Abstract
Double‐stranded (ds) RNA‐dependent protein kinase (PKR) is a ubiquitously expressed serine/threonine protein kinase. It was initially identified as an innate immune antiviral protein induced by interferon (IFN) and activated by dsRNA. PKR is recognized as a key executor of antiviral host defense. Moreover, it contributes to inflammation and immune regulation through several signaling pathways. In addition to IFN and dsRNA, PKR is activated by multiple stimuli and regulates various signaling pathways including the mitogen‐activated protein kinase (MAPK) and nuclear factor kappa‐light‐chain‐enhancer of activated B cells pathways. PKR was initially thought to be a tumor suppressor as a result of its ability to suppress cell growth and interact with major tumor suppressor genes. However, in several types of malignant disease, such as colon and breast cancers, its role remains controversial. In hepatocellular carcinoma, hepatitis C virus (HCV) is the main cause of liver cancer, and PKR inhibits HCV replication, indicating its role as a tumor suppressor. However, PKR is overexpressed in cirrhotic patients, and acts as a tumor promoter through enhancement of cancer cell growth by mediating MAPK or signal transducer and activator of transcription pathways. Moreover, PKR is reportedly required for the activation of inflammasomes and influences metabolic disorders. In the present review, we introduce the multifaceted roles of PKR such as antiviral function, tumor cell growth, regulation of inflammatory immune responses, and maintaining metabolic homeostasis; and discuss future perspectives on PKR biology including its potential as a therapeutic target for liver cancer.
Keywords: double‐stranded (ds) RNA‐dependent protein kinase (PKR), hepatitis C virus (HCV), hepatocellular carcinoma (HCC), interferon, mitogen‐activated protein kinase (MAPK)
Abbreviations
- ALL
acute lymphoblastic leukemia
- CLL
chronic lymphocytic leukemia
- eIF2α
eukaryotic initiation factor 2 alpha
- ER
endoplasmic reticulum
- ERK 1/2
extracellular signal‐regulated kinase 1/2
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HMGB1
high mobility group box 1
- IFN
interferon
- IKK
inhibitor of B (IkB) kinase
- IRF1
interferon regulatory factor 1
- ISG
interferon‐stimulated gene
- LC
liver cirrhosis
- MAPK
mitogen‐activated protein kinase
- NASH
non‐alcoholic steatohepatitis
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- PDGF
platelet‐derived growth factor
- PePHD
PKR‐eIF2α phosphorylation homology domain
- PERK
PKR‐like endoplasmic reticulum‐resident protein kinase
- pIC
poly(rI)·poly(rC)
- PKR
double‐stranded (ds) RNA‐dependent protein kinase
- STAT3
signal transducer and activator of transcription 3
1. INTRODUCTION
Double‐stranded PKR is a ubiquitously expressed serine/threonine protein kinase that was initially identified as an innate immune antiviral protein induced by IFN.1 It is also recognized as a host IFN‐stimulated gene.2 PKR was discovered after it was observed that cell extracts prepared from IFN‐treated vaccinia virus‐infected cells were sensitive to a translational block after the addition of exogenous mRNA3 and pIC, a synthetic analog of dsRNA.4 These studies led to the identification of a protein with dsRNA‐dependent kinase activity,5, 6 now known as PKR.7
PKR binds to dsRNA, resulting in a number of conformational changes, in which homodimerization appears to be most important according to biochemical and genetic analyses.8 PKR homodimerization leads to rapid autophosphorylation of a stretch of amino acids, namely Ser242, Thr255, Thr258, Ser83, Thr88, Thr89, Thr90, Thr446, and Thr451, termed the activation segment.9 Among others, residues Thr446 and Thr451 are consistently phosphorylated during activation,8, 10, 11 which further stabilizes the PKR dimer and increases its catalytic activity. Then, phosphorylated PKR phosphorylates Ser51 on the alpha subunit of eIF2α,12 and phosphorylated eIF2α inhibits the initiation of translation and decreases the rate of protein synthesis (Figure 1).
Figure 1.
