Abstract
Osimertinib is a potent, irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) selective for EGFR‑TKI sensitizing (EGFRm) and T790M resistance mutations. The primary objective of the cytology cohort in the AURA study was to investigate safety and efficacy of osimertinib in pretreated Japanese patients with EGFR T790M mutation‐positive non‐small cell lung cancer (NSCLC), with screening EGFR T790M mutation status determined from cytology samples. The cytology cohort was included in the Phase I dose expansion component of the AURA study. Patients were enrolled based on a positive result of T790M by using cytology samples, and received osimertinib 80 mg in tablet form once daily until disease progression or until clinical benefit was no longer observed at the discretion of the investigator. Primary endpoint for efficacy was objective response rate (ORR) by investigator assessment. Twenty‐eight Japanese patients were enrolled into the cytology cohort. At data cut‐off (February 1, 2016), 12 (43%) were on treatment. Investigator‐assessed ORR was 75% (95% confidence interval [CI] 55, 89) and median duration of response was 9.7 months (95% CI 3.8, not calculable [NC]). Median progression‐free survival was 8.3 months (95% CI 4.2, NC) and disease control rate was 96% (95% CI 82, 100). The most common all‐causality adverse events were paronychia (46%), dry skin (46%), diarrhea (36%) and rash (36%). Osimertinib provided clinical benefit with a manageable safety profile in patients with pretreated EGFR T790M mutation‐positive NSCLC whose screening EGFR T790M mutation‐positive status was determined from cytology samples. (ClinicalTrials.gov number NCT01802632).
Keywords: cytology, epidermal growth factor receptor, non‐small cell lung cancer, osimertinib, T790M
Abbreviations
- AE
adverse event
- ARMS
Amplification Refractory Mutation System
- AUCss
area under curve at steady state
- BAL
bronchoalveolar lavage
- BOR
best objective response
- CI
confidence interval
- Css,max
maximum plasma concentration at steady state
- DCR
disease control rate
- DoR
duration of response
- EGFR
epidermal growth factor receptor
- EGFRm
EGFR‐TKI‐sensitizing mutation
- EMA
European Medicines Agency
- FFPE
formalin‐fixed paraffin‐embedded
- ILD
interstitial lung disease
- NCI CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Event
- NC
not calculable
- NSCLC
non‐small cell lung cancer
- ORR
objective response rate
- PFS
progression‐free survival
- PK
pharmacokinetics
- PNA‐LNA
peptide nucleic acid‐locked nucleic acid
- RECIST
Response Evaluation Criteria in Solid Tumors
- SAE
serious adverse event
- T790M
mutation where threonine is replaced by methionine at position 790 of EGFR
- TKI
tyrosine kinase inhibitor
- TL
target lesion
- WHO
World Health Organization
1. INTRODUCTION
Epidermal growth factor receptor‐TKI, such as erlotinib, afatinib and gefitinib, are recommended as first‐line therapy and have shown clinical benefit in patients with advanced NSCLC harboring an EGFRm.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 However, the majority of patients who initially respond to EGFR‐TKI ultimately develop acquired resistance; approximately 60% of such patients harbor the EGFR T790M resistance mutation.11, 12
Osimertinib is an oral, potent, irreversible EGFR‐TKI selective for both EGFRm and T790M resistance mutations.13 Following positive outcomes from the AURA clinical programme (AURA Phase I with Phase II extension NCT01802632, AURA2 Phase II study NCT02094261, and AURA3 Phase III study NCT02151981),14, 15, 16, 17 osimertinib is recommended as a treatment choice for patients with EGFR T790M‐positive advanced NSCLC following disease progression on first‐line EGFR‐TKI.18
The Phase I part of the AURA study comprised dose escalation and dose expansion components. The dose expansion component enrolled patients based on confirmation of tumor EGFRm status from a biopsy sample. A cytology cohort was included as part of the dose expansion component at the osimertinib 80‐mg once‐daily dose and enrolled patients based on EGFR T790M mutation status derived from a cytology sample. The Phase II and III studies (AURA extension, AURA2 and AURA3) enrolled patients based upon central T790M mutation testing of a tumor biopsy sample following confirmed disease progression on a prior EGFR‐TKI.
Generally, patients who do not have solid tumor deposits suitable for tumor biopsy are excluded from clinical studies or access to licensed medicines that require prospective companion diagnostic testing. Cytology sampling may provide sufficient tumor material for molecular analysis and is a less invasive approach to tumor characterization than solid tumor biopsy. Studies have shown cytological samples to have equivalent or higher sensitivity and accuracy for identifying EGFR mutations in NSCLC when compared with surgical specimens.19, 20 Furthermore, cytological samples taken directly from tumors may provide higher sensitivity for EGFR mutations than plasma‐based testing as a result of higher tumor DNA load. In a recent analysis of plasma vs tumor tissue for the detection of EGFR T790M in patients with EGFRm advanced NSCLC and disease progression on a first‐line EGFR‐TKI, sensitivity (positive percent agreement) was 51%.21 However, plasma‐based testing is less invasive than cytological‐based testing.
