Abstract
The Kelch‐like ECH‐associated protein 1/nuclear factor erythroid‐derived 2‐like 2 (KEAP1‐NRF2) system is a pivotal defense mechanism against oxidative and electrophilic stress. Although transient NRF2 activation in response to stress is beneficial for health, persistent NRF2 activation in cancer cells has deleterious effects on cancer‐bearing hosts by conferring therapeutic resistance and aggressive tumorigenic activity on cancer cells. Because NRF2 increases the antioxidant and detoxification capability of cancer cells, persistently high levels of NRF2 activity enhance therapeutic resistance of cancer cells. NRF2 also drives metabolic reprogramming to establish cellular metabolic processes that are advantageous for cell proliferation in cooperation with other oncogenic pathways. As a result of these advantages, cancer cells with persistent activation of NRF2 often develop “NRF2 addiction” and show malignant phenotypes leading to poor prognoses in cancer patients. Inhibition of NRF2 is a promising therapeutic approach for NRF2‐addicted cancers and NRF2 inhibitors are being actively developed. However, giving systemic NRF2 inhibitors might have undesirable effects on cancer‐bearing hosts, considering the central roles of NRF2 in cytoprotection. To avoid these side‐effects, new therapeutic targets besides NRF2 for NRF2‐addicted cancers have been actively explored. This review introduces recent studies describing the development and characterization of NRF2‐addicted cancers, as well as their potential therapeutic targets. Expected advances in diagnostic and therapeutic interventions for NRF2‐addicted cancers are also discussed.
Keywords: KEAP1, metabolic reprogramming, NRF2, therapeutic resistance, tumor microenvironment
Abbreviations
- FH
fumarate hydratase
- GEMM
genetically engineered mouse model
- IHC
immunohistochemistry
- KEAP1
Kelch‐like ECH‐associated protein 1
- MEF
mouse embryonic fibroblast
- MDSC
myeloid‐derived suppressor cell
- NRF2
nuclear factor erythroid‐derived 2‐like 2
- ROS
reactive oxygen species
- SNP
single nucleotide polymorphism
- Treg
regulatory T cell
1. PHYSIOLOGICAL ROLES OF THE KEAP1‐NRF2 SYSTEM
Living organisms are constantly interacting with their surrounding environment. Appropriate environmental responses are relevant for the maintenance of homeostasis and optimal health at cellular as well as organismal levels. Many environmental stimuli disturb redox homeostasis and result in biomolecules undergoing chemical changes such as protein carbonylation, lipid peroxidation and nucleic acid oxidation, leading to functional alteration or impairment of biomolecules. The KEAP1‐NRF2 system is an important defense mechanism against redox disturbances.1 NRF2 is a potent transcription activator belonging to the Cap'n'Collar (CNC) transcription factor family, which is characterized by a unique CNC motif followed by a well‐conserved basic region‐leucine zipper (bZip) structure. Under normal conditions, NRF2 is constantly poly‐ubiquitinated by the CUL3‐KEAP1 E3 ubiquitin ligase complex and subjected to degradation by proteasomes. When cells are exposed to oxidative and/or electrophilic stress, highly reactive thiols in KEAP1 are directly modified, resulting in inactivation of the CUL3‐KEAP1 complex and stabilization of NRF2. NRF2 then translocates to the nucleus and induces a battery of cytoprotective genes by binding to the antioxidant response element (ARE) by heterodimerization with small MAF proteins (Figure 1, “Transient NRF2 activation”).1
Figure 1.
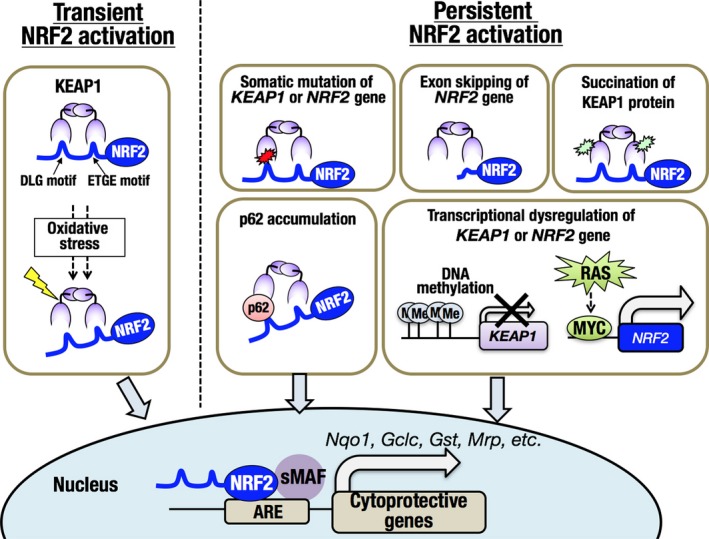
The KEAP1‐NRF2 system in physiological and pathological conditions in cancer cells. NRF2 activation is triggered by exposure to oxidative or electrophilic stress, resulting in upregulation of cytoprotective genes (left; transient NRF2 activation). In cancer cells, the KEAP1‐NRF2 regulatory system is frequently disrupted, resulting in the persistent activation of NRF2 (right; persistent NRF2 activation). ARE, antioxidant response element
Biochemical, biophysical and structural analyses showed that KEAP1 forms a cherry bob‐like homodimer and interacts with a single NRF2 molecule at two binding sites, namely a DLG motif and an ETGE motif in the N‐terminal region of NRF2 (Figure 1, “Transient NRF2 activation”).2, 3, 4 Appropriate interaction between KEAP1 and NRF2 is considered critical for efficient ubiquitination of NRF2, and modification of KEAP1 thiols by electrophiles is likely to induce conformational alterations in the overall structure of the CUL3‐KEAP1‐NRF2 complex and to suppress the ubiquitination of NRF2.
The physiological relevance of NRF2 has been shown by numerous studies using Nrf2‐deficient mice and human cohort studies of SNP in the promoter region of the NRF2 gene. Nrf2‐deficient mice are generally susceptible to redox disturbances and easily develop drug toxicity.5, 6 Oxidative tissue damage after ischemia and reperfusion, including that resulting in noise‐induced hearing loss, is effectively suppressed by NRF2 activation through its antioxidant function.7 Further, NRF2 possesses potent anti‐inflammatory activity and alleviates a variety of inflammatory conditions, including autoimmune diseases.8, 9
In line with these protective roles of the KEAP1‐NRF2 system, NRF2 activation effectively prevents chemical carcinogenesis by increasing antioxidant and detoxification capabilities.10, 11, 12, 13 NRF2 activation in cancer‐bearing hosts is also beneficial as a result of the fact that it potentiates anticancer immunity. NRF2 effectively inhibits the activity of MDSC and prevents apoptotic Treg cell‐mediated immunosuppression by protecting Treg cells from apoptosis.14, 15, 16 Thus, NRF2 activation in the host is beneficial as a result of its suppressive effect on cancer initiation and its anticancer‐cell activity.
