Abstract
When cerebral blood flow falls below a critical limit, syncope occurs and if prolonged ischemia leads to neuronal death. The cerebral circulation has its own complex finely tuned autoregulatory mechanisms to ensure blood supply to the brain can meet the high metabolic demands of the underlying neuronal tissue. This involves the interplay between myogenic and metabolic mechanisms, input from noradrenergic and cholinergic neurons, and the release of vasoactive substrates including adenosine from astrocytes and nitric oxide from the endothelium. The transcranial Doppler (TCD) is a non-invasive technique that provides real-time measurements of cerebral blood flow velocity. TCD can be very useful in the work up of a patient with recurrent syncope. Cerebral autoregulatory mechanisms help defend the brain against hypoperfusion when perfusion pressure falls on standing. Syncope occurs when hypotension is severe and susceptibility increases with hyperventilation, hypocapnia and cerebral vasoconstriction. We review clinical standards for the acquisition and analysis of TCD signals in the autonomic laboratory and the multiple methods available to assess cerebral autoregulation. We also describe the control of cerebral blood flow in autonomic disorders and functional syndromes.
Keywords: Transcranial Doppler, cerebral blood flow velocity, syncope, orthostatic hypotension, autonomic testing, autonomic failure, dysautonomia
1. INTRODUCTION
Cerebral blood flow (CBF) is normally 50–60 ml/min per 100 grams of brain tissue [52]. Despite the human brain weighing only 2% of the total body mass, it receives 15% of the cardiac output at rest and consumes 20% of the body’s oxygen [94]. Brain tissue has high metabolic demands and, in order to maintain consciousness, it must receive an adequate supply of blood flow to ensure that energy and oxygen demands are met. When blood flow falls below the critical limit, even for a few seconds, syncope (i.e., a reversible loss of consciousness with no neurological sequelae) occurs [46, 94]. The inbuilt capacity of the cerebral circulation to regulate its own flow to remain constant in the face of changes in perfusion pressure is known as cerebrovascular autoregulation.
The CBF autoregulation involves integrative interactions between brain tissue metabolism, systemic blood pressure, arterial blood gases, as well as neurogenic input from the central autonomic network. This interplay occurs at the level of the arterioles in the cerebrovasculature and at the neurovascular unit, and over multiple time scales from seconds to hours [40]. This adaptability ensures that the delivery of oxygen and nutrients can meet the high metabolic demands of the neuronal tissue in different regions of the brain [52, 75, 94]. The failure of cerebral autoregulation can occur at any age. The elderly population, in particular those with autonomic or cardiovascular disorders, are at a greater risk for dementia, stroke, long-term disability and death [13, 15, 69].
We focus on the transcranial Doppler (TCD) as a non-invasive method to evaluate cerebral hemodynamics and its usefulness in the outpatient autonomic clinic. We here review the available literature, using a PubMed search with the following keywords: cerebral blood flow, transcranial Doppler, syncope, orthostatic hypotension, autonomic testing and autonomic failure. Emphasis was given to articles published within the last ten years.
The current state-of-the-art assessment of cerebral autoregulation using TCD lacks validated tools and methodologies to reliably detect impaired blood flow regulation. Clinical validation will require a collaborative effort to organize a randomized well-powered control trial in a large population. International consensus guidelines exist to standardize TCD measures for best clinical practice [13], and should enable us to internationally standardize methodological approaches and together validate the tools necessary to assess cerebral autoregulation in the autonomic laboratory.
2. THE PHYSIOLOGY OF CEREBRAL BLOOD FLOW AUTOREGULATION
CBF autoregulation buffers variations of cerebral perfusion pressure to provide a constant supply of blood to the underlying brain tissue. Maintaining this steady-state requires balancing intracranial pressure (ICP), arterial blood pressure (ABP), and cerebrovascular resistance (CVR) in a limited intracranial space [31]. This relationship is depicted in the following formula:
The mechanisms involved in this process are illustrated in Figure 1. Myogenic and endothelial vascular responses play an important role in regulating CBF. The small arteries and arterioles within cerebral circulation have intrinsic mechanisms and contract when stretched to raise resistance [82]. The downstream resistance arterioles in the cerebral circulation are exquisitely sensitive to variations in arterial CO2 (PaCO2). This creates small shifts in pH, with hypercapnia inducing regional acidosis, dilatation of the smooth muscle, and an increase in blood flow to the underlying region, which flushes out the CO2.
Figure 1. Mechanisms involved the autoregulation of blood flow within cerebral arterioles.
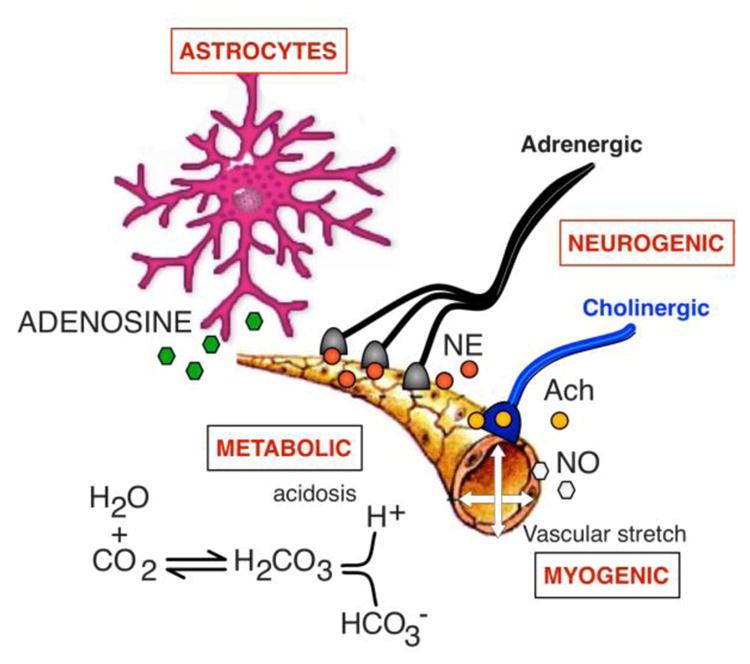
The resistance within small arterioles in the cerebral circulation is controlled by the interplay between metabolic factors (i.e., local changes in acid basis balance), the intrinsic myogenic ability of the smooth muscle to constrict when stretched, input from noradrenergic and cholinergic neurons and the release of vasoactive substrates including adenosine from glia, nitric oxide from the epithelium, among others. See text for details. NE = norepinephrine, AcH = acetylcholine, NO = nitric oxide.
Small vessels in the cerebral circulation respond less sensitively to hypoxia, particularly when chronic, as in patients with lung disease or congestive heart failure [61]. The changes in brain metabolism in responses to an increase in neuronal activity trigger the release of vasoactive compounds such as arachidonic acid, lactate, adenosine and nitric oxide within the neurovascular unit to address the increased energy demands. Glial cells, neurons and the vascular endothelium within the neurovascular unit can also re-direct local blood flow [20]. Astrocytes regulate the release adenosine and can elicit vasoconstriction (through changes in intracellular calcium concentration) or vasodilation of arterioles [27]. Similarly, an increase in glucose results in an increase in blood flow and oxygen supply. The cerebral arterioles are dually innervated by both parasympathetic and sympathetic fibers [32, 42], which are thought to play a role in buffering changes in perfusion. Activation of the sympathetic nerves presumably increases cerebrovascular tone, although there is uncertainty as to when these nerves are activated [26].
The assessment of cerebrovascular autoregulation evaluates how well the cerebral vessels respond to changes in arterial blood pressure to regulate blood supply constant. These changes can be evaluated by physical maneuvers that reduce venous return to the heart and lower perfusion pressure within the cerebral circulation (e.g., orthostatic stress or Valsalva straining). These challenges require an effective cerebral autoregulatory response to prevent cerebral blood flow from falling below critical limits [74]. Age and gender may also influence cerebral autoregulatory responses [10, 21].
Static versus dynamic cerebral autoregulation
Static autoregulation refers to the ability of the cerebral circulation to maintain a constant flow overtime in response to changes in blood pressure (BP). The evaluation of static autoregulation requires measurements of cerebral blood flow velocity (CBFv) and blood pressure under steady-state conditions. Typically, measurements are first obtained in the supine position to establish a baseline. BP is then manipulated, usually by infusion of systemically active vasoconstrictors (phenylephrine) and/or vasodilators (sodium nitroprusside) that increase or decrease BP. Once pressure is held constant and a different level, other steady state measurements are acquired over several minutes [75, 88]. If during the changes in blood pressure, CBF is maintained near to baseline levels, cerebral autoregulation is assumed to be intact [82].
Dynamic autoregulation is used to describe CBFv responses to spontaneous fluctuations in blood pressure at rest or by inducing small transient changes in blood pressure. Common scenarios include:
Transient changes in blood pressure while resting (not provoked, so-called spontaneous oscillations).
Transient increases or decreases in blood pressure induced pharmacological by systemically active pressor agents or vasodilators that do not cross the blood-brain barrier (e.g., intravenous administration of noradrenaline or sodium nitroprusside).
