Abstract
Purpose
Observational studies have reported increased colon cancer recurrence and mortality in patients with states of hyperinsulinemia, including type 2 diabetes, obesity, sedentary lifestyle, and high glycemic load diet. Nut intake has been associated with a lower risk of type 2 diabetes, metabolic syndrome, and insulin resistance. However, the effect of nut intake on colon cancer recurrence and survival is not known.
Patients and Methods
We conducted a prospective, observational study of 826 eligible patients with stage III colon cancer who reported dietary intake on food frequency questionnaires while enrolled onto a randomized adjuvant chemotherapy trial. Using Cox proportional hazards regression, we assessed associations of nut intake with cancer recurrence and mortality.
Results
After a median follow-up of 6.5 years, compared with patients who abstained from nuts, individuals who consumed two or more servings of nuts per week experienced an adjusted hazard ratio (HR) for disease-free survival of 0.58 (95% CI, 0.37 to 0.92; Ptrend = .03) and an HR for overall survival of 0.43 (95% CI, 0.25 to 0.74; Ptrend = .01). In subgroup analysis, the apparent benefit was confined to tree nut intake (HR for disease-free survival, 0.54; 95% CI, 0.34 to 0.85; Ptrend = .04; and HR for overall survival, 0.47; 95% CI, 0.27 to 0.82; Ptrend = .04). The association of total nut intake with improved outcomes was maintained across other known or suspected risk factors for cancer recurrence and mortality.
Conclusion
Diets with a higher consumption of nuts may be associated with a significantly reduced incidence of cancer recurrence and death in patients with stage III colon cancer.
INTRODUCTION
Recent prospective observational studies among patients with colon cancer suggest that diet and lifestyle factors may significantly influence the risk of colon cancer recurrence and death.1-6 In aggregate, these studies indicate that states of energy excess, including type 2 diabetes (T2D),7 obesity,6 sedentary lifestyle,2,5 Western-pattern diet,3 increased dietary glycemic load,1 and high intake of sugar-sweetened beverages,8 are associated with an increased risk of colon cancer recurrence and mortality. Moreover, increased cancer mortality was observed among patients with colorectal cancer with elevated plasma C-peptide or low insulin-like growth factor–binding protein-1 levels,9 suggesting that the association between energy excess and increased risk of colon cancer recurrence may be mediated, in part, by long-term hyperinsulinemia. In fact, increased coffee intake, which has been associated with decreased risk of T2D,10-14 lower plasma C-peptide levels,15,16 and increased insulin sensitization,17,18 conferred improved disease-free survival (DFS) in patients with stage III colon cancer.19
In prospective cohort studies, increased nut intake has been associated with a reduced risk of T2D and metabolic syndrome20-23 and a reduction in insulin resistance.20,24-27 Nuts are nutrient-dense foods that are rich in unsaturated fatty acids, fiber, vitamins, minerals, and other bioactive substances such as phenolic antioxidants and phytosterols.28-30
In light of evidence supporting a link between excess energy balance, hyperinsulinemia, and increased recurrence in patients with colon cancer, we prospectively examined the association of nut intake with cancer recurrence and mortality in a cohort of patients with stage III colon cancer enrolled onto a National Cancer Institute–sponsored randomized clinical trial of adjuvant chemotherapy. In the trial, detailed data on pathologic stage, performance status, postoperative treatment, and follow-up were prospectively captured. In addition, comprehensive data on diet and lifestyle were collected before any documentation of cancer recurrence.
PATIENTS AND METHODS
Study Population
Patients in this prospective cohort participated in the National Cancer Institute–sponsored Cancer and Leukemia Group B (now Alliance for Clinical Trials in Oncology) 89803 adjuvant therapy trial for stage III colon cancer, comparing therapy with weekly fluorouracil and leucovorin to weekly irinotecan, fluorouracil, and leucovorin (ClinicalTrials.gov identifier: NCT000038350).31 Between April 1999 and May 2001, 1,264 patients were enrolled. An amendment was introduced after enrolling 87 patients that required enrollees to complete a self-administered semiquantitative food frequency questionnaire (FFQ) that captured diet and lifestyle habits. The questionnaires were administered midway through adjuvant therapy (4 months after surgery; questionnaire 1 [Q1]) and again 6 months after completion of treatment (14 months after surgery; questionnaire 2 [Q2]).
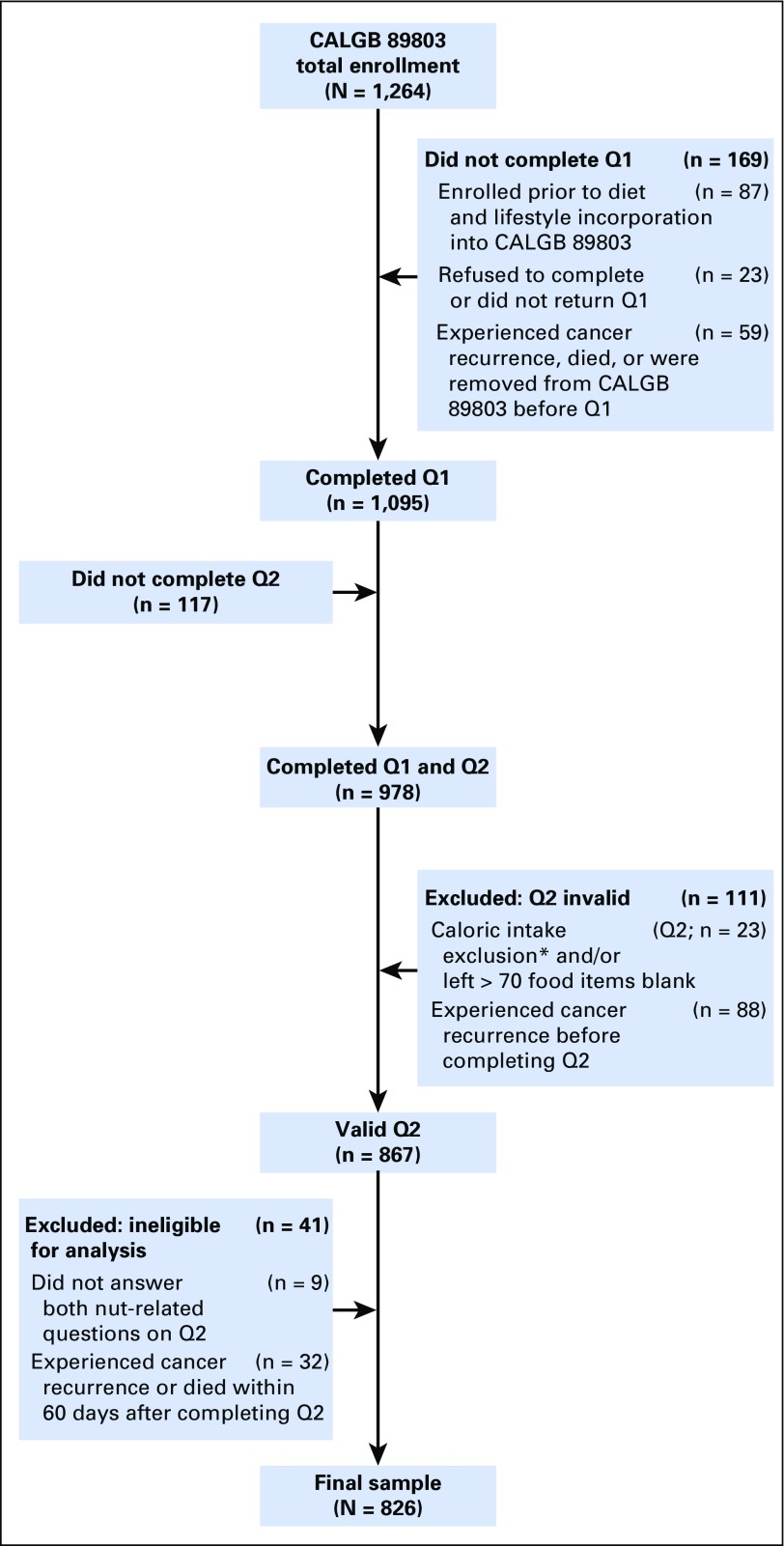
Patients were eligible if they underwent a complete surgical resection of the primary tumor within 56 days before trial entry, had no prior chemotherapy or radiation therapy for treatment of the tumor, had regional lymph node metastases without evidence of distant metastases, had a baseline Eastern Cooperative Oncology Group performance status of 0 to 2,32 and had adequate bone marrow, renal, and hepatic function.31 Because of possible dietary modifications immediately after colectomy,33 we a priori limited the primary analysis to nut intake reported on Q2. Patients were excluded if they reported significantly abnormal caloric intake (< 600 or > 4,200 calories per day for men; < 500 or > 3,500 calories per day for women), left > 70 food items blank, or left blank the nut consumption–related questions on Q2. Finally, patients were excluded if they had cancer recurrence or death before completion of Q2 or within 60 days of completing Q2 to avoid misattribution bias. The resulting cohort included 826 eligible patients (Fig 1). The protocol was reviewed by the institutional review board of each participating center, and all patients provided study-specific informed consent.
