Abstract
Purpose
Docetaxel added to androgen-deprivation therapy (ADT) significantly increases the longevity of some patients with metastatic hormone-sensitive prostate cancer. Herein, we present the outcomes of the CHAARTED (Chemohormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer) trial with more mature follow-up and focus on tumor volume.
Patients and Methods
In this phase III study, 790 patients with metastatic hormone-sensitive prostate cancer were equally randomly assigned to receive either ADT in combination with docetaxel 75 mg/m2 for up to six cycles or ADT alone. The primary end point of the study was overall survival (OS). Additional analyses of the prospectively defined low- and high-volume disease subgroups were performed. High-volume disease was defined as presence of visceral metastases and/or ≥ four bone metastases with at least one outside of the vertebral column and pelvis.
Results
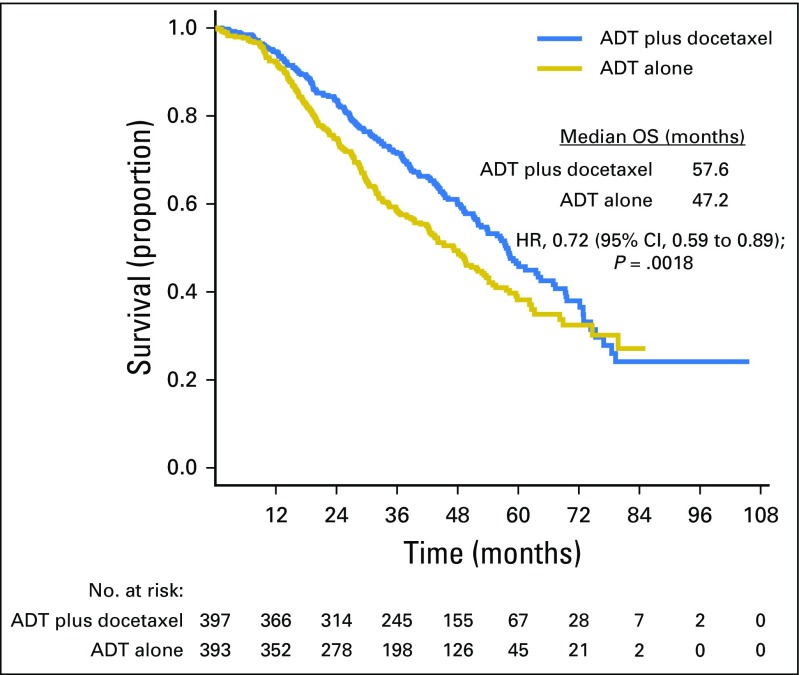
At a median follow-up of 53.7 months, the median OS was 57.6 months for the chemohormonal therapy arm versus 47.2 months for ADT alone (hazard ratio [HR], 0.72; 95% CI, 0.59 to 0.89; P = .0018). For patients with high-volume disease (n = 513), the median OS was 51.2 months with chemohormonal therapy versus 34.4 months with ADT alone (HR, 0.63; 95% CI, 0.50 to 0.79; P < .001). For those with low-volume disease (n = 277), no OS benefit was observed (HR, 1.04; 95% CI, 0.70 to 1.55; P = .86).
Conclusion
The clinical benefit from chemohormonal therapy in prolonging OS was confirmed for patients with high-volume disease; however, for patients with low-volume disease, no OS benefit was discerned.
INTRODUCTION
Although most patients with metastatic hormone-sensitive prostate cancer (mHSPC) initially respond to androgen-deprivation therapy (ADT),1 the duration of response is variable, and invariably, all patients develop castration-resistant prostate cancer (CRPC). Several therapies have been shown to improve the overall survival (OS) of men with CRPC; however, most patients eventually die as a result of CRPC within a few years.2 Combining novel therapies with ADT at the time of initiating systemic therapy for mHSPC has emerged as a strategy to potentially delay the development of CRPC and improve quality of life and OS.3-6
Docetaxel was the first drug shown to improve the OS of men with mHSPC.3,4 Early treatment with docetaxel is hypothesized to attack castration-resistant clones that may already be present at the time of presentation with metastatic disease, a phenomenon that is probably proportional to disease burden. Thus, early treatment with cytotoxic chemotherapy may delay progression to CRPC. The first phase III study of docetaxel with ADT versus ADT alone in mHSPC (GETUG-AFU 15 [Groupe d’Etudes des Tumeurs Uro-Génitales and Association Française d'Urologie 15]) failed to show a survival benefit from docetaxel,7 although with longer follow-up, there was a nonsignificant trend in favor of the combination arm (median OS of 48.6 months with ADT alone v 62.1 months with ADT plus docetaxel; P = .3).8 Then, the interim analysis of the CHAARTED (Chemohormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer) trial as well as that of the STAMPEDE (Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy) trial demonstrated a survival benefit.3,4 One notable difference between GETUG-AFU 15 versus STAMPEDE and CHAARTED was access to newer life-prolonging therapies for CRPC. Interestingly, the ADT alone arms of CHAARTED and metastatic STAMPEDE cohort had a median OS of 44 and 45 months, respectively, suggesting a similar mix between patients with low and high tumor burden. CHAARTED and GETUG-AFU 15 used the same definition for low- and high-volume disease, but those with low-volume disease comprised 35% (277 of 790) of patients in CHAARTED and 52.5% (202 of 385) of patients in GETUG-AFU 15. The long-term follow-up of GETUG-AFU 15 noted a trend in improvement for OS with the high-volume subgroup, in line with STAMPEDE and CHAARTED.8,9 In contrast, there was no suggestion of benefit from early docetaxel in low-volume patients (hazard ratio [HR], for death, 1.02; 95% CI, 0.67 to 1.55; P = .9).8
Another variable possibly associated with poorer outcome with ADT alone is de novo metastatic disease.10,11 Furthermore, retrospective studies have suggested that treatment of the primary tumor in patients with metastatic disease may confer a survival benefit.12-15 As such, several randomized phase III trials of systemic therapy with or without treatment of the primary tumor are under way (ClinicalTrials.gov identifiers NCT00268476, NCT01957436, NTR271, NCT01751438, and NCT02454543).
