Abstract
Purpose
Isolated locoregional recurrence (ILRR) predicts a high risk of developing breast cancer distant metastases and death. The Chemotherapy as Adjuvant for LOcally Recurrent breast cancer (CALOR) trial investigated the effectiveness of chemotherapy (CT) after local therapy for ILRR. A report at 5 years of median follow-up showed significant benefit of CT for estrogen receptor (ER)–negative ILRR, but additional follow-up was required in ER-positive ILRR.
Patients and Methods
CALOR was an open-label, randomized trial for patients with completely excised ILRR after unilateral breast cancer. Eligible patients were randomly assigned to receive CT or no CT and stratified by prior CT, hormone receptor status, and location of ILRR. Patients with hormone receptor–positive ILRR received adjuvant endocrine therapy. Radiation therapy was mandated for patients with microscopically involved margins, and anti–human epidermal growth factor receptor 2 therapy was optional. End points were disease-free survival (DFS), overall survival, and breast cancer-free interval.
Results
From August 2003 to January 2010, 162 patients were enrolled: 58 with ER-negative and 104 with ER-positive ILRR. At 9 years of median follow-up, 27 DFS events were observed in the ER-negative group and 40 in the ER-positive group. The hazard ratios (HR) of a DFS event were 0.29 (95% CI, 0.13 to 0.67; 10-year DFS, 70% v 34%, CT v no CT, respectively) in patients with ER-negative ILRR and 1.07 (95% CI, 0.57 to 2.00; 10-year DFS, 50% v 59%, respectively) in patients with ER-positive ILRR (Pinteraction = .013). HRs were 0.29 (95% CI, 0.13 to 0.67) and 0.94 (95% CI, 0.47 to 1.85), respectively, for breast cancer-free interval (Pinteraction = .034) and 0.48 (95% CI, 0.19 to 1.20) and 0.70 (95% CI, 0.32 to 1.55), respectively, for overall survival (Pinteraction = .53). Results for the three end points were consistent in multivariable analyses adjusting for location of ILRR, prior CT, and interval from primary surgery.
Conclusion
The final analysis of CALOR confirms that CT benefits patients with resected ER-negative ILRR and does not support the use of CT for ER-positive ILRR.
INTRODUCTION
The increased use of adjuvant radiation and systemic therapies and the improved efficacy of such therapies in the past two decades have resulted in a lower incidence of locoregional recurrence of breast cancer.1-5 However, after an isolated locoregional recurrence (ILRR) event of breast cancer, the risk of distant metastases and death is high.6-9 The Chemotherapy as Adjuvant for LOcally Recurrent breast cancer (CALOR) trial was designed as a prospective randomized study to determine the effectiveness of adjuvant chemotherapy (CT) after surgical excision of ILRR. Previously, we reported the results at a median follow-up of 5 years, which showed significant benefit of CT for estrogen receptor (ER)–negative ILRR, whereas for patients with ER-positive ILRR, the benefit of CT was uncertain.10
In a separate analysis, we found that a subset of patients developed a second ILRR during the first 5 years. These events occurred within a short interval of the first ILRR (median, 1.6 years), were uniquely progesterone-receptor (PR) negative, and were strong indicators of subsequent risk of distant recurrence and death.11 This report presents results at 9 years of median follow-up, focusing on the ER-status cohorts, with the aim of further clarifying the effect of CT in patients with ER-negative and ER-positive ILRR.