Fig 1.
Derivation of the study cohort. (*) Calorie exclusion: < 600 or > 4,200 calories per day for men and < 500 calories or > 3,500 calories per day for women. Q1, questionnaire 1 (midway through adjuvant therapy); Q2, questionnaire 2 (6 months after completion of adjuvant therapy). CALGB, Cancer and Leukemia Group B.
Dietary Assessment
Patients in this analysis completed semiquantitative FFQs that included 131 food items, vitamin and mineral supplements, and open-ended sections for other supplements and foods not specifically listed.34,35 Participants were asked how often, on average, over the previous 3 months they consumed a specific food portion size, with up to nine possible responses, which ranged from never to to six or more times per day. We computed nutrient intake by multiplying the frequency of consumption of each food by the nutrient content of the specified portions.36 Nutrient values were energy adjusted using the residuals methods.37
On the questionnaire, we separately assessed self-reported intake of 1-oz servings of tree nuts and peanuts with the following eight ordered categories for intake: none; less than once per month; one to three servings per month; one serving per week; two to four servings per week; five to six servings per week; one serving per day; and two or more servings per day. Total nut intake was calculated as the weighted proportional summation of tree nuts and peanuts. We similarly assessed self-reported peanut butter intake. In a previous cohort study evaluating the validity of our FFQ in measuring intake of nuts and peanut butter, the correlation coefficients between dietary records and the FFQs were 0.75 and 0.75, respectively.38
Tumor Assessments for KRAS, BRAF, PIK3CA, and TP53 Mutations, Microsatellite Instability, and PTGS2 Expression
Polymerase chain reaction (PCR) and pyrosequencing targeted for mutation hotspots in PIK3CA exons 9 and 20,39 BRAF codon 600,40 and KRAS codons 12 and 13 were performed, as previously described.41,42 Mutations in TP53 exons 5 to 8 were determined by Sanger sequencing and sequencing by hybridization, as previously described.43 Microsatellite instability (MSI) was assessed by PCR for 10 microsatellite markers; tumors with instability in ≥ 50% of the loci were classified as MSI-high; for 28 patients without PCR MSI results, those with loss of MLH1 or MSH2 expression were classified as MSI-high, as previously described.44 PTGS2 (COX-2) expression was assessed by immunohistochemistry, as previously described.45
Study End Points
The primary end point was DFS, defined as time from completion of Q2 to tumor recurrence, occurrence of a new primary colon tumor, or death from any cause. We also assessed recurrence-free survival (RFS), defined as time from completion of Q2 to tumor recurrence or occurrence of a new primary colon tumor. For RFS, patients who died without known tumor recurrence were censored at last documented physician evaluation. Finally, we assessed overall survival (OS), defined as the time from completion of Q2 to death from any cause.
Statistical Analysis
There was no statistically significant difference in OS or DFS in both arms of the randomized trial.31 Therefore, patient data from both arms were combined and analyzed according to frequency categories of dietary intake. The primary analysis was done with total nut intake, combining intake of tree nuts and peanuts. For consistency with prior published studies on nut intake and to conserve statistical power for the analysis, categories of nut intake were consolidated into the following five ordered categories: never, less than one serving per month, one to three servings per month, one serving per week, and two or more servings per week.46-49 Differences in distribution of baseline characteristics by nut consumption categories were evaluated using the χ2 test, except for continuous variables, which were evaluated using the Kruskal-Wallis test.
Cox proportional hazards regression analysis was used to determine the simultaneous affect of other variables potentially associated with each outcome.50 The covariates in the model were fixed as measured on Q2. To account for the caloric content of nuts, our initial model adjusted for total calorie intake.37 The final model further adjusted for potential confounders including age, sex, depth of invasion through bowel wall, number of positive lymph nodes, baseline performance status, treatment group, body mass index (calculated as weight in kilograms divided by height in meters squared), physical activity (measured in metabolic equivalent task hours per week), aspirin use, and glycemic load (as previously described).1 Selection of covariates for the final model was based on clinical significance, previous studies, and degree of correlation with the exposure. Covariates with missing variables were coded with indicator variables.
The statistical analysis was performed using nut consumption as a continuous measure to minimize bias created by selected categorization. We tested for linear trends across frequency categories of intake by assigning each participant the median value for each frequency category and modeling this value as a continuous variable, consistent with prior studies.1,2,8,19 P values for trend were calculated using the Wald test of the score variable. Secondary exploratory analysis was performed individually for peanuts and tree nuts; we further collapsed intake into four categories to conserve power (never, < one serving per month, one to three servings per month, and ≥ one serving per week). Stratified exploratory analyses were also performed for other risk factors; the likelihood ratio test was used to test for interaction. In addition, we performed several sensitivity analyses to confirm the robustness of the results. The Cox regression models were tested for and met the assumption of proportionality by both time-dependent covariate and Schoenfeld residuals methods. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A threshold level of significance of P < .05 was considered statistically significant. All P values are two-sided and were not adjusted for multiple comparisons. Data collection and management were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on November 9, 2009. See the Appendix (online only) for additional analyses.