We previously reported a survival benefit with the addition of docetaxel at an interim evaluation of the CHAARTED study.3 The survival benefit was noted for the study population as a whole (HR for death, 0.61; 95% CI, 0.47 to 0.80; P < .001), with the greatest benefit demonstrated in the high-volume subgroup (HR for death, 0.6; 95% CI, 0.45 to 0.81; P < .001), although the study was powered for the entire population and not the subgroups. At the time of the first report, there were only 44 deaths among 277 patients with low-volume disease, and there was a nonsignificant decrease in the risk of death (HR for death, 0.6; 95% CI, 0.32 to 1.13; P = .11). Here we present the updated analysis of the trial as a whole, by disease volume as prespecified, as well as by an unplanned analysis on the basis of prior local therapy (PLT) with or without curative intent.
PATIENTS AND METHODS
Study Design and Participants
As detailed previously,3 this multicenter, randomized, open-label, phase III National Cancer Institute study led by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network enrolled patients with mHSPC who had performance status and organ function suitable for docetaxel. All patients provided written informed consent before study entry. The study was conducted in accordance with the Declaration of Helsinki for human subject protection.
Procedures
Eligible patients were randomly assigned at a one-to-one ratio to ADT alone versus ADT plus docetaxel at a dose of 75 mg/m2 every 3 weeks for up to six cycles without daily prednisone. Randomization was stratified according to age (< 70 v ≥ 70 years), Eastern Cooperative Oncology Group performance status (0 to 1 v 2), duration of prior adjuvant therapy with ADT (> 12 v ≤ 12 months), planned use of combined androgen blockade for > 30 days (yes v no), and use of agents to prevent skeletal-related events (eg, zoledronic acid or denosumab). A key stratification factor was disease volume (high v low), with high-volume disease defined as presence of visceral metastases and/or at least four bone lesions with at least one lesion outside of the vertebral column and/or pelvis.
Neither dose modification nor intermittent ADT was permitted. Use of nonsteroidal antiandrogens was at the discretion of the investigators. Patients were allowed only two dose reductions for docetaxel, the first to 65 mg/m2 and, if indicated, a second to 55 mg/m2. Patients discontinued docetaxel permanently if there was a delay of > 3 weeks from the scheduled treatment day. Growth factor support was at the discretion of the investigators.
Study Assessments
Patients receiving chemohormonal therapy were evaluated every 3 weeks for the duration of treatment with chemotherapy and every 3 months thereafter, whereas patients receiving ADT alone were evaluated every 3 months. Radiographic disease assessment (with computed tomography [CT] of the abdomen and pelvis, x-ray or CT of the chest, and bone scan) was performed at baseline, at the time of development of CRPC, and/or as clinically indicated. For patients with measurable disease, disease evaluation was based on Response Evaluation Criteria in Solid Tumors (version 1.0). Serum prostate-specific antigen (PSA) concentration was checked with every visit. Complete PSA response was defined as a decrease to < 0.2 ng/mL confirmed by a second PSA 4 weeks later. Disease progression by PSA was defined as an increase in the PSA level by > 50% above nadir confirmed by a consecutive increase at least 2 weeks later. For patients with a PSA nadir of < 2 ng/mL, a PSA value of ≥ 2 ng/mL was required for disease progression by PSA only and qualified as CRPC.
The primary end point of the study was OS, defined as time from random assignment until death resulting from any cause. Secondary end points included time to development of CRPC, defined either serologically or clinically from the time of random assignment until PSA progression, development of worsening symptoms, evidence of radiographic progression, or patient’s deterioration as per investigator’s opinion.
Statistical Analysis
The Kaplan-Meier method16 was used to characterize event-time distributions. Cox proportional hazards models,17 stratified on stratification factors at random assignment, were used to estimate HRs and test for significance for time-to-event end points.