Transient increases in blood pressure induced by physical maneuvers (e.g., standing, squat to stand, periodic breathing, lower body negative pressure, or thigh cuff release) [87].
The feasibility of these techniques depends upon the expertise of the local laboratory, available experimental facilities, subject mobility and clinical risk. While lower body negative pressure has the advantage of providing a physiological rather than pharmacological hemodynamic stress, the application of suction below the level of the iliac crest may trigger other responses like hyperventilation [65]. The topic of dynamic cerebral autoregulation is covered in detail in the recent white paper [13] and the concepts covered extensively in excellent review articles [14]
3. THE TRANSCRANIAL DOPPLER METHOD
a. Basic Concepts
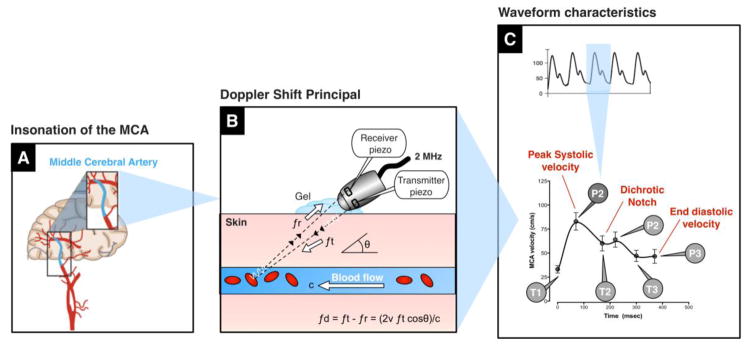
TCD ultrasonography provides real-time measurements of blood flow velocity in cerebral vessels. The technique can be used to measure changes in velocity within the large diameter arteries. Sonographers usually aim for the middle cerebral artery (MCA), which is easy to locate at depths around 50–56 mm. The MCA arises from the internal carotid artery and supplies the cerebral cortex and anterior temporal lobes with oxygenated blood.
By way of validation, measurements of blood flow velocity in the middle cerebral artery correlate closely with the “gold standard” intravenous Xenon133 washout technique [6, 89], magnetic resonance angiography [37], and perfusion computed tomography [96].
The Doppler probe has two piezotransducers, one to transmit a pulsed ultrasound beam and a second to receive back the scattered echoes from the moving red blood cells (Figure 2). The difference in the frequency of the transmitted beam and the frequency received from the backscattered beam (known as the Doppler shift, Figure 2B) is dependent on the motion of the red blood cells travelling within the vessel. Velocity is computed as follows [19]:
Figure 2. Principles of transcranial Doppler ultrasonography.
The middle cerebral artery is usually insonated through the temporal window with a Doppler probe (A). The transmitting piezotransducer sends a pulsed ultrasound beam at a frequency of 2 MHz, which is reflected back from the moving red blood cells and detected by the receiving piezotransducer. The difference in frequency (known as the Doppler shift) is used to calculate the average velocity of blood moving within the lamina of the MCA (B). Transcranial Doppler provides continuous measures of blood flow velocity. As depicted, the TCD waveform has a characteristic profile, with 3 peaks (P1, P2, P3) and 3 troughs (T1, T2, T3).fd = Doppler shift, ft = transmitted frequency, fr = received frequency, ν = velocity of the blood, θ = angle of insonation, c = speed of sound in tissue, P = peak, T = trough.
Where; fd = Doppler frequency shift; ft = transmitted frequency; fr = received frequency; ν = velocity of the blood; θ = angle of insonation; c = speed of sound in tissue.
Because flow within the vessel is laminar, the Doppler shift obtained contains a range of frequencies due to the range on velocities within the lumen. Mean flow velocity takes into account these variations, computing an average based on timing of the different frequencies and the proportion of red blood cells moving at that velocity [19]. Flow towards the probe appears as an upward deflection and flow away from the probe appears as a downward deflection. Transient periods of partial retrograde flow in diastole can occur when intracranial pressure rises, such as with cough syncope [56].
a. Technical standards for measurement
The transcranial Doppler uses a pulsed probe transmitting at a frequency of 2 mHz. The ultrasound beam is penetrated through the thinner skull areas, known as “windows” above the zygomatic arch or other areas, including via the transorbital or transoccipital approaches. Because autonomic testing requires TCD measurements in both the supine and upright positions, the temporal window provides the best location. The fingers can be used to feel for a thinning of the bone, above the zygomatic arch, between the ear and the orbit [19]. Ultrasound gel is used to facilitate conductivity. The angle of the probe has to be adjusted to find the strongest signal towards the probe. The MCA can usually be found at an insonation depth between 45 to 56 mm. Alternatively, the anterior cerebral artery can be insonated at depths of 70–75 mm [75] and the posterior cerebral artery can be insonated at depths of 55–75 mm [43]. The direction of the blood flow and sound can be used to locate the insonated artery. When insonating from the middle/anterior insonation window (above the zygomatic arch), velocity in the MCA flows toward the transducer, while both anterior cerebral artery velocity and posterior cerebral artery velocity flow away from the transducer. The occipital window is needed to insonate the vertebral arteries and transorbital window is used to insonate the ophthalmic artery. Sonographers must understand how to scan safely and adopt the practice of ALARA (as low as reasonably achievable power [1]) when optimizing the signal for recording. The gain should be reduced until all background noise is removed without compromising the signal envelope, which traces the waveform.
Flow velocity depends on two assumptions; first, that the diameter of the insonated vessel does not change [91] and second that the angle of insonation (at which the beam hits the vessel) remains at a constant. This is achieved by having the 2 MHz Doppler probes mounted on an adjustable headband holder to lock the angle in place at the optimal signal position. With meticulous care, it can be assumed that the angle of insonation remains constant. The subjects should be instructed to keep their head still as movement can cause the probe to shift and the signal to deteriorate. Artifacts/noise must be removed from the analysis.
b. TCD in autonomic testing
The autonomic laboratory provides a controlled environment to study cerebral autoregulation. The recent Cerebral Autoregulation Network (CARNet) white paper provides detailed consensus guidelines [13]. The laboratory should be temperature controlled with minimal distractions. Medications known to cross the blood brain barrier and modulate autonomic activity should be tapered and withdrawn, if safe to do so. Concomitant recordings of carbon dioxide (end-tidal) and beat-to-beat blood pressure are needed for clinical interpretation. Simultaneous video recordings can be very useful to correlate symptoms/behaviors [83]. Transcutaneous measures of carbon dioxide have poor temporal resolution and cannot provide the information needed to interpret parallel measurements of CBFv and BP. End-tidal CO2 measurements during a full tidal breath when gas-exchange equilibrium is achieved in the alveolar space are superior.
The hand with finger plethysmograph must always be supported at heart level or a height corrector used to accurately measure the distance between the transducer and the heart. Changes in position should be captured along with the TCD signals. The sampling rate for analog-digital conversion of the CBFv envelope signal should be at least 50 Hz with a low pass frequency cut off at 20 Hz, based on the Nyquist theorem (the sampling frequency should be at least double the largest frequency in the signal) [13]. A higher sampling frequency (e.g. 250 Hz, 500 Hz or higher) may be needed when CBFv is recorded together with other signals e.g. electrocardiogram (ECG), electromyogram (EMG) which have faster frequency components and the need for a higher sampling rate. A faster sampling rate also provides higher resolution for time-frequency, waveform analyses and time-shift between different waveforms. Ideally, all signals should be synchronized and the delay in timing of other signal outputs should be accounted for (e.g., ECG, BP, end-tidal CO2). Due to the hydrostatic pressure difference between the head and the heart when supine and upright, head-up tilt or standing from a supine position require using a correction factor to estimate perfusion pressure at brain level. The distance between the heart and TCD probe should be measured in cm and can be used to estimate cerebral perfusion pressure in the upright position with the following equation:
Where; estBPbrain = estimated blood pressure at brain level; BPheart = blood pressure at heart level, which must be corrected for the position of the transducer if placed on the finger; HD = height difference between height of the transducer (on the arm, finger, etc.) and the heart in cm.