RESULTS
Baseline Characteristics
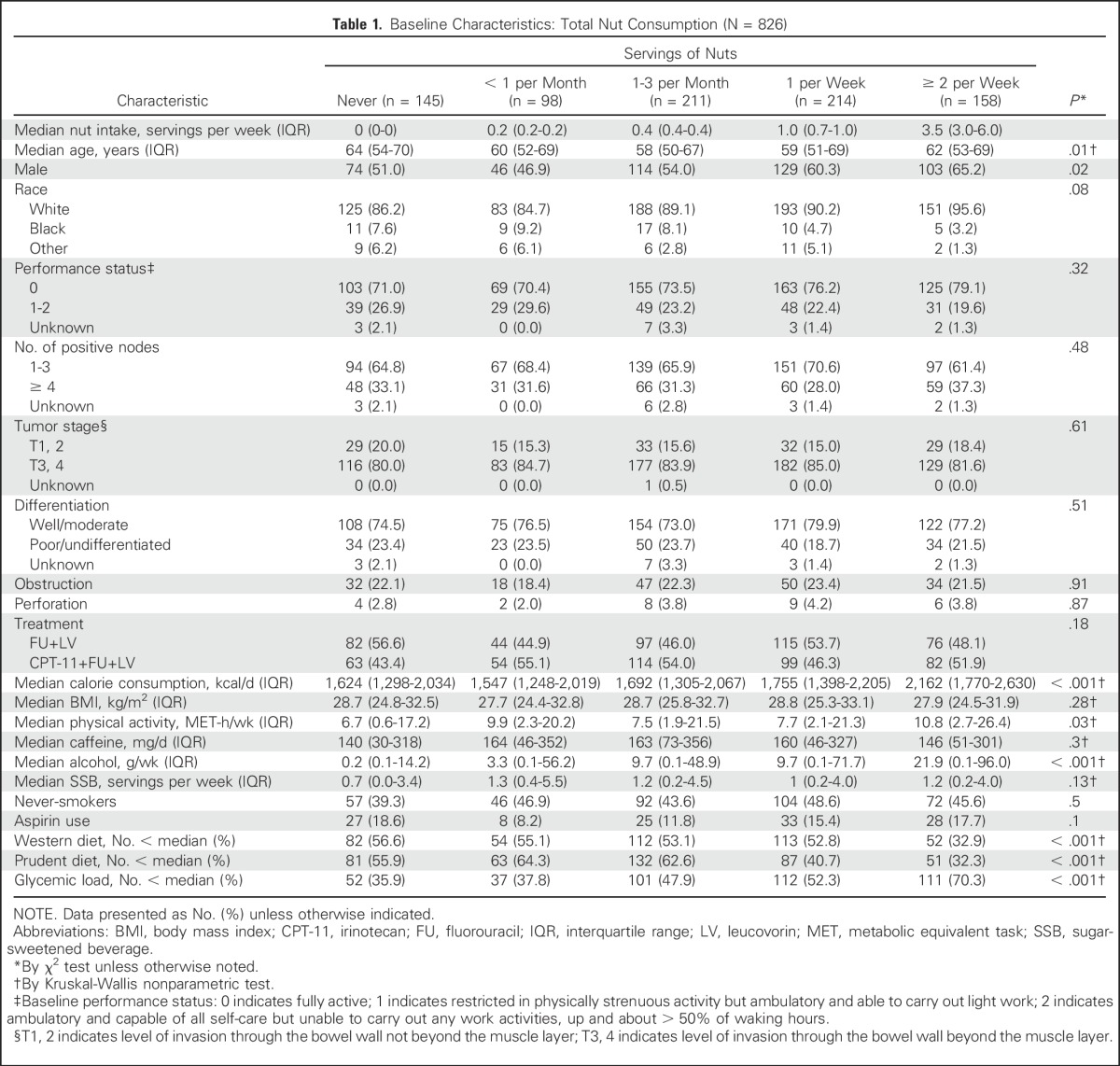
Baseline characteristics by frequency of total nut consumption are listed in Table 1. Patients who consumed more nuts were more frequently male, consumed more alcohol, and had a lower glycemic load diet. Although nut users consumed greater total calories, nut users did not present with a higher body mass index. There were statistical differences in age, Western and prudent dietary patterns, and dietary glycemic load by categories of nut intake, although clear trends in these factors by nut intake were less apparent.
Table 1.
Baseline Characteristics: Total Nut Consumption (N = 826)
Impact of Nut Intake on Cancer Recurrence and Death
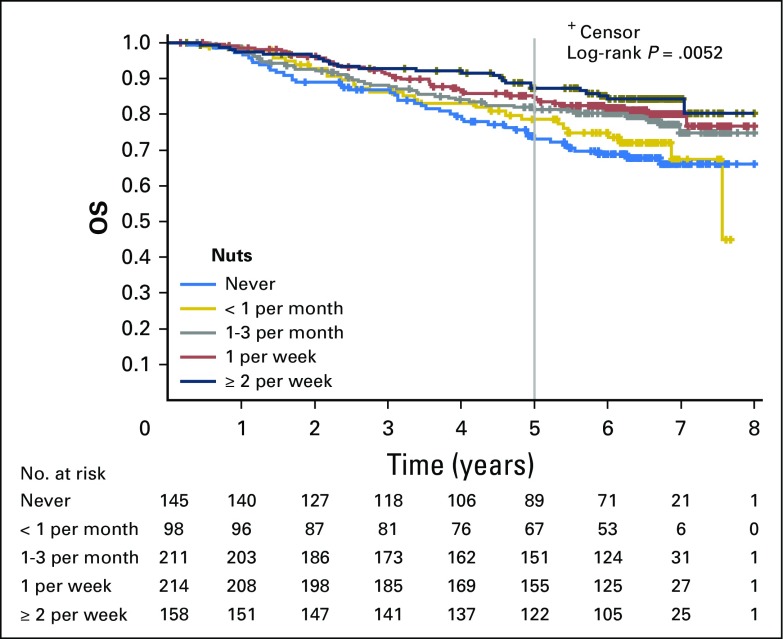
Median follow-up time from completion of Q2 was 6.5 years. During follow-up, 199 of the 826 patients experienced cancer recurrence or developed new primary tumors. Of the 826 patients, 177 patients died; of those, 39 patients died without documented cancer recurrence.
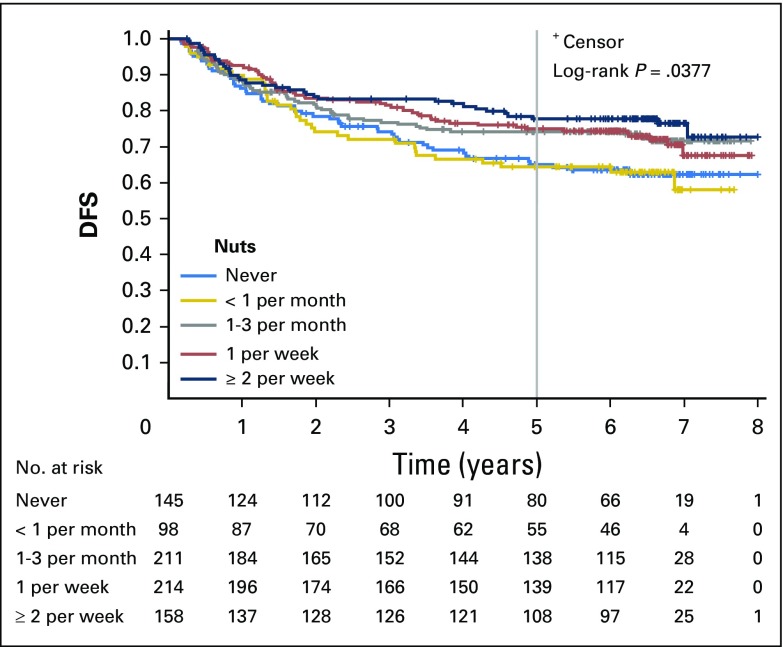
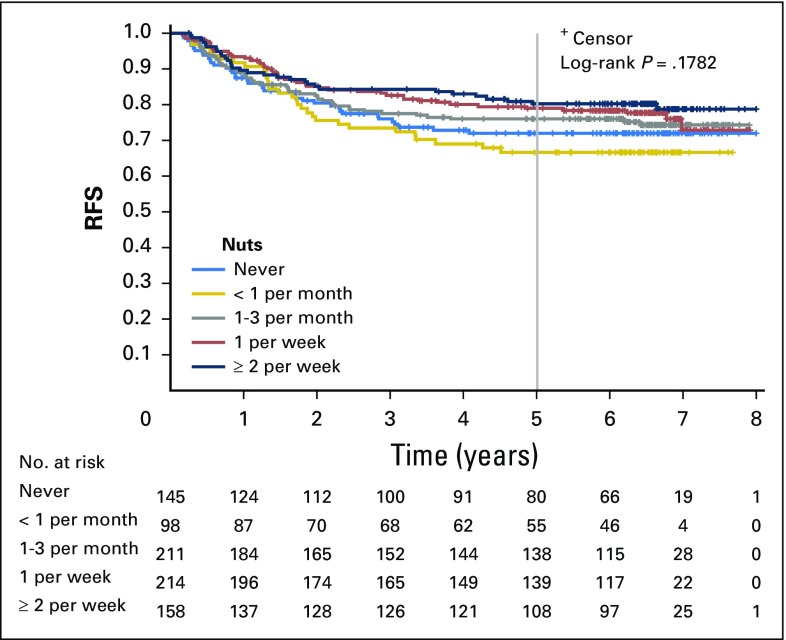
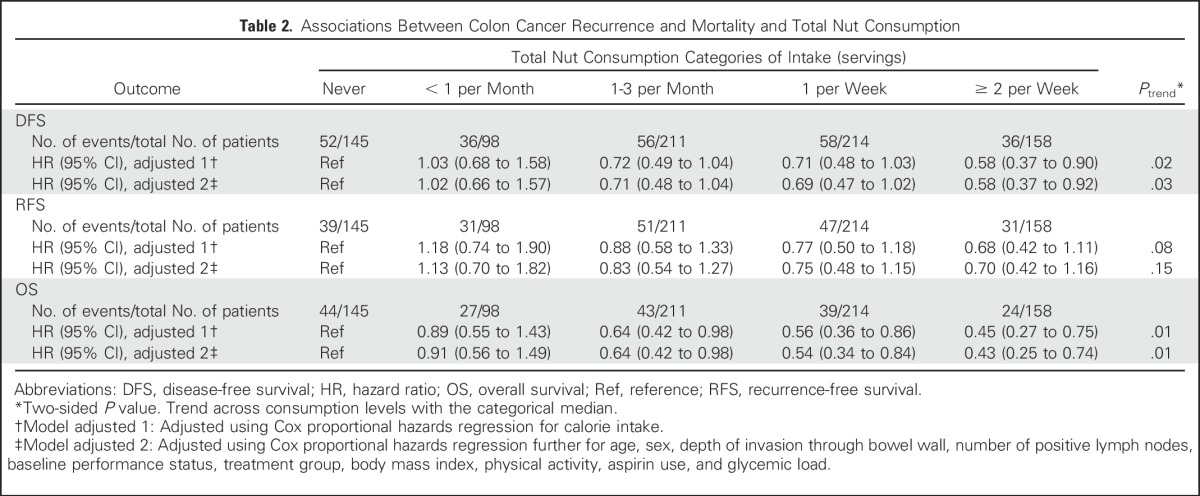
Increasing total nut intake was associated with significant reduction in recurrence and mortality after adjusting for other predictors of cancer recurrence (Table 2). Compared with patients who abstained, individuals who consumed diets with two or more servings of nuts per week experienced an adjusted hazard ratio (HR) for DFS of 0.58 (95% CI, 0.37 to 0.92; Ptrend = .03). Higher nut intake was also associated with a significant improvement in OS (HR, 0.43; 95% CI, 0.25 to 0.74; Ptrend = .01) and a trend toward improved RFS, which did not reach statistical significance (HR, 0.70; 95% CI, 0.42 to 1.16; Ptrend = .15). Unadjusted survival curves are presented in Appendix Figures A1, A2, and A3 (online only).
Table 2.
Associations Between Colon Cancer Recurrence and Mortality and Total Nut Consumption
We further examined the associations with nut intake models after controlling for other possible confounders (Western and prudent dietary patterns, race, smoking, and alcohol use) and found that our findings were largely unchanged (Appendix Table A1, online only).
Sensitivity Analyses
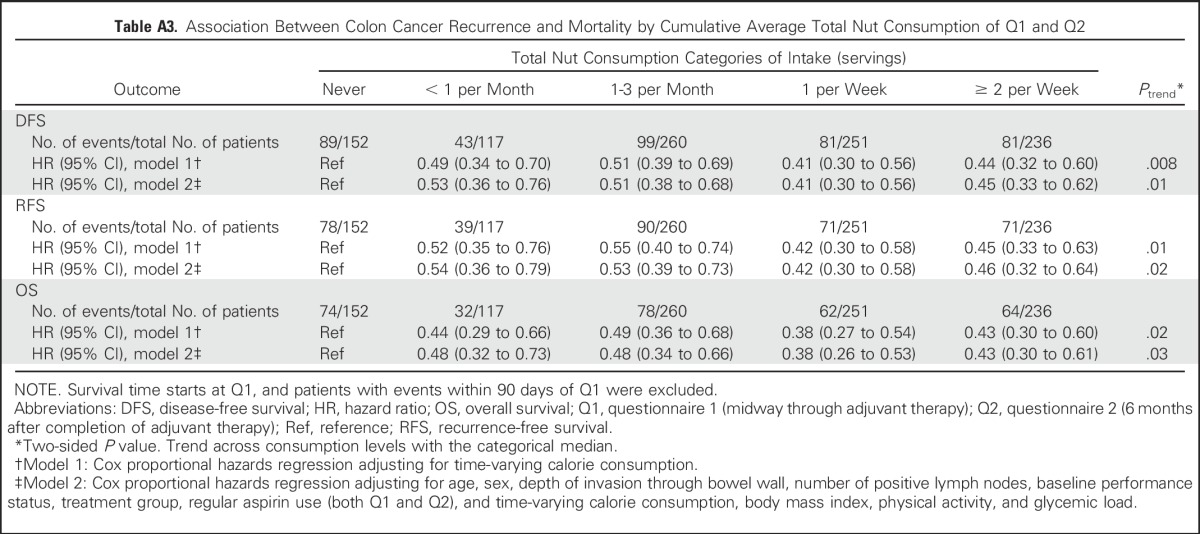
Because of possible dietary modifications immediately after colectomy,33 we a priori limited the primary analysis to nut intake reported on Q2. Nonetheless, we repeated our analyses using the cumulative average of nut intake on Q1 and Q2 and found that the association with DFS was unchanged (Appendix Tables A2 and A3, online only).