RESULTS
Patients
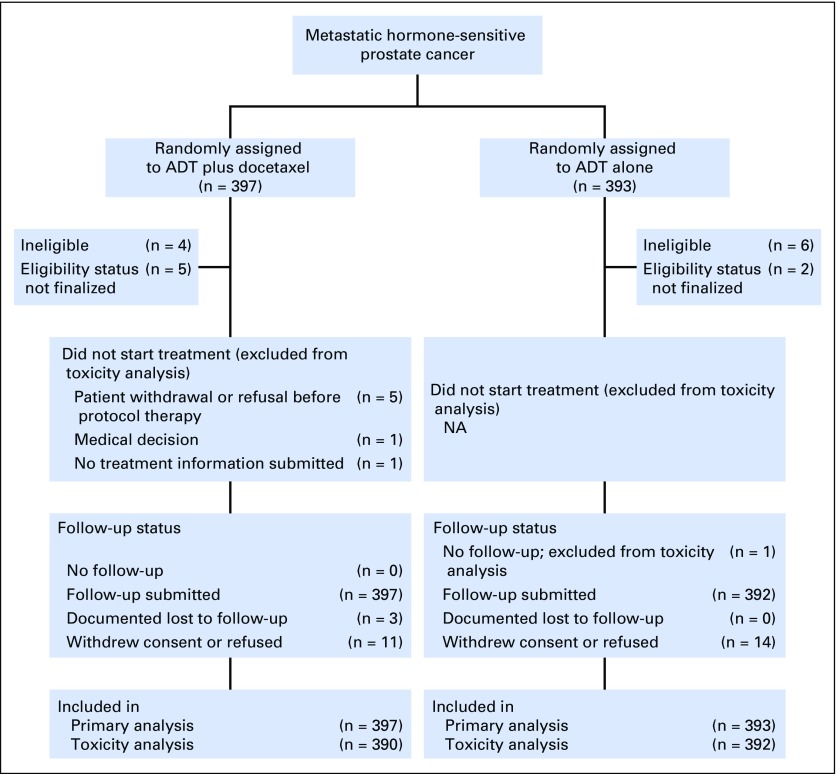
Between July 2006 and December 2012, 790 eligible patients were enrolled (Fig 1), including 397 in the ADT plus docetaxel arm and 393 in the ADT alone arm. This report represents data with a cutoff date for survival of April 23, 2016, resulting in a median follow-up of 53.7 months. Treatment groups were well balanced, as previously described.3 The median age was 64 years (range, 36 to 88 years) in the combination arm versus 63 years (range, 39 to 91 years) in the ADT alone arm. In terms of tumor volume, 263 patients (66.2%) in the combination arm and 250 patients (63.6%) in the ADT alone arm had high-volume disease. Furthermore, 289 patients (72.8%) in the combination arm versus 286 patients (72.8%) in the ADT alone arm had had no PLT and were considered to have de novo metastatic disease.
Fig 1.
CONSORT diagram. Overview of screened and randomly assigned patients. ADT, androgen-deprivation therapy; NA, not applicable.
Efficacy
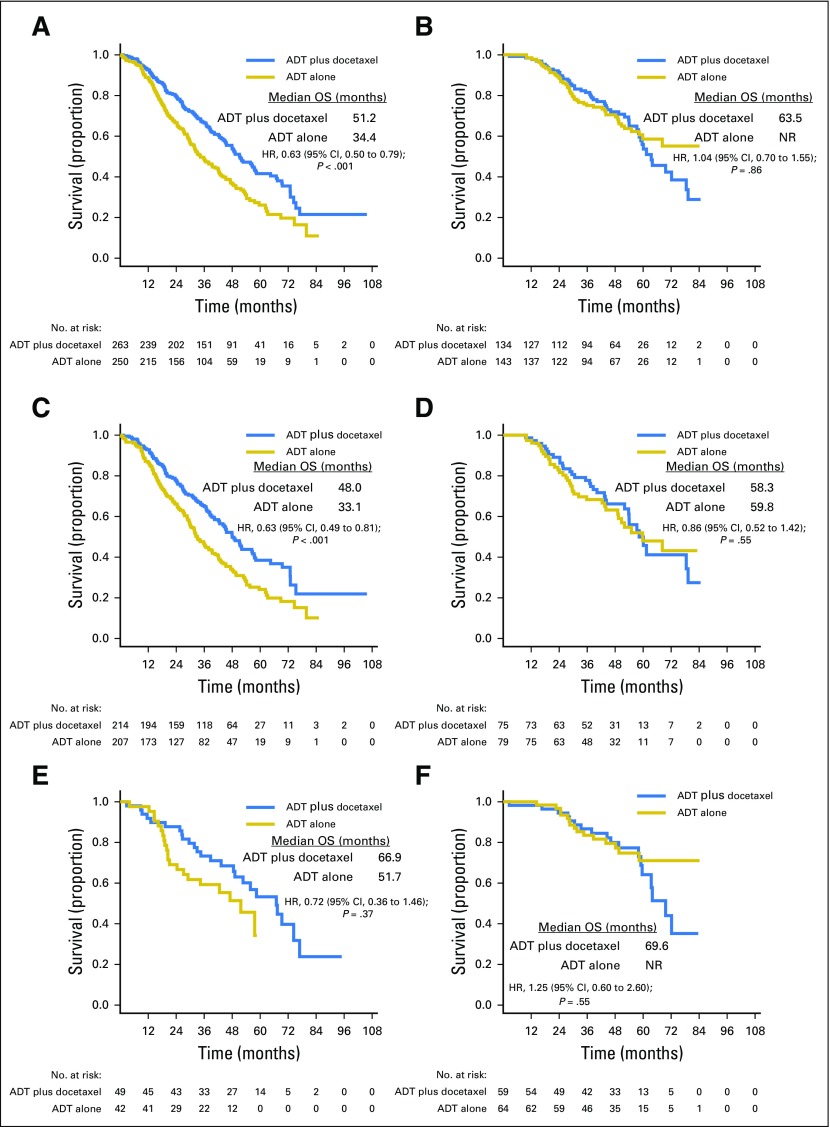
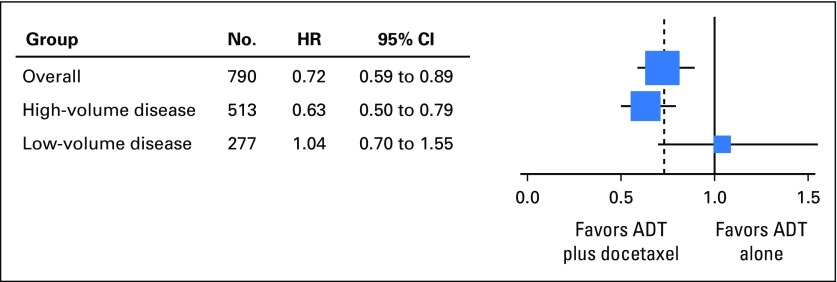
At the time of this analysis, there were 188 deaths in the combination arm and 211 deaths in the ADT alone arm. For the overall population, the median OS was 10.4 months longer in the combination arm (57.6 v 47.2 months; HR for death in the combination arm, 0.72; 95% CI, 0.59 to 0.89; P = .0018; Table 1; Fig 2). Longer follow-up confirmed that the effect of docetaxel was more pronounced for patients with high-volume disease. In this prospectively defined subgroup, with a median follow-up of 53.7 months, there