PATIENTS AND METHODS
Patients and Procedures
Briefly, CALOR is a pragmatic, open-label, randomized, multicenter and multinational trial for patients with completely excised ILRR after unilateral breast cancer.10 Eligible patients were randomly assigned to CT (selected by the investigator; multidrug for at least 3 months recommended) or no CT and stratified by prior CT, hormone receptor (ER, PR) status of ILRR, and location of ILRR. Patients with ER- and/or PR-positive ILRR were to receive adjuvant endocrine therapy and, if recurrence occurred while receiving endocrine therapy, a regimen change (eg, substitution of a selective ER modulator with an aromatase inhibitor) was recommended. Radiation therapy was mandated for patients with microscopically involved margins and recommended for all patients who had not received radiotherapy as part of their primary treatment. Human epidermal growth factor receptor 2 (HER2)–directed therapy was optional. Follow-up clinical examinations were required every 3 months during the first 2 years, every 6 months during years 3 to 5, and yearly thereafter. Annual mammography was required, but other laboratory or imaging studies were left to the discretion of the treating physicians. Participating institutions’ ethics committees or institutional review boards approved the trial according to local laws and regulations. All patients gave written informed consent, and the trial was conducted in compliance with the Helsinki Declaration. Patient data were anonymized.
International Breast Cancer Study Group (IBCSG) and National Surgical Adjuvant Breast and Bowel Project/NRG Oncology were responsible for the design of the study. IBCSG coordinated the collection and management of the data, medical review, and data analysis. The reporting of the results was performed jointly.
Outcomes
The primary end point was disease-free survival (DFS), defined as time from randomization to invasive local, regional, or distant recurrence, including invasive in-breast tumor recurrence, appearance of a second primary tumor, or death from any cause. In the absence of an event, DFS was censored at the date of the last follow-up visit. Overall survival (OS) and breast cancer–free interval (BCFI)12 were secondary end points. BCFI was defined as time from randomization to first invasive breast tumor recurrence, with second primary tumors ignored and death from causes other than breast cancer recurrence censored at the time of death. OS was defined as the time from randomization to death from any cause.
Statistical Analysis
The present analysis is the final update of the results of the CALOR trial within subgroups defined by the ER status of the ILRR. The subgroup analysis according to ER status was clinically motivated and prospectively specified in the protocol. Because the previously published results at 5 years of median follow-up indicated a significant interaction between CT effect and ER status of the ILRR,10 the focus of this update is to provide separate analyses within ER status cohorts.
We calculated Kaplan-Meier estimates13 of end points at 10 years, with SEs by Greenwood formula. Cox regression models were used to estimate HRs (95% CI) for treatment effects within cohorts, to adjust for the following covariables: location of ILRR, prior chemotherapy, interval from primary surgery, and ER status of ILRR, and to estimate HRs (95% CIs) for treatment effects across covariable subgroups by including treatment-by-covariable interaction in the model; the two-sided P values for treatment-by-covariable interaction was reported along with the HR (95% CI) in forest plots.14
The sample size and protocol modifications have been described elsewhere. Briefly, the original statistical design was modified because of lower-than-planned accrual, and CALOR closed on Jan 31, 2010, with 162 patients enrolled.10 All patients randomly assigned are included in this intention-to-treat analysis. The database lock was September 2016.