We considered the possibility that changes in dietary habits could reflect occult cancer or impending death; as such, our primary analyses excluded patients who developed cancer recurrence or died within 60 days of completing Q2. To further address this issue, we repeated the models after excluding recurrences or deaths within 180 days of Q2 completion (n = 783), and our results remained largely unchanged (HR, 0.54; 95% CI, 0.32 to 0.89; Ptrend = .02).
Furthermore, we considered that nut intake may be a marker of dentition and oral health, potential confounders that were not assessed in this cohort. To address this issue, we ran the models using a new reference group that combined the lowest intake categories of never and less than one serving per month; the results remained largely unchanged. Individuals reporting two or more servings of nuts per week demonstrated an improvement in DFS compared with the new reference group (HR, 0.57; 95% CI, 0.38 to 0.87; Ptrend = .03).
Stratified Analyses
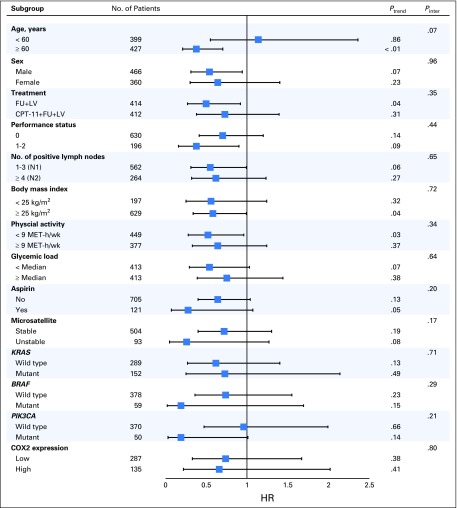
We evaluated the influence of total nut intake on DFS across strata of other potential predictors and confounders of patient outcome, comparing patients with an intake of two of more servings per week to nonconsumers (Fig 2). In this exploratory analysis, the association between total nut intake and DFS was consistent across strata of patient, disease, and treatment characteristics; these included strata of clinically relevant genomic alterations (MSI and KRAS, BRAF, and PIK3CA mutations). However, in these stratified analyses, statistical power to adequately detect differences was limited by the sample size, and such analyses should be considered exploratory.
Fig 2.
Multivariable hazard ratios (HRs) and 95% CIs for cancer recurrence or mortality across strata of various factors. The analyses used five categories of total nut intake (never, less than one serving per month, one to three servings per month, one serving per week, and two or more servings per week). The forest plot represents the HRs of the comparison of never nut consumers versus consumers of two or more servings of nuts per week, adjusting for calorie intake, age, sex, depth of invasion through bowel wall, number of positive lymph nodes, baseline performance status, treatment group, body mass index, physical activity, aspirin use, and glycemic load. P values are two-sided; Pinter indicates P for interaction between strata and nut intake; Ptrend indicates P for trend across levels of nut intake. COX2, cyclooxygenase-2; CPT-11, irinotecan; FU, fluorouracil; LV, leucovorin; MET-h/wk, metabolic equivalent task hours per week.