was a median OS benefit of 16.8 months (median OS, 51.2 v 34.4 months; HR for death in the combination arm, 0.63; 95% CI, 0.50 to 0.79; P < .001). In contrast, in the low-volume subgroup, with a median follow-up of 53.8 months, a survival benefit from docetaxel was not confirmed (median OS, 63.5 months for the chemohormonal arm v not reached for the ADT alone arm; HR for death in the combination arm, 1.04; 95% CI, 0.70 to 1.55; P = .86; Table 1; Fig 3). The interaction between treatment and disease volume was examined and showed heterogeneity between patients with high- and low-volume disease (P = .033; Fig 4). The benefit of docetaxel treatment was detected in all other subgroups analyzed and was similar to the initial analysis (Data Supplement).
Table 1.
OS and No. of Deaths by Subgroup
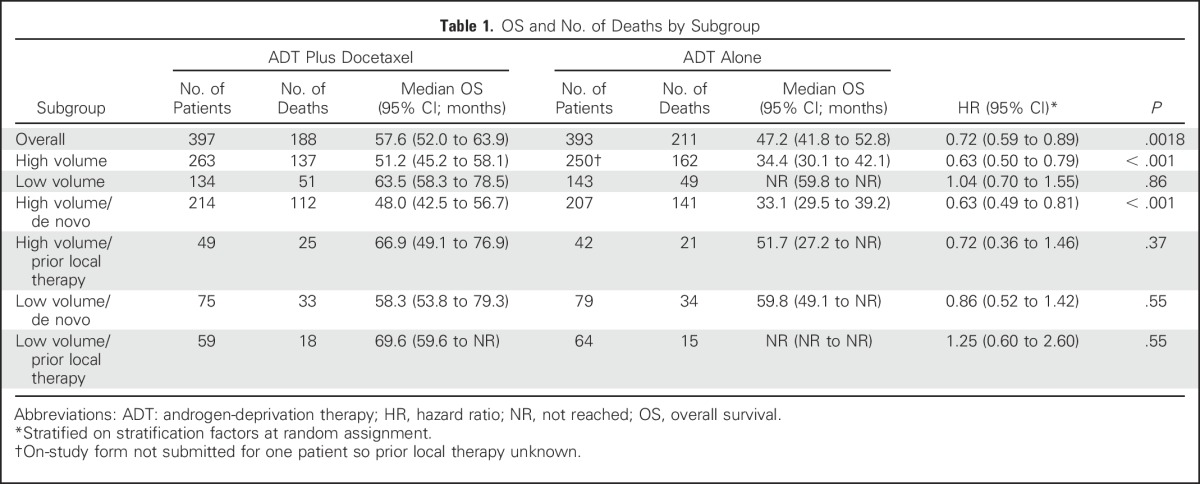
Fig 2.
Overall survival (OS) by treatment arm among all patients. ADT, androgen-deprivation therapy; HR, hazard ratio.
Fig 3.
Kaplan-Meier estimates of overall survival (OS) for (A) High-volume total patient population, (B) Low-volume total patient population, (C) High-volume de novo metastatic patients, (D) Low-volume de novo metastatic patients, (E) High-volume patients with prior local therapy, (F) Low-volume patients with prior local therapy. HR, hazard ratio; NR, not reached.
Fig 4.
Test of heterogeneity between patients with high- and low-volume disease. ADT, androgen-deprivation therapy. The size of the squares is proportional to the inverse of the variance of the log hazard ratio (small squares correspond to large variances).