2. ABERRANT ACTIVATION OF NRF2 AS A STRONG PROGNOSTIC FACTOR IN CANCER PATIENTS
In contrast to the protective roles described above, persistent activation of NRF2 at high levels in normal cells has deleterious effects. Keap1‐deficient mice die after weaning as a result of obstructive lesions in the upper digestive tract caused by epithelial hyperkeratosis.17 Keap1 deficiency in the developing kidney causes polyuria with low osmolality and bilateral hydronephrosis.18 Keap1 deficiency in bone marrow results in the exhaustion of hematopoietic stem cells.19 These deleterious effects are all canceled by Nrf2 disruption, indicating that excessive activation of NRF2 in normal cells is toxic and suggesting that appropriate regulation of NRF2 by KEAP1 is required for organismal health.
However, this scenario is not applicable to cancer cells. NRF2 activation in cancer cells confers therapeutic resistance and aggressive tumorigenic ability on cancer cells, driving their malignant progression. Many clinical studies have indeed shown strong correlations between NRF2 activation in tumor tissues and poor clinical outcomes of patients (Table 1). In many studies, NRF2 accumulation was examined using immunohistochemistry, and high levels of NRF2 accumulation were found to be commonly associated with poor prognosis in various cancer types. Somatic mutations of NRF2, KEAP1 and CUL3 are also prognostic markers of non‐small cell lung cancers, esophageal cancers and head and neck cancers.27, 28, 29, 31 Cancer tissue expression levels of NRF2 target genes, such as NQO1 and GCLC, and those of downstream effectors of NRF2, such as PHGDH, PSAT1 and SHMT2, are also well associated with clinical outcomes of cancer patients.21, 23, 27, 32, 41 Thus, NRF2 and its downstream effectors are important prognostic factors in a wide range of cancers.
Table 1.
Clinical studies analyzing the association between KEAP1‐NRF2 status and cancer patient prognosis
| Tissues | Study scale | Country | Prognosis marker (experiment) | Results | Reference | Reference no. | |
|---|---|---|---|---|---|---|---|
| Brain | Glioma | 75 | China | NRF2 (IHC) | High NRF2 expression is correlated with age, tumor grade and onset time. It is also correlated with short disease‐free survival and overall survival. | Zhao et al., 2015 | 20 |
| Anaplastic glioma | 95 | Japan | NQO1 and GCLC expression | Upregulation of NQO1 and GCLC correlates with short progression‐free survival. | Kanamori et al., 2015 | 21 | |
| Meningioma | 63 | Taiwan | NRF2 (IHC) | Meningioma patients with high NRF2 expression tend to show short overall survival. | Tsai et al., 2016 | 22 | |
| Lung | Non‐small cell lung cancer | 443 | USA | Serine biosynthesis enzyme (PHGDH, PSAT1 and SHMT2) | Patients with tumors expressing high levels of serine biosynthesis enzymes, which are induced downstream of NRF2, show poor prognosis. | DeNicola et al., 2015 | 23 |
| 235 | USA | NRF2 (IHC) | High NRF2 expression is associated with short overall survival and relapse‐free survival. | Solis et al., 2010 | 24 | ||
| 330 | USA | Somatic mutation of KEAP1 or NRF2 | Among patients with advanced KRAS mutant lung cancer, patients with co‐mutations in KEAP1/NRF2 have shorter survival, shorter duration of initial chemotherapy and shorter overall survival from initiation of immune therapy than those with single KRAS mutation. | Arbour et al., 2018 | 25 | ||
| 109 | Japan | NRF2 (IHC) | Among patients not receiving irradiation or chemotherapy, high NRF2 expression is associated with short overall survival and relapse‐free survival. | Inoue et al., 2012 | 26 | ||
| Adenocarcinoma | 458 | USA | NRF2 target gene signatureKEAP1 mutation | NRF2 target gene signature and KEAP1 mutation are correlated with short survival. | Romero et al., 2017 | 27 | |
| Squamous cell carcinoma | 48 | Japan | NRF2 mutation | Squamous cell carcinoma patients with NRF2 mutation have poor prognosis. | Shibata et al., 2008 | 28 | |
| 94 | USA | KEAP1 (IHC) | Low or absent KEAP1 expression is associated with short overall survival and relapse‐free survival. | Solis et al., 2010 | 24 | ||
| Esophageal squamous cell carcinoma | 82 | Japan | NRF2 mutation | Cancer patients with NRF2 mutation show short overall survival. | Shibata et al., 2011 | 29 | |
| 46 | Japan | NRF2 (IHC) | Among patients who have undergone chemotherapy and curative surgery, high expression of NRF2 is correlated with lymph node metastasis and poor postoperative outcome. | Kawasaki et al., 2014 | 30 | ||
| Head and neck squamous cell carcinoma | 302 | Canada | Somatic mutation of KEAP1, CUL3 and RBX1 | Mutations of KEAP1, CUL3 or RBX1 are associated with short median survival. | Martinez et al., 2015 | 31 | |
| 60 | Japan | NRF2 transcriptional profile (microarray) | NRF2‐activating transcriptional profiles are associated with poor prognosis. | Shibata et al., 2010 | 32 | ||
| Breast cancer | 106 | Japan | NRF2 (IHC) | Patients with NRF2 accumulation show short disease‐free survival and breast cancer‐specific survival. | Onodera et al., 2014 | 33 | |
| Hepatocellular carcinoma | 65 | China | NRF2 (IHC) | Among patients who have undergone surgical resection without any neoadjuvant or adjuvant chemotherapy, high expression of NRF2 is correlated with short overall survival and disease‐free survival. | Zhang et al., 2015 | 34 | |
| 107 | China | Pphosphorylated‐NRF2 (IHC) | Increased expression of phosphorylated NRF2 is associated with short overall survival and disease‐free survival. | Chen et al., 2016 | 35 | ||