In Japan, almost 40% of EGFR mutation testing is undertaken using cytological samples; Japanese clinicians treating NSCLC consider cytological samples to be valuable clinical specimens for testing EGFR mutations as they are collected less invasively than tissue samples.22 Therefore, the cytology cohort was planned to include Japanese patients only.
Assays available for cytology‐based EGFR testing include PCR‐Invader, peptide nucleic acid‐locked nucleic acid (PNA‐LNA) PCR clamp, PCR direct sequencing, Cycleave PCR and Scorpion ARMS. These techniques showed comparable capabilities when used to analyze FFPE samples and cytology samples.23
The cobas® EGFR Mutation Test (Roche Molecular Systems) was used to select eligible patients in clinical trials of osimertinib. The cobas® EGFR Mutation Test v2 is the approved companion diagnostic for osimertinib in the USA and Japan. This test is registered for use with FFPE tissue samples and also for use with plasma circulating‐free DNA (cfDNA) samples in some countries, including the European Union.
Herein we report clinical outcomes from the cytology cohort of the AURA Phase I study, dose expansion component. The inclusion of this cohort was designed to investigate the safety and efficacy of osimertinib in EGFR‐TKI‐pretreated patients with EGFR T790M mutation‐positive NSCLC and whose screening EGFR T790M mutation‐positive status was determined from cytology samples. The AURA study is registered with ClinicalTrials.gov, number NCT01802632.
2. MATERIALS AND METHODS
2.1. Study design and participants
The AURA study was a Phase I/II, open‐label, multicenter study of osimertinib given orally in patients with advanced NSCLC and disease progression following prior therapy with an EGFR‐TKI. The primary objective of the study was to determine the safety and efficacy of osimertinib. The Phase I component of the study consisted of dose escalation and dose expansion components and has been published previously.14
A cohort was added as part of the Phase I dose expansion component with the aim of exploring safety, tolerability and efficacy of osimertinib in Japanese patients with EGFR T790M mutation‐positive NSCLC, as determined from a cytology specimen (highlighted in Figure 1). Patients were screened for enrollment across sites in Japan. The first patient was enrolled in October 2014 and the last in February 2015. The data cut‐off reported here was February 1, 2016, and the study is ongoing for follow up.
Figure 1.
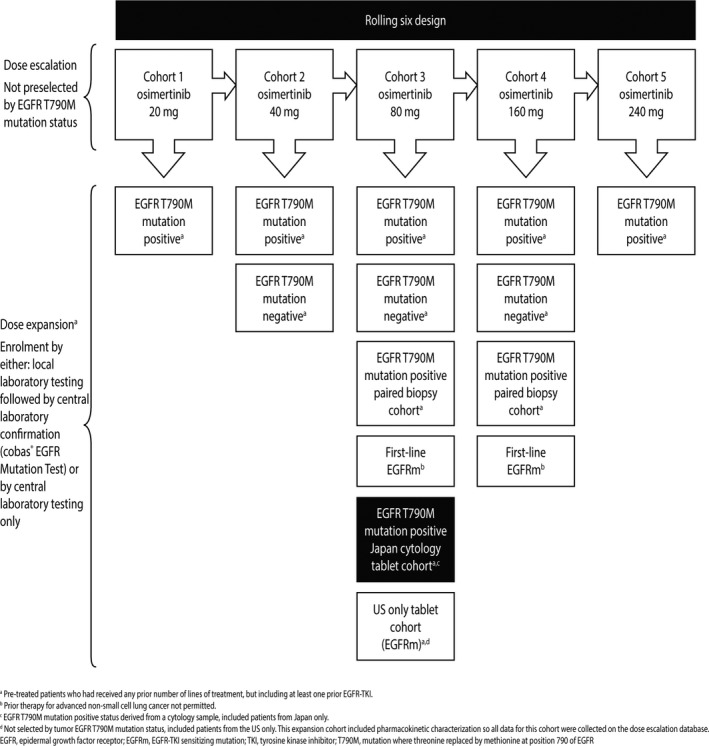
AURA Phase I study design, highlighting the Japanese expansion cohort
Patients enrolled into this cytology cohort were aged at least 20 years with histologically or cytologically confirmed diagnosis of NSCLC. Key inclusion criteria included at least one measurable lesion (≥10 mm diameter or ≥15 mm in lymph nodes at baseline) not previously irradiated or chosen for biopsy during study screening, confirmation of an EGFR mutation known to be associated with EGFR‐TKI sensitivity or experience of clinical benefit followed by progression while on continuous treatment with an EGFR‐TKI (as per Jackman criteria24) and local or central confirmation of tumor T790M mutation‐positive status from a cytology sample taken after disease progression on the most recent treatment (with either an EGFR‐TKI or chemotherapy). Patients were excluded from the study if they had received treatment with an EGFR‐TKI within 8 days or 5 half‐lives of the first dose of study treatment.
