Abstract
Purpose
To assess the retinal architecture changes which occur during epiretinal membrane (ERM) surgery, utilizing intraoperative optical coherence tomography (iOCT).
Design
Prospective multi-surgeon single center study.
Subjects/Participants
Subjects from the PIONEER iOCT study who underwent surgical intervention for management of ERM.
Methods
All subjects underwent vitrectomy with ERM peeling with optional internal limiting membrane (ILM) peeling. Preoperative, intraoperative, and postoperative quantitative and qualitative OCT assessments were performed. Clinical characteristics including visual acuity outcomes, central subfield thickness and complications including ERM recurrence and need for reoperation were assessed at 3, 6 and 12 months following surgery for membrane peeling, as available.
Main Outcome measures
Visual acuity outcomes, anatomic outcomes and complications including ERM recurrence. Microarchitectural alterations (i.e. retinal layer changes) following membrane peeling visualized with iOCT.
Results
Seventy-six were identified and included in this analysis of clinical outcomes and quantitative OCT assessment. Twenty-four eyes were excluded due to insufficient intraoperative OCT quality for quantitative assessment. The mean preoperative VA measured 20/63. The mean postoperative VA at 3 months was 20/41 (p<0.0001), at 6 months measured 20/36 (p < 0.0001), and at 12 months measured 20/33 (p < 0.0001). Preoperative mean central subfield thickness (CST) was 426 microns. At 3 months, the mean CST improved to 377 microns (p < 0.0001). The 6-month postoperative CST was 367 microns (p < 0.0001) and the 12-month postoperative CST measured 359 microns (p < 0.0001). Immediately following membrane peeling, the distance between the retinal pigment epithelium and the ellipsoid zone as well as the distance between the retinal pigment epithelium and the cone outer segment tips/interdigitation zone significantly increased (p < 0.001). iOCT identified occult residual membranes in 12% of cases and confirmed complete membrane peeling contrary to surgeon impression in 9% of cases. Reoperation was required for recurrent ERM in 1% of eyes.
Conclusions
iOCT-assisted ERM peeling resulted in significant improvement in visual acuity, reduction in macular thickness, and low recurrence rate. Additional research is needed with randomized clinical trials to better define the comparative success rates of image-guided ERM surgery to standard surgical visualization techniques.
INTRODUCTION
Optical coherence tomography (OCT) has become the diagnostic test of choice for assessing many macular diseases, including epiretinal membranes (ERMs).1, 2 Recent advances in the technology have allowed for increased speed and resolution. These advances combined with more portable solutions and microscope-integrated options have enabled its use in the operating room for in vivo retinal layer visualization.3 Intraoperative OCT (iOCT) has been demonstrated during surgical management in a number of vitreoretinal conditions, including epiretinal membrane, macular hole, vitreomacular traction, optic-pit related maculopathy, retinal detachment, and retinopathy of prematurity.4–12
The tangential traction and architectural distortion caused by an ERM may result in metamorphopsia and visual loss and increases in prevalence by age group.13 When the visual symptoms become significant, ERM is treated through a pars-plana vitrectomy (PPV) with membrane peeling.14 Several studies have pointed to OCT for insights into prognostic factors that can affect postoperative visual acuity, such as macular thickness, integrity of the photoreceptor ellipsoid zone (EZ; i.e., inner/outer segment junction),15, 16 and integrity of the interdigitation zone [IZ; i.e.., cone outer segment tips (COST) line].17 Previous small studies demonstrated the potential capabilities of iOCT during ERM surgery. 8–10 Changes in retinal architecture during ERM surgery can be noted with iOCT that are otherwise undetectable to a surgeon through the en face view of a microscope. Through the use of iOCT, more recent research has suggested that alterations occur in the outer retinal layers following membrane peeling.18 Two large-scale prospective studies have reported the significant discordance between surgeon perception and OCT findings in completeness of membrane peeling.19, 20 To date, functional and anatomic outcomes with iOCT-assisted surgery have not been reported to our knowledge.
