Identification of Epigenetically Active Antischizophrenia Drugs that Inhibit DNA Methylation
Progress in developing new, more effective, and less toxic drugs to treat the complex symptomatology of schizophrenia (SZ) and bipolar (BP) disorder has been hampered by the lack of objective diagnostic tools to assess prodromes, progression severity, and therapeutic responses to drugs. Additional fundamental barriers to the identification of new drugs to effectively treat SZ and BP disorder include the incomplete understanding of the etiopathogenetic mechanisms underlying the symptomatology of these diseases and the inability to reproduce the complex nature of these disorders in laboratory animals.
It is well established that SZ and BP disorders have a strong genetic component [1,2]. Environmental factors are thought to impact the genome through changes to the epigenome. Epigenetic studies indicate that altered genomic DNA methylation patterns likely play an important role in the neuro developmental pathogenesis of these diseases (Figure 1) and as a target mechanism for drug discoveries [3].
Figure 1.
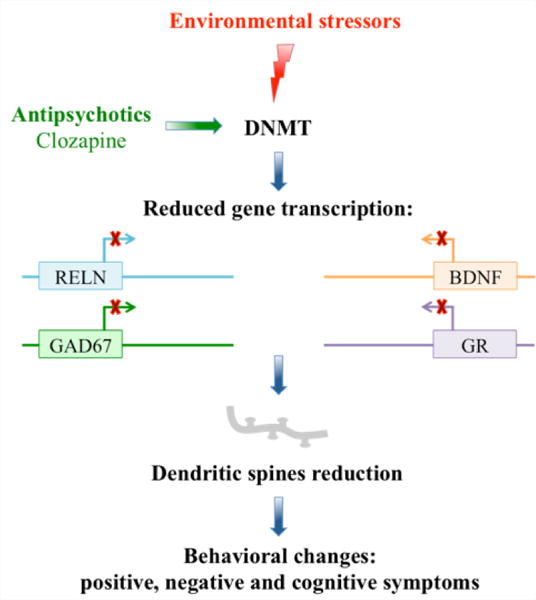
Epigenetic dysfunction triggered by prenatal environmental factors: stress, drugs, viral infection, or trauma.
Preclinical Evaluation of the Neuroleptic Properties of Drugs Active on Epigenetic Mechanisms: Rationale for the Experiments in Prenatally Stressed (PRS) Mice
The exposure of pregnant women to psychological stress, malnutrition, or viral infection has been associated with an increased incidence of psychosis in their children later in life [4–11]. It is plausible to hypothesize that both in human and mouse brain, chromatin remodeling induced by environmental stressors or inflammatory agents during embryonic life is the basis for a disturbance in the reciprocal interactions between GABA ergic, glutamatergic, and monoaminergic neurons [12,13]. This altered connectivity is likely the source of the cognitive and emotional disruptions underlying post-pubertal psychotic symptoms in SZ and BP disorder patients and in mice (PRS mice) born from mothers stressed during pregnancy. PRS mice are the offspring of dams that were stressed during prenatal days 7 through birth. Similar to humans, the SZ-like symptoms in mice can also be exacerbated by the administration of NMDA receptor antagonists [14].
We hypothesize that alterations in the equilibrium between DNA methylation and DNA demethylation network components at vulnerable chromatin domains in neocortical GABAergic, glutamatergic and monoaminergic neurons is a mechanism that contributes to the environmentally-induced dysregulation of brain circuitry and dendritic spine reduction in SZ and BP disorders [12–14] and (Figure 1). Based on the above premise, we directed our research efforts towards identifying the behavioral and molecular epigenetic signature that is common to SZ and BP disorder patients, in the offspring of mice (PRS mice) whose mothers were restraint-stressed during pregnancy [14]. PRS mice have behavioral and molecular endopheno types reminiscent of the behavioral and molecular alterations observed in psychotic patients (Table 1). The brains of PRS mice, like those of SZ and BP disorder patients [15], are characterized by a two fold increase of DNMT1 binding to the Gad1, Rein, and Bdnf promoters (Figure 2), [15] and a significant increase in 5mC and 5hmC at promoter regions corresponding to Gad1, Rein, and Bdnf (Table 1) [15]. The increases in promoter methylation are associated with reduced levels of synaptic remodeling proteins and with behavioral deficits reminiscent of the negative symptoms, positive symptoms, and cognitive impairment detected in SZ and BP disorder patients (Table1) [13–16]. These results and the reported observation that there are positive correlations between SZ-like behavioral phenotypes and levels of Bdnf, Gad1, and Rein promoter methylation in PFC of PRS mice [14–16], are consistent with a neuro developmental epigenetic mechanism underlying the SZ-like endophenotypic profile of these mice.
Table 1.