The AURA study Phase I component was approved by an independent institutional review board/independent ethics committee associated with each study center. The study was carried out in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with International Conference on Harmonisation/Good Clinical Practice and applicable regulatory requirements and the AstraZeneca policy on Bioethics. Informed consent was obtained from all patients prior to initiation of the study.
2.2. Procedures
Eligible patients received an osimertinib 80‐mg tablet once daily until RECIST version 1.1‐defined disease progression or until clinical benefit was no longer observed, as judged by the investigator.
All patients in the cohort were recruited based on confirmation of EGFR T790M mutation‐positive status through molecular analysis of a cytological sample, conducted with an acceptable method per institution guidance. Cytological samples were collected using a variety of methods, including by BAL, thoracocentesis, fine‐needle aspiration, bronchial brushing, or other methods (trans‐bronchial lung biopsy, washings of biopsy device or lumbar puncture) and EGFR T790M‐positive status was determined by local laboratory testing using various technology platforms. An overview of the cytological sampling and EGFR T790M testing methods used is shown in Table 1. If available, optional tissue and/or rest of the collected cytological samples were requested for provision for central testing by cobas® EGFR Mutation Test.
Table 1.
Summary of cytological samples used and method of T790M mutation testing overall
| No. (%) patients (N = 28) | ||
|---|---|---|
| Method of cytology | Bronchoalveolar lavage | 12 (42.9) |
| Thoracocentesis | 6 (21.4) | |
| Fine needle aspiration | 5 (17.9) | |
| Bronchial brushing | 2 (7.1) | |
| Other | 3 (10.7) | |
| Method of T790M testing | PNA‐LNA PCR clamp | 16 (57.1) |
| Scorpion‐ARMS | 7 (25.0) | |
| Cycleave | 5 (17.9) |
ARMS, Amplification Refractory Mutation System; PNA‐LNA, peptide nucleic acid‐locked nucleic acid.
Other includes trans‐bronchial lung biopsy (n = 1), washings of biopsy device (n = 1), lumbar puncture (n = 1).
Efficacy was primarily measured through ORR, defined by investigator assessment of radiological information using RECIST version 1.1. Additional efficacy endpoints included DoR, PFS, best percentage change from baseline in TL size and DCR. Analysis of overall survival was not planned for the AURA study Phase I component. The safety and tolerability profile was assessed throughout the study using AE graded according to the NCI CTCAE version 4, as well as through clinical chemistry, hematology, urinalysis, vital signs, electrocardiograms and physical examinations. PK data were investigated as a secondary outcome measure by venous blood samples and standard non‐compartmental analysis of plasma concentration data for osimertinib and its metabolites. Details of PK analysis are included as Doc. S1.
2.3. Statistical analysis
Thirty patients were planned for inclusion in the evaluable‐for‐response set to provide an estimate of the true response rate for the population. The evaluable‐for‐response data set and safety analysis included all patients who received at least one dose of osimertinib and had measureable disease at baseline. The PK analysis included all dosed patients who had at least one measurable plasma concentration collected after dosing.
ORR, BOR, DoR, DCR and maximum tumor shrinkage were conducted on the evaluable‐for‐response data set. Tumor size was defined as the sum of the lengths of the longest diameters of the RECIST TL. Percentage change in tumor size was determined for patients with measurable disease at baseline and was derived at each visit by the percentage change in the sum of the diameters of TL compared with baseline. The best change in TL size was the maximum reduction from baseline or the minimum increase from baseline, in the absence of a reduction. DoR was defined as the time from the date of first documented response until the date of documented progression and PFS as the time from the date of first dosing until the date of objective disease progression or death, as defined by RECIST (regardless of whether the patient withdrew from osimertinib therapy, received another anticancer therapy prior to progression or death occurred in the absence of progression). ORR (according to RECIST version 1.1) was defined as the percentage of patients with at least one confirmed complete or partial response prior to progression or subsequent therapy and DCR as complete response, partial response or stable disease for ≥6 weeks. A cycle of study treatment was defined as 21 days of continuous dosing.