It is recommended that the subject be given at least 20 minutes in the supine position to allow for a steady state. Steady-state values with good quality signals should be acquired for at least 5 minutes (300 seconds) for analysis. The subject should then be tilted to a 60-degree (or similar) angle with footplate support [48]. If the tilt table is not available, the subject should be instructed to stand immobile. All signals should be acquired in the upright position and symptoms documented in the recording file. The subject should remain upright for a minimum of 10 minutes or until syncope/near syncope develop. Prolonged tilt of 40 minutes or longer may be required to trigger and impending vasovagal episode. Lower body negative pressure can be applied while in the tilted position to increase orthostatic stress by exaggerating venous pooling [22].
c. Waveform analysis
The Doppler signal has a characteristic waveform with peak velocity in systole and lowest velocities in diastole (Figure 2C). The small downwards deflection, midway in the waveform is known as the dichotic notch, and occurs on closure of the aortic valve during the cardiac cycle. The characteristics of the waveform can be analyzed at by measuring flow velocity at six key inflection points, identifiable as three distinctive peaks (P1, P2 and P3) and three troughs (T1, T2 and T3)[2, 25]. Waveform analysis should be performed only when there is an artifact-free signal and values should be averaged over a several beats.
d. Derived indices
Area under the curve is a descriptive parameter that can be used to estimate overall changes in CBFv. Changes in the waveform are thought to reflect the overall tone of the vasculature. As the cerebral arterioles dilate, vascular resistance falls, allowing more flow pass through the vessels in diastole. This principle underlies many of the derived indices used to estimate cerebral hemodynamics. One common method is pulsatility index, calculated by subtracting end diastolic velocity from peak systolic velocity and dividing by the mean velocity. When systolic flow remains stable, a high pulsatility index suggests cerebral vasoconstriction and a low index suggests vasodilatation. High intracranial pressure result in a decrease in diastolic flow and an increase in pulsatility index [60]. Similarly, flow acceleration can be calculated by subtracting the peak systolic velocity from the end systolic velocity, and dividing by the systolic upstroke time. When the cerebral arterioles are constricted or there is underlying stenosis, the flow moves slower in systole and acceleration time is reduced [98].
e. Dynamic Cerebral Autoregulation analysis
Dynamic cerebral autoregulation is the mechanism involved in quickly buffering acute variations in perfusion pressure and restoring cerebral blood flow with everyday activities through rapid adaptation of cerebrovascular resistance. In recent years, mathematical models have been used to overcome some of the limitations in assessing cerebral autoregulation. They rely on indirect measures of critical parameters (e.g., intracranial pressure or to derive cerebral blood from arterial spin labeling MRI blood flow velocity [35]). For the present, these techniques remain experimental. Multiple methods exist to quantify dynamic cerebral autoregulation. While it is beyond the scope of this paper to review the methodology in vast detail, several excellent review papers cover this topic [73, 92]. Techniques to measure dynamic cerebral autoregulation include:
Correlation analysis
This method uses cerebral perfusion pressure and TCD-based blood flow velocity to predict the dynamics of cerebral autoregulation, which has shown promising results in clinical studies [16]. This coefficient is known as mean velocity index. Frequently, mean arterial pressure is used as a surrogate when intracranial pressure measurements are either low or not available.
Frequency domain analysis
One common method used in frequency domain analysis is the Transfer function analysis (TFA) between cerebral perfusion pressure and blood flow velocity derived from the Fourier transform. Since invasive intracranial measurements of cerebral perfusion pressure are not available outside the intensive care setting, systemic blood pressure is often used as a surrogate.
There are several potential limitations that have to be considered with TFA. First, a recent meta-analysis highlights the importance of standardizing measurements to enable the findings to be generally applicable across clinical practices [57]. Second, TFA assumes a linear association between the two signals, but it is well known that the pressure-flow relationship in cerebral autoregulation is nonlinear [77]. Finally, TFA assumes stationary oscillations with constant amplitude and period, an assumption that may be unreliable or even invalid for analysis of non-stationary blood pressure and blood flow velocity signals [12, 55]. Standards for TFA analysis are covered by the Cerebral Autoregulation Network white paper [13] aimed to 1) minimize variability in the data acquisition; 2) quality of recordings; 3) preprocessing and TFA parameters and 4) data interpretation and prevent a large spread of results that makes data interpretation difficult.
There are a number of methods in the time-frequency analyses that have been used experimentally to assess dynamic autoregulation including; autoregressive–moving-average modeling with shifting windows, sub-component analysis, Laguerre-Volterra network, neural networks, cross-correlation, principal dynamic modes, wavelet phase synchronization, and support vector machines. Collectively, results with these techniques have shown that dynamic autoregulation is not a stationary process, and therefore a key priority for future work is the development and validation of multivariate time-varying techniques to minimize the influence of co-variates that influence dynamic autoregulation on multiple time scales. Detailed description of these approaches exceeds to scope of this review, and we direct the interested readers to explore an excellent review of these techniques [76].
Non-linear/multimodal pressure-flow analysis
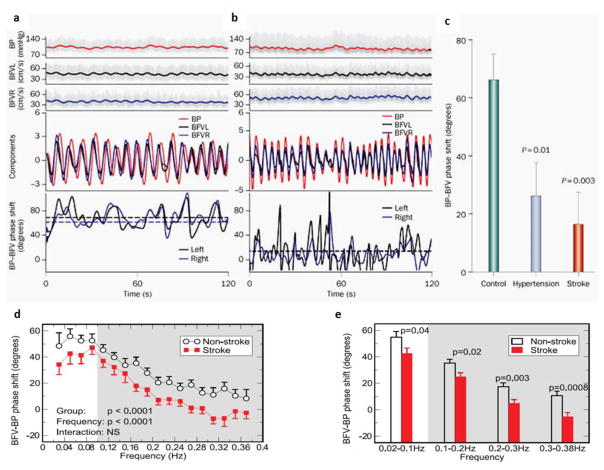
The nonlinear pressure–pressure-flow method is a novel computational tool to assess cerebral autoregulation based on the nonlinear dynamic theory of empirical mode decomposition (EEMD)[40]. The method assesses the relationship between BP and CBFv without assuming that these are stationary signals [40, 71]. Multimodal pressure-flow (MMPF) relationships-based phase shift has greater sensitivity and specificity to detect abnormalities in dynamic cerebral autoregulation in people with chronic infarcts and type 2 diabetes, which were missed using the TFA method [40]. Figure 3 demonstrates the frequency dependency of BP-CBFv phase relationship at slower and faster frequencies (Figure 3D); and compares the phase shift between the stroke and non-stroke subjects at multiple time scales (Figure 3E) [40]. A recent MMPF modification overcomes many limitations of the MMPF and TFA by examining the phase shift of intrinsic cycle-by-cycle BP-CBFv oscillations at different time scales. It also uses a spectrum to describe frequency-dependent phase interaction to better account for non-stationarities and noise in the BP and CBFv recordings by filtering out data without matched BP-CBFv cycles [18, 55].
Figure 3. Dynamic cerebral autoregulation measure based on pressure-flow phase shift.
Spontaneous oscillations in blood pressure (BP) and blood flow velocity (BFV) and dominant decomposed signals from older (A) healthy (B) diabetic subjects and instantaneous phases of BP and BFV oscillations (solid lines, bottom graphs). The mean BP–BFV phase shift (dashed lines) was reduced between (C) controls, hypertension patients and stroke patients. In stroke subjects, the phase shift was reduced across multiple frequencies from 0.02–0.38 Hz as compared to controls (D, E). This phase shift reduction indicates impaired cerebral autoregulation among the groups and across multiple time scales. Figures reprinted from [39–41, 68]
3. CLINICAL APPLICATIONS OF TCD IN THE AUTONOMIC CLINIC
Over one million patients are evaluated for syncope in the US each year. This accounts for around 1% of emergency visits [94]. TCD measurements can be very useful in the clinical work up of a patient with recurrent transient loss of consciousness. Symptoms of cerebral perfusion usually occur when blood flow velocity is reduced by 50% [31]. In cases of pseudo/psychogenic syncope there is unresponsiveness with no change in cerebral flow velocity [66]. Panic, hyperventilation and presyncope produce hypocapnia and strong constriction of the cerebral vessels [65, 70]. These are normal physiological responses to stress and are reversible by CO2 re-breathing [70]. However, orthostatic intolerance symptoms may persist despite improvement in blood flow velocity. There is substantial evidence supporting the usefulness of physical counter maneuvers that increase venous return as a technique to abort and impending vasovagal episode [49, 50, 97]. These maneuvers have been shown to increase cerebral blood flow velocity [34].
a. Syncope
Syncope is defined as global cerebral hypoperfusion that results in transient loss of consciousness characterized by rapid onset, short duration, and spontaneous complete recovery [85]. Syncope occurs when cerebral blood flow falls below a critical limit and consciousness can no longer be maintained [11]. Rapid loss of postural tone physically restores blood supply back to the brain, most likely due to a hardwired protective mechanism [7]. The overall goal of clinical autonomic testing is to determine whether orthostatic symptoms are a result of an autonomic abnormality and whether the autonomic nerves are transiently switched off [95], not properly activated [44] or functionally impaired [29, 45]. TCD studies in disorders of orthostatic intolerance and syncope have used different methods to assess cerebral autoregulation and the results are heterogeneous. Previous reviews have covered early work describing TCD findings in the evaluation of syncope [75]. Table 1 provides a description of key studies examining the TCD in syncope. It was not intended to be an exhaustive literature review, but to provide an update and summarize the more recent relevant studies.
Table 1. List of selected studies examining TCD in syncope.