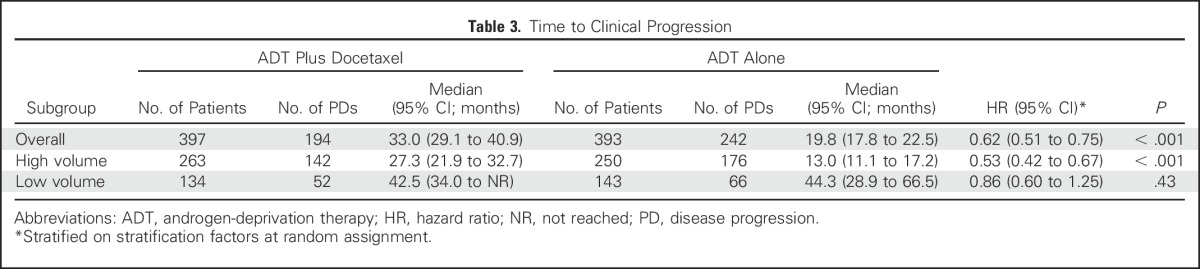
Other clinical end points assessed were time to development of CRPC (Table 2) and time to clinical progression (Table 3). The time to CRPC was 19.4 months in the combination arm versus 11.7 months in the ADT alone arm (HR in the combination arm, 0.61; 95% CI, 0.52 to 0.73; P < .001). For high-volume disease, the median time to CRPC was 14.9 months for the combination arm versus 8.6 months for the ADT alone arm (HR for the combination arm, 0.58; 95% CI, 0.47 to 0.71; P < .001); for low-volume disease, it was 31.0 months for the combination arm versus 22.7 months for the ADT alone arm (HR for the combination arm, 0.70; 95% CI, 0.50 to 0.96; P = .03). Similarly, the median time to clinical progression was 33.0 months for the combination arm versus 19.8 months in the ADT alone arm (HR in the combination arm, 0.62; 95% CI, 0.51 to 0.75; P < .001), a difference that was confirmed for patients with high-volume disease (median time to clinical progression, 27.3 months for the combination arm v 13.0 months for the ADT alone arm; HR in the combination arm, 0.53; 95% CI, 0.42 to 0.67; P < .001) but not for those with low-volume disease (median time to clinical progression, 42.5 months in the combination arm v 44.3 months in the ADT alone arm; HR in the combination arm, 0.86; 95% CI, 0.60 to 1.25; P = .43). An unplanned analysis of progression-free survival defined by survival and CRPC as well as survival and clinical progression was conducted and was consistent with the results presented (Data Supplement).
Table 2.
Time to CRPC (PSA rise or clinical progression)
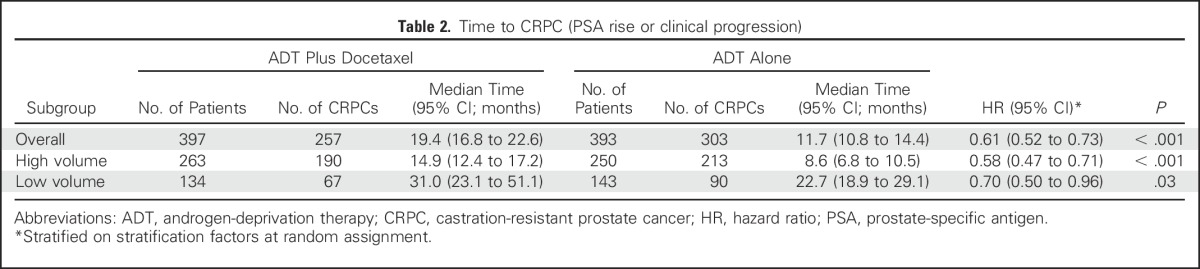
Table 3.
Time to Clinical Progression
When outcome was analyzed by type of presentation, patients with high-volume de novo metastatic disease (n = 421) were found to have a survival benefit of 14.9 months from addition of docetaxel to ADT (median OS, 48.0 v 33.1 months; HR for death in the combination arm, 0.63; 95% CI, 0.49 to 0.81; P < .001). In the smaller subgroup of patients with high-volume disease with PLT (n = 91), those who received ADT alone had a better outcome compared with their counterparts with de novo metastatic disease. When docetaxel was added, there was a numeric improvement in OS; however, this was not statistically significant (median OS, 66.9 v 51.7 months; HR for death in the combination arm, 0.72; 95% CI, 0.36 to 1.46; P = .37; Table 1; Fig 3). In contrast, for the low-volume subgroups, neither patients with de novo metastatic disease nor patients with PLT were found to derive a survival benefit from docetaxel (de novo metastatic low-volume disease: median OS, 58.3 months for the combination arm v 59.8 months for the ADT alone arm; HR for death in the combination arm, 0.86; 95% CI, 0.52 to 1.42; P = .55; low-volume metastatic disease with PLT: median OS, 69.6 months for the combination arm v not reached for the ADT alone arm; HR for death in the combination arm, 1.25; 95% CI, 0.60 to 2.60; P = .55; Table 1; Fig 3).
Safety
A summary of the adverse effects observed in the combination arm was included in the initial publication; these were in accordance with previous experience with docetaxel.9 No additional long-term adverse effects were observed.
DISCUSSION
The updated analysis of the CHAARTED trial with a median follow-up of 53.7 months confirms the OS benefit from early treatment with docetaxel seen at the interim analysis with a median follow-up of 28.9 months, which resulted in an early data release. In addition, all the secondary end points were in favor of the combination arm. With longer follow-up, the clinical benefit observed with chemohormonal therapy was confirmed for patients with high-volume disease regardless of whether they had relapsed after PLT of the prostate with or without curative intent. In contrast, the subgroup with low-volume disease showed no evidence of survival benefit when docetaxel was added (HR, 1.04 with 100 deaths), despite the early analysis showing a nonsignificant HR of 0.60 with 44 deaths.