3. RESULTS
3.1. Demographics
Overall, 28 patients from 13 institutions were enrolled into this cohort. Baseline characteristics are summarized in Table 2. The majority of patients were female (75%), with adenocarcinoma histology (86%), a World Health Organization (WHO) performance status score of 1 (75%) and had never smoked (64%). All patients were of Japanese ethnicity.
Table 2.
Baseline demographic and clinical characteristics
| Characteristic, n (%) | N = 28 |
|---|---|
| Sex | |
| Male | 7 (25) |
| Female | 21 (75) |
| Age in years, median (range) | 62.5 (38‐81) |
| Histological type | |
| Adenocarcinoma | 24 (86) |
| Squamous‐cell carcinoma | 2 (7) |
| Large‐cell carcinoma | 1 (4) |
| Missing data | 1 (4) |
| Smoking status | |
| Never | 18 (64) |
| Current | 1 (4) |
| Former | 9 (32) |
| No. prior EGFR‐TKI at baseline, median (range) | 2.0 (1‐6) |
| EGFR mutation type by local cytology sample testing T790Ma | 28 (100) |
| Exon 19 deletion | 10 (36) |
| L858R | 8 (29) |
| EGFRm subtype unspecified | 1 (4) |
| Sensitizing mutation status unavailable | 9 (32) |
| WHO performance status | |
| 0 | 7 (25) |
| 1 | 21 (75) |
EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; EGFRm, EGFR‐TKI‐sensitizing mutation; WHO, World Health Organization.
Two patients had tissue samples available, enabling confirmation of T790M‐positive status by central cobas® test in both patients.
3.2. Duration of treatment
As of February 1, 2016, data cut‐off, 12 patients (43%) were on study treatment. Median total treatment duration was 9.6 months, with a treatment exposure range of 0.7‐15.4 months. Median treatment duration (without dose interruptions) was 8.6 months, with a treatment exposure range of 0.7‐14.7 months. A total of 16 patients (57%) discontinued the study drug: 10 (36%) as a result of progressive disease, 5 (18%) as a result of an AE and 1 (4%) as a result of physician decision to switch cancer therapy following progressive disease.
3.3. Cytology
Cytology samples were collected from BAL (n = 12), thoracocentesis (n = 6), fine‐needle aspiration (n = 5), bronchial brushing (n = 2) or other (n = 3). T790M testing was by PNA‐LNA PCR clamp (n = 16), Scorpion‐ARMS (n = 7) or Cycleave (n = 5). An overview of the methods used is shown in Table 1 with details of patient characteristics by cytological sampling and T790M testing methods included as Table S1. Of the 2 optional tissue samples locally confirmed as T790M positive by cytology sampling, both were confirmed as T790M positive by the cobas® EGFR Mutation Test.
3.4. Efficacy
3.4.1. Tumor response
The ORR was 75% (95% CI 55, 89) with confirmed partial response in 21 of 28 patients. Overall, 81% of patients with a response had a documented initial response at the first RECIST scan (ie, 6 weeks from the start of treatment); the median time to onset of response was 6.1 weeks. Disease progression subsequently occurred in 12 patients (57%) with a response. Six patients (21%) reported stable disease for ≥6 weeks. A reduction in tumor TL size occurred in all but 1 patient (27/28; 96%) with a mean percentage change from baseline at 6 weeks of −39% (SD 21.5) (Figure 2). DCR was 96% (95% CI 82, 100). Median DoR was 9.7 months (95% CI 3.8, NC). A summary of individual patient DoR is shown in Figure 3 and a DoR Kaplan‐Meier curve is included as Figure S1. Response rate at 6 months was 62% (95% CI 38, 79), 52% (95% CI 29, 70) at 9 months and 41% (95% CI 20, 61) at 12 months.
Figure 2.
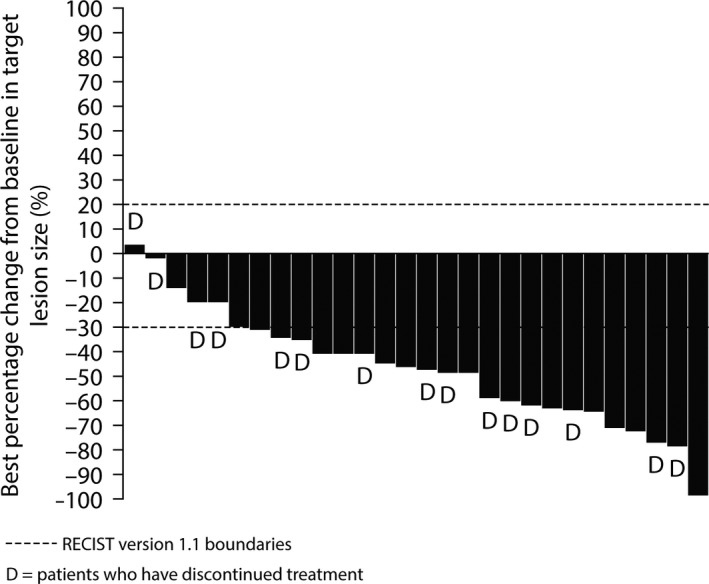
Waterfall plot for best percentage change in target lesion size (investigator assessed)
Figure 3.