For additional details see references [9, 17, 21, 26, 30, 38, 53, 54, 59, 70, 86, 90]. Arrows indicate the main response. CA= Cerebral autoregulation, HR = heart rate, CBF = cerebral blood flow, MCA = middle cerebral artery, VMR = Vasomotor reactivity, NR = not reported, LBNP = lower body negative pressure, BP = blood pressure, Pet = partial pressure of end tidal gases.
| RESPONSES TO SYNCOPE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CARDIO- VASCULAR |
CEREBRO-VASCULAR | RESPIRATORY | ||||||||||
| FIRST AUTHOR and REFERENCE |
MEAN AGE years |
POPULATION and N | STIMULI | TCD ANALYSIS METHOD |
PRIMARY STUDY GOAL |
HR | MAP | Systolic MCA velocity |
Diastolic MCA velocity |
Pet CO2 |
Resp. rate |
OUTCOME |
| Murrel [59] | 27 to 65 |
Exercise trained young and elderly vs. controls Young healthy & trained, n=9 Young healthy & untrained, n=12 Older healthy & trained, n=9 Older healthy & untrained, n=9 |
60° Tilt | Static CA No model to correlate BP/CBF velocity | Effect of physical fitness on orthostatic tolerance | ↑ | ↑ | ↓ | ↓ | ↓ | NR | Orthostatic tolerance did not differ with age or fitness |
| Lewis [53] | 25 |
Healthy controls Group 1: (+) alpha blockade, n=6 Group 2: (−) alpha blockade, n=6 |
Supine to standing | Dynamic CA TFA analysis | Effect of alpha- 2 blockade on CBF | ↓ | ↓ | ↓ | ↓ | ↓ | NR | Sympathetic response contributes to CBF regulation |
| Lewis [54] | 25 |
Healthy controls Group 1a: Hypocapnia(−) & LBNP(+), n=7 Group 2a: Hypocapnia(+) & LBNP(+), n=5 Group 1b: Acetazolamide(−) & LBNP(+), n=6 Group 2b: Acetazolamide(+) & LBNP(+), n=4 |
LBNP + Induced-hypocapnia (acetazolamid e) | Static CA No model to correlate BP/CBF velocity | Effect of hypocapnia and decreased CBF on orthostatic tolerance | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | Hypocapnia does not affect orthostatic tolerance |
| Edgell [21] | 27 vs. 57 |
Healthy controls Group1: Young women, n=7 Group 2: Post-menopausal women, n=11 Group 3: Young men, n=10 Group 4: Older men, n=9 |
Supine to standing | Static CA No model to correlate BP/CBF velocity | CBF responses to orthostatic stress in younger and older women vs. men | ↑ | ↑ | ↓ | ↓ | ↓ | (=) | Sex differences in cerebral autoregulation |
| Deegan [17] | 28 |
Healthy controls Volunteers, n=9 |
70° Tilt with LBNP | Static CA No model to correlate BP/CBF velocity | CBF changes in the anterior and posterior circulation | ↑ | ↓ | ↓ | ↓ | ↓ | NR | No differences between anterior and posterior cerebral circulation |
| Gierthmuhlen [26] | 42.8+−16.7 |
Central sympathetic deficit in stroke vs controls Healthy controls, n= 21 Subjects with stroke having central sympathetic deficit, n=17 |
65° Tilt with LBNP | Dynamic CA ARI analysis | Functional role of sympathetic innervation on CA | ↑ | ↓ | ↓ | NR | NR | NR | Sympathetic innervation is not involved on CA |
| Novak [70] | 61.7+−2.4 |
Orthostatic hypotension vs controls Healthy controls, n= 14 Multiple system atrophy, n=8 Pure autonomic failure, n=3 Diabetic neuropathy, n=6 Idiopathic autonomic neuropathy, n=4 |
80° Tilt + hyperventilati on | Static CA No model to correlate BP/CBF velocity | CBF changes when BP decreases | ↑ | ↓ | ↓ | ↓ | ↓ | (=) | Normal or impaired CA in OH |
| Tugba [90] | 7–17 |
Vasovagal vs. controls Group 1: Syncopal history(+) & HUT(+), n=31 Group 2: Syncopal history(+) & HUT(−), n= 21 Group 3: Healthy children, n=22 |
80° Tilt | Static CA No BP/CBF velocity correlation | Describe CBF in vasovagal syncope | NR | NR | ↓ | ↓ | NR | NR | Decreased CBF when syncope and tilt (=) occur |
| Thomas [86] | 25 +−5 |
Syncope vs. controls Healthy controls, n=37 37 Healthy Volunteers Group 1: Syncope & venular dysfunction, n=15 Group 2: Syncope & arteriolar dysfunction, n=1 Group 3: Syncope + mixed dysfunction, n=21 |
70° Tilt with LBNP | Static CA No model to correlate BP/CBF velocity | CBF changes in syncope +/− impaired systemic vascular resistance | ↑ | ↓ | ↓ | ↓ | ↓ | NR | No changes in CBF velocity if syncope classified based on SVR |
| Gur [30] | 69 to 77 |
Synucleinopathies Parkinson disease, n=15 Multiple system atrophy, n=9 Pure autonomic failure, n=5 Group 1: Syncopal history(+) Group 2: No syncopal history (−) |
70° Tilt with acetazolamide | Static CA No model to correlate BP/CBF velocity | Cerebral vasomotor reactivity in syncope | NR | NR | ↓ | ↓ | NR | NR | Association between syncope and decreased calculated VMR |
| Brooks [9] | 52.8 |
Autonomic failure Multiple system atrophy, n=4 Pure autonomic failure, n=4 Dopamine- β-Hydroxylase deficiency, n=2 |
45° Tilt, Ephedrine, 113Xe washout technique | Static CA No BP/CBF velocity correlation | Effect of Autonomic failure in CA | ↑ | ↓ | ↓ | NR | NR | NR | CA is preserved in autonomic failure |
| Horowitz [38] | 72 |
Autonomic failure Multiple system atrophy, n=3 Pure autonomic failure, n=6 |
60° Tilt | Static CA No BP/CBF velocity correlation | CBF in autonomic failure when hypotension occurs | NR | ↓ | ↓ | NR | NR | NR | OH induces autoregulatory cerebral vasodilation |
b. Vasovagal Syncope
Vasovagal syncope (also known as neurally mediated or reflex syncope) is the most common cause of transient loss of consciousness. It is characterized by the sudden withdrawal of sympathetic activity to the systemic circulation accompanied by an increase in parasympathetic activity and slowing of the heart [84]. Emotional factors play a significant role in many vasovagal episodes [93] and hyperventilation-induced hypocapnia is common prior to loss of consciousness. A reduction in CBFv usually occurs before the fall in BP, as a physiological consequence of the hyperventilation-induced hypocapnia that occurs in habitual fainters.
Hypocapnia causes dilatation in the peripheral circulation and constriction within the cerebral circulation and individual susceptibility to vasovagal syncope may depend on vascular sensitivity to alterations in CO2 [65]. Dynamic cerebral autoregulation appears to be intact in patients with vasovagal syncope (Figure 4 and 5) [80]. Cerebrovascular responses are similar in vasodepressor, cardio-vagal and mixed forms of vasovagal syncope [66]. Vasovagal syncope after exercise can occur due to cerebral hypoperfusion. Simple behavioral techniques that minimize hypocapnia with hypoventilation may be helpful [54], as this helps the vessels in the cerebral circulation remain dilated [11]. Studies show that although prolonged bed rest lowers CO2 levels and increases susceptibility to vasovagal syncope, the cerebral autoregulatory capacity appears to adequately compensate [28] and cerebral responses to nitroglycerin challenge are preserved [100].
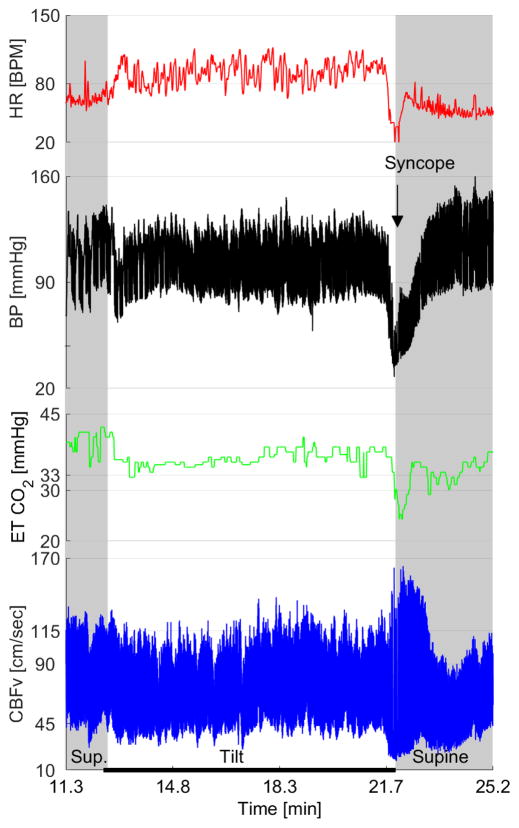
Figure 4. TCD and autonomic findings on tilt test in syncope.
