Abstract
Objective
Studies in adults have shown a significant association between obesity and psoriatic arthritis, however the association of obesity with pediatric psoriatic arthritis is unknown. We aimed to evaluate obesity in pediatric psoriatic arthritis.
Methods
We conducted a cross-sectional study of children with psoriasis and psoriatic arthritis evaluated at a single center between 6/2010 and 9/2014. Two healthy reference populations were utilized: 1) local reference population from the surrounding community and 2) a national reference population derived from NHANES. Age and sex-specific z-scores for weight, height, and body mass index (BMI) were calculated. Differences in clinical and demographic characteristics between groups were assessed.
Results
During the study period, 48 children with psoriatic arthritis and 231 patients with psoriasis were evaluated. Three (6.2%) and 5 (10.4%) of the children with psoriatic arthritis were overweight or obese, respectively. In comparison to the reference healthy groups and psoriasis patients, the mean BMI z-score of children with psoriatic arthritis was not statistically different. However, patients with psoriasis were more likely to be obese than the community reference population (p-value <0.001).
Conclusions
Children with psoriasis were more obese than the healthy reference population, however there was no statistically significant difference in obesity between children with psoriatic arthritis and psoriasis or psoriatic arthritis and the reference population. This lack of association may be reflective of true differences in pediatric and adult-onset disease and warrants further investigation.
Keywords: psoriatic arthritis, pediatric rheumatic diseases, obesity
Introduction
Psoriasis affects approximately 2–4% of the pediatric population and up to one-third of affected children develop arthritis, however publications focused on juvenile psoriatic arthritis are exceedingly rare. According to the International League of Associations for Rheumatology (ILAR) criteria [1] children are classified as having psoriatic arthritis if they are age 16 or younger and have arthritis and psoriasis or if they have arthritis plus at least two of the following: dactylitis, nail pits, or psoriasis in a first degree relative. The inflammatory joint disease in these children has the potential for severe destruction. Additional studies are greatly needed to help understand which patients with psoriasis are at risk of developing arthritis and how best to identify and manage this understudied population.
Risk factors for the development of psoriatic arthritis among adults with psoriasis have been examined in several studies and include obesity [2, 3] and elevated body mass index (BMI) [4]. It is postulated that patients with large amounts of adipose tissue produce increased quantities of pro-inflammatory cytokines (such as leptin, IL-6, and TNF-alpha) and decreased amounts of anti-inflammatory cytokines (such as adiponectin), leading to an increased risk of developing inflammatory joint disease [4, 5]. Additionally, weight loss may improve the response to treatment in adults with psoriatic arthritis [5].
In a prior study of 101 children with juvenile arthritis, 4 of whom had juvenile psoriatic arthritis, BMI z-score was not statistically different than the healthy reference population [6]. In a sub-analysis, children with polyarticular and systemic juvenile arthritis had significantly lower and higher BMI z-scores, respectively, compared to healthy controls. Another study failed to demonstrate an association between obesity and increased disease activity in children with all categories of juvenile arthritis [7]. Similar to adult psoriasis, juvenile psoriasis is associated with an increased prevalence of obesity [8]. The association of obesity with the development of arthritis in children with psoriasis has not been previously evaluated.
In this study, we aimed to test the association between obesity and juvenile psoriatic arthritis in a single tertiary care center. In addition, we aimed to further characterize the clinical manifestations of this rare disease in children.
Materials and Methods
The protocol for the conduct of this study was reviewed and approved by the Children's Hospital of Philadelphia Committee for the Protection of Human Subjects (IRB 14-011409).
Study design and subjects
A cross-sectional study was performed utilizing data obtained from the electronic medical records at a single tertiary care hospital. The cohort consisted of children with an ICD-9-CM code of 696.0 (“psoriatic arthritis”) given at initial diagnosis or a subsequent follow-up visit evaluated between June 2010 and September 2014. A comparison group of psoriasis patients included children with an ICD-9-CM code of 696.1 (“psoriasis”) evaluated at the same institution during the same period. Two healthy reference populations were used. The first reference population consisted of healthy participants recruited by the Nutrition and Growth Laboratory at our institution from general pediatric clinics and advertising in the surrounding community (Reference Data Project on Skeletal Development). The second reference population included participants derived from the National Health and Nutrition Examination Survey (NHANES) [9].