Subgroup Analyses
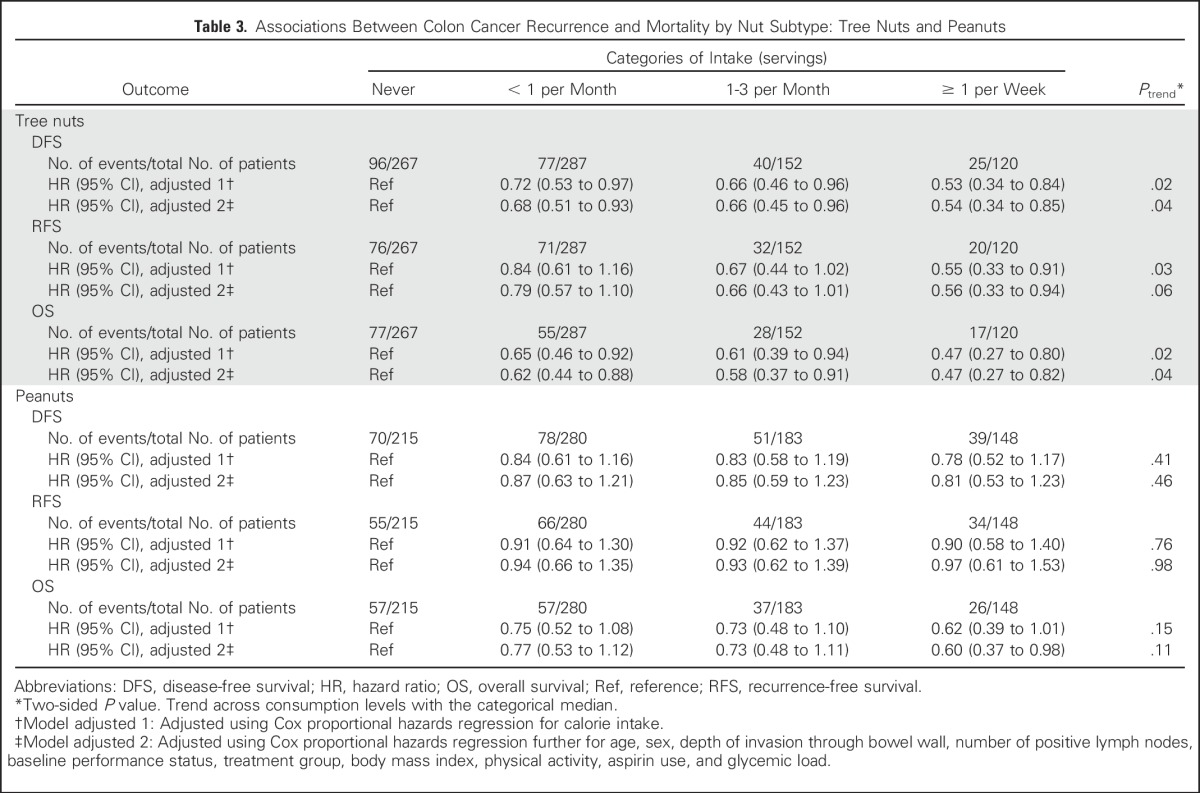
In separate analyses of the subtypes of nuts, we observed a significant improvement in both DFS and OS with increasing tree nut consumption, whereas the associations with peanut intake did not reach statistical significance (Table 3). We similarly examined the influence of peanut butter consumption on patient outcome and found no association between peanut butter intake and DFS (Ptrend = .15), OS (Ptrend = .12), or RFS (Ptrend = .09).
Table 3.
Associations Between Colon Cancer Recurrence and Mortality by Nut Subtype: Tree Nuts and Peanuts
DISCUSSION
In this prospective cohort of patients with stage III colon cancer who received adjuvant chemotherapy, a diet with increased total nut intake was associated with a significant improvement in cancer recurrence or mortality (DFS) and all-cause mortality (OS). Moreover, these associations seemed to be independent of other predictors of patient outcome, diet, and lifestyle factors, and the effect of total nut consumption was maintained across other known or suspected risk factors of cancer recurrence. In contrast to tree nuts, we did not observe a significant association with peanuts, which are legumes, although the statistical power to assess peanut intake was limited.
To our knowledge, this is the first study to examine the association between nut intake and colon cancer recurrence and survival. Prior observational cohort studies have explored the relationship between nut intake and risk of developing colorectal cancer, finding inconsistent results.48,51,52 Other studies have reported reduced cancer-related mortality in association with increased nut intake49,53-55; however, the attributable mortality reductions in colorectal cancer or other individual cancer types were not described in these studies.
Our data are consistent with a wealth of existing observational and clinical trial data reporting health benefits of nut consumption on many chronic diseases, including reductions in the risk of T2D, insulin resistance, metabolic syndrome, cardiovascular disease, and all-cause mortality.49,56-65 Nutrients in nuts, such as unsaturated fatty acids, high-quality protein, fiber, vitamins, minerals, phytochemicals, and other bioactive substances, may confer potential anticarcinogenic, anti-inflammatory, and antioxidant properties.28-30 Clinical trials have demonstrated beneficial effects of nuts on relevant intermediate markers including oxidation,25,66 endothelial dysfunction,67 hyperglycemia,25 and insulin resistance.20,68 In addition, preclinical studies suggest that the contents of nuts may inhibit the growth of a colon cancer cell line69 and may decrease the rate of colorectal cancer growth and angiogenesis in mice.70
Multiple prior observational studies in patients with colorectal cancer, including analyses from our study population, suggest that states of excess energy balance (eg, obesity, T2D, sedentary lifestyle, Western-pattern diet, greater glycemic load diet, and high sugar-sweetened beverage intake) are associated with greater risk of cancer recurrence and mortality.1-3,8 Therefore, we hypothesize that the effect of nuts on hyperinsulinemia and energy balance may, in part, explain the association between nut intake and improved patient outcome in our study. Nonetheless, there may be other possible mechanisms through which nut intake may influence survival in patients with colon cancer.
There are several strengths of our analysis. Embedding the cohort within a randomized trial ensures equal distribution of potential confounders. All patient had stage III colon cancer, minimizing the effect of heterogeneity of the disease stage on the outcomes. Treatment and patient follow-up were rigorous because they were prescribed and monitored by the clinical trial; the outcomes of death and cancer recurrence were prospectively collected through regular detailed medical examinations during the course of the follow-up period. Furthermore, detailed information about potential confounders and effect modifiers was collected prospectively.
However, our study has a few limitations. Nut intake was self-reported, potentially subject to measurement errors, and somewhat limited in range of intake in this patient population. However, in previous validation studies, nut intake as recorded on our questionnaire showed good concordance with independent dietary records.38 Moreover, because dietary data were collected prospectively, any misclassification of nut intake would underestimate a true association. Given the observational nature of this study, we cannot exclude the possibility that the associations between nut intake and improved outcome are a result of confounding variables or that error in the measurement of adjusted confounding variables could result in residual confounding. However, these associations persisted even after controlling for known or suspected predictors of patient outcome. Furthermore, the associations remained largely consistent on multiple sensitivity analyses.
Our cohort did not collect data on dentition. Poor dentition may influence the intake of foods such as nuts, which are hard and require significant mastication. Observational studies have shown an association between dental disease and increased incidence and mortality of cardiovascular disease.71-77 Periodontal disease has also been linked with a higher incidence of some solid cancers,78-85 although studies on the association with colon cancer incidence have yielded conflicting results79,82,86 and the effect on colon cancer outcomes is largely unknown. Our additional sensitivity analysis to address this potential confounder, collapsing the two lowest categories into a new control group, yielded consistent results. Nonetheless, as with many nutritional epidemiology studies, the potential for residual confounding remains.
