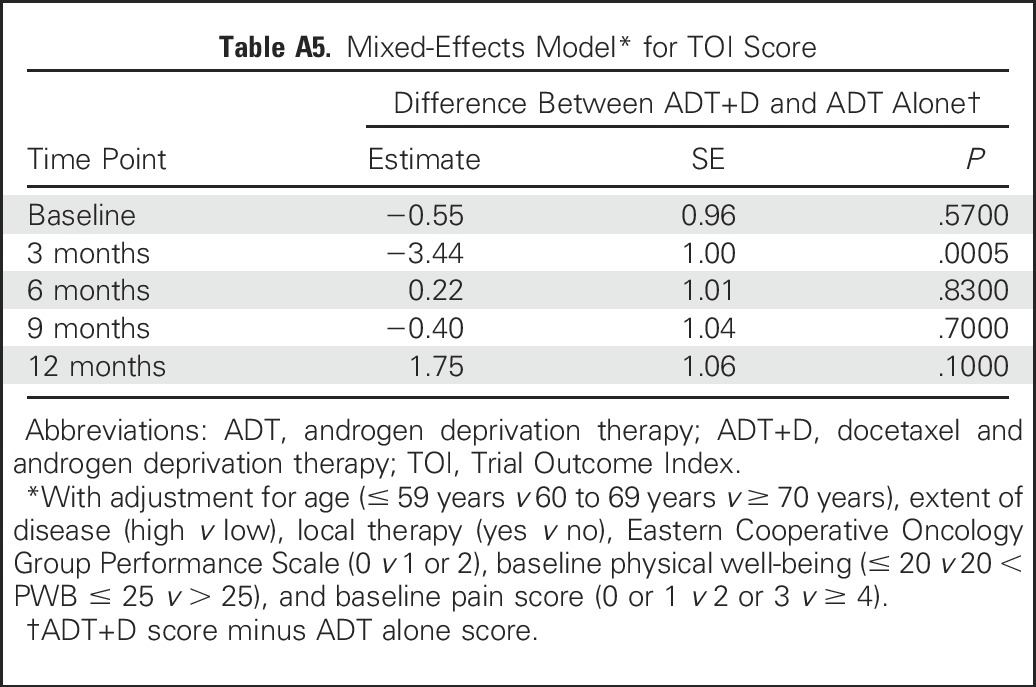
Table A6.
Distribution of Additional QOL Scores Over Time by Treatment Arm, Including FACT-Taxane, FACIT-Fatigue, and Brief Pain Index Pain and Interference Scores
Table A7.
Distribution of FACT-P Total Score* Over Time by Arm and Disease Volume
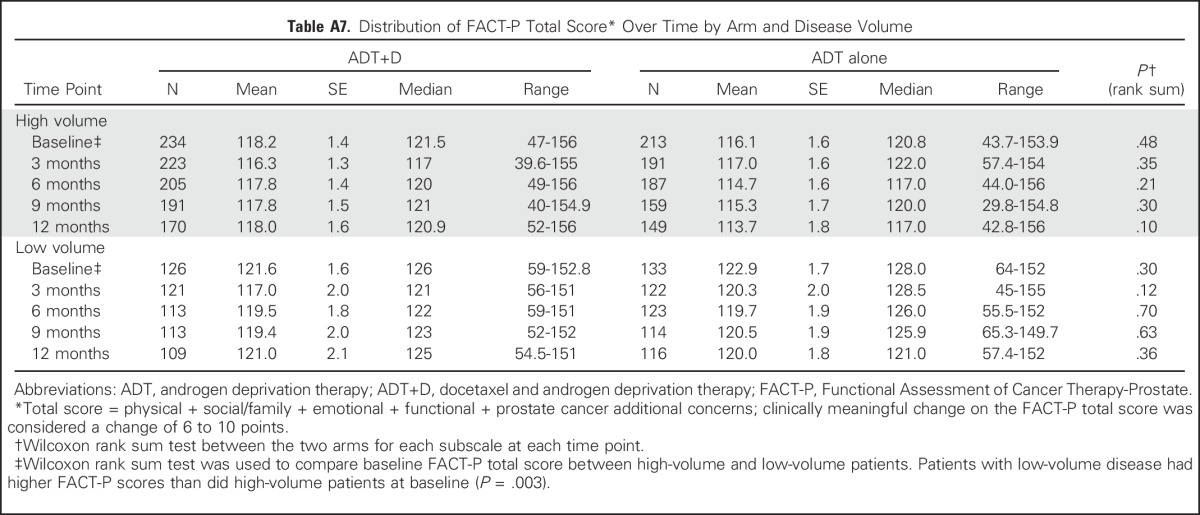
Table A8.