Clinical data and measures
Pediatric psoriatic arthritis was defined according to the ILAR criteria [1]. Per these criteria, patients have psoriatic arthritis if they have both arthritis and psoriasis or if they have arthritis plus at least two of the following: dactylitis, nail pits, or psoriasis in a 1st degree relative. Patients must be diagnosed with arthritis before age 16 years.
For children who met the diagnostic criteria, the following data was abstracted from the medical record: age at diagnosis, sex, race, active joint count at time of diagnosis, tender enthesis count at diagnosis, juvenile arthritis disease activity score 3–10 (JADAS3-10) score at diagnosis, presence of dactylitis during disease course, presence of psoriasis during disease course, family history of psoriasis in a 1st degree relative, oligoarticular vs. polyarticular disease course during the first 6 months after diagnosis, and serologic status (ANA and/or HLA B27 positivity). The JADAS3 is a validated disease activity measure [10] calculated using the active joint count (0–10), physician global assessment, and parent global evaluation. JADAS3-10 values range from 0–30, with higher values indicating higher disease activity. Cut-offs for disease activity have been developed for the JADAS3-10 and are as follows: (a) oligoarthritis course: inactive disease ≤1, low disease activity ≤1.5, moderate disease activity 1.51–4, and high disease activity >4, and (b) polyarthritis course: inactive disease ≤1, low disease activity ≤2.5, moderate disease activity 2.51–8.5, and high disease activity >8.5 [11]. Patient data was reported for the total patient population as well as stratified by patient age at diagnosis. In addition, information regarding which joints were affected in each patient during the study period was collected and tabulated to assess the most commonly affected joints in this population.
Age- and sex-specific z-scores for weight, height, and BMI were calculated based on the 2000 CDC Growth data for the patient’s initial visit [12]. Overweight or obese status was defined as a BMI ≥85th and <95th percentile and a BMI ≥ 95th percentile, respectively.
Statistical analysis
Clinical and demographic characteristics were summarized by mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables, and count and percent for categorical variables. Differences between patient groups were assessed using t-test, Wilcoxon rank sum, chi-squared, or Fisher’s exact test, as appropriate. The association of age, sex, race, active joint count, and presence of psoriasis with being overweight or obese was tested using univariate logistic regression. Stata 14 was used for all statistical analysis (Stata Corp, College Station, TX).
Results
During the study period, 48 children with psoriatic arthritis were evaluated (Table 1). Thirty four (71%) of the patients were female and the mean age at diagnosis was 8.8 years (SD 5.2 years). Forty one (85.4%) of the children with psoriatic arthritis were Caucasian. The median active joint count at diagnosis was 3 (IQR 1–7). The median JADAS3-10 was 12.5 (IQR 10–17) for polyarticular disease course and 9 (IQR 3–10) for oligoarticular disease course; both scores indicate high disease activity. Thirty (62.5%) and 19 (39.6%) of children had psoriasis or dactylitis at diagnosis, respectively. Arthritis was most often oligoarticular in course, asymmetric, and most commonly affected the finger proximal interphalangeal (PIP) joints, knees, and finger metacarpophalangeal (MCP) joints (Table 2).
Table 1.
Psoriatic Arthritis Features at Diagnosis
| All psoriatic arthritis (n=48), N (%) |
Age at onset < 7 years (n=18), N (%) |
Age at onset ≥7 years (n=30), N (%) |
p-value | |
|---|---|---|---|---|
| Age in years, mean ±SD | 8.8 ± 5.2 | 3.1 ± 1.9 | 12.2 ± 3.0 | <0.001 |
| Sex, Female | 34 (70.8) | 14 (77.8) | 20 (66.7) | 0.41 |
| Race | ||||
| Caucasian | 41 (85.4) | 16 (88.9) | 25 (83.3) | 1.00 |
| AA | 1 (2.1) | 0 (0) | 1 (3.3) | |
| Other | 6 (12.5) | 2 (11.1) | 4 (13.3) | |
| Psoriasis | 30 (62.5) | 11 (61.1) | 19 (63.3) | 0.88 |
| Dactylitis | 19 (39.6) | 8 (44.4) | 11 (36.7) | 0.76 |
| Family History of Psoriasis | 17 (35.4) | 9 (50.0) | 8 (26.7) | 0.13 |
| Active Joint Count, median (IQR) | 3 (1–7) | 3 (1–7) | 2 (1–7) | 0.58 |
| Polyarticular Course | 18 (37.5) | 6 (33.3) | 12 (40.0) | 0.76 |
| Tender Enthesis Count, median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–3) | 0.07 |
| JADAS3-10 polyarticular, median (IQR) | 12.5 (10–17) | 14 (14–14) | 11 (10–17) | 0.77 |
| JADAS3-10, oligoarticular median (IQR) | 9 (3–10) | 11 (10–14) | 5 (3–9) | 0.02 |
| ANA positive | 22 (45.8) | 12 (66.7) | 10 (33.3) | 0.02 |
| HLA B27 positive | 6 (12.5) | 3 (16.7) | 3 (10.0) | 0.66 |
Legend. Age cut-off of 7yo chosen based on two peaks of onset in Figure 1. AA: African American. Cut-offs for disease activity for the JADAS3-10: (a) oligoarthritis course: inactive disease ≤1, low disease activity ≤1.5, moderate disease activity 1.51–4, and high disease activity >4, (b) polyarthritis course: inactive disease ≤1, low disease activity ≤2.5, moderate disease activity 2.51–8.5, and high disease activity >8.5 (11).