Finally, patients in randomized trials may differ from the population at large. However, the distribution of dietary and lifestyle habits reported by our cohort was similar to that of other US cohorts,87 supporting generalizability of our results. Still, it is imperative to replicate our findings in other cohorts.
In conclusion, this prospective study of patients with stage III colon cancer suggests that a diet with increased nut consumption is associated with a significant reduction in cancer recurrence and mortality. Although the findings of our observational study do not establish causality, the results offer further support of the role of diet and lifestyle as modifiable risk factors of outcomes in patients with colon cancer.
Appendix
Methods
In supplemental analyses, we offer additional multivariable models using Cox proportional hazards regression, whereby additional covariates were added as part of sensitivity analyses. In successive models, Western and prudent dietary patterns (model 3) and race (as a categorical variable: white, black, or other), smoking as binary (ever or never), and alcohol (as a continuous variable in grams per day; model 4) were added to the final model that included age, sex, depth of invasion through bowel wall, number of positive lymph nodes, baseline performance status, treatment group, body mass index (calculated as weight in kilograms divided by height in meters squared), physical activity (measured in metabolic equivalent task hours per week), aspirin use, and glycemic load. Glycemic load and Western and prudent dietary patterns were determined by methods previously described by our study cohort (Wu K, et al: Cancer Causes Control 15:853-862, 2004; Fung T, et al: Arch Intern Med 163:309-314, 2003).1,3
Because of possible dietary modifications immediately after colectomy, we a priori limited the primary analysis to nut intake reported on questionnaire 2 (Q2). In secondary analyses, we repeated the models using cumulative nut intake from questionnaire 1 (Q1) and Q2. Cumulative nut exposure average was calculated based on weighting proportional to the time between Q1 and Q2, and then the time between Q2 and the outcome survival period, using previously published methods (Hu FB, et al: Am J Epidemiol 149:531-540, 1999).1 In this analysis, the follow-up period began from the completion of Q1. Consistent with previous analyses in our study cohort, patients were excluded if they experienced cancer recurrence or death within 90 days of completing Q1 to prevent misattribution bias.1,8,18 Survival estimate curves for disease-free survival, recurrence-free survival, and overall survival by nut intake categories were calculated using the Kaplan-Meier methods, and the log-rank testing was used to compare survival between groups (Kaplan EL, Meier P: J Am Stat Assoc 53:457-481, 1958).
Fig A1.
Kaplan-Meier curves for disease-free survival (DFS) by total nut consumption. Curves depict survival for five categories of total nut intake (never, less than one serving per month, one to three servings per month, one serving per week, and two or more servings per week).
Fig A2.
Kaplan-Meier curves for recurrence-free survival (RFS) by total nut consumption. Curves depict survival for five categories of total nut intake (never, less than one serving per month, one to three servings per month, one serving per week, and two or more servings per week).
Fig A3.
Kaplan-Meier curves for overall survival (OS) by total nut consumption. Curves depict survival for five categories of total nut intake (never, less than one serving per month, one to three servings per month, one serving per week, and two or more servings per week).
Table A1.
Sensitivity Analyses Further Controlling for Additional Confounders: Dietary Pattern, Race, Smoking, and Alcohol Use
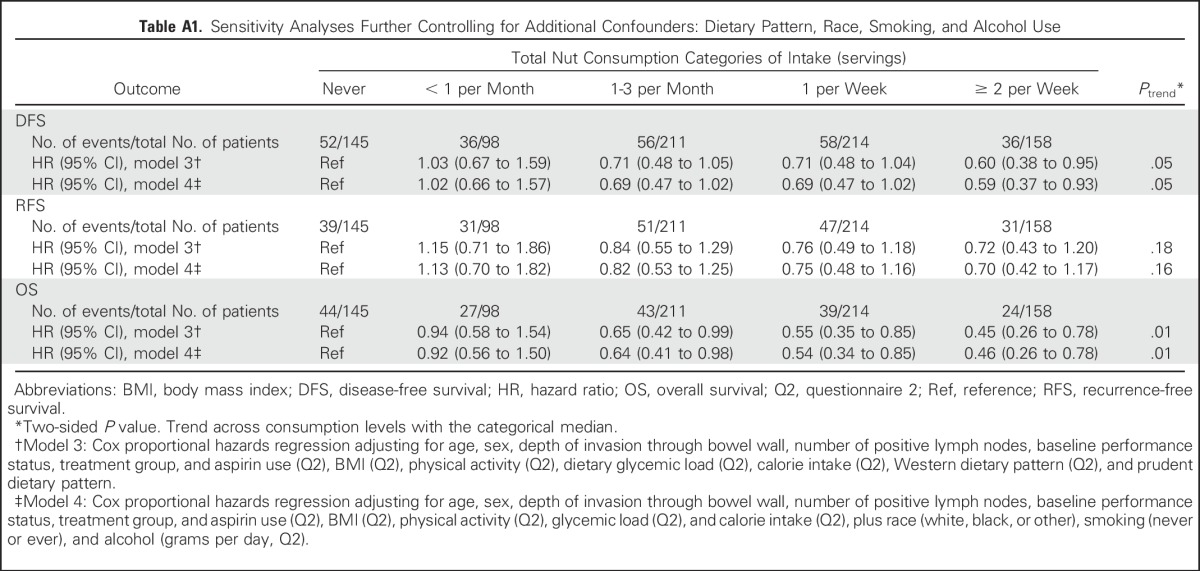
Table A2.
Consumption of Nuts on Q1 and Q2 in Servings per Day
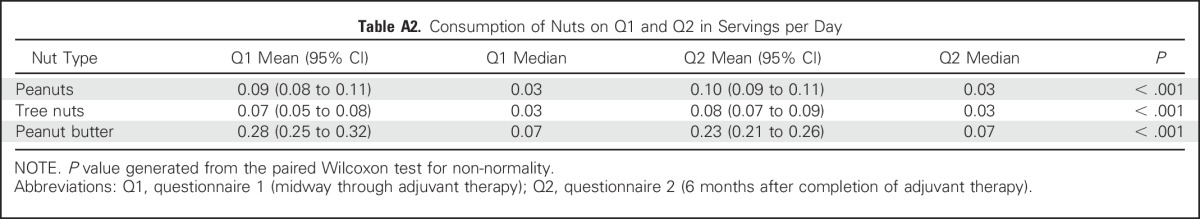
Table A3.
Association Between Colon Cancer Recurrence and Mortality by Cumulative Average Total Nut Consumption of Q1 and Q2
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health under Awards No. U10CA180821 and U10CA180882 (to Alliance for Clinical Trials in Oncology), U10CA180820 (to Eastern Cooperative Oncology Group–American College of Radiology Imaging Network), and U10CA180888 (to Southwest Oncology Group). Also supported by Grants No. U10CA60138, U10CA138561, U10CA180791, U10CA180836, U10CA180867, and UG1CA189858, and the Pharmacia & Upjohn Company, now Pfizer Oncology. C.S.F., K.N., J.M., D.N., and X.Y. are supported in part by grants from the National Cancer Institute (Grants No. K07 CA148894, R01 CA118553, R01 CA149222, R01 CA169141, and P50 CA127003). Y.B. is supported in part by Grant No. P30 DK046200 from the National Institutes of Health and the International Tree Nut Council Nutrition Research and Education Foundation. S.O. is supported in part by Grant No. R35 CA197735 from the National Institutes of Health.
Nonfederal sponsors did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article. The views expressed in this article are those of the authors and not an official position of their affiliated institutions or funders.
Clinical trial information: NCT00003835.