The Prospective Intraoperative and Perioperative Ophthalmic Imaging with Optical Coherence Tomography (PIONEER) study is a large prospective multi-surgeon study that included subjects undergoing vitrectomy for the treatment of a vitreomacular interface disorder.19 In this report, we examine eyes from the PIONEER study that underwent membrane peeling with concurrent iOCT imaging to assess clinical outcomes in ERM patients and to better define the retinal architecture changes that occur during surgical intervention for ERM.
METHODS
PIONEER is an IRB-approved prospective multi-surgeon single center study that examined the feasibility and utility of iOCT for ophthalmic surgery. The methods of the PIONEER study have been previously described.22 For this report, subjects in the PIONEER study were identified that underwent surgical repair for a preoperative diagnosis of epiretinal membrane. Preoperative, intraoperative, and postoperative quantitative and qualitative OCT assessments were performed. Clinical characteristics including visual acuity outcomes, central subfield thickness and complications including ERM recurrence and need for reoperation were assessed at 3, 6 and 12 months following surgery for membrane peeling, as available. ERM recurrence was defined as OCT-based visualization of an ERM that involved the foveal center or resulted in significant foveal distortion. Microsoft Excel was utilized for statistical analysis. T-test was utilized for comparing continuous variables.
Surgical Procedure
All patients underwent standard 3-port small-gauge PPV (23 or 25-gauge) for surgical ERM peel by one of four surgeons (JPE, SKS, PKK, RS). Following completion of the core PPV with elevation of the hyaloid, as needed. Indocyanine green (ICG) and/or triamcinolone was applied prior to membrane peeling at surgeon discretion. This staining was performed based on surgeon technique rather than iOCT findings. The membrane peeling technique was based on surgeon preference and performed with either vitreoretinal forceps or a diamond-dusted membrane scraper combined with vitreoretinal forceps for peel completion. Internal limiting membrane (ILM) was peeling was optional and performed at surgeon discretion, often based on iOCT findings following initial ERM peeling.
iOCT Scanning System
A microscope mounted iOCT system was utilized for intraoperative imaging. The Bioptigen Envisu SDOIS (Bioptigen, Research Triangle Park, NC) portable probe was attached to the ophthalmic microscope utilizing a custom, microscope-mounted system, as previously described.11, 12 iOCT was performed at various surgical milestones as determined by the surgeon, including pre-peel and following membrane peeling (e.g., post-peel). If a second peel attempt was deemed necessary, a second iOCT image was also obtained for confirmation of completion. A consistent image acquisition protocol was utilized, including cubic 10×10 mm volume scans (at 0 and 90 degrees), 10×5 mm volume scans with oversampling for averaging, and 10mm radial volume scans. Each scan consisted of 100 B-scans distributed across the area with 1000 A-scans per B-scan. The 10×10 mm cube scans translated to a scan density of one B-scan every 0.1 mm.
iOCT Image Analysis
Qualitative and quantitative analysis of all scanning sequences were performed. Quantitative analysis was performed using a custom OCT analysis software platform. For quantitative assessment of retinal layer thickness, image analysis was performed of retinal zone measurements including inner retinal thickness (i.e. nerve fiber layer), middle retinal thickness (ganglion cell layer, inner plexiform later, outer plexiform layer) and outer retinal thickness (i.e. outer nuclear layer, EZ, retinal pigment epithelium). Only eyes with sufficient scans of both preincision and post-peel measurements were included in the analysis. Each scan was assessed for several variables prior to and following surgical peeling: subretinal hyporeflectivity [i.e., EZ to RPE distance], central foveal thickness (CFT), and cone outer segment tips [COST; or interdigitation zone (IZ)] to RPE distance. The outer boundary utilized for measurement was the middle of the RPE. The inner boundary varied based on location of interest (e.g., ILM, middle of EZ). The pre-incision and post-peel measurements were compared using paired t-test using Microsoft Excel. Surgical peeling technique, as well as specific surgeon was compared to extent of architectural alterations.