RESULTS
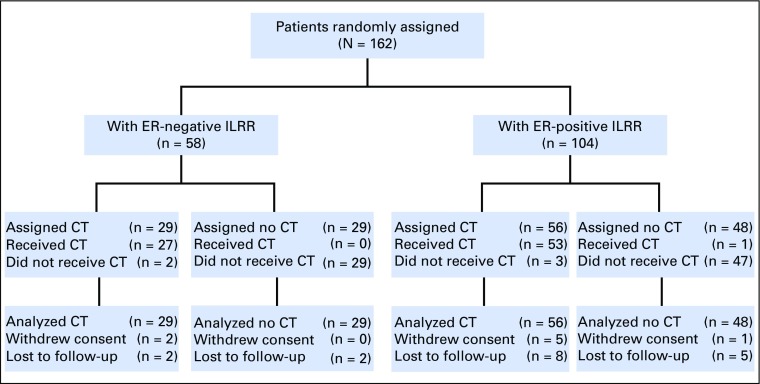
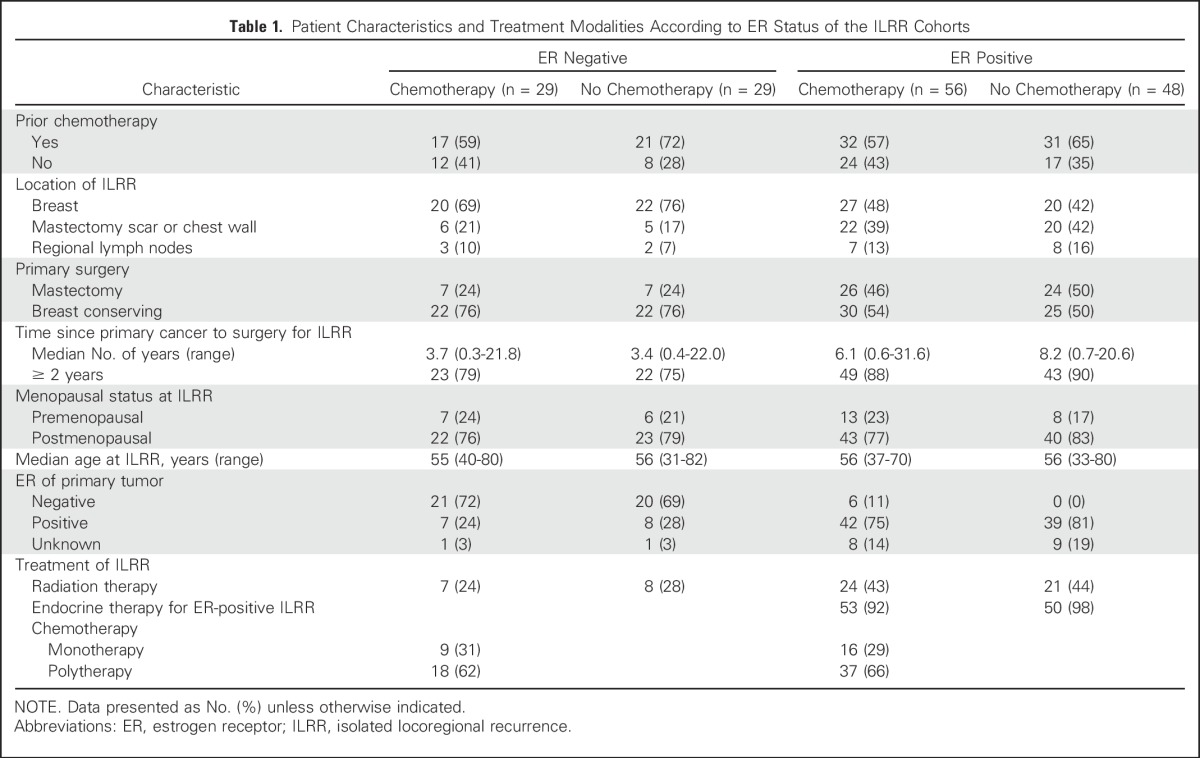
The study cohort consisted of 162 women enrolled from August 2003 to January 2010 (Fig 1). The patient and treatment characteristics according to ER status are listed in Table 1. The median time to recurrence from primary cancer to ILRR was 3.6 years for the ER-negative cohort compared with 6.8 years for the ER-positive cohort; 94% of patients with ER-positive ILRR received prior endocrine therapy, and only a small proportion of patients (9%) with ER-positive primary cancers were receiving such treatment at the time of the ILRR diagnosis.
Fig 1.
CONSORT diagram of Chemotherapy as Adjuvant for LOcally Recurrent breast cancer (CALOR) trial update according to estrogen receptor (ER) status of the ILRR. CT, chemotherapy; ILRR, isolated locoregional recurrence; LFU, lost to follow-up; no CT, no chemotherapy.
Table 1.