It shows physiological responses of the subjects to head-up tilt when recording blood pressure, heart rate, end-tidal CO2 and cerebral blood flow velocity in syncope. BP = Blood pressure, HR = Heart rate, ETCO2 = End tidal CO2, CBFv = Cerebral blood flow velocity. Image courtesy of Dr. Peter Novak.
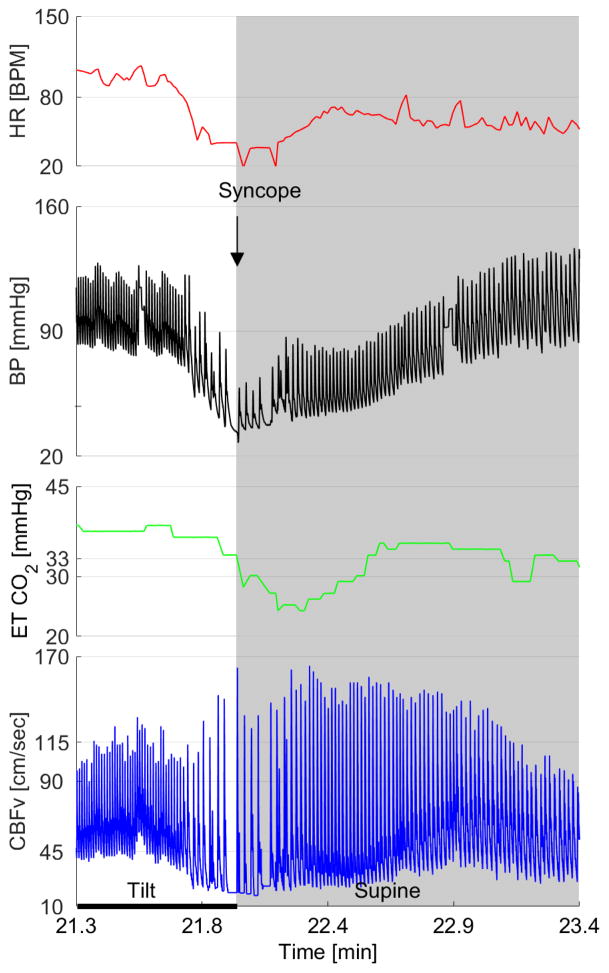
Figure 5. TCD and autonomic findings on tilt test in syncope – A detailed recording when syncope occurs.
It shows physiological responses of the subjects to head-up tilt when recording when recording blood pressure, heart rate, end-tidal CO2 and cerebral blood flow velocity in syncope. Image courtesy of Dr. Peter Novak.
c. Orthostatic Intolerance
Postural Tachycardia Syndrome (PoTS) is a common disorder encountered in the autonomic clinic that affects children, young and middle age adults, and is more prevalent in women. It is defined as orthostatic symptoms including light-headedness, generalized weakness and palpitations accompanied by a sustained increase in heart rate of >40 bpm in a child (or >30 bpm an adult) within 10 minutes upright [24]. There are usually multiple mechanisms involved (including drugs that increase heart rate, anemia, hypovolemia, hyperadrenergic states, peripheral neuropathies) and comorbid disorders are frequent (e.g., psychiatric somatic sensory disorders [78], anxiety, fibromyalgia, chronic headache, etc. [4]). Children and young adults with PoTS appear to have intact cerebral autoregulation [23]. Symptoms on standing can be triggered when cardiovascular responses are normal and cerebral vasodilatation is intact [67]. A recent study in patients with orthostatic intolerance of mixed causes suggested that simultaneous measurements of TCD and near-infrared spectroscopy maybe of additional use to monitor cerebral hypoperfusion and correlate symptoms [51].
d. Chronic Autonomic Failure
Chronic failure of the autonomic nervous system results in neurogenic orthostatic hypotension (nOH), defined as a fall in systolic blood pressure ≥20 mmHg or diastolic blood pressure ≥10 mmHg within three minutes of standing or tilt [24]. It occurs because of a failure to increase sympathetic activity when upright. Accompanying symptoms are the result of tissue ischemia and include lightheadedness, visual difficulties and weakness [47]. nOH is the hallmark of autonomic failure. Underlying causes include neurodegenerative synucleinopathies (Parkinson disease, dementia with Lewy bodies, multiple system atrophy, pure autonomic failure), toxic/metabolic/inherited neuropathies (post chemotherapy, diabetes, familial dysautonomia) or autoimmune conditions (ganglionopathies, paraneoplastic syndromes).
The key studies to define cerebral autoregulation in patients with chronic autonomic failure were performed almost 2-decades ago [8, 38, 69]. Overall, the findings show preserved autoregulatory capacity. Regression analysis shows that in order to withstand periods of low blood pressure standing, most patients with chronic autonomic failure have intact static autoregulation or an expanded autoregulatory range [69]. Others show marked cerebral vasodilatation on standing and syncope occurring when this adaptation is overridden by orthostatic dyspnea and ensuing hypocapnia [8]. Once blood pressure is passively restored in the supine position, most patients with autonomic failure show a dynamic overshoot in cerebral blood flow velocity (i.e., a hyperemic response) suggesting intact vasodilatation in response to hypotension [38]. A small minority with synucleinopathies or diabetes may have autoregulatory failure, which impairs their tolerance to standing [69]. A study of dynamic autoregulation showed that in some patients with multiple system atrophy, cerebral blood flow velocity may be slow to return to baseline after standing [99]. However, none of these studies took into account if the patient had underlying vascular disease, supine hypertension, or was being treated with fludrocortisone or other vasoactive agents. A recent large study in patients with Parkinson disease showed normal cerebrovascular responses to hypocapnia, suggesting that metabolic autoregulation was intact [33]. TCD and autonomic findings in recent studies of patients with synucleinopathies have mixed results and are described in table 2.
Table 2. Transcranial Doppler and autonomic findings on tilt table testing in orthostatic syndromes and synucleinopathies.
Highlighted arrows indicate the main autonomic abnormality found in the syndrome. (↔) normal, (↓) decreased, (↑) increased, (↕) not specific, (↔ ↓) normal or decreased, (↔ ↑) normal or increased, (~ ↑) very low variability, (HR) heart rate, (HUT) Head up-tilt, (MAP) median arterial pressure, cerebral blood flow velocity (BFV). (*) Cerebrovascular resistance is increased in OCHOS compared to controls, but decreased in OINH. Table modified from [67]. For further detail about autonomic findings in synucleinopathies see their respective references [3, 9, 34, 36, 48, 58, 81, 99].
| Syndrome | HR | MAP | CBFv | ||||
|---|---|---|---|---|---|---|---|
| Supine | HUT | Supine | HUT | Supine | HUT | ||
| Orthostatic hypotension (OH) | ↔ | ↕ | ↕ | ↓ | ↔ | ↔ ↓ | |
| Postural tachycardia syndrome (PoTS) | ↔ | ↑ | ↔ ↓ | ↔ ↑ | ↔ | ↔ ↓ | |
| Syncope, cardiovagal | ↔ | ↓ | ↔ | ↓ | ↔ | ↓ | |
| Syncope, vasodepressor | ↔ | ↔ ↑ | ↔ | ↓ | ↔ | ↓ | |
| Syncope, mixed | ↔ | ↓ | ↔ | ↓ | ↔ | ↓ | |
| Primary Cerebral Autoregulatory Failure (pCAF) | ↔ | ↔ | ↔ ↑ | ↔ | ↓ | ↔ ↓ | |
| Orthostatic cerebral hypoperfusion syndrome (OCHOS) | ↔ | ↔ | ↕ | ↔ | ↔ | ↓ * | |
| Orthostatic intolerance with normal HUT (OINH) [81] | ↔ | ↑ | ↔ | ↔ | ↔ | ↓ * | |
| Pure autonomic failure (PAF) [48] | ↔ ↓ | ~ ↑ | ↔ | ↓ | ↔ | ↔ [9] | ↓ [34] |
| Parkinson’s disease (PD) [58] | ↔ ↓ | ~ ↑ | ↔ | ↓ | ↔ | ↔ [3] | ↓ [36] |
| Multiple system atrophy (MSA) [5] | ↔ | ~ ↑ | ↔ | ↓ | ↔ | ↓ | |
4. Afferent Baroreflex Failure
Afferent baroreflex failure (not to be confused with efferent autonomic failure) occurs when there are acquired or genetic lesions in the nerves relaying information from the arterial baroreceptors in the peripheral circulation to the brainstem [62]. As a result, patients have unstable blood pressure with hypotension alternating with stress-induced hypertension [63]. Inherited congenital lesions in the IXth and Xth cranial nerves do not impair cerebral autoregulation [64]. Patients retain a remarkable ability to withstand hypotension when upright without developing cerebral hypoperfusion and do not develop hypocapnia when upright [25]. They seldom complain of orthostatic symptoms and syncope usually only occurs in the setting of additional stressors including hypovolemia or hypoxia [72]. It is thought that negative pressure within the sinuses may help suctioning blood to the cerebral circulation. There are no TCD studies in patients with acquired afferent baroreflex lesions due to cancer, surgery or radiotherapy of the neck.