RESULTS
Clinical Characteristics and Demographics
One hundred eyes were identified in the PIONEER study to have undergone surgical intervention for the treatment of epiretinal membrane. . The median age was 68.4 years (range 29 to 86 years). There were 39 males (51%) and 37 females (49%). The mean preoperative visual acuity (VA) was 20/63 (range: 20/25 to 20/2000). Fifty-two eyes (68%) were phakic and 24 (32%) were pseudophakic prior to surgery. All eyes underwent small-gauge PPV. ICG was utilized in 71 cases (93%) and triamcinolone was utilized in 27 cases (36%). Technique utilized for membrane peeling included combined diamond dusted membrane scraper/forceps 39 eyes (51%) and forceps alone for 37 eyes (49%). No intraoperative complications were noted.
Clinical and Safety Outcomes
The mean preoperative VA measured 20/63 (range: 20/25 to 20/2000). The mean VA was 20/41 at 3 months (range: 20/20 to 20/400, p<0.0001, n =76), 20/37 at 6 months (range: 20/15 to 20/500, p < 0.0001, n=64), and 20/34 (range: 20/15 to 20/200, p < 0.0001, n=60) at 12 months. Preoperative mean central subfield thickness (CST) was 434 microns (range: 283 to 649). The mean CST improved to 377 microns at 3 months (range 209 to 559, p < 0.0001, n=73), 367 microns at 6 months (range: 211 to 592, p < 0.0001, n=57), and 359 microns at 12 months (range 215 to 531, p < 0.0001, n=48).
Cataract progression was reported in 3 subjects that remained phakic following the initial surgery following membrane peeling. One patient developed a retinal detachment after sustaining direct ocular trauma. OCT-based assessment of ERM recurrence revealed significant recurrent ERM in 2 of 76 eyes (3%). Of those 2 subjects with significant recurrent ERM, 1 subject underwent reoperation (1%).
iOCT Impact on Surgical Decision-making and Architectural Alterations
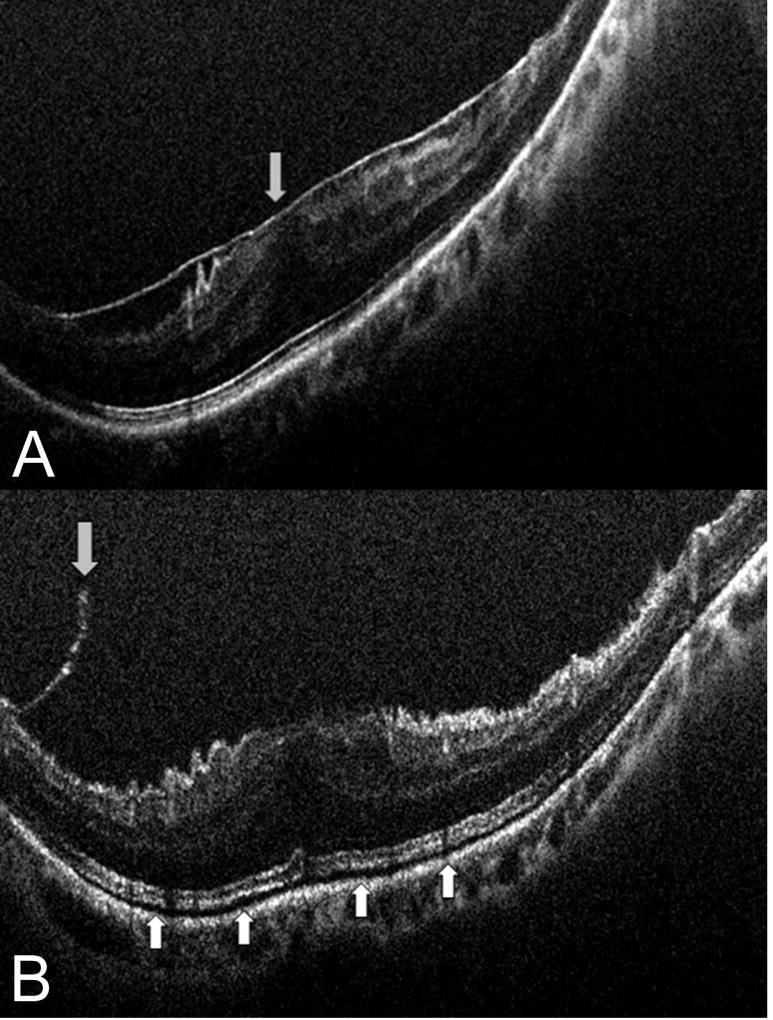
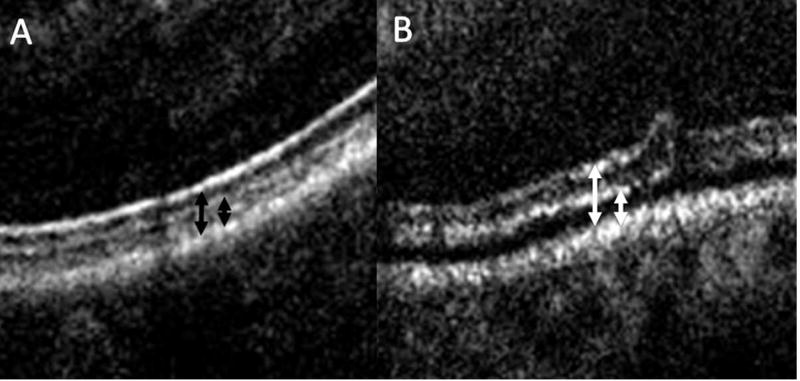