Table 2.
Most Commonly Affected Joints in Children with Psoriatic Arthritis
| Joint | No. of Affected Joints, N | Unilateral, N (%) |
Bilateral, N (%) |
|---|---|---|---|
| Finger PIPs | 35 | 9 (19) | 13 (27) |
| Knee | 27 | 17 (35) | 5 (10) |
| Finger MCPs | 18 | 6 (12) | 6 (12) |
| Ankle | 14 | 8 (17) | 3 (6) |
| Toe PIPs | 10 | 8 (17) | 1 (2) |
| Wrist | 9 | 3 (6) | 3 (6) |
| Finger DIPs | 8 | 6 (12) | 1 (2) |
| Subtalar | 4 | 4 (8) | 0 (0) |
| Hip | 3 | 1 (2) | 1 (2) |
| Elbow | 3 | 1 (2) | 1 (2) |
| Thumb IP | 3 | 3 (6) | 0 (0) |
| Toe MCPs | 3 | 1 (2) | 1 (2) |
| Toe DIPs | 2 | 2 (4) | 0 (0) |
| Cervical | 2 | 2 (4) | - |
| Midfoot | 1 | 1 (2) | 0 (0) |
| Great toe IP | 1 | 1 (2) | 0 (0) |
Legend. PIP: proximal interphalangeal joint; MCP: metacarpophalangeal joint; DIP: distal interphalangeal joint; IP: interphalangeal joint
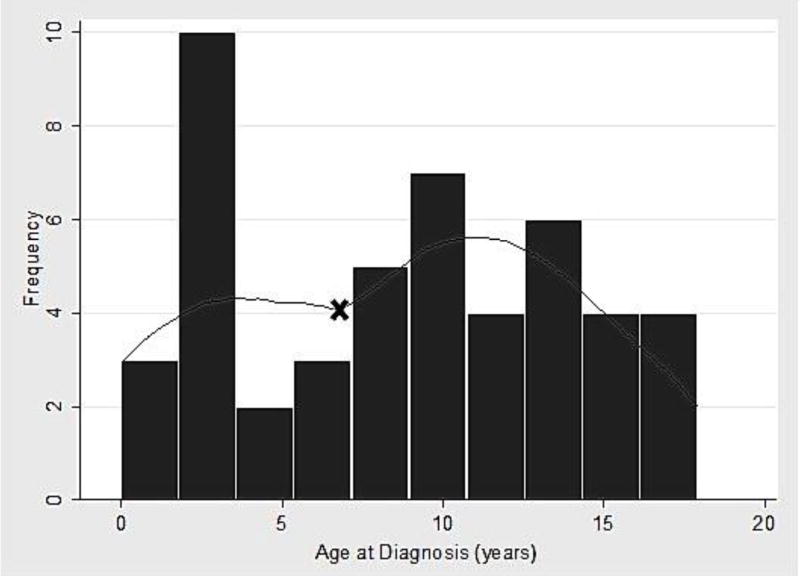
There were two peaks of disease onset (Figure 1), with the first occurring at 3 years and the second at 12 years. Based on the age distribution in Figure 1, a sub-analysis was performed stratifying patients into those diagnosed at less than 7 years old and those diagnosed at age 7 or older. Children in the younger age group were significantly more likely to be ANA positive than those diagnosed at an older age (p-value 0.02). There was no significant difference between the age groups in terms of the remaining disease features assessed in Table 1 (all p-values >0.05).
Figure 1. Age at Onset of Psoriatic Arthritis.