Patient Characteristics and Treatment Modalities According to ER Status of the ILRR Cohorts
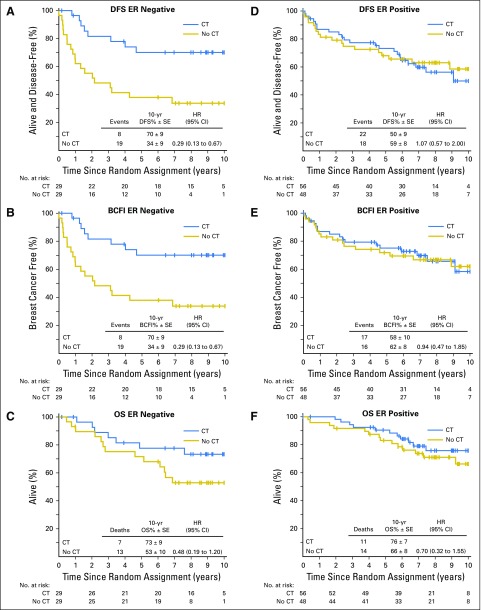
At 9 years of median follow-up, CT improved DFS substantially in patients with ER-negative ILRR (Fig 2A): 10-year DFS was 70% (SE, 9%) in patients with and 34% (SE, 9%) in patients without CT (hazard ratio [HR] 0.29; 95% CI, 0.13 to 0.67). In contrast, CT had no benefit in patients with ER-positive ILRR: 10-year DFS was 50% (SE, 9%) in patients with and 59% (SE, 8%) in patients without CT (HR, 1.07; 95% CI, 0.57 to 2.00; Fig 2D). Similarly, BCFI was prolonged by CT in patients with ER-negative ILRR (breast cancer free at 10 years, 70% v 34%; HR,0.29; 95% CI, 0.13 to 0.67), but not in patients with ER-positive ILRR (58% v 62%; HR, 0.94; 95% CI, 0.47 to 1.85; Figs 2B and 2E). OS at 10 years in patients with ER-negative ILRR was 73% with CT versus 53% without CT (HR, 0.48; 95% CI, 0.19 to 1.20); among patients with ER-positive ILRR, 10-year OS was 76% versus 66%, respectively (HR, 0.70; 95% CI, 0.32 to 1.55; Figs 2C and 2F). Interaction tests comparing CT effect for ER-positive ILRR versus ER-negative ILRR are Pinteraction = .013 for DFS (Fig 2A v 2D); Pinteraction = .034 for BCFI (Fig 2B v 2E); and Pinteraction = .53 for OS (Fig 2C v Fig 2F). The overall DFS, BCFI, and OS results, not separated by ER status of the ILRR, are presented in the Data Supplement.
Fig 2.
Disease-free survival (DFS), breast cancer–free interval (BCFI), and overall survival (OS) for patients with (A-C) estrogen-receptor (ER)-negative and (D-F) ER-positive isolated locoregional recurrence (ILRR). Interaction tests comparing the effect of chemotherapy (CT) for patients with ER-negative ILRR versus ER-positive ILRR are Pinteraction = .013 for DFS (A v D); Pinteraction = .034 for BCFI (B v E); and Pinteraction = .53 for OS (C v F). No CT, not assigned to chemotherapy; HR, hazard ratio.
Overall, there were 67 DFS events, as listed in Table 2. The site of first recurrence after randomization and therefore after the primary ILRR recurrence was local and regional for 16 patients, six in the CT arm and 10 in the no-CT arm. Distant disease was the first site of recurrence for 41 patients, 18 patients in the CT arm and 23 patients in the no-CT arm. In the ER-positive cohort, visceral and bone metastasis rates were similar overall and between treatment groups. In the ER-negative cohort, bone metastases as site of first recurrence after ILRR were rare (two of 58 patients, one in each treatment group), whereas visceral metastases were more common and differed according to treatment group (four of 29 [14%] in the CT arm; 10 of 29 [34%] no CT arm). All 10 nonbreast cancer DFS events occurred in the ER-positive cohort; thus, DFS and BCFI outcomes were the same in the ER-negative cohort (Table 2; Figs 2A and 2B).