iOCT identified residual membranes that required additional membrane peeling in 12% of cases (Figure 1). In 7 of 76 cases (9.2%), the intraoperative OCT findings minimized unnecessary surgical maneuvers based on the surgeon indicating that the OCT information confirmed peel completion that was contrary to their clinical impression (Supplemental video 1). Intraoperative and postoperative OCT images were used to compare retinal zone measurements including inner and full thickness retinal elevations. Mean pre-peel CFT was 421 microns (range: 138 to 702 microns). Following surgical peel, the mean CFT was 419 microns (range: 226 to 785 microns, p = 0.71). The mean pre-peel EZ to RPE distance was 46.7 microns. The mean post-peel EZ to RPE distance was 53.5 microns (15% increase, p<0.001). This alteration resulted in the appearance of increased subretinal hyperreflectivity (Figure 2).
Figure 1. Intraoperative optical coherence tomography and epiretinal membrane peeling.
(A) Intraoperative optical coherence tomography (OCT) prior to membrane peeling with clearly identified epiretinal membrane. (B) Post-peel intraoperative OCT with occult residual membrane (down arrow) and increased subretinal hyporeflectance (up arrows).
Figure 2. Outer retinal changes following membrane peeling.
(A) High magnification view of outer retina prior to membrane peeling with preoperative relationship of ellipsoid zone to retinal pigment epithelium (left black arrow) and the cone outer segment tips to retinal pigment epithelium (right black arrow). (B) Following membrane peeling, increased subretinal hyporeflectivity is visualized with an expansion between the outer retinal bands and the retinal pigment epithelium (white arrows).
Sixty-three of 76 eyes had scans that were of sufficient quality to visualize the COST line. The mean pre-peel COST/RPE distance was 24.1 microns and increased following membrane peeling to 29.2 microns (p<0.001). Architectural alterations including increased subretinal hyporeflectivity, EZ-RPE expansion and COST-RPE expansion were not associated with surgical peel technique (e.g., forceps only vs diamond dusted membrane scraper).