| Bladder cancer | 44 | UK | NRF2 (IHC) | Among patients treated with radical cystectomy and chemotherapy, positive NRF2 staining is associated with short overall survival, bladder cancer‐specific survival and recurrence‐free survival. | Hayden et al., 2014 | 36 | |
| Pancreatic adenocarcinoma | 69 | Finland | KEAP1 (IHC) | Decreased KEAP1 expression is associated with short relapse‐free survival and pancreatic cancer‐specific survival. | Isohookana et al., 2015 | 37 | |
| 103 | Finland | NRF2 (IHC) | Nuclear staining of NRF2 is associated with poor prognosis. | Soini et al., 2014 | 38 | ||
| Cervical cancer | 89 | China | NRF2/KEAP1 (IHC) | Positive NRF2 staining and negative KEAP1 staining are both associated with poorly differentiated histology, lymph node metastasis and advanced FIGO stage. | Ma et al., 2015 | 39 | |
| Melanoma | 121 | Finland | NRF2 (IHC) | Nuclear NRF2 expression correlates with greater Breslow's depth, invasive phenotype, nodular growth and short melanoma‐specific survival. | Hintsala et al., 2016 | 40 | |
| Ovarian cancer | 64 | USA | NRF2 target gene expression (microarray) | Patients with NRF2 pathway activation have high resistance to platinum‐based therapy and have short overall survival. | Konstantinopoulos et al., 2011 | 41 | |
| 108 | Taiwan | NRF2 (IHC) | High NRF2 expression is associated with short disease‐free survival and overall survival. | Liew et al., 2015 | 42 | ||
| Gastric cancer | 175 | Japan | NRF2 (IHC) | Positive NRF2 staining is associated with clinicopathological factors, including tumor size, tumor depth, lymph node metastases, lymphovascular invasion, undifferentiated histology, advanced stage, and chemoresistance.Positive NRF2 staining is associated with poor overall postoperative survival. | Kawasaki et al., 2015 | 43 | |
| 186 | China | NRF2 (IHC) | NRF2 accumulation correlates with short overall survival and disease‐free survival. | Hu et al., 2013 | 44 | ||
| Colorectal cancer | 76 | China | NRF2/NQO1 (IHC) | High NRF2 or NQO1 expression correlates with Duke's stage and poor prognosis. | Ji et al., 2014 | 45 | |
FIGO, The International Federation of Gynecology and Obstetrics; GEMM, genetically engineered mouse model; IHC, immunohistochemistry; KEAP1, Kelch‐like ECH‐associated protein 1; NRF2, nuclear factor erythroid‐derived 2‐like 2.
3. VARIOUS CAUSES OF ABERRANT ACTIVATION OF NRF2
Multiple mechanisms that cause aberrant persistent activation of NRF2 have been reported, including genetic changes, epigenetic effects and altered protein‐protein interactions (Figure 1, “Persistent NRF2 activation”).
3.1. Somatic mutations of KEAP1 and NRF2
Somatic mutations of KEAP1 and NRF2 genes are one of the main causes of constitutive NRF2 activation. Mutations in KEAP1, which are generally mutually exclusive with those in NRF2, are frequently found in solid tumors, especially in the head and neck, lung and bladder.46 Although KEAP1 mutations are found in various positions in the coding region, most NRF2 mutations are located in the DLG and ETGE motifs, which are critical for binding with KEAP1. The functional impacts of these mutations have been analyzed by co‐crystallization of KEAP1 and the DLG/ETGE motifs of NRF2.47, 48
3.2. Exon skipping in NRF2
Aberrant NRF2 transcripts with recurrent loss of exon 2 have been found in lung, head and neck squamous cell carcinoma and hepatocellular carcinoma.49 NRF2 mutants that are translated from mRNA lacking exon 2 do not interact with KEAP1, resulting in persistent localization in the nucleus.
3.3. KEAP1 promoter methylation
Epigenetic alteration has been suggested as another cause of dysregulation of the KEAP1‐NRF2 system. Inverse correlation between DNA methylation levels and KEAP1 expression levels was reported in renal cell carcinoma.50
3.4. p62 (SQSTM1) accumulation
p62 is one of the adaptor proteins that recognizes ubiquitinated substrate proteins for selective autophagy. Phosphorylated p62 has a higher affinity for KEAP1 than the non‐phosphorylated form of p62, and competes with NRF2 for KEAP1 binding.51 Aberrant accumulation of p62 is frequently observed in hepatocellular carcinoma, and causes persistent activation of NRF2.52, 53
3.5. Fumarate hydratase mutation
Fumarate hydratase is a Krebs cycle enzyme that catalyzes the conversion from fumarate to malate. Fumarate, which accumulates in FH deficiency, modifies KEAP1 thiols as a result of its electrophilic property and stabilizes NRF2. Type II papillary renal cell carcinoma, which is accompanied by FH mutations, shows elevated expression of NRF2 target genes and highly malignant phenotypes.54, 55
3.6. Transcriptional activation of the NRF2 gene
Transcription levels of the NRF2 gene influence protein levels of NRF2 in basal and induced conditions.56 RAS signal activation induces the recruitment of MYC to the NRF2 promoter and upregulates NRF2 transcription, which is suggested to enhance the tumorigenesis induced by the oncogenic KRAS mutant.57
4. ESTABLISHMENT OF NRF2‐ADDICTED CANCER CELLS
Because NRF2 confers great advantages on cancer cells, including therapeutic resistance, increased antioxidant capacity and aggressive tumorigenic ability, cancer cells with NRF2 activation often develop “NRF2 addiction”, which is one of the forms of non‐oncogene addiction. This state has been shown in human cancer cell lines and mouse cancer models with abundant accumulation of NRF2 (Table 2). Although persistent activation of NRF2 confers growth and survival advantages on cancer cells, leading to NRF2 addiction, excessive activation of NRF2 in normal cells is rather toxic, as described in Section 2. These results imply that certain prerequisites, which are not fully understood, enable the establishment of NRF2‐addicted cancers.
Table 2.