Comparison of the behavioral and epigenetic, molecular abnormalities between a mouse model of psychiatric disorder (PRS mouse) and schizophrenia patients.
| Subjects | Reln, GAD67 and BDNF | Symptoms | |||||
|---|---|---|---|---|---|---|---|
| DNMT binding | Promotermethylation | mRNA | Protein | Positive | Negative | Cognitive perfomances | |
| PRS mice | ↑ | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ |
| Schizophrenia Patients | ↑ | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ |
Prenatally restraint stressed (PRS); DNA methyltransferase (DNMT); Reelin (Reln); glutamate decarboxylase (GAD67); Brain-Derived Neurotrophic Factor (BDNF).
Figure 2.
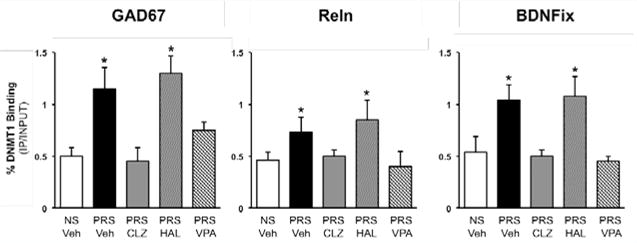
Increased levels of DNMT1 binding at Bdnf-ix, Rein, and Gad1 promoter regions in the frontal cortex of PRS mice are reduced by treatment with clozapine (5mg/kg twice a day for 5 days) but not haloperidol (1mg/kg twice a day for 5 days). The animals (PND 75) were tested on Day 6, 18hr after the last injection of neuroleptic. Data are expressed as mean +/− SEM of 5 animals per group and analyzed with one-way ANOVA followed by Student Newman-Keuls multiple comparisons. ANOVA for Bdnf-ix (7.13, P <0.001), Rein (F3,20 5.4, p <0.01), Gad1 (F3,20 5.8, p <0.001); (data from Dong et al[15]).
Recently, we have shown that prenatally stressed mice (PRS mice) have behavioral, and molecular SZ-like characteristics that can be used to preclinically test the potency and efficacy of antipsychotic drugs acting on altered epigenetic mechanisms [15]. This model responds with behavioral and neuroepigenetic improvements to the protracted administration of clozapine but not to that of haloperidol. For the behavioral experiments, adult (PND 75–90 days) PRS mice were treated subcutaneously with clozapine (5mg/kg/twice a day/5days) or haloperidol (1mg/kg/twice a day/5days). Clozapine but not haloperidol treated mice show a correction to the positive and negative symptoms and cognitive deficits (Figure 3). Interestingly, valproic acid, a histone deacetylase inhibitor used to treat BP disorder patients, shows correction of the behavioral parameters altered in the PRS mice (Figure 3). The same animals used for the behavioral studies show that clozapine but not haloperidol corrects the two-fold increased binding of DNMT to promoters of psychiatric candidate genes (GAD, RELN, BDNF) (Figure 2) [15,16]. We are now testing whether the behavioral and neuro epigenetic effects of clozapine are correlated and mimicked by the intracerebro ventricular injection of the DNMT inhibitor N-phthaloyl-L-tryptophan (RG108) [18], or subtype specific histone deacetylase inhibitors [19], suggesting that PRS mice may help to identify novel families of neuroleptic drugs able of reducing DNA-hypermethylation either directly (RG108) or indirectly by inducing chromatin remodeling (HDAC inhibitors), and to correct positive, negative symptoms, and cognitive deficits.
Figure 3.
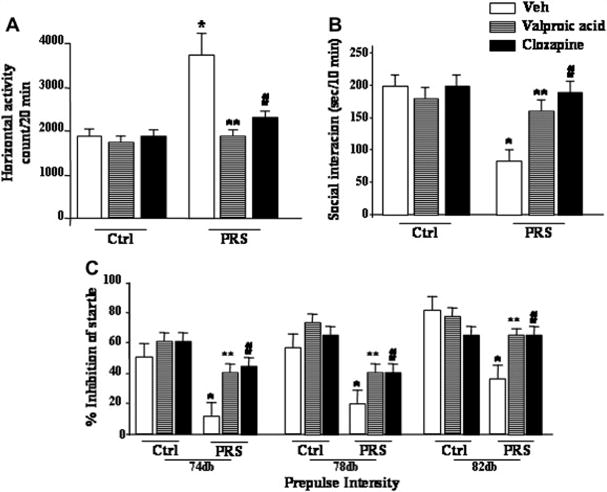
Schizophrenia-like behavioral abnormalities in PRS mice are reversed by treatment with valproic acid (70 mg/kg, i.p.; twice a day for 5 days), or clozapine (5 mg/kg, s.c.; twice a day, for 5 days). Data of locomotor activity (A), social interaction (B), and PPI (C), 24h after the last drug injection, are shown. One way ANOVA comparing controls (NS) veh, PRS veh, PRS VPA and PRS clozapine: (A) locomotor activity (F3,33 = 8.9 1 9, p = 0.001), (B) social interaction (F3,28 = 17.233, p <0.001, and (C) PPI (74 dbF3,42 = 5.189, p = 0.004; 78 db F3,42 = 9.625, p <0.001; 82 dbF3,42 = 6.582, p <0.001). Post-hoc Newman-Keuls comparisons indicated that PRS veh mice differed from controls veh mice (*), and from PRS mice treated with VPA (**) or clozapine (#). Analysis for PRS mice considered yielded a similar result while ANOVAs for controls were not significant.