Table 2.
Sites of First Failure (DFS events) After Randomization According to Treatment Group Within ER Status of the ILRR Cohorts
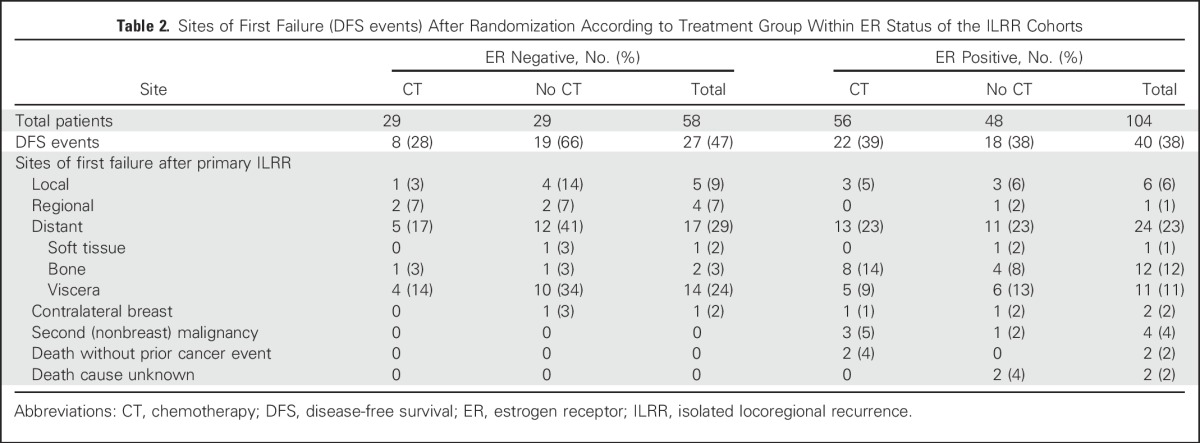
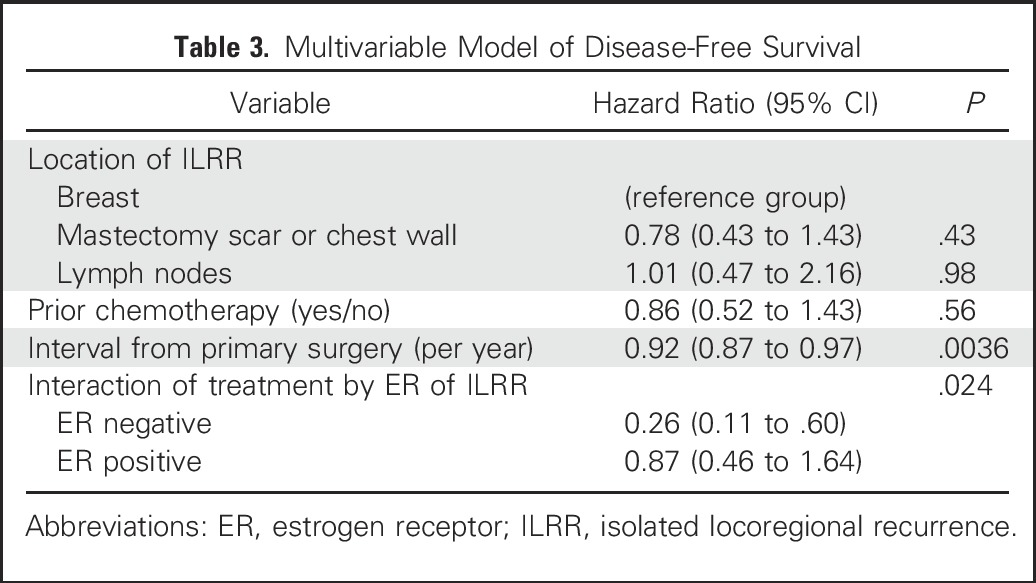
The improvement of DFS by CT remained significant in a multivariable proportional hazards model that included factors for ER status of ILRR, location of ILRR, previous CT use, and interval from primary surgery. The interaction between ER status and CT effect was statistically significant, confirming the differential efficacy of CT depending on ER expression of the ILRR (Table 3). The multivariable analysis of BCFI gave similar results, again with a statistically significant interaction between ER status and efficacy of CT (data not shown).