Histogram demonstrating two peaks of age at disease diagnosis
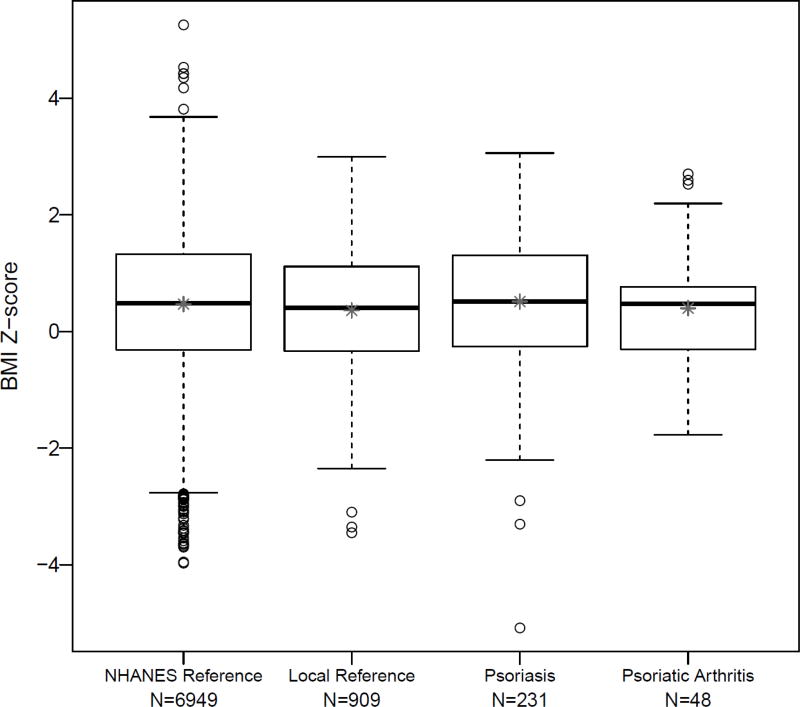
The mean BMI z-score of children with psoriatic arthritis was not statistically different in comparison to the reference groups (Tables 3 and 4, Figure 2). Three (6.2%) and 5 (10.4%) of the children with psoriatic arthritis were overweight or obese, respectively. Thirty-four (14.7%) of the psoriasis patients were overweight and 41 (17.7%) were obese. Children with psoriatic arthritis were no more likely than the reference patients to be obese or overweight, however, children with psoriasis were significantly more obese than the community-derived reference population (p-value <0.001, Table 3). This difference persisted when comparing children with psoriasis to the nationally-derived reference population, although the difference was no longer statistically significant (Table 4). There was no statistically significant difference in mean BMI z-score between psoriatic arthritis patients with and without psoriasis (p-value 0.33). Comparisons remained non-significant between those with and without psoriasis after stratifying by age at diagnosis (p-value 0.57 for patients diagnosed at < 7 years old and 0.47 for patients diagnosed at age 7 years or older).
Table 3.
Comparison of Overweight and Obese Status (Local Reference Population)
| Psoriatic Arthritis n=48 |
Psoriasis n=231 |
Healthy Reference n=909 |
Psoriatic Arthritis vs. Reference p-value |
Psoriasis vs. Reference p-value |
Psoriatic Arthritis vs. Psoriasis p-value |
|
|---|---|---|---|---|---|---|
| Race, N (%) | ||||||
| Non-AA | 47 (97.9) | 212 (91.8) | 511 (56.2) | <0.001 | <0.001 | 0.13 |
| AA | 1 (2.1) | 19 (8.2) | 398 (43.8) | |||
| BMI Z-score, mean ±SD | 0.40 ±1.0 | 0.51 ±1.2 | 0.36 ±1.0 | 0.79 | 0.05 | 0.55 |
| Non-AA | 0.39 ±1.0 | 0.53 ±1.2 | 0.20 ±0.9 | 0.17 | <0.001 | 0.46 |
| AA | 0.59 (N/A) | 0.28 ±1.2 | 0.57 ±1.1 | N/A | 0.26 | N/A |
| Overweight, N (%) | 3 (6.2) | 34 (14.7) | 152 (16.7) | 0.06 | 0.46 | 0.12 |
| Obese, N (%) | 5 (10.4) | 41 (17.7) | 89 (9.8) | 0.89 | <0.001 | 0.21 |
Legend. Overweight: BMI 85–95%; Obese: BMI≥95%. AA: African American.
Table 4.