Control dams were left undisturbed throughout gestation, whereas stressed dams were subjected to repeated episodes of restraint stress, as described previously [14,15]. The stress procedure consisted of restraining the pregnant dam in a transparent tube (12 × 3 cm) under a bright light for 45 min three times per day from the seventh day of pregnancy until delivery. After weaning (postnatal day [PND] [21], male mice were selected for the study and housed four to five per cage separately by condition.
CONCLUSIONS
The PRS mouse model may be useful in preclinically screening for the potential efficacy of neuroleptic drugs acting on altered epigenetic mechanisms. The model is insensitive to neuroleptics (i.e., haloperidol) that do not have an epigenetic action but is sensitive to drugs like clozapine, valproate [14,15], and RG-108 because they modulate chromatin function. In this context, PRS mice behave like “antipsychotic-resistant” SZ patients predicting treatment responses to an as yet unexplored group of neuroleptic agents. It seems likely that congeners of clozapine (olanzapine, quetiapine), or related molecules that exhibit monoamine receptor efficacy and that facilitate alterations in the epigenome may prove promising in the treatment of these devastating diseases.
Acknowledgments
This work was supported in part by grants R01 MH093348 and R01 MH101043 to AG and P50 AA022538 to AG and DRG.
References
- 1.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38:38–66. doi: 10.1038/npp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Higher risk of offspring for schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 5.Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology. 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One. 2011;6:14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Os J, Kenis G, Rutten BPF. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 9.Zhang TY, Labonte b, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsycopharmacology. 2013;38:111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao-Lei L, Elgbeili G, Massart R, Laplante DP, Szyf M, King S. Pregnant women’s cognitive appraisal of a natural disaster affects DNA methylation in their children 13 years later: Project Ice Storm. Transl Psychiatry. 2015;5:515. doi: 10.1038/tp.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao-Lei L, Veru F, Elgbeili G, Szyf M, Laplante D, King S. DNA Methylation Mediates the Effect of Exposure to Prenatal Maternal Stress on Cytokine Production in Children at Age 13½ Years: Project Ice Storm. Clin Epigenetics. 2016;8:54. doi: 10.1186/s13148-016-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun K, Bock J, Wainstock T, Matas E, Gaisler-Salomon I, Fegert J, et al. Experience-induced transgenerational re-programming of neuronal structure and functions: Impact of stress prior and during pregnancy. Neurosci Biobehav Rev. 2017 doi: 10.1016/j.neubiorev.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry. 2014;19:641–651. doi: 10.1038/mp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, et al. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–194. doi: 10.1016/j.neuropharm.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong E, Tueting P, Matrisciano F, Grayson DR, Guidotti A. Behavioral and molecular neuroepigenetic alterations in prenatally stressed mice: relevance for the study of chromatin remodeling properties of antipsychotic drugs. Transl Psychiatry. 2016;6:711. doi: 10.1038/tp.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong E, Dzitoyeva S, Matrisciano F, Tueting P, Grayson DR, Guidotti A. BDNF epigenetic modifications associated with schizophrenia-like phenotype induced by prenatal stress in mice. Biol Psych. 2015;77:589–596. doi: 10.1016/j.biopsych.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong E, Ruzicka WB, Grayson DR, Guidotti A. DNA-methyltransferase1 (DNMT1) binding to CpG rich GABAergic and BDNF promoters is increased in the brain of schizophrenia and bipolar disorder patients. Schizophr Res. 2015;167:35–41. doi: 10.1016/j.schres.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asgatay S, Champion C, Marloie G, Drujon T, Senamaud-Beaufort C, Ceccaldi A, et al. Synthesis and evaluation of analogues of N-phthaloyl-l-tryptophan (RG108) as inhibitors of DNA methyltransferase 1. J Med Chem. 2014;57:421–434. doi: 10.1021/jm401419p. [DOI] [PubMed] [Google Scholar]
- 19.Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, et al. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60:1007–1016. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