Table 3.
Multivariable Model of Disease-Free Survival
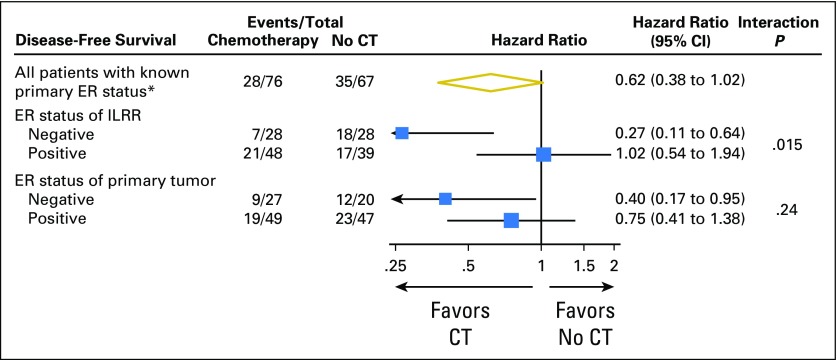
The interaction between ER expression and CT effect was strong and statistically significant if the ER status of the ILRR tissue was considered. In contrast, the ER status of the primary tumor tissue was less predictive of the efficacy of CT, and the interaction was not statistically significant (Fig 3).
Fig 3.
Subgroup analysis of disease-free survival according to estrogen-receptor (ER) status of isolated locoregional recurrence (ILRR) and ER status of primary breast cancer tissue among 143 patients with known primary ER status. The size of the boxes is proportional to the number of events. The x-axis is on a log scale. CT, assigned to chemotherapy; no CT, not assigned to chemotherapy. (*) 143 of the 162 randomly assigned patients.
DISCUSSION
The long-term follow-up results of the CALOR trial confirm the reported findings of the 5-year analysis10: the statistically significant benefit of CT for the cohort of patients with ER-negative ILRR was sustained. The extended follow-up now available strengthens conclusions for the ER-positive ILRR cohort: no benefit of CT was observed for these patients. Interactions between ER expression of the ILRR and the use of CT were significant for DFS and BCFI. These results were confirmed in multivariable analyses adjusting for location of ILRR, prior CT, and interval from primary surgery. This updated analysis demonstrates that patients with an ILRR should be managed according to the endocrine molecular profile of the recurrent cancer and not the primary cancer.
The CALOR trial investigated the role of pragmatically chosen CT, at the discretion of treating physicians. Seemingly, oncologists selected effective CT regimens for their patients on the basis of prior cytotoxic agent exposure and in consideration of experienced toxicities. Although not a trial question, endocrine therapy was mandated for patients with ER-positive ILRR. In fact, the protocol recommended a switch of therapy, for example, from a selective ER modulator to an aromatase inhibitor, especially for the few patients whose recurrence happened while receiving endocrine treatment. Because CT did not reduce the number of failures or any of the measured end points in patients with ER-positive ILRR, it is reasonable to conclude that endocrine therapy is the mainstay treatment of such patients.