Although prospectively defined, the subgroups were not powered to be analyzed separately. The test for heterogeneity supports the observation that there was a differential effect of docetaxel in the high- and low-volume subgroups. The different outcomes between the two subgroups (namely, longer OS with ADT alone in the low- v high-volume subgroups and no clear benefit with early chemotherapy for the former) can guide biologic studies and future clinical trial design. Notably, previous studies in mHSPC have identified higher tumor volume and presence of de novo metastatic disease as risk factors associated with shorter OS with ADT alone.18,19 In CHAARTED, the presence of both risk factors was associated with a median OS of approximately 3 years with ADT alone; only one risk factor (de novo presentation or high-volume disease) generated a median OS of approximately 5 years, and absence of both was associated with a median OS of approximately 70 months. This was also noted in the GETUG-AFU 15 analysis.20 Moreover, the more indolent behavior of the low-volume subgroup was associated with no clear OS benefit from early docetaxel despite a modest delay in time to CRPC (mostly PSA rise). This is not to say that some patients with low-volume disease, especially those with de novo presentation, do not benefit. When studied as a unique subgroup, although there was a suggestion of benefit for a few patients in the early phase, there was no clear OS improvement for the subgroup as a whole with longer follow-up. More precise biomarkers are urgently needed to determine the patient phenotype that might benefit from early chemotherapy in this setting. The findings also suggest that patients with delayed presentation of metastatic disease and lower tumor burden probably have a distinct underlying biology and thus may have a differential response to a given treatment. For example, patients with late relapse and low tumor burden may benefit from more intense androgen receptor (AR) pathway inhibition. In contrast, these data indicate that patients with a higher disease volume are more likely to benefit from early docetaxel with ADT than patients with a lower tumor burden.
The benefit from early docetaxel in terms of delay to CRPC and subsequent clinical progression was more pronounced in the high-volume subgroup than in the low-volume subgroup. This suggests that there is a greater effect of secondary therapies for CRPC after early docetaxel, and this may contribute to the OS benefit in high-volume disease and may explain why no clear benefit was seen in GETUG-AFU 15, where there was less access to newer therapies for CRPC during the study conduct. In contrast, sequential therapies after ADT alone seem more effective as salvage treatments for patients with low-volume disease. This observation leads to the hypothesis that greater tumor debulking and attacking of AR-driven and AR-independent disease with upfront chemothormonal therapy in patients with greater tumor burden at baseline facilitate the greater benefit derived from the agents shown to prolong OS in the CRPC setting. Efforts are planned to more accurately capture treatment response to subsequent therapies in CHAARTED.
Despite the limitations of subgroup analyses, the information derived can inform future trial design in terms of projections for outcomes to determine sample size, especially for oligometastatic disease. Moreover, the use of conventional CT and technetium bone scan imaging, although crude, provides a cut point that identifies patients who benefit from early docetaxel and can serve as a benchmark for future molecular and radiologic biomarker work. This knowledge can also help inform retrospective studies, such as whether treatment of the primary tumor in de novo metastatic disease is associated with better outcomes because patients with lower burden of disease may have been selected for treatment of the primary tumor.
Finally, these results need to be interpreted in light of the recent data demonstrating a significant benefit from the addition of abiraterone to ADT to the same degree as docetaxel in high-volume disease (OS, not reached v 34.7 months; HR, 0.62; 95% CI, 0.51 to 0.76; P < .001 and progression-free survival, 33 v 14.8 months; HR, 0.47; 95% CI, 0.39 to 0.55; P < .001 [both in favor of the abiraterone plus ADT arm in the LATITUDE study]; HR for death, 0.63; 95% CI, 0.52 to 0.76; P < .001 and HR for treatment failure events, 0.29; 95% CI, 0.25 to 0.34; P < .001 [both in favor of the abiraterone plus ADT arm in the STAMPEDE trial]).5,6 Notably, the predominant population accrued to these studies had de novo metastatic disease. The future question will be whether to add docetaxel to ADT and newer AR-targeting agents such abiraterone (or enzalutamide if proven to confer OS benefit in the mHSPC setting). Fortunately, three studies have or have nearly completed accrual and are stratified by docetaxel use and will allow an analysis of ADT plus docetaxel with or without abiraterone, enzalutamide, or apalutamide (ClinicalTrials.gov identifiers NCT02446405, NCT01957436, and NCT02489318).
In conclusion, the updated data confirm the benefit of docetaxel in combination with ADT for patients with mHSPC, which is clearly defined for patients with high-volume disease. Although burden of metastases determined by conventional imaging can assist in patient selection for treatment with docetaxel, additional studies should focus on identifying more accurate biomarkers and gaining a better understanding of the underlying mechanisms of resistance to ADT and the biologic basis for AR targeting and cytotoxics in prostate cancer.21-23
Footnotes
Supported in part by Public Health Service Grants No. CA180790, CA180794, CA180795, CA180799, CA180801, CA180802, CA180820, CA180821, CA180833, CA180847, CA180853, CA180867, CA180888, and CA189829 and by the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services and coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN) Cancer Research Group. Sanofi provided docetaxel and a grant to ECOG-ACRIN.
Presented in part at the Annual Congress of the European Society of Medical Oncology, Copenhagen, Denmark, October 7-11, 2016.