Aberrant activation of NRF2 in cancer cells and their NRF2 dependency
| Tissue | Experiment | Cancer cell line/mouse cancer model with NRF2 activation | Method of modulating the KEAP1‐NRF2 pathway | Observations (with suggested mechanisms) | Reference | Reference no. |
|---|---|---|---|---|---|---|
| Brain | Xenograft | U251MG glioblastoma cell line | NRF2 knockdown by shRNA | NRF2 knockdown suppresses tumorigenic activity of U251MG cells. | Ji et al., 2013 | 58 |
| Dish culture Soft agar growth | F98/U87 glioblastoma cell line | NRF2 overexpression KEAP1 knockdown by shRNA | NRF2 activation promotes cell proliferation, anchorage‐independent growth and inhibits ferroptosis. | Fan et al., 2017 | 59 | |
| Xenograft | Human primary glioblastoma | NRF2 knockdown by shRNA | NRF2 knockdown suppresses tumorigenic activity of glioma stem cells. | Zhu et al., 2013 | 60 | |
| Lung | Soft agar growthXenograft | NSCLC cell line | PHGDH knockdown by shRNA | Inhibition of PHGDH that is induced by NRF2 by ATF4 activation suppresses soft agar growth and tumorigenic activity of NSCLC cells. | DeNicola et al., 2015 | 23 |
| GEMM | Kras LSL‐G12D/+ mouse + Adeno‐Cre | Mating with Nrf2 knockout mice | NRF2 deficiency inhibits KrasG12D‐mediated lung carcinogenesis. | DeNicola et al., 2011 | 57 | |
| Dish culture Xenograft | A549, ABC1, COR‐L105 (NSCLC cell lines) KYSE70 (esophageal squamous cell carcinoma cell line) | NRF2 inhibitor (halofuginone) | NRF2 inhibition by halofuginone alleviates resistance to chemotherapy. | Tsuchida et al., 2017 | 61 | |
| GEMM | Kras LSL‐G12D/+:CCSP‐Cre mouse | NRF2 inhibitor (brusatol) | NRF2 inhibition by brusatol sensitizes Kras G12D‐induced lung cancer to cisplatin treatment. | Tao et al., 2014 | 62 | |
| Dish culture Xenograft | A549 NSCLC cell line | NRF2 knockdown by siRNA NRF2 knockdown by direct injection of siRNA into tumors | NRF2 knockdown inhibits cell growth and suppresses tumorigenesis. | Mitsuishi et al., 2012 | 63 | |
| GEMM | Kras LSL‐G12D/+:Tp53 f/f mouse + Adeno‐Cre | Keap1 disruption by CRISPR‐CAS9 system | Keap1 deletion accelerates lung tumorigenesis, and depends on glutaminolysis. | Romero et al., 2017 | 27 | |
| Dish culture Xenograft | CALU1, HCC364, HCC827, MGH‐065 (NSCLC cell line) | Keap1 disruption by CRISPR‐CAS9 system | Keap1 deletion promotes cell survival in the presence of multiple inhibitors targeting the RT/Ras/MAPK pathway. | Krall et al., 2017 | 64 | |
| GEMM | Tp53 f/f:Keap1 f/f mouse + Adeno‐Cre/Krt5 CreERT2 | Nrf2 knockdown by shRNA | Keap1 deletion enhances therapeutic resistance and promotes aggressive proliferation and metastasis, which are canceled by NRF2 knockdown. | Jeong et al., 2017 | 65 | |
| Head and Neck | GEMM | TetO‐Hras G12V mouse | Nrf2/Keap1 knockdown by shRNA | NRF2 activation protects cancer stem cells from cisplatin treatment by glutathione production. | Oshimori et al., 2015 | 66 |
| Breast |
3D culture Soft agar growth Xenograft |
MCF10A expressing active mutant of AKT2 MDA‐MB‐231, T47D, ZE‐75‐1 (breast cancer cell lines) |
Inhibition of glutathione synthesis by BSO treatment | Glutathione synthesis stimulated by NRF2 activation contributes to spheroid formation, anchorage‐independent growth and tumorigenesis and confers chemoresistance on cancer cells. | Lien et al., 2016 | 67 |
| Soft agar growth | Transformed human mammary epithelial cells | NRF2 knockdown by shRNA | NRF2 knockdown decreases proteasome subunit gene expression, impairing misfolded protein degradation, and suppresses anchorage‐independent growth. | Chen et al., 2017 | 68 | |
| Liver | Xenograft | Huh1 HCC cell line | p62 disruption and re‐introduction of wild‐type and serine mutant of p62 | Phosphorylated p62 stabilizes NRF2 and promotes tumorigenesis. | Ichimura et al., 2013 | 51 |
| Pancreas |
Sphere formation Xenograft GEMM |
MIA PaCa2, Capan 2 (pancreatic cancer cell lines) Kras LSLG12D/+:IKKa ∆pan:Pdx1‐Cre mouse |
NRF2 disruption by CRISPR‐CAS9 system Mating with Nrf2 knockout mice | NRF2 knockdown decreases sphere formation and tumorigenic activity. p62 accumulation extends survival period of Kras LSLG12D/+:IKKa ∆pan:Pdx1‐Cre mice through NRF2 activation. | Todoric et al., 2017 | 69 |
|
Organoid culture Xenograft GEMM |
Human primary and metastatic tumor cells Suit2 PDA cell line Kras LSLG12D/+:Tp53 R172H/+:Pdx1‐Cre mouse |
NRF2 knockdown by shRNA Mating with Nrf2 knockout mice |
NRF2 deletion reduces tumor size and proliferation of organoids by protecting translation factors from oxidative stress. | Chio et al., 2016 | 70 | |
| GEMM | Kras LSLG12D/+:Ptf1a‐Cre mouse | Mating with Nrf2 knockout mice | NRF2 deletion reduces numbers of total PanIN‐1a cells and their Ki67‐positive ratios. | DeNicola et al., 2011 | 57 | |
| Melanocyte |
Dish culture Allograft |
B16 melanoma cells | BSO treatment | Glutathione synthesis promoted by NRF2 confers temozolomide resistance. | Rocha et al., 2016 | 71 |
| Prostate |
Dish culture Xenograft |
DU‐145 prostate cancer cell line | NRF2 knockdown by shRNA | Attenuation of NRF2 expression enhances the efficacy of chemotherapeutic drugs and ionizing radiation and reduces tumor volume. | Zhang et al., 2010 | 72 |
| Kidney | GEMM | FH‐deficient mouse | FH re‐expression | FH deficiency induces NRF2 activation by KEAP1 inactivation. | Adam et al., 2011 | 54 |
| Dish culture | FH‐deficient UOK262 cells derived from hereditary leiomyomatosis and renal cell carcinoma (HLRCC) patients | Ooi et al., 2011 | 55 | |||
| Xenograft | CCF‐RC1 renal cell carcinoma cell line | iASPP knockdown by siRNA | NRF2 activation by iASPP accumulation confers 5‐FU resistance and promotes proliferation and tumorigenesis. | Ge et al., 2017 | 73 | |
| MEF | Allograft |
NRF2‐addicted cancer model cell (MEF with SV40 and HRAS G12V) |
Nrf2 knockdown by shRNA | Constitutive NRF2 activation as a result of Keap1 deletion enhances tumorigenesis by IL11 upregulation under the influence of the tumor microenvironment. | Kitamura et al., 2017 | 74 |
5‐FU, fluorouracil; FH, fumarate hydratase; GEMM, genetically engineered mouse model; IHC, immunohistochemistry; KEAP1, Kelch‐like ECH‐associated protein 1; NRF2, nuclear factor erythroid‐derived 2‐like 2; NSCLC, non‐small cell lung cancer.