Comparison of Overweight and Obese Status (NHANES Reference Population)
| Psoriatic Arthritis n=48 |
Psoriasis n=231 |
Healthy Reference n= 6949 |
Psoriatic Arthritis vs. Reference p-value |
Psoriasis vs. Reference p-value |
Psoriatic Arthritis vs. Psoriasis p- value |
|
|---|---|---|---|---|---|---|
| Race, N (%) | ||||||
| Non-AA | 47 (97.9) | 212 (91.8) | 5018 (72.2) | <0.001 | <0.001 | 0.13 |
| AA | 1 (2.1) | 19 (8.2) | 1931 (27.8) | |||
| BMI Z-score, mean ± SD | 0.40 ±1.0 | 0.52 ±1.2 | 0.47 ±1.2 | 0.69 | 0.53 | 0.55 |
| Non-AA | 0.39 ±1.0 | 0.53 ±1.2 | 0.44 ±1.2 | 0.78 | 0.28 | 0.46 |
| AA | 0.59 (N/A) | 0.28 ±1.2 | 0.51 ±1.2 | N/A | 0.45 | N/A |
| Overweight, N (%) | 3 (6.2) | 34 (14.7) | 1035 (14.9) | 0.09 | 0.94 | 0.12 |
| Obese, N (%) | 5 (10.4) | 41 (17.7) | 1213 (17.4) | 0.20 | 0.91 | 0.21 |
Legend. Overweight: BMI 85–95%; Obese: BMI≥95%. AA: African American.
Figure 2. Comparison of Body Mass Index Z-score.
Comparison of BMI z-scores across four populations. * mean of the BMI z-score.
Clinical features between those children with psoriatic arthritis who were overweight or obese and not overweight were examined, including age, sex, race, active joint count, presence of psoriasis, and presence of dactylitis. In univariate logistic regression, only male sex was associated with increased odds of being overweight or obese (Odds ratio: 5.74, 95% CI: 1.15–28.8).
Discussion
This is the first study to evaluate the association of obesity with psoriatic arthritis in children. Patients with psoriatic arthritis showed no statistically significant difference from the healthy reference group in terms of BMI z-score or proportion of those who were obese or overweight. Similar to a prior study [8], we found that children with psoriasis were more likely to be obese than the healthy reference population. However in contrast, psoriatic arthritis patients who also had psoriasis did not show an increased prevalence of obesity.
Juvenile psoriatic arthritis is a highly heterogeneous disease. A previous study showed that there are two peaks of onset and that these age groups represent clinically distinct subtypes of psoriatic arthritis [13]. Our patient population also demonstrated two peaks of disease onset, one in young children and a second in early adolescence. In comparison to the study by Stoll et al. (2006), ANA positivity was the only feature to differ significantly between the two age groups. We hypothesized that the association of obesity with psoriatic arthritis may depend on the age of the patient or race, but this lack of association with obesity held true even when the group was stratified by the presence/absence of psoriasis (since not all children with psoriatic arthritis have psoriasis), age at diagnosis, and race.
It is important to keep in mind several limitations when interpreting these results. This was a retrospective single center study and there could be unmeasured confounders. However, we included all children seen at our institution with psoriasis and psoriatic arthritis and we expect that these confounders are non-differential. Importantly, both dermatology and rheumatology at our institution are major referral centers and have a similar distribution of urban and suburban patients and racial composition. We included two separate healthy reference populations in our analysis to ensure that we were using an appropriate comparison group. Both analyses showed no significant difference in BMI z-score or overweight/obese status between psoriatic arthritis patients and healthy children. Given the retrospective nature of this study, we can only show evidence of association, not causation. In this cross-sectional study, we looked at the patient’s BMI at time of diagnosis of psoriatic arthritis or psoriasis, and it is possible that change in BMI over time, rather than at one isolated time point, is associated with the development of psoriatic arthritis in children. This will need to be tested in future studies. Lastly, our small sample size is limited in regards to the number of patients with psoriatic arthritis, so the ability to detect a statistically significant difference between groups is also limited. However, our results do not even show a trend towards those with psoriatic arthritis being more overweight or obese. These results will need to be replicated in population-based studies.