The strengths of the CALOR trial included its prospective randomized design and the pragmatic assignment of individual CT by the participating oncologist. The median follow-up is now sufficiently long to capture the effects of adjuvant CT. The individualized choice of CT may seem to be a weakness, but the robust benefit of CT in patients with ER-negative ILRR points to a generally beneficial effect of CT as chosen by the investigators. In addition, the availability of ER status for both ILRR and primary tumor enabled analyses showing that ILRR ER status was the better predictor of CT benefit. The main weakness of the trial was its small sample size due to the lower-than-anticipated accrual rate, which precludes definitive evaluation of several secondary questions. For example, given the limited number of ER-positive ILRR participants and the 12% lost to follow-up rate for this subgroup, a modest benefit of CT in patients with luminal recurrences could not be excluded. Furthermore, the benefit of CT in patients with luminal B-like (eg, ER-positive, PR-negative) recurrences could not be evaluated. Whereas the interval between primary breast cancer and ILRR is prognostic, the number of trial participants was insufficient to evaluate whether the time from primary adjuvant CT to ILRR was predictive of CT benefit after ILRR. In particular, because of small numbers and a median interval of 3.5 years between primary diagnosis and ER-negative ILRR, the question of whether CT effectiveness diminished for short intervals could not be addressed. The hypothesis of a more pronounced efficacy of CT in patients who experienced the ER-positive ILRR while receiving adjuvant endocrine therapy also could not be investigated because of the low number of patients with these characteristics. Similarly, although adjuvant taxanes became standard practice during the accrual period, the influence of their use before ILRR could not be evaluated. Furthermore, fewer than 5% of the participants received HER2-directed adjuvant therapy; thus, this trial cannot shed any light on the question of HER2-directed therapy for ILRR.
In conclusion, the CALOR trial indicates that at present, CT offers the best prospect of prolonged DFS in patients with ER-negative first ILRR, whereas adding CT to endocrine therapy seems to offer no benefit to patients with ER-positive ILRR.
ACKNOWLEDGMENT
We thank the patients, physicians, nurses, trial coordinators, and pathologists who participated in the Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer (CALOR) trial. We thank Helmut Rauschecker, MD, for his support of this international collaboration, and Karolyn Scott for central data management. The authors are grateful for the intellectual contributions that John Bryant, former director of the National Surgical Adjuvant Breast and Bowel Project Biostatistical Center, made to this study.
Footnotes
The Chemotherapy as Adjuvant for LOcally Recurrent Breast Cancer (CALOR) trial was supported in part by Public Service Grants U10-CA-180868, UG1-CA-189867, U10-180822 (to NRG Oncology), U24-CA-075362 (to International Breast Cancer Study Group Statistical Center) from the National Cancer Institute, Department of Health and Human Services. The International Breast Cancer Study Group is supported in part by the Swiss Group for Clinical Cancer Research, Frontier Science and Technology Research Foundation, Australia and New Zealand Breast Cancer Trials Group, Swedish Cancer Society, Cancer Research Switzerland/Oncosuisse, Cancer Association of South Africa, and Foundation for Clinical Cancer Research of Eastern Switzerland. Spanish participation was funded by GEICAM Spanish Breast Cancer Group and Dutch participation by BOOG, the Dutch Breast Cancer Trialists' Group.
Presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2-6, 2017.
Clinical trial information: NCT00074152.