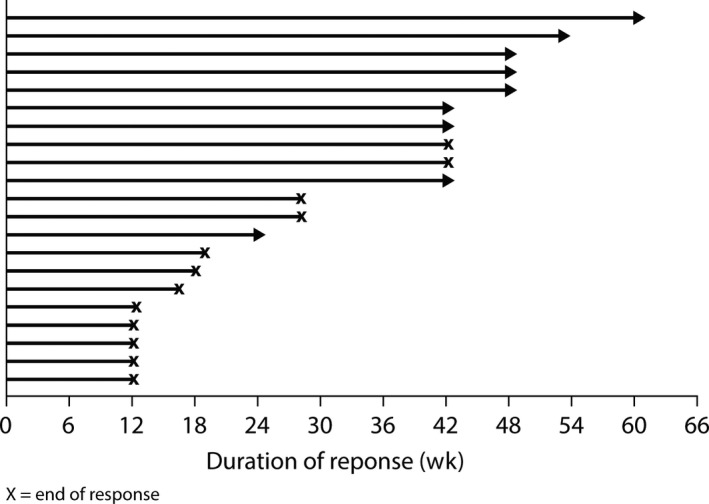
Duration of patient response
3.4.2. Progression‐free survival
There were a total of 17 progression events (61%). Median PFS was 8.3 months (95% CI 4.2, NC) and median length of RECIST follow up was 8.1 months. The proportion of patients progression‐free at 6 and 12 months was 57% (95% CI 37, 73) and 37% (95% CI 19, 55), respectively. A Kaplan‐Meier curve showing PFS is included as Figure S2.
3.5. Safety
All 28 patients (100%) reported at least one AE, with 27 patients (96%) reporting a possibly causally related AE (as assessed by the investigator). A description of AE reported in ≥15% of patients is shown in Table 3. The most common all‐causality AE were dermatological or gastrointestinal in nature: paronychia (46%), dry skin (46%), diarrhea (36%) and rash (36%). ILD‐like AE were reported in 5 patients (18%), with three (11%) cases of pneumonitis and two (7%) of ILD. There were five QT prolongation events, four classified as CTCAE grade 1 and one classified as CTCAE grade 2.
Table 3.
Safety summary
| AE categorya | No. (%) patientsb (N = 28) |
|---|---|
| Any AE | 28 (100.0) |
| Any possibly causally related AEc | 27 (96) |
| Any Grade ≥3 AE | 16 (57) |
| Any Grade ≥3 possibly causally related AEc | 10 (36) |
| Any AE with outcome = death | 0 |
| Any SAE | 4 (14) |
| Any possibly causally related SAEc | 3 (11) |
| Any AE leading to interruption of osimertinib | 9 (32) |
| Any AE leading to reduction of osimertinib | 6 (21) |
| Any AE leading to discontinuation of osimertinib | 5 (18) |
| Any possibly causally related AE leading to discontinuation of osimertinibc | 5 (18) |
| Most common AE (all causality), occurring in ≥15% of patients overall | ||||
|---|---|---|---|---|
| n (%) | Grade 1 | Grade 2 | Grade ≥3 | Total (N = 28) |
| Dry skind | 6 (21) | 7 (25) | 0 | 13 (46) |
| Paronychiad | 9 (32) | 4 (14) | 0 | 13 (46) |
| Diarrhea | 9 (32) | 1 (4) | 0 | 10 (36) |
| Rashd | 7 (25) | 2 (7) | 1 (4) | 10 (36) |
| Stomatitis | 7 (25) | 3 (11) | 0 | 10 (36) |
| Anemia | 2 (7) | 3 (11) | 1 (4) | 6 (21) |
| Neutrophil count decreased | 0 | 3 (11) | 3 (11) | 6 (21) |
| White blood cell count decreased | 0 | 4 (14) | 2 (7) | 6 (21) |
| Decreased appetite | 2 (7) | 3 (11) | 0 | 5 (18) |
| Electrocardiogram QT prolongation | 4 (14) | 1 (4) | 0 | 5 (18) |
| ILD‐like AEd | 1 (4) | 1 (4) | 3 (11) | 5 (18) |
| Nausea | 0 | 4 (14) | 1 (4) | 5 (18) |
| Neutropenia | 0 | 3 (11) | 2 (7) | 5 (18) |
| Upper respiratory tract infection | 2 (7) | 3 (11) | 0 | 5 (18) |
AE, adverse event; ILD, interstitial lung disease; SAE, serious adverse event.