DISCUSSION
Until recently, surgical management of ERM with vitrectomy and membrane peeling has been solely performed with en face visualization of the tissues of interest by the surgeon. iOCT allows for the visualization of significant retinal architectural details that would otherwise be impossible to assess with standard visualization. In this study, a quantitative analysis of the architectural alterations that occur following membrane peeling is provided. In addition, this report provides an assessment of iOCT utility during ERM peeling. In 12% of cases, iOCT revealed residual membranes that required additional membrane peeling. This is important as it has the potential to improve visual outcomes as well as spare the patient the risks and expenses of a repeated operation. In contrast, in nearly 10% of cases the iOCT reduced unnecessary surgical maneuvers and may have increased surgical efficiency and safety. Additional potential areas of impact of iOCT on surgical maneuvers included minimizing unnecessary surgical maneuvers beyond peeling, such as restaining with ICG.
iOCT allowed for not only determining the presence or extent of ERM while in the operating room, but also for assessment of changes in microarchitectural following membrane peeling. Other studies have documented these qualitative changes in membrane peeling surgery.8, 11, 12, 18 Our group has previously described the quantitative changes in the outer retina following ILM peeling in MH surgery.11 The functional significance of these findings remain unknown and further research is needed to better understand the potential role in long visual/anatomic recovery. Interestingly, there was no correlation between architectural alterations and peeling technique or surgeon experience.
Several studies have demonstrated the importance of postoperative outer retinal features and visual recovery. A recent study has found that retinal restoration following surgical peeling is dependent on the rehabilitation of all retinal layers, coming to the conclusion that retinal function and retinal morphology are both important functions to determining visual recovery after ERM peeling.21 The COST line or interdigitation zone is believed to occur due to scattering from the tips of the cone OS within the region of the of RPE/photoreceptor interaction.14 One recent study has demonstrated that BCVA after ERM surgery largely depended on the status of the COST line and was more significant if the EZ line was also disrupted.22
Our OCT-based assessment revealed recurrent ERM in 2 of 76 eyes (2.6%) during the course of this study. Of those 2 subjects with recurrent ERM, 1 subject underwent reoperation (1%). This is lower than prior reported recurrence rate of 12% and reoperation rates of about 3% following ERM peeling without iOCT guidance. 23 One of the presumed causes of recurrence of ERM is incomplete peeling of ERM due to lack of visualization of this semi-transparent membrane. 24, 25 In fact, histopathological studies have shown that even when ERM was determined to be completely peeled using various staining methods intraoperatively such as ICG26, 27 and trypan blue27, 28, microscopic ERM components persisted on the ILM. In addition, brilliant blue is frequently used globally for ILM staining. To this end, our iOCT technology holds great potential since we found that in 12% of cases, iOCT revealed residual membrane that prompted the surgeon to undertake additional peeling. A larger study is needed to highlight differences in recurrence and potential reoperation rates following ERM peeling with iOCT guidance versus without.
Study limitations include the lack of a control group and lack of standardized surgical method. Technical limitations include the lack of tracking and co-localization during imaging sequences, limiting the ability to precisely correlate preoperative and postoperative images to the identical cross-section. Furthermore, variations in image quality may affect measurement precision. iOCT images were analyzed in a masked fashion by two independent reviewers in order to make results as objective as possible, however, in the near future, we can expect computer algorithms for more precise and objective measurement of imaging dimensions. This study assessed iOCT identified changes that occurred immediately following membrane peeling. The high utilization of ICG and lack of histologic data makes it difficult to tease out the true frequency of ILM peeling in this series, making it challenging to appropriately attribute the impact of iOCT.