Distributions of Changes From Baseline to Follow-Up for FACT-P Total Scores by Arm and Disease Volume*
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA180794, CA180888, CA180847, CA180867, CA180799, [CA189859], CA180802, and CA180853. Also sponsored by Sanofi via a grant to Eastern Cooperative Oncology Group-American College of Radiology Imaging Network.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Clinical trial information: NCT00309985.
Listen to the podcast by Dr Alibhai at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Christopher J. Sweeney, David F. Jarrard, Benjamin A. Gartrell, Michael A. Carducci, Linda J. Patrick-Miller
Provision of study materials or patients: Michael A. Carducci
Collection and assembly of data: Alicia K. Morgans, Yu-Hui Chen, Christopher J. Sweeney, Benjamin A. Gartrell, Michael A. Carducci, Maha Hussain
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Quality of Life During Treatment With Chemohormonal Therapy: Analysis of E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Alicia K. Morgans
Honoraria: Genentech, Janssen Pharmaceuticals
Consulting or Advisory Role: Genentech, AstraZeneca, Sanofi
Travel, Accommodations, Expenses: Genentech, AstraZeneca, Janssen Pharmaceuticals
Yu-Hui Chen
Employment: Constellation Pharmaceuticals (I)
Christopher J. Sweeney
Stock or Other Ownership: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer AG, Genentech/Roche, AstraZeneca, Pfizer
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer AG (Inst), Sotio (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide. Exelixis: Abiraterone plus cabozantinib combination
David F. Jarrard
No relationship to disclose
Elizabeth R. Plimack
Consulting or Advisory Role: AstraZeneca/MedImmune, Bristol-Myers Squibb, Clovis Oncology, Exelexis, AstraZeneca/MedImmune, Synergene, Acceleron Pharma, Eli Lilly, Horizon Pharma, Pfizer, Inovio Biomedical
Research Funding: Bristol-Myers Squibb (Inst), Acceleron Pharma (Inst), AstraZeneca (Inst), Pfizer (Inst), Eli Lilly (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), Peloton Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: US patent No: 14/588,503, filed January 2, 2015 (Inst); US patent No: 15/226,474 filed on July 1, 2015 (Inst)
Benjamin A. Gartrell
Consulting or Advisory Role: Pfizer, Exelixis, Bayer AG
Michael A. Carducci
Consulting or Advisory Role: Astellas Pharma, Churchill Pharmaceuticals, Abbvie, Roche/Genentech, Pfizer
Research Funding: Bristol-Myers Squibb (Inst), Pfizer (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
Maha Hussain
Honoraria: Onclive, Sanofi
Research Funding: Genentech (Inst), Pfizer (Inst), PCCTC (Inst), Bayer AG (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Title: Systems and Methods for Tissue Imaging, 3676 Our file: Serial No. UM-14437/US-1/PRO 60/923,385 UM-14437/US-2/ORD 12/101,753US 8,185,186 (US patent No.) Systems and methods for tissue imaging (issued patent) EP 08745653.9 (EP application No.) Systems and methods for tissue imaging (pending) CA 2683805 (Canadian application No.) Systems and methods for tissue imaging (pending) US 13/362,500 (US application No.) Systems and Methods for Tissue Imaging (continuation application of US 8,185,186); Title: Method of Treating Cancer, Docket No: Serial No. 224990/10-016P2/311733 61/481/671 Application filed May 2, 2011; Title: Dual Inhibition of MET and VEGF for the treatment of castration resistant prostate cancer and osteoblastic bone metastases. Applicant/Proprietor Exelexis. Application No./Patent No. 11764665.4-1464 Application No./Patent No. 11764656.2-1464 Application Filed September 9, 2011
Travel, Accommodations, Expenses: Sanofi
Jorge A. Garcia
Honoraria: Astellas Pharma, Sanofi, Bayer AG, Pfizer
Consulting or Advisory Role: Sanofi, Pfizer, Bayer AG, Medivation
Speakers' Bureau: Bayer AG, Sanofi, Medivation/Astellas Pharma
Research Funding: Pfizer (Inst), Astellas Pharma (Inst), Orion Pharma GmbH (Inst), Bayer AG (Inst), Janssen Oncology (Inst), Genentech/Roche (Inst), Eli Lilly (Inst)
Travel, Accommodations, Expenses: Pfizer, Bayer AG, Sanofi, Exelixis, Eisai, Medivation/Astellas, Genentech/Roche
David Cella
No relationship to disclose
Robert S. DiPaola
No relationship to disclose
Linda J. Patrick-Miller
No relationship to disclose
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, et al: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11, Lyon, France, International Agency for Research on Cancer, 2013. [Google Scholar]
- 2. Key statistics for prostate cancer, in American Cancer Society 2017. [Google Scholar]
- 3.Sweeney CJ, Chen YH, Carducci M, et al. : Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737-746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James ND, Sydes MR, Clarke NW, et al. : Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387:1163-1177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sweeney C, Chen YH, Liu G, et al: Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial. European Society for Medical Oncology Annual Congress 2016, Copenhagen, Denmark, 2016 (abstr 720PD) [Google Scholar]