Includes AE with an onset date on or after the date of first dose and up to and including 28 days following the date of the last dose of study medication.
Patients with multiple events in the same category are counted only once in that category. Patients with events in more than 1 category are counted once in each of those categories.
As assessed by the investigator.
This category represents a grouped term for the event.
Overall, 16 (57%) patients experienced an AE of Grade 3 or higher, most commonly neutrophil count decrease (3 patients [11%]), followed by pneumonitis and white blood cell count decrease (each reported in 2 patients [7%]). SAE were reported in 4 patients (14%) (Table 3), three of which were considered to be related to the study drug; these were two reported cases of pneumonitis and one of ILD. AE leading to dose reduction occurred in 6 patients (21%) and AE leading to dose interruption occurred in 9 patients (32%) (Table 3). AE leading to dose discontinuation occurred in 5 patients (18%); these were three reported cases of pneumonitis and two of ILD (all five were considered by the investigator as possibly related to osimertinib treatment). No deaths were reported in this cohort.
3.6. Pharmacokinetics
Pharmacokinetic data were obtained from 25/28 patients; a summary of primary PK parameters has been included as Table S2. The geometric mean AUCss and Css,max of osimertinib (obtained on Cycle 2, Day 1 [Day 22]) were 12 670 nmol/h/L and 660.7 nmol/L, respectively, with a steady state apparent clearance of 13.1 L/h. The AZ5104 and AZ7550 metabolite ratios for AUCss and Css,max to osimertinib AUCss and Css,max were between 10% and 12%.
4. DISCUSSION
Osimertinib is recommended as a treatment choice for patients with EGFR T790M‐positive advanced NSCLC following progression on first‐line EGFR‐TKI.25, 26, 27 Data from the current cytology cohort of the AURA study suggest that osimertinib 80 mg orally once daily provides clinical benefit with a manageable safety profile in Japanese patients with pretreated EGFR T790M mutation‐positive NSCLC whose screening EGFR T790M mutation status was determined from cytology samples.
Although there have been advances in the early detection of NSCLC, the majority (80%‐85%) of advanced‐stage patients have unresectable disease.28 In addition, the remaining 15%‐20% of patients with resectable disease are often not appropriate for surgical resection as a result of significant comorbidities.28 In these non‐surgical situations, cytological materials may be the only diagnostic specimens available.28 Furthermore, patients who do not have solid tumor deposits suitable for biopsy are generally excluded from clinical studies or access to licensed medicines that require prospective testing. A cytology sample may offer sufficient material for molecular analysis and characterization of a tumor by a less invasive approach. Comparative studies of cytology specimens and FFPE tissue in the analysis of genetic mutations in patients with cancer reported a high concordance rate between the samples and concluded cytological preparations to be a reliable source for molecular oncology testing.29, 30, 31 This highlights the importance of investigating cytological samples for diagnostic and molecular characterization purposes. The current cohort in the Phase I expansion component of the AURA study was included to explore the efficacy, safety and tolerability of osimertinib in patients enrolled using a cytology sample for detection of the EGFR T790M mutation.
The most common method of cytological sampling used in this cohort was BAL, used in 12 patients (43%). For T790M testing of cytology samples, PNA‐LNA PCR clamp was most commonly used (57% of patients). Although comparison of the limits of EGFR mutation detection was out of the scope of this study, the relative test sensitivities may be a consideration when choosing an EGFR mutation test. This is particularly important in the use of diagnostic tests, where a false‐negative result could delay or impede patients receiving effective treatments.
The primary efficacy endpoint, investigator‐assessed ORR, reported in this cohort (75%) is comparable to high response rates reported for osimertinib 80 mg orally once daily treatment in patients with EGFR T790M mutation‐positive advanced NSCLC following disease progression on an EGFR‐TKI: AURA Phase I ORR 71%,32 AURA Phase II extension 62%,15 AURA2 70%,16 and AURA3 71%.17 In the current cohort, all but 1 patient experienced a reduction in tumor size, with a DCR of 96%. Median DoR was 9.7 months and median PFS 8.3 months, which were comparable to the results from AURA Phase II extension, AURA2 and AURA3 studies.15, 16, 17 The PK results observed in Japanese patients enrolled into a cytology expansion cohort of the AURA study were shown to be broadly comparable with those reported in the rest of the global AURA Phase I study.14, 33 Likewise, the safety and tolerability observed in this study are consistent with those reported in previous trials of osimertinib in pretreated patients with EGFR T790M mutation‐positive advanced NSCLC.14, 15, 16, 17, 34 The frequency of ILD in this study was higher than that previously reported in Japanese patients with EGFR T790M mutation‐positive NSCLC; in the pooled analysis from AURA Phase II extension and AURA2, 6% (5/80) of patients reported ILD25, 26 but the difference may have arisen by chance as a result of the small sample size of this cohort.