True “real-time” iOCT was not possible given that the OCT system used in this study is not integrated into the microscope optics. Other studies examining true real-time interactions of the tissue and instruments may be illustrative regarding the impact of specifics of peeling (e.g., angle of tension) on the outer retinal changes. Microscope integrated OCT systems allow for this real-time visualization of surgical motion. 5, 6, 9, 22, 29
Despite the limitations, this study finds that iOCT is of potential practical value in the operating room for the determination of peel completion. The use of OCT-based visualization of ophthalmic anatomy in the operating room continues to expand and may provide a transformation in surgical visualization during surgical procedures. In this study, iOCT feasibility and potential utility has been explored in ERM surgery. The use of iOCT provides new insight into retinal alteration changes during epiretinal membrane procedures. Further study is warranted to determine the potential impact of intraoperative changes on visual recovery and outcomes. Randomized controlled clinical trials are also needed to more clearly define the impact of iOCT-guided membrane peeling and overall outcomes in ERM surgery.
Supplementary Material
Case example demonstrating complete removal of epiretinal membrane during elevation of the hyaloid. This finding was contrary to the en face impression of the surgeon. The intraoperative OCT finding confirmed completion of surgical objectives and omitted the need for additional maneuvers (e.g., unnecessary membrane peeling, use of adjuvant dyes). Three-dimensional reconstruction shows pre-peel epiretinal membrane in red and then demonstrates the change in epiretinal membrane location following hyaloid elevation. The OCT finding following hyaloid elevation strongly suggests that this was an example of posterior hyaloidal traction rather than a typical idiopathic epiretinal membrane; however, this was not apparent without the intraoperative OCT confirmation.
Acknowledgments
Financial Support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE, SKS);
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
JPE: Bioptigen/Leica (P, C), Thrombogenics (C, R), Synergetics/Bausch and Lomb (P), Genentech (R), Regeneron (R), Alcon (C,R); MK: None; DP: None; LS: None; PKK: Carl Zeiss Meditec (C), Topcon (C), Alcon (C), Novartis (C), Bausch and Lomb (C); RPS: Carl Zeiss Meditec (C); JLR: None. SKS: Bausch and Lomb (C, R); Bioptigen (P); Allergan (R); Synergetics (P)
References
- 1.Wojtkowski M, Srinivasan V, Fujimoto JG, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2005;112(10):1734–46. doi: 10.1016/j.ophtha.2005.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt-Erfurth U, Leitgeb RA, Michels S, et al. Three-dimensional ultrahigh-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci. 2005;46(9):3393–402. doi: 10.1167/iovs.05-0370. [DOI] [PubMed] [Google Scholar]
- 3.Chen TC, Cense B, Pierce MC, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005;123(12):1715–20. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 4.Ehlers JP, Kernstine K, Farsiu S, et al. Analysis of pars plana vitrectomy for optic pit-related maculopathy with intraoperative optical coherence tomography: a possible connection with the vitreous cavity. Arch Ophthalmol. 2011;129(11):1483–6. doi: 10.1001/archophthalmol.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52(6):3153–9. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlers JP, Tao YK, Farsiu S, et al. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013;33(1):232–6. doi: 10.1097/IAE.0b013e31826e86f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee LB, Srivastava SK. Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg Lasers Imaging. 2011;42:e71–4. doi: 10.3928/15428877-20110804-05. Online. [DOI] [PubMed] [Google Scholar]
- 8.Ray R, Baranano DE, Fortun JA, et al. Intraoperative Microscope-Mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy during Macular Surgery. Ophthalmology. 2011;118(11):2212–7. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Binder S, Falkner-Radler CI, Hauger C, et al. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332–6. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 10.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29(10):1457–68. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers JP, Xu D, Kaiser PK, et al. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014;34(2):213–21. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers JP, Tam T, Kaiser PK, et al. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014;34(7):1341–6. doi: 10.1097/IAE.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty DJ, Mukesh BN, Chikani V, et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol. 2005;140(2):288–94. doi: 10.1016/j.ajo.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 14.McDonald HR, Verre WP, Aaberg TM. Surgical management of idiopathic epiretinal membranes. Ophthalmology. 1986;93(7):978–83. doi: 10.1016/s0161-6420(86)33635-2. [DOI] [PubMed] [Google Scholar]