An important observation for understanding the dominant role of NRF2 in driving aggressive cell proliferation is that nuclear accumulation of NRF2 is greatly enhanced in the presence of proliferative signals.63, 75, 76 Whereas NRF2 is trapped by the CUL3‐KEAP1 complex in the cytoplasm and ubiquitinated for degradation, NRF2 is also ubiquitinated by the CUL1‐βTrCP complex after being phosphorylated by GSK3.77, 78 As GSK3 is phosphorylated by AKT and inactivated, CUL1‐βTrCP complex‐mediated degradation of NRF2 is inhibited in proliferating cells in which the PI3K‐AKT pathway is active. For instance, PI3K‐AKT activation caused by Pten deficiency in combination with Keap1 deficiency in the mouse liver results in massive accumulation of NRF2 and NRF2‐dependent proliferation of hepatocytes and cholangiocytes.63, 75, 76 Thus, quantitative increases of the NRF2 protein under proliferative signals substantiates the dominant role played by NRF2 in leading cancer cells to NRF2 addiction.
Consistent with this observation, oncogenic mutations inducing proliferative signals are likely to convert the role of NRF2 from cellular guardian to cancer driver. Experimental results using genetically engineered mouse models have shown that simple stabilization and accumulation of NRF2 are not sufficient for making NRF2 a cancer driver.18, 19, 79 Because Keap1‐deficient mice are resistant to carcinogenesis, establishment of NRF2‐addicted cancer models by Keap1 mutation requires combining Keap1 mutation with additional oncogenic mutations, such as activating mutations of KRAS/HRAS and loss‐of‐function of TP53.27, 65, 74 These results suggest that NRF2 is a facultative cancer driver, able to confer malignant phenotypes on cancer cells only in the presence of active oncogenic signaling.
Intriguingly, the frequency of NRF2‐addicted cancers possessing somatic mutations of KEAP1 or NRF2 is likely to vary from tissue to tissue. In The Cancer Genome Atlas (TCGA) database, KEAP1 and NRF2 genes are mutated in approximately 10%‐30% of lung cancers, in combination with oncogenic mutations such as KRAS and TP53, whereas no mutations have been found in KEAP1 or NRF2 genes in the case of pancreatic cancers. In good agreement with these clinical observations, Kras:Tp53:Keap1 triple mutations in the lung cause cancers showing aggressive proliferation,27 whereas these triple mutations in the pancreas do not cause cancers but result in fibrosis instead.80 These observations suggest that tissue‐specific factors are likely to determine the prerequisites for NRF2‐addicted cancer development.
5. CHARACTERISTICS OF NRF2‐ADDICTED CANCER CELLS
Although NRF2 inhibitors are expected to be promising therapeutic drugs for NRF2‐addicted malignant cancers, giving systemic NRF2 inhibitors might cause undesirable effects as a result of the impaired protective functions of NRF2. Detailed characterization of NRF2‐addicted cancers has been conducted to identify effective therapeutic targets besides NRF2 for NRF2‐addicted cancers.
Several metabolic features of NRF2‐addicted cancers have been described (Figure 2, left side). In proliferating cancer cells, NRF2 stabilization is enhanced and its transcriptional activation ability is augmented, resulting in the transcriptional activation of a wider range of NRF2 target genes (i.e., metabolic genes in addition to cytoprotective genes).63 NRF2 activates genes encoding enzymes for NADPH production and the pentose phosphate pathway, and subsequently facilitates the metabolic flux of glucose into purine nucleotide synthesis and that of glutamine into glutaminolysis and glutathione synthesis. An NRF2‐addicted lung cancer model generated by triple mutations of the Kras, Tp53 and Keap1 genes in mice consistently showed a heavy dependence on glutaminolysis, showing a robust sensitivity to inhibition of SLC1A5, a glutamine transporter.27 NRF2 also promotes serine synthesis from glucose by indirectly inducing genes in the serine synthesis pathway, namely PHDGH, PSAT1 and SHMT4, through ATF4 activation.23
Figure 2.
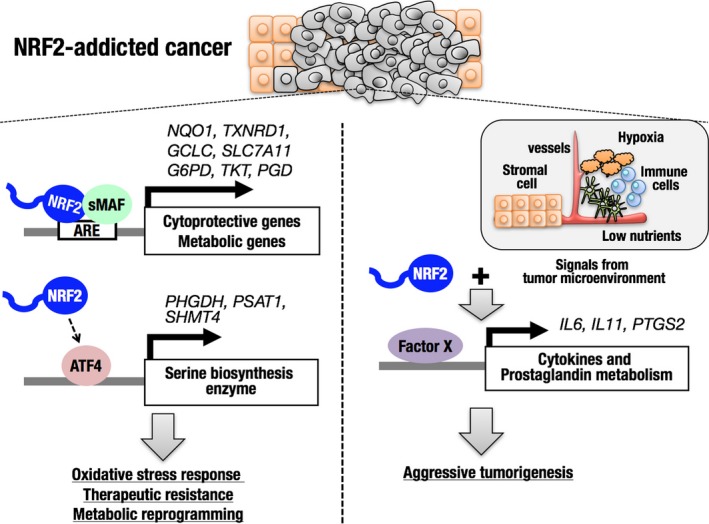
Transcriptional regulation by nuclear factor erythroid‐derived 2‐like 2 (NRF2) and its impacts on NRF2‐addicted cancer cells. Canonical (left) and non‐canonical (right) downstream genes of NRF2 in NRF2‐addicted cancer cells. Direct target genes of NRF2 are upregulated by NRF2 irrespective of signals from the microenvironment (left). Non‐canonical downstream genes are upregulated downstream of NRF2 only in the presence of signals from the microenvironment (right). ARE, antioxidant response element; IL, interleukin
Novel downstream effectors of NRF2 in NRF2‐addicted cancer models have been identified through the comparison of gene expression profiles in ordinary dish culture conditions and allograft tumor‐forming conditions.74 MEF obtained from wild‐type and Keap1‐null embryos were transformed by SV40 T antigen and oncogenic HRAS to establish WT‐TR MEF and Keap1 −/−‐TR MEF, respectively. Although cell growth in the culture‐dish condition was comparable between WT‐ and Keap1 −/−‐TR MEF, tumorigenic activity of Keap1 −/−‐TR MEF was dramatically enhanced compared with WT‐TR MEF, and the increased tumorigenic activity of Keap1 −/−‐TR MEF was verified as NRF2 dependent. When gene expression profiles were compared between WT‐TR MEF and Keap1 −/−‐TR MEF in the culture‐dish and tumor‐forming conditions, canonical NRF2 target genes were all upregulated in Keap1 −/−‐TR MEF in both conditions, whereas non‐canonical genes encoding cytokines and prostaglandin‐metabolizing enzymes were highly upregulated in Keap1 −/−‐TR MEF in the tumor‐forming condition only (Figure 2, right side). Among the non‐canonical genes, Il11 was found to be critical for the aggressive tumorigenic activity of Keap1 −/−‐TR MEF, which is consistent with a clinical observation that expression levels of NRF2 and IL‐11 are significantly correlated in breast cancer cases.74
Another intriguing difference in the gene expression profiles of WT‐TR MEF and Keap1 −/−‐TR MEF, unique to the tumor‐forming condition, was the significant downregulation of genes encoding MHC class I and antigen‐presentation factors in the tumors generated from Keap1 −/−‐TR MEF. This result suggests that Keap1 −/−‐TR MEF are likely to evade anticancer immunity, which might be an alternative advantage supporting the aggressive tumorigenesis of Keap1 −/−‐TR MEF. Thus, the tumor microenvironment has a substantial impact on the expression levels of downstream effectors of NRF2 in NRF2‐addicted cancer cells. Detailed mechanisms of the NRF2 contribution to tumorigenesis under various tumor microenvironments need to be clarified in future studies.