In conclusion, results from this cohort suggest that an EGFR T790M‐positive status from a cytology sample predicts clinical benefit with osimertinib treatment.
CONFLICTS OF INTEREST
K Kiura reports research funding from AstraZeneca, Chugai, Boehringer Ingelheim, Daiichi Sankyo and Shionogi; honoraria from Novartis, Chugai, Pfizer, Eli Lilly, and Taiho; travel expenses from AstraZeneca. K Yoh reports research funding and travel expenses from AstraZeneca; grants from Bayer, Novartis and Takeda; lecture fees from Chugai, Eli Lilly Japan, Boehringer Ingelheim, Pfizer, Taiho Pharmaceutical, Bristol‐Myers Squibb and Ono Pharmaceutical. N Katakami reports research funding from AstraZeneca, MSD, Astellas, Eisai, Ono, Amgen, Shionogi, Daiichi Sankyo, Chugai, Eli Lilly, Boehringer Ingelheim, Bristol‐Myers Squibb, Maruishi and Merck Serono; lecture fees from AstraZeneca, Eli Lilly, Pfizer, Boehringer Ingelheim, Ono, Taiho and Novartis Pharma K.K. N Nogami reports research funding and travel expenses from AstraZeneca; grants from Taiho, Ono, Nippon Boehringer Ingelheim, Chugai and Kyowa Hakko Kirin; lecture fees from Taiho, Ono, Nippon Boehringer Ingelheim, Chugai, Kyowa Hakko Kirin, Eli Lilly and AstraZeneca. K Kasahara reports research funding and lecture fees from AstraZeneca. T Takahashi reports research funding and travel expenses from AstraZeneca; grants from AstraZeneca, Pfizer, Eli Lilly, Chugai, Ono, Boehringer Ingelheim, Takeda and Taiho; honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, Chugai, Ono and Eli Lilly. I Okamoto reports research funding and travel expenses from AstraZeneca. M Cantarini is a former employee of AstraZeneca and is a shareholder in AstraZeneca. R Hodge is an employee of, and shareholder in, AstraZeneca. H Uchida is an employee of, and shareholder in, AstraZeneca.
Supporting information
ACKNOWLEDGMENTS
Natasha Cary BSc from iMed Comms, an Ashfield Company, provided medical writing support funded by AstraZeneca. We thank the investigators and staff at all 13 sites that participated (Yasuhito Fujisaka, Takashi Seto, Takayasu Kurata, Norihiko Ikeda, Tomonori Hirashima, Hiroaki Okamoto).
Kiura K, Yoh K, Katakami N, et al. Osimertinib in patients with epidermal growth factor receptor T790M advanced non‐small cell lung cancer selected using cytology samples. Cancer Sci. 2018;109:1177–1184. https://doi.org/10.1111/cas.13511
Funding information
This study was funded by AstraZeneca. The study sponsor contributed to the design of the study, was involved in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Mireille Cantarini is a former employee.