- 15.Suh MH, Seo JM, Park KH, Yu HG. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147(3):473–80. e3. doi: 10.1016/j.ajo.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Oster SF, Mojana F, Brar M, et al. Disruption of the photoreceptor inner segment/outer segment layer on spectral domain-optical coherence tomography is a predictor of poor visual acuity in patients with epiretinal membranes. Retina. 2010;30(5):713–8. doi: 10.1097/IAE.0b013e3181c596e3. [DOI] [PubMed] [Google Scholar]
- 17.Shimozono M, Oishi A, Hata M, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012;153(4):698–704. e1. doi: 10.1016/j.ajo.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Ehlers JP, Han J, Petkovsek D, et al. Membrane Peeling-Induced Retinal Alterations on Intraoperative OCT in Vitreomacular Interface Disorders from the PIONEER Study. Invest Ophthalmol Vis Sci. 2015;56(12):7324–30. doi: 10.1167/iovs.15-17526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg With Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am J Ophthalmol. 2014;158(5):999–1007. e1. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers JP, Goshe J, Dupps WJ, et al. Determination of Feasibility and Utility of Microscope-Integrated Optical Coherence Tomography During Ophthalmic Surgery: The DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015 doi: 10.1001/jamaophthalmol.2015.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichi F, Alkabes M, Nucci P, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging. 2012;43(6 Suppl):S54–60. doi: 10.3928/15428877-20121001-08. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98(10):1329–32. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grewing R, Mester U. Results of surgery for epiretinal membranes and their recurrences. Br J Ophthalmol. 1996;80(4):323–6. doi: 10.1136/bjo.80.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margherio RR, Cox MS, Jr, Trese MT, et al. Removal of epimacular membranes. Ophthalmology. 1985;92(8):1075–83. doi: 10.1016/s0161-6420(85)33902-7. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson CP. Recurrent macular pucker. Am J Ophthalmol. 1979;88(6):1029–31. doi: 10.1016/0002-9394(79)90411-2. [DOI] [PubMed] [Google Scholar]
- 26.Kwok AK, Lai TY, Li WW, et al. Indocyanine green-assisted internal limiting membrane removal in epiretinal membrane surgery: a clinical and histologic study. Am J Ophthalmol. 2004;138(2):194–9. doi: 10.1016/j.ajo.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Kwok AK, Lai TY, Li WW, et al. Trypan blue- and indocyanine green-assisted epiretinal membrane surgery: clinical and histopathological studies. Eye (Lond) 2004;18(9):882–8. doi: 10.1038/sj.eye.6701359. [DOI] [PubMed] [Google Scholar]
- 28.Gibran SK, Flemming B, Stappler T, et al. Peel and peel again. Br J Ophthalmol. 2008;92(3):373–7. doi: 10.1136/bjo.2007.129965. [DOI] [PubMed] [Google Scholar]
- 29.Tao YK, Srivastava SK, Ehlers JP. Microscope-integrated intraoperative OCT with electrically tunable focus and heads-up display for imaging of ophthalmic surgical maneuvers. Biomed Opt Express. 2014;5(6):1877–85. doi: 10.1364/BOE.5.001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case example demonstrating complete removal of epiretinal membrane during elevation of the hyaloid. This finding was contrary to the en face impression of the surgeon. The intraoperative OCT finding confirmed completion of surgical objectives and omitted the need for additional maneuvers (e.g., unnecessary membrane peeling, use of adjuvant dyes). Three-dimensional reconstruction shows pre-peel epiretinal membrane in red and then demonstrates the change in epiretinal membrane location following hyaloid elevation. The OCT finding following hyaloid elevation strongly suggests that this was an example of posterior hyaloidal traction rather than a typical idiopathic epiretinal membrane; however, this was not apparent without the intraoperative OCT confirmation.