6. FUTURE PERSPECTIVES OF DIAGNOSTIC AND THERAPEUTIC STRATEGIES FOR NRF2‐ADDICTED CANCERS
Several NRF2 inhibitors have been reported for the treatment of NRF2‐addicted cancers.61, 62 For example, brusatol, which is a plant‐derived natural quassinoid, promotes poly‐ubiquitination of NRF2, which reduces the NRF2 protein level without changing the transcription level of the NRF2 gene.62 Another NRF2 inhibitor, halofuginone, was found to exert a chemosensitizing effect on NRF2‐addicted cancer cells.61 Halofuginone represses prolyl‐tRNA synthetase activity leading to translational inhibition. NRF2 protein level is effectively reduced by halofuginone, which is consistent with a short half‐life of the NRF2 protein.
New potential therapeutic targets of NRF2‐addicted cancers are being identified in addition to NRF2 inhibitors (Table 2). Some of them, such as glutathione synthesis, serine synthesis, the pentose phosphate pathway, and IL‐11, are direct or indirect downstream effectors of NRF2 for mediating malignant phenotypes. In contrast to these downstream effectors of NRF2, upstream regulators causing NRF2 activation, including p62 accumulation, FH mutation, and iASPP, are also possible therapeutic targets.
Recently, enhancement of anticancer immunity in cancer‐bearing hosts has been shown to be very effective for eradicating cancers. Because NRF2 activation inhibits immunosuppressive events directed by MDSC and apoptotic Treg cells,14, 15, 16 giving NRF2 inducers to cancer‐bearing hosts is expected to be an immunostimulatory therapy against cancer cells. A concern in treating cancer patients with NRF2 inducers is possible malignant progression as a result of NRF2 activation in cancer cells. However, the effects of NRF2 inducers on NRF2‐addicted cancer cells are expected to be minimal, as NRF2 is already maximally activated in NRF2‐addicted cancer cells, although intratumor heterogeneity must be carefully considered. Appropriate animal models need to be developed to evaluate the indication for NRF2 inducers for NRF2‐addicted cancers.
Compared to active exploratory and mechanistic research on therapeutic targets, diagnostic biomarkers and surrogate markers have yet to be developed for NRF2‐addicted cancers. Based on the unique metabolic activities of NRF2‐addicted cancers, detailed metabolite analysis might lead to the identification of useful diagnostic markers. Diagnostic and therapeutic advances await further studies and technological improvements.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
We thank all of the members of our laboratory for their daily contributions. This work was supported in part by the Japan Society for the Promotion of Science (KAKENHI Grant No. 17K15591 to H.K. and 15H04692 to H.M.), the Uehara Memorial Foundation (H.M.), the Mitsubishi Foundation (H.M.), the Naito Foundation (H.M.), and the Princess Takamatsu Cancer Research Fund 15‐24728 (H.M.). The funders had no role in the design of the study, data collection and analysis, decision to publish or preparation of the manuscript.
Kitamura H, Motohashi H. NRF2 addiction in cancer cells. Cancer Sci. 2018;109:900–911. https://doi.org/10.1111/cas.13537
Funding information
Princess Takamatsu Cancer Research Fund Grant/Award Number: ‘15‐24728’, Uehara Memorial Foundation (Grant/Award Number: na), Mitsubishi Foundation (Grant/Award Number: na), Japan Society for the Promotion of Science (Grant/Award Numbers: ‘15H04692’, ‘17K15591’), Naito Foundation (Grant/Award Number: na).