5. Other Disorders
A recent retrospective review in 669 patients, described primary cerebral autoregulatory failure as a low CBFv in the supine position without systemic hypotension [67] and orthostatic cerebral hypoperfusion syndrome as an abnormal drop of mean blood flow velocity without orthostatic hypotension nor tachycardia [67].
Orthostatic cerebral hypoperfusion syndrome (OCHOS) has low CBFv with normal cardiovascular reflex responses to standing [66]. Patients are predominately women (59%), with comorbid hypertension (21%) and migraine (up to 35%). Cerebral blood flow velocity decreases by 20% or more in the standing position compared to controls. This is presumably caused by cerebral vasoconstriction and ineffective compensation [67] (See figure 5). Another similar syndrome is orthostatic intolerance with normal head up tilt defined as orthostatic symptoms in patients with normal blood pressure and heart rate responses to tilt. These patients had a decreased cerebrovascular resistance and CBFv (mainly systolic) compared with controls, but no significant cerebral blood flow velocity change when compared with patients diagnosed with PoTS or nOH [81]. Expected cardiovascular responses and TCD velocity changes in the syndromes are described in table 2.
Recognizing different TCD profiles with autonomic testing can be useful to correlate orthostatic symptoms in patients with normal cardiovascular responses. Further studies are needed to better define the spectrum of these disorders and reach consensus.
6. LIMITATIONS
Acquiring TCD data requires a rigorous approach with proper insonation and good quality blood flow velocity recordings, especially during head-up tilt. TCD measurements are limited by the operator’s ability to detect an optimal insonation window, to maintain the probe stable, and to properly identify and remove artifacts caused by signal loss due to improper probe positioning. Newer devices allow automatic detection and maintain maximal flow velocity and proper probe positioning. Transcranial color-coded duplex ultrasonography (TCDD) is a relatively novel instrument that can also be used to assess the cerebral circulation. The method is based upon TCD parameters, and has similar limitations due to the insonation window. However, it allows direct visualization of the insonated artery, which may be beneficial for angle correction. TCDD is not typically used for longitudinal recordings. Most of the studies that have assessed cerebral autoregulation in autonomic disorders have used TCD, hence further studies are warranted to assess the advantages of TCDD in the autonomic clinic [79].
TCD measures flow velocity within the large vessels, which is poorly correlated with the tissue perfusion within the insonated territory [35]. Flow velocity measurements are also affected by underlying small vessel disease and gray and white matter abnormalities. Therefore, a decline in MCA blood flow velocity may be compensated for by redistribution of perfusion to other vascular territories, rather than representing global hypoperfusion.
Clinical studies in autonomic disorders are limited due to method variability and a small sample size. International guidelines for cerebral autoregulation measurements and analyses have been proposed, but are still lacking validation in a large sample size [13]. Conducting multicenter studies using unifying criteria would provide a gold standard to characterize normal and abnormal cerebral autoregulatory responses. Validated methods for cerebral autoregulatory assessment and interpretation would enhance clinical autonomic testing diagnosis and treatment options.
6. CONCLUSIONS AND FUTURE DIRECTIONS
The transcranial Doppler provides an excellent way to study acute changes in cerebral blood flow velocity in the autonomic clinic. Cerebral autoregulation is a well-defined physiological mechanism essential in everyday life. Standardization of TCD recordings is essential.
Acknowledgments
Funding: National Institutes of Health NINDS (U54- NS065736 to LN-K) and by NIH-NIDDK (R01-DK13902-01A2 to VN)
Footnotes
Conflict of interests: None
BIBLIOGRAPHY
- 1.Abramowicz JS, Kremkau FW, Merz E. Obstetrical ultrasound: can the fetus hear the wave and feel the heat? Ultraschall Med. 2012;33:215–217. doi: 10.1055/s-0032-1312759. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Brooks DM, Kang Y, Linden PK, Patzer JF., 2nd Noninvasive monitoring of cerebral perfusion pressure in patients with acute liver failure using transcranial doppler ultrasonography. Liver Transpl. 2008;14:1048–1057. doi: 10.1002/lt.21499. [DOI] [PubMed] [Google Scholar]
- 3.Angeli S, Marchese R, Abbruzzese G, Gandolfo C, Conti M, Gasparetto B, Del Sette M. Tilt-table test during transcranial Doppler monitoring in Parkinson’s disease. Parkinsonism & related disorders. 2003;10:41–46. doi: 10.1016/s1353-8020(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 4.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87:1214–1225. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman JR, Berman LA, Werbin TJ, Goldstein I. Female sexual dysfunction: anatomy, physiology, evaluation and treatment options. Curr Opin Urol. 1999;9:563–568. doi: 10.1097/00042307-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17:913–915. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- 7.Blanc JJ, Benditt DG. Vasovagal Syncope: Hypothesis Focusing on Its Being a Clinical Feature Unique to Humans. J Cardiovasc Electrophysiol. 2016;27:623–629. doi: 10.1111/jce.12945. [DOI] [PubMed] [Google Scholar]
- 8.Bondar RL, Dunphy PT, Moradshahi P, Kassam MS, Blaber AP, Stein F, Freeman R. Cerebrovascular and cardiovascular responses to graded tilt in patients with autonomic failure. Stroke. 1997;28:1677–1685. doi: 10.1161/01.str.28.9.1677. [DOI] [PubMed] [Google Scholar]
- 9.Brooks DJ, Redmond S, Mathias CJ, Bannister R, Symon L. The effect of orthostatic hypotension on cerebral blood flow and middle cerebral artery velocity in autonomic failure, with observations on the action of ephedrine. J Neurol Neurosurg Psychiatr. 1989;52:962–966. doi: 10.1136/jnnp.52.8.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwers PJ, Vriens EM, Musbach M, Wieneke GH, van Huffelen AC. Transcranial pulsed Doppler measurements of blood flow velocity in the middle cerebral artery: reference values at rest and during hyperventilation in healthy children and adolescents in relation to age and sex. Ultrasound Med Biol. 1990;16:1–8. doi: 10.1016/0301-5629(90)90079-r. [DOI] [PubMed] [Google Scholar]
- 11.Carey BJ, Eames PJ, Panerai RB, Potter JF. Carbon dioxide, critical closing pressure and cerebral haemodynamics prior to vasovagal syncope in humans. Clin Sci (Lond) 2001;101:351–358. [PubMed] [Google Scholar]
- 12.Chen Z, Hu K, Stanley HE, Novak V, Ivanov PC. Cross-correlation of instantaneous phase increments in pressure-flow fluctuations: applications to cerebral autoregulation. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;73:031915. doi: 10.1103/PhysRevE.73.031915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB international Cerebral Autoregulation Research N. Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab. 2016;36:665–680. doi: 10.1177/0271678X15626425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocritical care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 15.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 16.Czosnyka M, Smielewski P, Lavinio A, Pickard JD, Panerai R. An assessment of dynamic autoregulation from spontaneous fluctuations of cerebral blood flow velocity: a comparison of two models, index of autoregulation and mean flow index. Anesth Analg. 2008;106:234–239. doi: 10.1213/01.ane.0000295802.89962.13. table of contents. [DOI] [PubMed] [Google Scholar]
- 17.Deegan BM, Cooke JP, Lyons D, Olaighin G, Serrador JM. Cerebral autoregulation in the vertebral and middle cerebral arteries during combine head upright tilt and lower body negative pressure in healthy humans. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2505–2508. doi: 10.1109/IEMBS.2010.5626647. [DOI] [PubMed] [Google Scholar]
- 18.Devault K, Gremaud PA, Novak V, Olufsen MS, Vernieres G, Zhao P. Blood Flow in the Circle of Willis: Modeling and Calibration. Multiscale Model Simul. 2008;7:888–909. doi: 10.1137/07070231X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeWitt LD, Wechsler LR. Transcranial Doppler. Stroke. 1988;19:915–921. doi: 10.1161/01.str.19.7.915. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly J, Budohoski KP, Smielewski P, Czosnyka M. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care. 2016;20:129. doi: 10.1186/s13054-016-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgell H, Robertson AD, Hughson RL. Hemodynamics and brain blood flow during posture change in younger women and postmenopausal women compared with age-matched men. Journal of applied physiology. 2012;112:1482–1493. doi: 10.1152/japplphysiol.01204.2011. [DOI] [PubMed] [Google Scholar]