REFERENCES
- 1. Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S. Metastatic non‐small‐cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2014;25:iii27‐iii39. [DOI] [PubMed] [Google Scholar]
- 2. Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non‐small‐cell lung cancer considering first‐line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29:2121‐2127. [DOI] [PubMed] [Google Scholar]
- 3. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29:2866‐2874. [DOI] [PubMed] [Google Scholar]
- 4. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380‐2388. [DOI] [PubMed] [Google Scholar]
- 5. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121‐128. [DOI] [PubMed] [Google Scholar]
- 6. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13:239‐246. [DOI] [PubMed] [Google Scholar]
- 7. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327‐3334. [DOI] [PubMed] [Google Scholar]
- 8. Wu Y‐L, Zhou C, Hu C‐P, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15:213‐222. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Zhou C, Liam CK, et al. First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: analyses from the phase III, randomized, open‐label. ENSURE study. Ann Oncol. 2015;26:1883‐1889. [DOI] [PubMed] [Google Scholar]
- 10. Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802). Ann Oncol. 2015;26:1877‐1883. [DOI] [PubMed] [Google Scholar]
- 11. Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res. 2013;19:2240‐2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med. 2015;372:1689‐1699. [DOI] [PubMed] [Google Scholar]
- 15. Yang JC‐H, Ahn M‐J, Kim D‐W, et al. Osimertinib in pretreated T790M‐positive advanced non–small‐cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35:1288‐1296. [DOI] [PubMed] [Google Scholar]
- 16. Goss G, Tsai C‐M, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met‐positive advanced non‐small‐cell lung cancer (AURA2): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2016;17:1643‐1652. [DOI] [PubMed] [Google Scholar]
- 17. Mok TS, Wu Y‐L, Ahn M‐J, et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376:629‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novello S, Barlesi F, Califano R, et al. Metastatic non‐small‐cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v1‐v27. [DOI] [PubMed] [Google Scholar]
- 19. Billah S, Stewart J, Staerkel G, Chen S, Gong Y, Guo M. EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol. 2011;119:111‐117. [DOI] [PubMed] [Google Scholar]
- 20. da Cunha Santos G, Liu N, Tsao MS, Kamel‐Reid S, Chin K, Geddie WR. Detection of EGFR and KRAS mutations in fine‐needle aspirates stored on Whatman FTA cards: is this the tool for biobanking cytological samples in the molecular era? Cancer Cytopathol. 2010;118:450‐456. [DOI] [PubMed] [Google Scholar]
- 21. Wu YL, Jenkins S, Ramalingam S, et al. MA08.03 osimertinib vs platinum‐pemetrexed for T790M‐mutation positive advanced NSCLC (AURA3): plasma ctDNA analysis. J Thorac Oncol. 2017;12(Suppl):S386. [Google Scholar]
- 22. Hagiwara K, Kobayashi K. Importance of the cytological samples for the epidermal growth factor receptor gene mutation test for non‐small cell lung cancer. Cancer Sci. 2013;104:291‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goto K, Satouchi M, Ishii G, et al. An evaluation study of EGFR mutation tests utilized for non‐small‐cell lung cancer in the diagnostic setting. Ann Oncol. 2012;23:2914‐2919. [DOI] [PubMed] [Google Scholar]
- 24. Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non‐small‐cell lung cancer. J Clin Oncol. 2010;28:357‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Japan MHLW TAGRISSO™ (osimertinib) prescribing information. TAGRISSO® 40 mg and 80 mg tablets. 2016.
- 26. European Medicines Agency . EPAR summary for the public: Tagrisso. Osimertinib. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/004124/WC500202025.pdf. Accessed January 31, 2017.
- 27. Highlights of prescribing information: TAGRISSO™ (osimertinib) tablet, for oral use. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208065s000lbl.pdf. Accessed January 31, 2017.
- 28. Cai G, Wong R, Chhieng D, et al. Identification of EGFR mutation, KRAS mutation, and ALK gene rearrangement in cytological specimens of primary and metastatic lung adenocarcinoma. Cancer Cytopathol. 2013;121:500‐507. [DOI] [PubMed] [Google Scholar]
- 29. Gailey MP, Stence AA, Jensen CS, Ma D. Multiplatform comparison of molecular oncology tests performed on cytology specimens and formalin‐fixed, paraffin‐embedded tissue. Cancer Cytopathol. 2015;123:30‐39. [DOI] [PubMed] [Google Scholar]
- 30. Hida N, Misumi Y, Agemi Y, et al. A comparison of bronchofiberscopic (BFS) washing cytology (BWC) and formalin‐fixed paraffin‐embedded tissue (PPFE) in the analysis of EGFR mutations in advanced non‐small cell lung cancer (NSCLC). J Clin Oncol. 2013;31 (Suppl.):Abstract No: 8054. [Google Scholar]
- 31. Satouchi M, Tanaka H, Yoshioka H, et al. Detection of epidermal growth factor receptor gene T790M mutation in cytology samples using the cobas(R) EGFR mutation test. Lung Cancer. 2017;111:190‐194. [DOI] [PubMed] [Google Scholar]
- 32. Yang J, Ramalingam SS, Jänne PA, Cantarini M, Mitsudomi T. LBA2_PR: Osimertinib (AZD9291) in pre‐treated pts with T790M‐positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol. 2016;11:S152‐S153. [Google Scholar]
- 33. Planchard D, Brown KH, Kim DW, et al. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol. 2016;77:767‐776. [DOI] [PubMed] [Google Scholar]
- 34. Jänne PA, Ahn M‐J, Kim D‐W, et al. Phase I study of AZD9291 in patients with EGFR‐TKI‐resistant advanced NSCLC ‐ updated progression free survival and duration of response data. Ann Oncol. 2015;26:i60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