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. Sanofi was not involved in the design, analysis, or reporting of the results.
Clinical trial information: NCT00309985.
See accompanying Editorial on page 1060
AUTHOR CONTRIBUTIONS
Conception and design: Michael A. Carducci, Robert S. DiPaola, Christopher J. Sweeney
Provision of study materials or patients: Yu-Hui Chen, Michael A. Carducci, Glenn Liu, David F. Jarrard, Noah M. Hahn, Daniel H. Shevrin, Robert Dreicer, Maha Hussain, Mario Eisenberger, Manish Kohli, Elizabeth R. Plimack, Nicholas J. Vogelzang, Joel Picus, Matthew M. Cooney, Jorge A. Garcia, Robert S. DiPaola, Christopher J. Sweeney
Collection and assembly of data: Christos E. Kyriakopoulos, Yu-Hui Chen, Christopher J. Sweeney
Data analysis and interpretation: Christos E. Kyriakopoulos, Yu-Hui Chen, Michael A. Carducci, Glenn Liu, David F. Jarrard, Noah M. Hahn, Daniel H. Shevrin, Robert Dreicer, Maha Hussain, Mario Eisenberger, Manish Kohli, Elizabeth R. Plimack, Nicholas J. Vogelzang, Joel Picus, Matthew M. Cooney, Jorge A. Garcia, Christopher J. Sweeney
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Christos E. Kyriakopoulos
Consulting or Advisory Role: Exelixis
Yu-Hui Chen
Employment: Constellation Pharmaceuticals (I)
Michael A. Carducci
Consulting or Advisory Role: Astellas Pharma, Churchill Pharmaceuticals, AbbVie, Roche/Genentech, Pfizer
Research Funding: Bristol-Myers Squibb (Inst), Pfizer (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), EMD Serono (Inst)
Glenn Liu
Leadership: AIQ Solutions
Stock or Other Ownership: AIQ Solutions
Consulting or Advisory Role: Sanofi
Research Funding: Johnson & Johnson (Inst), Novartis (Inst), Millennium Pharmaceuticals (Inst), Cellectar (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Coinventor on utility patient licensed by AIQ Solutions
David F. Jarrard
No relationship to declare
Noah M. Hahn
Consulting or Advisory Role: Bristol-Myers Squibb, Oncogenex, AstraZeneca/MedImmune, Pieris Pharmaceuticals, Inovio Pharmaceuticals, Genentech/Roche, Health Advances, Merck, Seattle Genetics, Rexahn Pharmaceuticals
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Mirati Therapeutics (Inst), Oncogenex (Inst), Merck (Inst), Heat Biologics (Inst), Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Principa Biopharma (Inst), Acerta Pharma (Inst)
Travel, Accommodations, Expenses: Merck, Seattle Genetics, Genentech, AstraZeneca
Daniel H. Shevrin
No relationship to disclose
Robert Dreicer
Consulting or Advisory Role: Medivation, Astellas Pharma, Asana Biosciences, Exelixis, AstraZeneca, Bristol-Myers Squibb, Eisai, Genzyme, Genentech/Roche, EMD Serono
Research Funding: Genentech (Inst), Asana Biosciences (Inst)
Maha Hussain
Honoraria: Sanofi
Research Funding: Genentech (Inst), Pfizer (Inst), AstraZeneca (Inst), Bayer HealthCare Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Title: Systems and Methods for Tissue Imaging, 3676 Our File: Serial No.: UM-14437/US-1/PRO 60/923,385 UM-14437/US-2/ORD 12/101,753US 8,185,186 (US patent No.) Systems and methods for tissue imaging (issued patent) EP 08745653.9 (EP application No.) Systems and methods for tissue imaging (pending) CA 2683805 (Canadian application No.) Systems and methods for tissue imaging (pending) US 13/362,500 (US application No.) Systems and methods for tissue imaging (continuation application of US 8,185,186); Title: Dual Inhibition of MET and VEGF for the Treatment of Castration Resistant Prostate Cancer and Osteoblastic Bone Metastases, applicant/proprietor Exelexis, application No./Patent No. 11764665.4-1464, application No./patent No. 11764656.2-1464, application filed on September 26, 2011
Travel, Accommodations, Expenses: Sanofi
Mario Eisenberger
Honoraria: Sanofi, Pfizer
Consulting or Advisory Role: Astellas Pharma, Bayer HealthCare Pharmaceuticals, Sanofi, Pfizer, Veru
Research Funding: Sanofi, Tokai Pharmaceuticals, Genentech
Travel, Accommodations, Expenses: Bayer HealthCare Pharmaceuticals, Astellas Pharma, Sanofi, Pfizer
Manish Kohli
No relationship to disclose
Elizabeth R. Plimack
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Novartis, Horizon Pharma, Inovio Pharmaceuticals, Merck, Clovis Oncology, Eli Lilly, Pfizer
Research Funding: Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), Merck Sharp & Dohme (Inst), Peloton Therapeutics (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: US patent No. 14/588,503, filed on January 2, 2015 (Inst); US patent No. 15/226,474, filed on July 1, 2015 (Inst)
Nicholas J. Vogelzang
Stock or Other Ownership: Caris Life Sciences
Honoraria: UpToDate, Pfizer
Consulting or Advisory Role: Amgen, Cerulean Pharma, Pfizer, Bayer HealthCare Pharmaceuticals, Genentech/Roche, Churchill Pharmaceuticals, Heron Therapeutics, AstraZeneca, Caris Life Sciences, Fujifilm, Tolero Pharmaceuticals
Speakers’ Bureau: Bayer HealthCare Pharmaceuticals, Sanofi, Genentech/Roche, Bristol-Myers Squibb, Exelixis
Travel, Accommodations, Expenses: Genentech/Roche, US Oncology, Pfizer, Bayer HealthCare Pharmaceuticals/Onyx Pharmaceuticals, Exelixis, AstraZeneca/MedImmune
Joel Picus
Consulting or Advisory Role: Novo Nordisk
Research Funding: Novartis (Inst), BioClin Therapeutics (Inst), Altor BioScience (Inst), Agensys (Inst), Oncogenex (Inst), Mirati Therapeutics (Inst), Astex Pharmaceuticals (Inst), Innocrin Pharma (Inst), Rexahn Pharmaceuticals (Inst)