Several studies in adults have shown an association between obesity and the development of psoriatic arthritis in patients with psoriasis and it is curious that these findings were not replicated in our pediatric cohort. Our results are in line with one prior study of juvenile arthritis which demonstrated that children with spondyloarthritis (grouping enthesitis related arthritis, undifferentiated arthritis and psoriatic arthritis together; N of psoriatic arthritis= 4) are no more obese than the general population [6]. This lack of association may reflect a difference in prevalence and severity of psoriasis in adult and pediatric populations. It is also possible that children with psoriatic arthritis lose lean mass while maintaining fat mass, as seen in children with systemic inflammation due to Crohn’s disease [14]. In this case, the BMI may not adequately reflect a patient’s body composition, however one would have expected this finding to be present in adults also. On the other hand, this finding may demonstrate a fundamental difference in the pathophysiology of psoriatic arthritis in children. Just as juvenile idiopathic arthritis has been shown to be a distinct disease from adult rheumatoid arthritis [15], it is important to recognize that juvenile psoriatic arthritis may be a unique entity.
In summary, this is the first study to evaluate the association between juvenile psoriatic arthritis and obesity. Although adult studies have shown that psoriasis patients who are obese are more likely to develop psoriatic arthritis than their normal weight counterparts, this association was not found to exist in our pediatric psoriatic arthritis cohort. Due to the rarity of this disease, the use of a large healthcare database may prove useful in future studies aimed at better characterizing this patient population and understanding the factors associated with development of inflammatory arthritis in children with psoriasis.
Acknowledgments
Funding
Dr. Manos is supported by Mallinckrodt Pharmaceuticals grant 421439-A01. Dr. Weiss is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) NIH grant 1-R03-AR062665.
References
- 1.Stoll ML, Lio P, Sundel RP, Nigrovic PA. Comparison of Vancouver and International League of Associations for rheumatology classification criteria for juvenile psoriatic arthritis. Arthritis Rheum. 2008;59:51–8. doi: 10.1002/art.23240. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Han J, Qureshi AA. Obesity and risk of incident psoriatic arthritis in US women. Ann Rheum Dis. 2012;71:1267–72. doi: 10.1136/annrheumdis-2011-201273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Love TJ, Zhu Y, Zhang Y, Wall-Burns L, Ogdie A, Gelfand JM, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71:1273–7. doi: 10.1136/annrheumdis-2012-201299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soltani-Arabshahi R, Wong B, Feng BJ, Goldgar DE, Duffin KC, Krueger GG. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol. 2010;146:721–6. doi: 10.1001/archdermatol.2010.141. [DOI] [PubMed] [Google Scholar]
- 5.Russolillo A, Iervolino S, Peluso R, Lupoli R, Di Minno A, Pappone N, et al. Obesity and psoriatic arthritis: from pathogenesis to clinical outcome and management. Rheumatology (Oxford) 2013;52:62–7. doi: 10.1093/rheumatology/kes242. [DOI] [PubMed] [Google Scholar]
- 6.Burnham JM, Shults J, Dubner SE, Sembhi H, Zemel BS, Leonard MB. Bone density, structure, and strength in juvenile idiopathic arthritis: importance of disease severity and muscle deficits. Arthritis Rheum. 2008;58:2518–27. doi: 10.1002/art.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelajo CF, Lopez-Benitez JM, Miller LC. Obesity and disease activity in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2012;10:3. doi: 10.1186/1546-0096-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustin M, Radtke MA, Glaeske G, Reich K, Christophers E, Schaefer I, et al. Epidemiology and Comorbidity in Children with Psoriasis and Atopic Eczema. Dermatology. 2015;231:35–40. doi: 10.1159/000381913. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Accessed 29 March 2016];National Health and Nutrition Examination Survey Data 2010–2014. Available via http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 10.McErlane F, Beresford MW, Baildam EM, Chieng SE, Davidson JE, Foster HE, et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis. 2013;72:1983–8. doi: 10.1136/annrheumdis-2012-202031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aless, Consolaro r, Cal S, ra, Robbiano C, Ravelli A. Treating Juvenile Idiopathic Arthritis According to JADAS-Based Targets. Ann Paediatr Rheum. 2014;3:4–10. [Google Scholar]
- 12.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Stoll ML, Nigrovic PA. Subpopulations within juvenile psoriatic arthritis: a review of the literature. Clin Dev Immunol. 2006;13:377–80. doi: 10.1080/17402520600877802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnham JM, Shults J, Semeao E, Foster BJ, Zemel BS, Stallings VA, et al. Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr. 2005;82:413–20. doi: 10.1093/ajcn.82.2.413. [DOI] [PubMed] [Google Scholar]
- 15.Prahalad SGD. Is juvenile rheumatoid arthritis/juvenile idiopathic arthritis different from rheumatoid arthritis? Arthritis Research & Therapy. 2002;4:303–10. [Google Scholar]