AUTHOR CONTRIBUTIONS
Conception and design: Leonard B. Saltz, Robert J. Mayer, Rex B. Mowat, Alexander Hantel, Michael Messino, Hedy L. Kindler, Alan Venook, Walter Willett, Edward Giovannucci, Charles S. Fuchs
Financial support: Shuji Ogino
Administrative support: Robert J. Mayer, Charles S. Fuchs
Provision of study materials or patients: Renaud Whittom, Michael Messino, Hedy L. Kindler
Collection and assembly of data: Renaud Whittom, Daniel M. Atienza, Shuji Ogino, Charles S. Fuchs
Data analysis and interpretation: Temidayo Fadelu, Sui Zhang, Donna Niedzwiecki, Xing Ye, Al B. Benson, Alan Venook, Shuji Ogino, Kimmie Ng, Kana Wu, Walter Willett, Edward Giovannucci, Jeffrey Meyerhardt, Ying Bao, Charles S. Fuchs
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nut Consumption and Survival in Patients With Stage III Colon Cancer: Results From CALGB 89803 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Temidayo Fadelu
No relationship to disclose
Sui Zhang
No relationship to disclose
Donna Niedzwiecki
No relationship to disclose
Xing Ye
No relationship to disclose
Leonard B. Saltz
Consulting or Advisory Role: Eli Lilly, McNeil PPC (I), Abbvie
Research Funding: Taiho Pharmaceutical
Robert J. Mayer
Honoraria: Taiho Pharmaceutical
Consulting or Advisory Role: CASI Pharmaceuticals
Rex B. Mowat
No relationship to disclose
Renaud Whittom
Honoraria: AstraZeneca
Consulting or Advisory Role: Boehringer Ingelheim, AstraZeneca, Pfizer, Bristol-Myers Squibb
Research Funding: Astellas (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Seattle Genetics, Celgene
Alexander Hantel
Travel, Accommodations, Expenses: Genentech
Al B. Benson
Consulting or Advisory Role: Genentech, Bristol-Myers Squibb, Merck Serono, Celgene, Vicus Therapeutics, Pharmacyclics, Precision Therapeutics, Taiho Pharmaceutical, Bayer, Alchemia, Infinity Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, EMD Serono, IntegraGen, Guardant Health, Opsona Therapeutics, Lexicon, Novartis, Boston Biomedical, Helsinn, Guerbet, Eli Lilly, Spectrum Pharmaceuticals, Advanced Accelerator Applications, Bayer/Onyx, TRM Oncology, DAVA Pharmaceuticals, Sanofi, Eli Lilly/ImClone
Research Funding: Gilead Sciences (Inst), Amgen (Inst), Astellas Pharma (Inst), Advanced Accelerator Applications (Inst), Bayer/Onyx (Inst), Novartis (Inst), Alchemia (Inst), AVEO (Inst), Infinity Pharmaceuticals (Inst), Genentech (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst)
Travel, Accommodations, Expenses: Genentech, Eli Lilly/ImClone, Bayer, Sanofi, Spectrum Pharmaceuticals, AVEO, Gilead Sciences, Astellas Pharma, Boehringer Ingelheim, DAVA Oncology, TRM Oncology, Guardant Health, Helsinn, Guerbet, Boston Biomedical
Daniel M. Atienza
Employment: Virginia Oncology Associates
Michael Messino
No relationship to disclose
Hedy L. Kindler
Consulting or Advisory Role: Aduro Biotech, MedImmune, Bayer, Genentech, Verastem, Celgene, GlaxoSmithKline, AstraZeneca, Merck
Research Funding: Aduro Biotech, AstraZeneca, Bayer, GlaxoSmithKline, Merck, MedImmune, Verastem, Bristol-Myers Squibb, Eli Lilly
Expert Testimony: Aduro Biotech
Alan Venook
Consulting or Advisory Role: Genentech
Research Funding: Genentech (Inst), Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Now-UptoDate for authoring and maintaining two chapters
Travel, Accommodations, Expenses: Genentech, Taiho Pharmaceutical, Roche
Shuji Ogino
No relationship to disclose
Kimmie Ng
Consulting or Advisory Role: Bayer, Tarrex, Genentech, Eli Lilly
Research Funding: Gilead Sciences, Tarrex, Genentech (Inst)
Kana Wu
No relationship to disclose
Walter Willett
No relationship to disclose
Edward Giovannucci
No relationship to disclose
Jeffrey Meyerhardt
Honoraria: Chugai Pharma
Consulting or Advisory Role: Genentech (Inst)
Ying Bao
No relationship to disclose
Charles S. Fuchs
Leadership: CytomX Therapeutics
Consulting or Advisory Role: Eli Lilly, Sanofi, Merck, Entrinsic Health, Five Prime Therapeutics, Agios, Gilead Sciences, Merrimack, Taiho Pharmaceutical, KEW Group, Genentech
REFERENCES
- 1.Meyerhardt JA, Sato K, Niedzwiecki D, et al. : Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Natl Cancer Inst 104:1702-1711, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. : Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol 24:3535-3541, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. : Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298:754-764, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. : Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: Findings from Cancer and Leukemia Group B 89803. J Clin Oncol 26:4109-4115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. : Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24:3527-3534, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dignam JJ, Polite BN, Yothers G, et al. : Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 98:1647-1654, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt JA, Catalano PJ, Haller DG, et al. : Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol 21:433-440, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs MA, Sato K, Niedzwiecki D, et al. : Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS One 9:e99816, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolpin BM, Meyerhardt JA, Chan AT, et al. : Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol 27:176-185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhupathiraju SN, Pan A, Manson JE, et al. : Changes in coffee intake and subsequent risk of type 2 diabetes: Three large cohorts of US men and women. Diabetologia 57:1346-1354, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M, Bhupathiraju SN, Chen M, et al. : Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 37:569-586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhupathiraju SN, Pan A, Malik VS, et al. : Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr 97:155-166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam RM, Hu FB: Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA 294:97-104, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Salazar-Martinez E, Willett WC, Ascherio A, et al. : Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med 140:1-8, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Wu T, Willett WC, Hankinson SE, et al. : Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in U.S. women. Diabetes Care 28:1390-1396, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Fung TT, Hu FB, Schulze M, et al. : A dietary pattern that is associated with C-peptide and risk of colorectal cancer in women. Cancer Causes Control 23:959-965, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wedick NM, Brennan AM, Sun Q, et al. : Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: A randomized controlled trial. Nutr J 10:93, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CJ, Fargnoli JL, Hwang JJ, et al. : Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: A prospective cohort study. Diabetes Care 31:504-507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guercio BJ, Sato K, Niedzwiecki D, et al. : Coffee intake, recurrence, and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). J Clin Oncol 33:3598-3607, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casas-Agustench P, López-Uriarte P, Bulló M, et al. : Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis 21:126-135, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Holligan SD, West SG, Gebauer SK, et al. : A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. Br J Nutr 112:744-752, 2014 [DOI] [PubMed] [Google Scholar]
- 22.O’Neil CE, Fulgoni VL, III, Nicklas TA: Tree nut consumption is associated with better adiposity measures and cardiovascular and metabolic syndrome health risk factors in U.S. adults: NHANES 2005-2010. Nutr J 14:64, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ros E: Health benefits of nut consumption. Nutrients 2:652-682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-Alonso P, Salas-Salvadó J, Baldrich-Mora M, et al. : Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: A randomized clinical trial. Diabetes Care 37:3098-3105, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Jenkins DJ, Kendall CW, Josse AR, et al. : Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr 136:2987-2992, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Jiang R, Jacobs DR, Jr, Mayer-Davis E, et al. : Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol 163:222-231, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Sweazea KL, Johnston CS, Ricklefs KD, et al. : Almond supplementation in the absence of dietary advice significantly reduces C-reactive protein in subjects with type 2 diabetes. J Funct Foods 10:252-259, 2014 [Google Scholar]
- 28.Kris-Etherton PM, Hu FB, Ros E, et al. : The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J Nutr 138:1746S-1751S, 2008 [DOI] [PubMed] [Google Scholar]
- 29.González CA, Salas-Salvadó J: The potential of nuts in the prevention of cancer. Br J Nutr 96:S87-S94, 2006. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 30.Pfundstein B, Haubner R, Würtele G, et al. : Pilot walnut intervention study of urolithin bioavailability in human volunteers. J Agric Food Chem 62:10264-10273, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Saltz LB, Niedzwiecki D, Hollis D, et al. : Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 25:3456-3461, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Zubrod CG, Schneiderman M, Frei E, et al. : Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis 11:7-33, 1960 [Google Scholar]
- 33. Sturrock PR, Maykel JA: Postoperative management, in colorectal surgery. https://www-clinicalkey-com.ezp-prod1.hul.harvard.edu/#!/content/book/3-s2.0-B9781437717242000040?scrollTo=%23hl0000956.