REFERENCES
- 1. Motohashi H, Yamamoto M. NRF2‐Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549‐557. [DOI] [PubMed] [Google Scholar]
- 2. Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two‐site molecular recognition model. Mol Cell Biol. 2006;26:2887‐2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tong KI, Padmanabhan B, Kobayashi A, et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27:7511‐7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogura T, Tong KI, Mio K, et al. Keap1 is a forked‐stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C‐terminal domains. Proc Natl Acad Sci USA. 2010;107:2842‐2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enomoto A, Itoh K, Nagayoshi E, et al. High sensitivity of NRF2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE‐regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169‐177. [DOI] [PubMed] [Google Scholar]
- 6. Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte‐specific deletion of the keap1 gene activates NRF2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79‐88. [DOI] [PubMed] [Google Scholar]
- 7. Honkura Y, Matsuo H, Murakami S, et al. NRF2 is a key target for prevention of noise‐induced hearing loss by reducing oxidative damage of cochlea. Sci Rep. 2016;6:19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi EH, Suzuki T, Funayama R, et al. NRF2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki T, Murakami S, Biswal SS, et al. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol Cell Biol. 2017;37:e00063‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramos‐Gomez M, Kwak MK, Dolan PM, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in NRF2 transcription factor‐deficient mice. Proc Natl Acad Sci USA. 2001;98:3410‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iida K, Itoh K, Kumagai Y, et al. NRF2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424‐6431. [DOI] [PubMed] [Google Scholar]
- 12. Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. NRF2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158‐4168. [DOI] [PubMed] [Google Scholar]
- 13. Satoh H, Moriguchi T, Saigusa D, et al. NRF2 intensifies host defense systems to prevent lung carcinogenesis, but after tumor initiation accelerates malignant cell growth. Cancer Res. 2016;76:3088‐3096. [DOI] [PubMed] [Google Scholar]
- 14. Satoh H, Moriguchi T, Taguchi K, et al. NRF2‐deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis. 2010;31:1833‐1843. [DOI] [PubMed] [Google Scholar]
- 15. Hiramoto K, Satoh H, Suzuki T, et al. Myeloid lineage‐specific deletion of antioxidant system enhances tumor metastasis. Cancer Prev Res (Phila). 2014;7:835‐844. [DOI] [PubMed] [Google Scholar]
- 16. Maj T, Wang W, Crespo J, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD‐L1‐blockade resistance in tumor. Nat Immunol. 2017;18:1332‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wakabayashi N, Itoh K, Wakabayashi J, et al. Keap1‐null mutation leads to postnatal lethality due to constitutive NRF2 activation. Nat Genet. 2003;35:238‐245. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki T, Seki S, Hiramoto K, et al. Hyperactivation of NRF2 in early tubular development induces nephrogenic diabetes insipidus. Nat Commun. 2017;8:14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murakami S, Suzuki T, Harigae H, Romeo PH, Yamamoto M, Motohashi H. NRF2 activation impairs quiescence and bone marrow reconstitution capacity of hematopoietic stem cells. Mol Cell Biol. 2017;37:e00086‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao M, Xu H, Zhang B, Hong B, Yan W, Zhang J. Impact of nuclear factor erythroid‐derived 2–like 2 and p62/sequestosome expression on prognosis of patients with gliomas. Human Pathol. 2015;46:843‐849. [DOI] [PubMed] [Google Scholar]
- 21. Kanamori M, Higa T, Sonoda Y, et al. Activation of the NRF2 pathway and its impact on the prognosis of anaplastic glioma patients. Neuro Oncol. 2015;17:555‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsai WC, Hueng DY, Lin CR, Yang TC, Gao HQ. NRF2 expressions correlate with WHO grades in gliomas and meningiomas. Int J Mol Sci. 2016;17:E722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeNicola GM, Chen PH, Mullarky E, et al. NRF2 regulates serine biosynthesis in non‐small cell lung cancer. Nat Genet. 2015;47:1475‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solis LM, Behrens C, Dong W, et al. NRF2 and Keap1 abnormalities in non‐small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743‐3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arbour KC, Jordan E, Kim HR, et al. Effects of co‐occurring genomic alterations on outcomes in patients with KRAS‐mutant non‐small cell lung cancer. Clin Cancer Res. 2018;24:334‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoue D, Suzuki T, Mitsuishi Y, et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romero R, Sayin VI, Davidson SM, et al. Keap1 loss promotes Kras‐driven lung cancer and results in dependence on glutaminolysis. Nat Med. 2017;23:1362‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shibata T, Ohta T, Tong KI, et al. Cancer related mutations in NRF2 impair its recognition by Keap1‐Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA. 2008;105:13568‐13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibata T, Kokubu A, Saito S, et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawasaki Y, Okumura H, Uchikado Y, et al. NRF2 is useful for predicting the effect of chemoradiation therapy on esophageal squamous cell carcinoma. Ann Surg Oncol. 2014;21:2347‐2352. [DOI] [PubMed] [Google Scholar]
- 31. Martinez VD, Vucic EA, Thu KL, Pikor LA, Lam S, Lam WL. Disruption of KEAP1/CUL3/RBX1 E3‐ubiquitin ligase complex components by multiple genetic mechanisms: association with poor prognosis in head and neck cancer. Head Neck. 2015;37:727‐734. [DOI] [PubMed] [Google Scholar]
- 32. Shibata T, Saito S, Kokubu A, et al. Global downstream pathway analysis reveals a dependence of oncogenic NF‐E2–related factor 2 mutation on the mtor growth signaling pathway. Cancer Res. 2010;70:9095‐9105. [DOI] [PubMed] [Google Scholar]
- 33. Onodera Y, Motohashi H, Takagi K, et al. NRF2 immunolocalization in human breast cancer patients as a prognostic factor. Endocr Relat Cancer. 2014;21:241‐252. [DOI] [PubMed] [Google Scholar]
- 34. Zhang M, Zhang C, Zhang L, et al. NRF2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer. 2015;15:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J, Yu Y, Ji T, et al. Clinical implication of Keap1 and phosphorylated NRF2 expression in hepatocellular carcinoma. Cancer Med. 2016;5:2678‐2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayden A, Douglas J, Sommerlad M, et al. The NRF2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol Oncol. 2014;32:806‐814. [DOI] [PubMed] [Google Scholar]
- 37. Isohookana J, Haapasaari KM, Soini Y, Karihtala P. Keap1 expression has independent prognostic value in pancreatic adenocarcinomas. Diagn Pathol. 2015;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soini Y, Eskelinen M, Juvonen P, et al. Nuclear NRF2 expression is related to a poor survival in pancreatic adenocarcinoma. Pathol Res Pract. 2014;210:35‐39. [DOI] [PubMed] [Google Scholar]
- 39. Ma JQ, Tuersun H, Jiao SJ, Zheng JH, Xiao JB, Hasim A. Functional role of NRF2 in cervical carcinogenesis. PLoS ONE. 2015;10:e0133876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hintsala HR, Jokinen E, Haapasaari KM, et al. NRF2/Keap1 pathway and expression of oxidative stress lesions 8‐hydroxy‐2’‐deoxyguanosine and nitrotyrosine in melanoma. Anticancer Res. 2016;36:1497‐1506. [PubMed] [Google Scholar]