- 22.El-Bedawi K, Hainsworth R. Combined head-up tilt and lower body suction: a test of orthostatic tolerance. Clin Autonom Res. 1994;4:41–47. doi: 10.1007/BF01828837. [DOI] [PubMed] [Google Scholar]
- 23.Endo A, Fujita Y, Fuchigami T, Takahashi S, Mugishima H, Skatani K. Changes in cerebral blood oxygenation induced by active standing test in children with POTS and NMS. Adv Exp Med Biol. 2014;812:253–261. doi: 10.1007/978-1-4939-0620-8_34. [DOI] [PubMed] [Google Scholar]
- 24.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 25.Fuente Mora C, Palma JA, Kaufmann H, Norcliffe-Kaufmann L. Cerebral autoregulation and symptoms of orthostatic hypotension in familial dysautonomia. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16667524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gierthmuhlen J, Allardt A, Sawade M, Baron R, Wasner G. Dynamic cerebral autoregulation in stroke patients with a central sympathetic deficit. Acta Neurol Scand. 2011;123:332–338. doi: 10.1111/j.1600-0404.2010.01424.x. [DOI] [PubMed] [Google Scholar]
- 27.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greaves DK, Arbeille P, Hughson RL. WISE 2005: altered cerebrovascular autoregulation after 60 day head-down bed rest. J Gravit Physiol. 2007;14:P61–62. [PubMed] [Google Scholar]
- 29.Gupta A, Harris S, Vernino S, Naina HV. Rituximab-based therapy and long-term control of autoimmune autonomic ganglionopathy. Clin Auton Res. 2015;25:255–258. doi: 10.1007/s10286-015-0299-5. [DOI] [PubMed] [Google Scholar]
- 30.Gur AY, Auriel E, Korczyn AD, Gadoth A, Shopin L, Giladi N, Bornstein NM, Gurevich T. Vasomotor reactivity as a predictor for syncope in patients with orthostatism. Acta Neurol Scand. 2012;126:32–36. doi: 10.1111/j.1600-0404.2011.01591.x. [DOI] [PubMed] [Google Scholar]
- 31.Hainsworth R. Pathophysiology of syncope. Clin Auton Res. 2004;14(Suppl 1):18–24. doi: 10.1007/s10286-004-1004-2. [DOI] [PubMed] [Google Scholar]
- 32.Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. J Physiol. 2012;590:6343–6352. doi: 10.1113/jphysiol.2012.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanby MF, Panerai RB, Robinson TG, Haunton VJ. Is cerebral vasomotor reactivity impaired in Parkinson disease? Clin Auton Res. 2017 doi: 10.1007/s10286-017-0406-x. [DOI] [PubMed] [Google Scholar]
- 34.Harms MP, Wieling W, Colier WN, Lenders JW, Secher NH, van Lieshout JJ. Central and cerebrovascular effects of leg crossing in humans with sympathetic failure. Clin Sci (Lond) 2010;118:573–581. doi: 10.1042/CS20090038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart J, Novak V, Saunders C, Gremaud PA. Transcranial Doppler-Based Surrogates for Cerebral Blood Flow: A Statistical Study. PLoS One. 2016;11:e0165536. doi: 10.1371/journal.pone.0165536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haubrich C, Pies K, Dafotakis M, Block F, Kloetzsch C, Diehl RR. Transcranial Doppler monitoring in Parkinson’s disease: cerebrovascular compensation of orthostatic hypotension. Ultrasound Med Biol. 2010;36:1581–1587. doi: 10.1016/j.ultrasmedbio.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Helton KJ, Adams RJ, Kesler KL, Lockhart A, Aygun B, Driscoll C, Heeney MM, Jackson SM, Krishnamurti L, Miller ST, Sarnaik SA, Schultz WH, Ware RE, Investigators SW. Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood. 2014;124:891–898. doi: 10.1182/blood-2013-12-545186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horowitz DR, Kaufmann H. Autoregulatory cerebral vasodilation occurs during orthostatic hypotension in patients with primary autonomic failure. Clin Auton Res. 2001;11:363–367. doi: 10.1007/BF02292768. [DOI] [PubMed] [Google Scholar]
- 39.Hu K, Lo M-T, Peng CK, Novak V, Schmidt EA, Kumar A, Czosnyka M. Nonlinear pressure-flow relationship is able to detect asymmetry of brain blood circulation associated with midline shift. J Neurotrauma. 2009;26:227–233. doi: 10.1089/neu.2008.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu K, Lo MT, Peng CK, Liu Y, Novak V. A nonlinear dynamic approach reveals a long-term stroke effect on cerebral blood flow regulation at multiple time scales. PLoS Comput Biol. 2012;8:e1002601. doi: 10.1371/journal.pcbi.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu K, Peng CK, Huang NE, Wu Z, Lipsitz LA, Cavallerano J, Novak V. Altered Phase Interactions between Spontaneous Blood Pressure and Flow Fluctuations in Type 2 Diabetes Mellitus: Nonlinear Assessment of Cerebral Autoregulation. Physica A. 2008;387:2279–2292. doi: 10.1016/j.physa.2007.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itakura T, Yamamoto K, Tohyama M, Shimizu N. Central dual innervation of arterioles and capillaries in the brain. Stroke. 1977;8:360–365. doi: 10.1161/01.str.8.3.360. [DOI] [PubMed] [Google Scholar]
- 43.Jatuzis D, Zachrisson H, Blomstrand C, Ekholm S, Holm J, Volkmann R. Evaluation of posterior cerebral artery blood flow with transcranial Doppler sonography: value and risk of common carotid artery compression. J Clin Ultrasound. 2000;28:452–460. doi: 10.1002/1097-0096(200011/12)28:9<452::aid-jcu2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Jordan J, Shibao C, Biaggioni I. Multiple system atrophy: using clinical pharmacology to reveal pathophysiology. Clin Auton Res. 2015;25:53–59. doi: 10.1007/s10286-015-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaufmann H, Hague K, Perl D. Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology. 2001;56:980–981. doi: 10.1212/wnl.56.7.980. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann H, Hainsworth R. Why do we faint? Muscle Nerve. 2001;24:981–983. doi: 10.1002/mus.1102. [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22:79–90. doi: 10.1007/s10286-011-0146-2. [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann H, Norcliffe-Kaufmann L, Palma JA, Biaggioni I, Low PA, Singer W, Goldstein DS, Peltier AC, Shibao CA, Gibbons CH, Freeman R, Robertson D Autonomic Disorders C. Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol. 2017;81:287–297. doi: 10.1002/ana.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krediet CT, de Bruin IG, Ganzeboom KS, Linzer M, van Lieshout JJ, Wieling W. Leg crossing, muscle tensing, squatting, and the crash position are effective against vasovagal reactions solely through increases in cardiac output. J Appl Physiol. 2005;99:1697–1703. doi: 10.1152/japplphysiol.01250.2004. [DOI] [PubMed] [Google Scholar]
- 50.Krediet CT, van Dijk N, Linzer M, van Lieshout JJ, Wieling W. Management of vasovagal syncope: controlling or aborting faints by leg crossing and muscle tensing. Circulation. 2002;106:1684–1689. doi: 10.1161/01.cir.0000030939.12646.8f. [DOI] [PubMed] [Google Scholar]
- 51.Lankford J, Numan M, Hashmi SS, Gourishankar A, Butler IJ. Cerebral blood flow during HUTT in young patients with orthostatic intolerance. Clin Auton Res. 2015;25:277–284. doi: 10.1007/s10286-015-0295-9. [DOI] [PubMed] [Google Scholar]
- 52.Lassen NA, Christensen MS. Physiology of cerebral blood flow. Br J Anaesth. 1976;48:719–734. doi: 10.1093/bja/48.8.719. [DOI] [PubMed] [Google Scholar]
- 53.Lewis NC, Ainslie PN, Atkinson G, Jones H, Grant EJ, Lucas SJ. Initial orthostatic hypotension and cerebral blood flow regulation: effect of alpha1-adrenoreceptor activity. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304:R147–154. doi: 10.1152/ajpregu.00427.2012. [DOI] [PubMed] [Google Scholar]
- 54.Lewis NC, Bain AR, MacLeod DB, Wildfong KW, Smith KJ, Willie CK, Sanders ML, Numan T, Morrison SA, Foster GE, Stewart JM, Ainslie PN. Impact of hypocapnia and cerebral perfusion on orthostatic tolerance. J Physiol. 2014;592:5203–5219. doi: 10.1113/jphysiol.2014.280586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo MT, Hu K, Liu Y, Peng CK, Novak V. Multimodal Pressure Flow Analysis: Application of Hilbert Huang Transform in Cerebral Blood Flow Regulation. EURASIP J Adv Signal Process. 2008;2008:785243. doi: 10.1155/2008/785243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattle HP, Nirkko AC, Baumgartner RW, Sturzenegger M. Transient cerebral circulatory arrest coincides with fainting in cough syncope. Neurology. 1995;45:498–501. doi: 10.1212/wnl.45.3.498. [DOI] [PubMed] [Google Scholar]