- 34.Willett WC, Reynolds RD, Cottrell-Hoehner S, et al. : Validation of a semi-quantitative food frequency questionnaire: Comparison with a 1-year diet record. J Am Diet Assoc 87:43-47, 1987 [PubMed] [Google Scholar]
- 35.Willett WC, Sampson L, Stampfer MJ, et al. : Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122:51-65, 1985 [DOI] [PubMed] [Google Scholar]
- 36.US Department of Agriculture : Composition of Foods: Raw, Processed, and Prepared, 1963-1988. Washington, DC, Department of Agriculture, US Government Printing Office, 1989 [Google Scholar]
- 37.Willett W, Stampfer MJ: Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol 124:17-27, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Salvini S, Hunter DJ, Sampson L, et al. : Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol 18:858-867, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Ogino S, Liao X, Imamura Y, et al. : Predictive and prognostic analysis of PIK3CA mutation in stage III colon cancer intergroup trial. J Natl Cancer Inst 105:1789-1798, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogino S, Shima K, Meyerhardt JA, et al. : Predictive and prognostic roles of BRAF mutation in stage III colon cancer: Results from intergroup trial CALGB 89803. Clin Cancer Res 18:890-900, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogino S, Kawasaki T, Brahmandam M, et al. : Sensitive sequencing method for KRAS mutation detection by pyrosequencing. J Mol Diagn 7:413-421, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogino S, Meyerhardt JA, Irahara N, et al. : KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res 15:7322-7329, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren RS, Atreya CE, Niedzwiecki D, et al. : Association of TP53 mutational status and gender with survival after adjuvant treatment for stage III colon cancer: Results of CALGB 89803. Clin Cancer Res 19:5777-5787, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. doi: 10.1200/JCO.2008.18.2071. Bertagnolli MM, Niedzwiecki D, Compton CC, et al: Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B protocol 89803. J Clin Oncol 27:1814-1821, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan AT, Ogino S, Fuchs CS: Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356:2131-2142, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Bao Y, Hu FB, Giovannucci EL, et al. : Nut consumption and risk of pancreatic cancer in women. Br J Cancer 109:2911-2916, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Yang M, Kenfield SA, et al. : Nut consumption and prostate cancer risk and mortality. Br J Cancer 115:371-374, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang M, Hu FB, Giovannucci EL, et al. : Nut consumption and risk of colorectal cancer in women. Eur J Clin Nutr 70:333-337, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao Y, Han J, Hu FB, et al. : Association of nut consumption with total and cause-specific mortality. N Engl J Med 369:2001-2011, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox DR: Regression models and life-tables. J R Stat Soc B 34:187-220, 1972 [Google Scholar]
- 51.Yeh C-C, You S-L, Chen C-J, et al. : Peanut consumption and reduced risk of colorectal cancer in women: A prospective study in Taiwan. World J Gastroenterol 12:222-227, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenab M, Ferrari P, Slimani N, et al. : Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 13:1595-1603, 2004 [PubMed] [Google Scholar]
- 53.Guasch-Ferré M, Bulló M, Martínez-González MÁ, et al. : Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med 11:164, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández-Montero A, Bes-Rastrollo M, Barrio-López MT, et al. : Nut consumption and 5-y all-cause mortality in a Mediterranean cohort: The SUN project. Nutrition 30:1022-1027, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Bonaccio M, Di Castelnuovo A, De Curtis A, et al. : Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: Prospective results from the Moli-sani study. Br J Nutr 114:804-811, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Jiang R, Manson JE, Stampfer MJ, et al. : Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 288:2554-2560, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Villegas R, Gao Y-T, Yang G, et al. : Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am J Clin Nutr 87:162-167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan A, Sun Q, Manson JE, et al. : Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr 143:512-518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-Montero A, Bes-Rastrollo M, Beunza JJ, et al. : Nut consumption and incidence of metabolic syndrome after 6-year follow-up: The SUN (Seguimiento Universidad de Navarra, University of Navarra Follow-up) cohort. Public Health Nutr 16:2064-2072, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh PN, Fraser GE: Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol 148:761-774, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Djoussé L, Rudich T, Gaziano JM: Nut consumption and risk of hypertension in US male physicians. Clin Nutr 28:10-14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai C-J, Leitzmann MF, Hu FB, et al. : A prospective cohort study of nut consumption and the risk of gallstone disease in men. Am J Epidemiol 160:961-968, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Tsai C-J, Leitzmann MF, Hu FB, et al. : Frequent nut consumption and decreased risk of cholecystectomy in women. Am J Clin Nutr 80:76-81, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Strate LL, Liu YL, Syngal S, et al. : Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA 300:907-914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gopinath B, Buyken AE, Flood VM, et al. : Consumption of polyunsaturated fatty acids, fish, and nuts and risk of inflammatory disease mortality. Am J Clin Nutr 93:1073-1079, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Torabian S, Haddad E, Rajaram S, et al. : Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J Hum Nutr Diet 22:64-71, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Ma Y, Njike VY, Millet J, et al. : Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care 33:227-232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tapsell LC, Batterham MJ, Teuss G, et al. : Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 63:1008-1015, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Lux S, Scharlau D, Schlörmann W, et al. : In vitro fermented nuts exhibit chemopreventive effects in HT29 colon cancer cells. Br J Nutr 108:1177-1186, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Nagel JM, Brinkoetter M, Magkos F, et al. : Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 28:67-75, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeStefano F, Anda RF, Kahn HS, et al. : Dental disease and risk of coronary heart disease and mortality. BMJ 306:688-691, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshipura KJ, Hung H-C, Rimm EB, et al. : Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke 34:47-52, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Jimenez M, Krall EA, Garcia RI, et al. : Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol 66:505-512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watt RG, Tsakos G, de Oliveira C, et al. : Tooth loss and cardiovascular disease mortality risk: Results from the Scottish Health Survey. PLoS One 7:e30797, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desvarieux M, Demmer RT, Rundek T, et al. : Relationship between periodontal disease, tooth loss, and carotid artery plaque: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke 34:2120-2125, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dietrich T, Jimenez M, Krall Kaye EA, et al. : Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation 117:1668-1674, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tu Y-K, Galobardes B, Smith GD, et al. : Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart 93:1098-1103, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Migliorati CA: Periodontal diseases and cancer. Lancet Oncol 9:510-512, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Michaud DS, Liu Y, Meyer M, et al. : Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol 9:550-558, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abnet CC, Qiao Y-L, Mark SD, et al. : Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control 12:847-854, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Garrote LF, Herrero R, Reyes RMO, et al. : Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer 85:46-54, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hiraki A, Matsuo K, Suzuki T, et al. : Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev 17:1222-1227, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Abnet CC, Kamangar F, Dawsey SM, et al. : Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol 40:681-687, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Boylan MR, Khalili H, Huang ES, et al. : A prospective study of periodontal disease and risk of gastric and duodenal ulcer in male health professionals. Clin Transl Gastroenterol 5:e49, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hujoel PP, Drangsholt M, Spiekerman C, et al. : An exploration of the periodontitis-cancer association. Ann Epidemiol 13:312-316, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Momen-Heravi F, Babic A, Tworoger SS, et al. : Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. Int J Cancer 140:646-652, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michaud DS, Fuchs CS, Liu S, et al. : Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 14:138-147, 2005 [PubMed] [Google Scholar]