- 41. Konstantinopoulos PA, Spentzos D, Fountzilas E, et al. Keap1 mutations and NRF2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71:5081‐5089. [DOI] [PubMed] [Google Scholar]
- 42. Liew PL, Hsu CS, Liu WM, Lee YC, Lee YC, Chen CL. Prognostic and predictive values of NRF2, Keap1, p16 and E‐cadherin expression in ovarian epithelial carcinoma. Int J Clin Exp Pathol. 2015;8:5642‐5649. [PMC free article] [PubMed] [Google Scholar]
- 43. Kawasaki Y, Ishigami S, Arigami T, et al. Clinicopathological significance of nuclear factor (erythroid‐2)‐related factor 2 (NRF2) expression in gastric cancer. BMC Cancer. 2015;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu XF, Yao J, Gao SG, et al. NRF2 overexpression predicts prognosis and 5‐FU resistance in gastric cancer. Asian Pac J Cancer Prev. 2013;13:5231‐5235. [DOI] [PubMed] [Google Scholar]
- 45. Ji L, Wei Y, Jiang T, Wang S. Correlation of NRF2, NQO1, MRP1, cmyc and p53 in colorectal cancer and their relationships to clinicopathologic features and survival. Int J Clin Exp Pathol. 2014;7:1124‐1131. [PMC free article] [PubMed] [Google Scholar]
- 46. Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Padmanabhan B, Tong KI, Ohta T, et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689‐700. [DOI] [PubMed] [Google Scholar]
- 48. Fukutomi T, Takagi K, Mizushima T, Ohuchi N, Yamamoto M. Kinetic, thermodynamic, and structural characterizations of the association between NRF2‐DLGex degron and Keap1. Mol Cell Biol. 2014;34:832‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldstein LD, Lee J, Gnad F, et al. Recurrent loss of NFE2L2 exon 2 is a mechanism for NRF2 pathway activation in human cancers. Cell Rep. 2016;16:2605‐2617. [DOI] [PubMed] [Google Scholar]
- 50. Fabrizio FP, Costantini M, Copetti M, et al. Keap1/NRF2 pathway in kidney cancer: frequent methylation of KEAP1 gene promoter in clear renal cell carcinoma. Oncotarget. 2017;8:11187‐11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ichimura Y, Waguri S, Sou YS, et al. Phosphorylation of p62 activates the Keap1‐NRF2 pathway during selective autophagy. Mol Cell. 2013;51:618‐631. [DOI] [PubMed] [Google Scholar]
- 52. Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor NRF2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213‐223. [DOI] [PubMed] [Google Scholar]
- 53. Inami Y, Waguri S, Sakamoto A, et al. Persistent activation of NRF2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adam J, Hatipoglu E, O'Flaherty L, et al. Renal cyst formation in Fh1‐deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and NRF2 signaling. Cancer Cell. 2011;20:524‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ooi A, Wong JC, Petillo D, et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell. 2011;20:511‐523. [DOI] [PubMed] [Google Scholar]
- 56. Suzuki T, Shibata T, Takaya T, et al. Regulatory nexus of synthesis and degradation deciphers cellular NRF2 expression levels. Mol Cell Biol. 2013;33:2402‐2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene‐induced NRF2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ji X, Chen SH, Zhu L, et al. Knockdown of NF‐E2‐related factor 2 inhibits the proliferation and growth of U251MG human glioma cells in a mouse xenograft model. Oncol Rep. 2013;30:157‐164. [DOI] [PubMed] [Google Scholar]
- 59. Fan Z, Wirth AK, Chen D, et al. NRF2‐Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6:e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu J, Wang H, Sun Q, et al. NRF2 is required to maintain the self‐renewal of glioma stem cells. BMC Cancer. 2013;13:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsuchida K, Tsujita T, Hayashi M, et al. Halofuginone enhances the chemo‐sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic Biol Med. 2017;103:236‐247. [DOI] [PubMed] [Google Scholar]
- 62. Tao S, Wang S, Moghaddam SJ, et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 2014;74:7430‐7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mitsuishi Y, Taguchi K, Kawatani Y, et al. NRF2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66‐79. [DOI] [PubMed] [Google Scholar]
- 64. Krall EB, Wang B, Munoz DM, et al. KEAP1 loss modulates sensitivity to kinase targeted therapy in lung cancer. eLife 2017;6:e18970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jeong Y, Hoang NT, Lovejoy A, et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. 2017;7:86‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oshimori N, Oristian D, Fuchs E. TGF‐β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160:963‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lien EC, Lyssiotis CA, Juvekar A, et al. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt‐driven breast cancer. Nat Cell Biol. 2016;18:572‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen L, Brewer MD, Guo L, et al. Enhanced degradation of misfolded proteins promotes tumorigenesis. Cell Rep. 2017;18:3142‐3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Todoric J, Antonucci L, Di Caro G, et al. Stress‐activated NRF2‐MDM2 cascade controls neoplastic progression in pancreas. Cancer Cell. 2017;32:824‐839.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chio IIC, Jafarnejad SM, Ponz‐Sarvise M, et al. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell. 2016;166:963‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rocha CR, Kajitani GS, Quinet A, Fortunato RS, Menck CF. NRF2 and glutathione are key resistance mediators to temozolomide in glioma and melanoma cells. Oncotarget. 2016;7:48081‐48092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang P, Singh A, Yegnasubramanian S, et al. Loss of Kelch‐like ECH‐associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther. 2010;9:336‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ge W, Zhao K, Wang X, et al. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with NRF2 for Keap1 binding. Cancer Cell. 2017;32:561‐573. [DOI] [PubMed] [Google Scholar]
- 74. Kitamura H, Onodera Y, Murakami S, Suzuki T, Motohashi H. IL‐11 contribution to tumorigenesis in an NRF2 addiction cancer model. Oncogene. 2017;36:6315‐6324. [DOI] [PubMed] [Google Scholar]
- 75. Taguchi K, Hirano I, Itoh T, et al. NRF2 enhances cholangiocyte expansion in Pten‐deficient livers. Mol Cell Biol. 2014;34:900‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shirasaki K, Taguchi K, Unno M, Motohashi H, Yamamoto M. NF‐E2‐related factor 2 promotes compensatory liver hypertrophy after portal vein branch ligation in mice. Hepatology. 2014;59:2371‐2382. [DOI] [PubMed] [Google Scholar]
- 77. Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}‐TrCP promotes glycogen synthase kinase 3‐dependent degradation of the NRF2 transcription factor in a Keap1‐independent manner. Mol Cell Biol. 2011;31:1121‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. NRF2 is controlled by two distinct β‐TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK‐3 activity. Oncogene. 2013;32:3765‐3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol. 2010;30:3016‐3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hamada S, Shimosegawa T, Taguchi K, Nabeshima T, Yamamoto M, Masamune A. Simultaneous K‐ras activation and Keap1 deletion cause atrophy of pancreatic parenchyma. Am J Physiol Gastrointest Liver Physiol. 2018;314:G65‐G74. ajpgi.00228.2017. [DOI] [PubMed] [Google Scholar]