- 57.Meel-van den Abeelen AS, van Beek AH, Slump CH, Panerai RB, Claassen JA. Transfer function analysis for the assessment of cerebral autoregulation using spontaneous oscillations in blood pressure and cerebral blood flow. Med Eng Phys. 2014;36:563–575. doi: 10.1016/j.medengphy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Mihci E, Dora B, Balkan S. Transcranial Doppler ultrasonographic evaluation of cerebral circulation during passive tilting in patients with Parkinson’s disease. J Clin Ultrasound. 2007;35:138–143. doi: 10.1002/jcu.20309. [DOI] [PubMed] [Google Scholar]
- 59.Murrell CJ, Cotter JD, George K, Shave R, Wilson L, Thomas K, Williams MJ, Ainslie PN. Cardiorespiratory and cerebrovascular responses to head-up tilt II: influence of age, training status and acute exercise. Exp Gerontol. 2011;46:1–8. doi: 10.1016/j.exger.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Nicoletto HA, Burkman MH. Transcranial Doppler series part III: interpretation. Am J Electroneurodiagnostic Technol. 2009;49:244–259. [PubMed] [Google Scholar]
- 61.Norcliffe LJ, Rivera-Ch M, Claydon VE, Moore JP, Leon-Velarde F, Appenzeller O, Hainsworth R. Cerebrovascular responses to hypoxia and hypocapnia in high-altitude dwellers. J Physiol. 2005;566:287–294. doi: 10.1113/jphysiol.2005.086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–1911. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norcliffe-Kaufmann L, Palma JA, Kaufmann H. Mother-induced hypertension in familial dysautonomia. Clin Auton Res. 2016;26:79–81. doi: 10.1007/s10286-015-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norcliffe-Kaufmann L, Slaugenhaupt SA, Kaufmann H. Familial dysautonomia: History, genotype, phenotype and translational research. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Norcliffe-Kaufmann LJ, Kaufmann H, Hainsworth R. Enhanced vascular responses to hypocapnia in neurally mediated syncope. Ann Neurol. 2008;63:288–294. doi: 10.1002/ana.21205. [DOI] [PubMed] [Google Scholar]
- 66.Novak P. Cerebral Blood Flow, Heart Rate, and Blood Pressure Patterns during the Tilt Test in Common Orthostatic Syndromes. Neurosci J. 2016;2016:6127340. doi: 10.1155/2016/6127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novak P. Orthostatic Cerebral Hypoperfusion Syndrome. Front Aging Neurosci. 2016;8:22. doi: 10.3389/fnagi.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke. 1998;29:104–111. doi: 10.1161/01.str.29.1.104. [DOI] [PubMed] [Google Scholar]
- 70.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29:1876–1881. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 71.Novak V, Yang AC, Lepicovsky L, Goldberger AL, Lipsitz LA, Peng CK. Multimodal pressure-flow method to assess dynamics of cerebral autoregulation in stroke and hypertension. Biomed Eng Online. 2004;3:39. doi: 10.1186/1475-925X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palma JA, Norcliffe-Kaufmann L, Fuente-Mora C, Percival L, Mendoza-Santiesteban C, Kaufmann H. Current treatments in familial dysautonomia. Expert Opin Pharmacother. 2014;15:2653–2671. doi: 10.1517/14656566.2014.970530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panerai RB. Assessment of cerebral pressure autoregulation in humans--a review of measurement methods. Physiol Meas. 1998;19:305–338. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- 74.Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Phys. 2003;25:621–632. doi: 10.1016/s1350-4533(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 75.Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2009;19:197–211. doi: 10.1007/s10286-009-0011-8. [DOI] [PubMed] [Google Scholar]
- 76.Panerai RB. Nonstationarity of dynamic cerebral autoregulation. Med Eng Phys. 2014;36:576–584. doi: 10.1016/j.medengphy.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Panerai RB, Sammons EL, Smith SM, Rathbone WE, Bentley S, Potter JF, Samani NJ. Continuous estimates of dynamic cerebral autoregulation: influence of non-invasive arterial blood pressure measurements. Physiol Meas. 2008;29:497–513. doi: 10.1088/0967-3334/29/4/006. [DOI] [PubMed] [Google Scholar]
- 78.Pederson CL, Brook JB. Health-related quality of life and suicide risk in postural tachycardia syndrome. Clin Auton Res. 2017 doi: 10.1007/s10286-017-0399-5. [DOI] [PubMed] [Google Scholar]
- 79.Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Seminars in neurology. 2012;32:411–420. doi: 10.1055/s-0032-1331812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schondorf R, Stein R, Roberts R, Benoit J, Cupples W. Dynamic cerebral autoregulation is preserved in neurally mediated syncope. Journal of applied physiology. 2001;91:2493–2502. doi: 10.1152/jappl.2001.91.6.2493. [DOI] [PubMed] [Google Scholar]
- 81.Shin KJ, Kim SE, Park KM, Park J, Ha SY, Kim SE, Kwon OY. Cerebral hemodynamics in orthostatic intolerance with normal head-up tilt test. Acta Neurol Scand. 2016;134:108–115. doi: 10.1111/ane.12516. [DOI] [PubMed] [Google Scholar]
- 82.Strandgaard S, Paulson OB. Regulation of cerebral blood flow in health and disease. J Cardiovasc Pharmacol. 1992;19(Suppl 6):S89–93. doi: 10.1097/00005344-199219006-00014. [DOI] [PubMed] [Google Scholar]
- 83.Tannemaat MR, Thijs RD, van Dijk JG. Managing psychogenic pseudosyncope: Facts and experiences. Cardiol J. 2014;21:658–664. doi: 10.5603/CJ.a2014.0070. [DOI] [PubMed] [Google Scholar]
- 84.Tellez MJ, Norcliffe-Kaufmann LJ, Lenina S, Voustianiouk A, Kaufmann H. Usefulness of tilt-induced heart rate changes in the differential diagnosis of vasovagal syncope and chronic autonomic failure. Clin Auton Res. 2009;19:375–380. doi: 10.1007/s10286-009-0039-9. [DOI] [PubMed] [Google Scholar]
- 85.Thijs RD, Wieling W, Kaufmann H, van Dijk G. Defining and classifying syncope. Clin Auton Res. 2004;14(Suppl 1):4–8. doi: 10.1007/s10286-004-1002-4. [DOI] [PubMed] [Google Scholar]
- 86.Thomas KN, Galvin SD, Williams MJ, Willie CK, Ainslie PN. Identical pattern of cerebral hypoperfusion during different types of syncope. J Hum Hypertens. 2010;24:458–466. doi: 10.1038/jhh.2009.93. [DOI] [PubMed] [Google Scholar]
- 87.Tiecks FP, Douville C, Byrd S, Lam AM, Newell DW. Evaluation of impaired cerebral autoregulation by the Valsalva maneuver. Stroke. 1996;27:1177–1182. doi: 10.1161/01.str.27.7.1177. [DOI] [PubMed] [Google Scholar]
- 88.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 89.Trivedi UH, Patel RL, Turtle MR, Venn GE, Chambers DJ. Relative changes in cerebral blood flow during cardiac operations using xenon-133 clearance versus transcranial Doppler sonography. Ann Thorac Surg. 1997;63:167–174. doi: 10.1016/s0003-4975(96)01017-x. [DOI] [PubMed] [Google Scholar]
- 90.Tugba B, Zubeyir K, Nevzat U, Ali Y, Birsen U, Tevfik D. Cerebral blood flow of children with vasovagal syncope. Cardiol Young. 2015;25:267–273. doi: 10.1017/S1047951113002059. [DOI] [PubMed] [Google Scholar]
- 91.Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhaupl KM. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. AJNR Am J Neuroradiol. 1997;18:1929–1934. [PMC free article] [PubMed] [Google Scholar]
- 92.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 93.van Dijk JG. Fainting in animals. Clin Auton Res. 2003;13:247–255. doi: 10.1007/s10286-003-0099-1. [DOI] [PubMed] [Google Scholar]
- 94.Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94:833–848. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- 95.Wallin B, Sundlof G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- 96.Westermaier T, Pham M, Stetter C, Willner N, Solymosi L, Ernestus RI, Vince GH, Kunze E. Value of transcranial Doppler, perfusion-CT and neurological evaluation to forecast secondary ischemia after aneurysmal SAH. Neurocrit Care. 2014;20:406–412. doi: 10.1007/s12028-013-9896-0. [DOI] [PubMed] [Google Scholar]
- 97.Wieling W, van Lieshout JJ, van Leeuwen AM. Physical manoeuvres that reduce postural hypotension in autonomic failure. Clin Auton Res. 1993;3:57–65. doi: 10.1007/BF01819146. [DOI] [PubMed] [Google Scholar]
- 98.Wilterdink JL, Feldmann E, Furie KL, Bragoni M, Benavides JG. Transcranial Doppler ultrasound battery reliably identifies severe internal carotid artery stenosis. Stroke. 1997;28:133–136. doi: 10.1161/01.str.28.1.133. [DOI] [PubMed] [Google Scholar]
- 99.Xu WH, Wang H, Hu YH, Wang B, Chen J, Gao S. Supine-to-standing transcranial Doppler test in patients with multiple system atrophy. Parkinsonism & related disorders. 2013;19:539–542. doi: 10.1016/j.parkreldis.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 100.Zuj KA, Edgell H, Shoemaker JK, Custaud MA, Arbeille P, Hughson RL. WISE 2005: responses of women to sublingual nitroglycerin before and after 56 days of 6 degrees head-down bed rest. Journal of applied physiology. 2012;113:434–441. doi: 10.1152/japplphysiol.00445.2012. [DOI] [PubMed] [Google Scholar]