Matthew M. Cooney
Speakers’ Bureau: Potomac Center for Medical Education
Jorge A. Garcia
Honoraria: Astellas Pharma, Sanofi, Eisai, Exelixis, Bayer HealthCare Pharmaceuticals, Genentech
Consulting or Advisory Role: Sanofi, Pfizer, Bayer HealthCare Pharmaceuticals, Eisai, Exelixis, Medivation, Genentech/Roche
Speakers’ Bureau: Bayer HealthCare Pharmaceuticals, Sanofi, Medivation/Astellas Pharma, Genentech/Roche
Research Funding: Pfizer (Inst), Astellas Pharma (Inst), Orion Pharma GmbH (Inst), Bayer HealthCare Pharmaceuticals (Inst), Janssen Oncology (Inst), Genentech/Roche (Inst), Eli Lilly (Inst)
Travel, Accommodations, Expenses: Pfizer, Bayer HealthCare Pharmaceuticals, Sanofi, Exelixis, Eisai, Medivation/Astellas, Genentech/Roche
Robert S. DiPaola
No relationship to disclose
Christopher J. Sweeney
Stock or Other Ownership: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer HealthCare Pharmaceuticals, Genentech/Roche, AstraZeneca, Pfizer
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer HealthCare Pharmaceuticals (Inst), Sotio (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix: parthenolide, dimethylaminoparthenolide; Exelixis: abiraterone plus cabozantinib combination
REFERENCES
- 1.Huggins C, Hodges CV: Studies on prostate cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1:293-297, 1941 [Google Scholar]
- 2.Crawford ED, Higano CS, Shore ND, et al. : Treating patients with metastatic castration resistant prostate cancer: A comprehensive review of available therapies. J Urol 194:1537-1547, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Sweeney CJ, Chen YH, Carducci M, et al. : Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737-746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James ND, Sydes MR, Clarke NW, et al. : Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387:1163-1177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352-360, 2017 [DOI] [PubMed] [Google Scholar]
- 6.James ND, de Bono JS, Spears MR, et al. : Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377:338-351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravis G, Fizazi K, Joly F, et al. : Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol 14:149-158, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Gravis G, Boher JM, Joly F, et al. : Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: Impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol 70:256-262, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Vale CL, Burdett S, Rydzewska LHM, et al. : Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: A systematic review and meta-analyses of aggregate data. Lancet Oncol 17:243-256, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finianos AN, Gupta K, Meshikhes M, et al: Characterization of differences between prostate cancer (PCa) patients presenting as de novo versus primary progressive metastatic disease. J Clin Oncol 33, 2015 (suppl; abstr 285) [DOI] [PubMed]
- 11. Gupta K, Finianos AN, Clark B, et al: Survival outcomes for de novo versus primary progressive metastatic prostate cancer. J Clin Oncol 35, 2017 (suppl; abstr 258) [DOI] [PubMed]
- 12.Culp SH, Schellhammer PF, Williams MB: Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol 65:1058-1066, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Gratzke C, Engel J, Stief CG: Role of radical prostatectomy in metastatic prostate cancer: Data from the Munich Cancer Registry. Eur Urol 66:602-603, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Rusthoven CG, Jones BL, Flaig TW, et al. : Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol 34:2835-2842, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Satkunasivam R, Kim AE, Desai M, et al. : Radical prostatectomy or external beam radiation therapy vs no local therapy for survival benefit in metastatic prostate cancer: A SEER-Medicare analysis. J Urol 194:378-385, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P: Nonparametric estimation of incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 17.Cox DR: Regression models and life tables (with discussion). J R Stat Soc B 34:187-220, 1972 [Google Scholar]
- 18.Crawford ED, Eisenberger MA, McLeod DG, et al. : A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 321:419-424, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Eisenberger MA, Blumenstein BA, Crawford ED, et al. : Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 339:1036-1042, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Gravis G, Boher JM, Chen YH, et al: Burden of metastatic hormone-sensitive prostate cancer to identify men more likely to benefit from early docetaxel. J Clin Oncol 35, 2017 (suppl; abstr 136)
- 21.Polkinghorn WR, Parker JS, Lee MX, et al. : Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 3:1245-1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin JF, Schiewer MJ, Dean JL, et al. : A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov 3:1254-1271, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komura K, Jeong SH, Hinohara K, et al. : Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression. Proc Natl Acad Sci USA 113:6259-6264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]