- 6. Mohler JL, Lee RJ, Antonarakis ES, et al: NCCN Clinical Practice Guidelines in Oncology, Version 1.2016: Prostate Cancer. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, et al. : Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502-1512, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Stockler MR, Osoba D, Corey P, et al. : Convergent discriminitive, and predictive validity of the Prostate Cancer Specific Quality of Life Instrument (PROSQOLI) assessment and comparison with analogous scales from the EORTC QLQ-C30 and a trial-specific module. European Organisation for Research and Treatment of Cancer. Core Quality of Life Questionnaire. J Clin Epidemiol 52:653-666, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Esper P, Mo F, Chodak G, et al. : Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 50:920-928, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Nichol MB, Eton D, et al. : Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy--Prostate: Results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 12:124-129, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Basch E, Autio K, Ryan CJ, et al. : Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: Patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol 14:1193-1199, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Nilsson S, Cislo P, Sartor O, et al. : Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol 27:868-874, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harland S, Staffurth J, Molina A, et al. : Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer 49:3648-3657, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Peterman A, Hudgens S, et al. : Measuring the side effects of taxane therapy in oncology: The functional assessment of cancer therapy-taxane (FACT-taxane). Cancer 98:822-831, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Yellen SB, Cella DF, Webster K, et al. : Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 13:63-74, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Nordin Å, Taft C, Lundgren-Nilsson Å, et al. : Minimal important differences for fatigue patient reported outcome measures-a systematic review. BMC Med Res Methodol 16:62, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daut RL, Cleeland CS, Flanery RC: Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 17:197-210, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS: The measurement of pain from metastatic bone disease: Capturing the patient’s experience. Clin Cancer Res 12:6236s-6242s, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Wong K, Zeng L, Zhang L, et al. : Minimal clinically important differences in the brief pain inventory in patients with bone metastases. Support Care Cancer 21:1893-1899, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Mathias SD, Crosby RD, Qian Y, et al. : Estimating minimally important differences for the worst pain rating of the Brief Pain Inventory-Short Form. J Support Oncol 9:72-78, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Cox DR: Analysis of Binary Data (Monographs on Applied Probability & Statistics). London, United Kingdom, Methuen and Co, 1970. [Google Scholar]
- 22.Wilcoxon F: Individual comparisons by ranking methods. Biom Bull 1:80-83, 1945 [Google Scholar]
- 23.Fitzmaurice G, Laird N, Ware J: Applied Longitudinal Analysis . Hoboken, NJ, John Wiley & Sons, 2004. [Google Scholar]
- 24.Loriot Y, Miller K, Sternberg CN, et al. : Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol 16:509-521, 2015 [DOI] [PubMed] [Google Scholar]
- 25. Reference deleted. [Google Scholar]
- 26.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352-360, 2017 [DOI] [PubMed] [Google Scholar]
- 27.James ND, de Bono JS, Spears MR, et al. : Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377:338-351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sydes MR, Mason MD, Spears MR, et al: LBA31_PR-Adding abiraterone acetate plus prednisolone (AAP) or docetaxel for patients (pts) with high-risk prostate cancer (PCa) starting long-term androgen deprivation therapy (ADT): Directly randomised data from STAMPEDE ( NCT00268476). Ann Oncol 28: v605-v649, 2017 (suppl 5) [Google Scholar]
- 29.Fallowfield L, Payne H, Jenkins V: Patient-reported outcomes in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 13:643-650, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Resnick MJ, Koyama T, Fan KH, et al. : Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 368:436-445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen RC, Basak R, Meyer AM, et al. : Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA 317:1141-1150, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith DC, Esper P, Strawderman M, et al. : Phase II trial of oral estramustine, oral etoposide, and intravenous paclitaxel in hormone-refractory prostate cancer. J Clin Oncol 17:1664-1671, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Chi K Protheroe A, Rodriguez Antolin A, et al: Benefits of abiraterone acetate plus prednisone (AA+P) when added to androgen deprivation therapy (ADT) in LATITUDE on patient reported outcomes. Ann Oncol 28:v269-v294, 2017 (suppl 5) [Google Scholar]
- 34.Ringash J, O’Sullivan B, Bezjak A, et al. : Interpreting clinically significant changes in patient-reported outcomes. Cancer 110:196-202, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Revicki D, Hays RD, Cella D, et al. : Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 61:102-109, 2008 [DOI] [PubMed] [Google Scholar]