See accompanying Editorial on page 1058
AUTHOR CONTRIBUTIONS
Conception and design: Karen N. Price, Beat Thürlimann, Alan S. Coates, Richard D. Gelber, Stefan Aebi
Administrative support: Charles E. Geyer Jr, Priya Rastogi, Norman Wolmark
Provision of study materials or patients: Irene L. Wapnir, André Robidoux, Miguel Martín, Johan W.R. Nortier, Alexander H.G. Paterson, Mothaffar F. Rimawi, István Láng, José Manuel Baena-Cañada, Beat Thürlimann, Eleftherios P. Mamounas, Charles E. Geyer Jr, Stefan Aebi
Collection and assembly of data: Irene L. Wapnir, Karen N. Price, André Robidoux, Miguel Martín, Johan W.R. Nortier, Alexander H.G. Paterson, Mothaffar F. Rimawi, István Láng, José Manuel Baena-Cañada, Beat Thürlimann, Eleftherios P. Mamounas, Charles E. Geyer Jr, Shari Gelber, Richard D. Gelber, Priya Rastogi, Norman Wolmark, Stefan Aebi,
Data analysis and interpretation: Irene L. Wapnir, Karen N. Price, Stewart J. Anderson, Shari Gelber, Richard D. Gelber, Meredith M. Regan, Stefan Aebi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy of Chemotherapy for ER-Negative and ER-Positive Isolated Locoregional Recurrence of Breast Cancer: Final Analysis of the CALOR Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Irene L. Wapnir
Consulting or Advisory Board: Genomic Health, Amgen
Research Funding: OncoSec, Novadaq Technologies
Karen N. Price
No relationship to disclose
Stewart J. Anderson
No relationship to disclose
André Robidoux
No relationship to disclose
Miguel Martín
Consulting or Advisory Role: Roche/Genentech, Novartis, Amgen, Pfizer, Eli Lilly, PharmaMar
Research Funding: Novartis (Inst)
Johan W.R. Nortier
No relationship to disclose
Alexander H.G. Paterson
Consulting or Advisory Role: Pfizer
Research Funding: Amgen
Mothaffar F. Rimawi
Consulting or Advisory Role: Celgene, Genentech (Inst)
Research Funding: Genentech (Inst)
István Láng
No relationship to disclose
José Manuel Baena-Cañada
No relationship to disclose
Beat Thürlimann
No relationship to disclose
Eleftherios Mamounas
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Genomic Health, Pfizer, Novartis, bioTheranostics, Celcuity, GRAIL, Macrogenics
Speakers' Bureau: Genomic Health, Genentech/Roche
Travel, Accommodations, Expenses: Genomic Health, Genentech/Roche
Charles E. Geyer Jr
Consulting or Advisory Role: Stemnion, Myriad Genetics, Celgene, Heron Therapeutics
Research Funding: Incyte, Merck
Travel, Accommodations, Expenses: AstraZeneca, Abbvie, Genentech/Roche
Shari Gelber
No relationship to disclose
Alan S. Coates
No relationship to disclose
Richard D. Gelber
Research Funding: AstraZeneca (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Merck (Inst), Pfizer (Inst), Ipsen (Inst), Ferring (Inst)
Priya Rastogi
No relationship to disclose
Meredith M. Regan
Consulting or Advisory Role: Merck, Ipsen (Inst)
Research Funding: Veridex (Inst), OncoGenex (Inst), Pfizer (Inst), Ipsen (Inst), Novartis (Inst), Merck (Inst), Ferring (Inst), Celgene (Inst), AstraZeneca (Inst), Pierre Fabre (Inst), Ipsen (Inst)
Norman Wolmark
No relationship to disclose
Stefan Aebi
No relationship to disclose
REFERENCES
- 1.Dowsett M, Cuzick J, Ingle J, et al. : Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28:509-518, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, et al. : Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707-1716, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peto R, Davies C, Godwin J, et al. : Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432-444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGale P, Taylor C, Correa C, et al. : Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127-2135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez EA, Romond EH, Suman VJ, et al. : Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32:3744-3752, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher B, Anderson S, Fisher ER, et al. : Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet 338:327-331, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Anderson SJ, Wapnir I, Dignam JJ, et al. : Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol 27:2466-2473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson P, Cole BF, Chua BH, et al. : Patterns and risk factors for locoregional failures after mastectomy for breast cancer: An International Breast Cancer Study Group report. Ann Oncol 23:2852-2858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wapnir IL, Anderson SJ, Mamounas EP, et al. : Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol 24:2028-2037, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Aebi S, Gelber S, Anderson SJ, et al. : Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): A randomised trial. Lancet Oncol 15:156-163, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wapnir IL, Gelber S, Anderson SJ, et al. : Poor prognosis after second locoregional recurrences in the CALOR trial. Ann Surg Oncol 24:398-406, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudis CA, Barlow WE, Costantino JP, et al. : Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol 25:2127-2132, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P: Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 14.Cox DR: Regression models and life-tables. JR Stat Soc B (Methodology) 34:187-220, 1972 [Google Scholar]