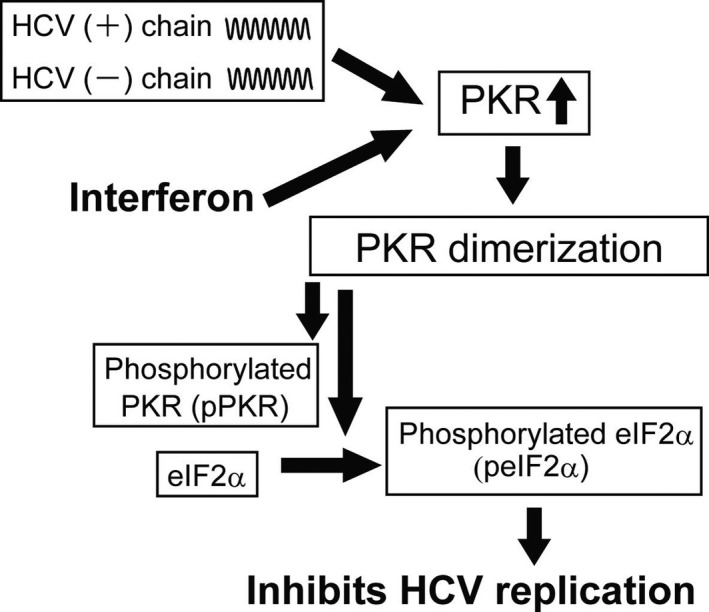
Antiviral effects of double‐stranded RNA‐dependent protein kinase (PKR). Double stranded‐RNA produced by RNA viral replication and interferon are potent activators of PKR. Activated PKR induces PKR dimerization and PKR phosphorylation. Then, PKR phosphorylates eukaryotic initiation factor‐2 alpha (eIF2α), which inhibits protein synthesis, including that of virally encoded proteins. HCV, hepatitis C virus
In addition to its established role in antiviral activities, PKR contributes to the regulation of inflammation and immune responses through several signaling pathways. In addition to IFN and dsRNA, PKR is activated by multiple stimuli, including cytokines such as tumor necrosis factor alpha (TNF), interleukin 1 (IL‐1),13, 14 LPS, through the Toll‐interleukin 1 receptor domain‐containing adaptor protein (TIRAP) and Toll‐like receptor 4 (TLR4) signaling pathways,15 PDGF through STAT3 and ERK 1/2 phosphorylation,16 heat shock protein 90,17 and some cellular stressors including arsenite, thapsigargin, and hydrogen peroxide.18, 19
PKR regulates various signal transduction pathways such as the MAPK, STAT,20, 21 and NF‐κB pathways,22 IRF123 or IRF3,24 and activating transcription factors.25 PKR has also been implicated as a general transducer of apoptosis,26, 27 and was shown to trigger autophagy through eIF2α‐mediated activation of microtubule‐associated protein light chain 3 (LC3).28 Thus, it is clear that PKR has multi‐functional roles in the regulation of inflammatory and immune signaling.
PKR has been implicated as an ER stress‐regulated kinase, as well as a PERK. Both PKR and PERK are activated by autophosphorylation, after which they phosphorylate eIF2α.29 Several studies have reported the probable role of PKR in ER stress‐induced neural cell death in Alzheimer's disease and Huntington's disease.30, 31 Moreover, Lee et al32 found that PKR plays a significant role in ER stress‐induced apoptosis mediated by protein activator of interferon‐induced protein kinase (PACT).
2. PKR AND HUMAN HEPATITIS C VIRUS
Hepatitis C virus infection is a major public health concern. About 150 million individuals are infected worldwide, and each year, 3‐4 million individuals become infected with the virus.33, 34 HCV is the leading cause of chronic liver disease and the most common indication for liver transplantation. HCV establishes persistent infection and induces chronic hepatitis, which leads to LC and frequently to HCC. However, the detailed mechanisms underlying the progression of LC to HCC remain unknown. Both dsRNA produced by HCV replication and the HCV core protein can activate PKR.35 It is likely that PKR activation by the core protein is associated with the similar ability of the latter to bind dsRNA, thereby providing the PKR activator substrate and possible mechanism of PKR activation during HCV infection.36 In the liver tissue of patients with chronic HCV, PKR mRNA is significantly increased compared with that in patients with other etiologies.37, 38 In contrast, consistent with the important role of PKR in the control of HCV infection, HCV has several PKR‐inactivation strategies (Figure 2). For example, the IFN sensitivity‐determining region in the HCV‐1b NS5A region within the PKR‐binding domain inhibits IFN‐induced PKR, thereby influencing responses to IFN‐based therapies.39, 40 In addition, the PKR‐eIF2α phosphorylation homology domain of the HCV E2 gene also inhibits IFN‐induced PKR.41 However, it remains unclear whether these proteins contribute to persistent HCV or are resistant to the antiviral activities of PKR.9, 42 Tokumoto et al43 showed that HCV protein expression is directly dependent on PKR expression. In this report, HCV core protein levels significantly increased upon knockdown of PKR; conversely, overexpression of PKR significantly suppressed HCV core levels in cell lines transfected with full‐length HCV constructs. Moreover, PKR expression was responsible for the antiviral effects of IFN against HCV. Hence, in contrasting actions, PKR suppressed HCV replication, and HCV suppressed PKR function. In chronic HCV infection, the functions of HCV and PKR are balanced. In patients with chronic HCV infection, we speculate that HCV function against PKR is greater than the anti‐HCV function of PKR, so active inflammation is maintained. However, in liver cancer, PKR is overexpressed and its functions dominate, resulting in cancer progression (Figure 3).
Figure 2.
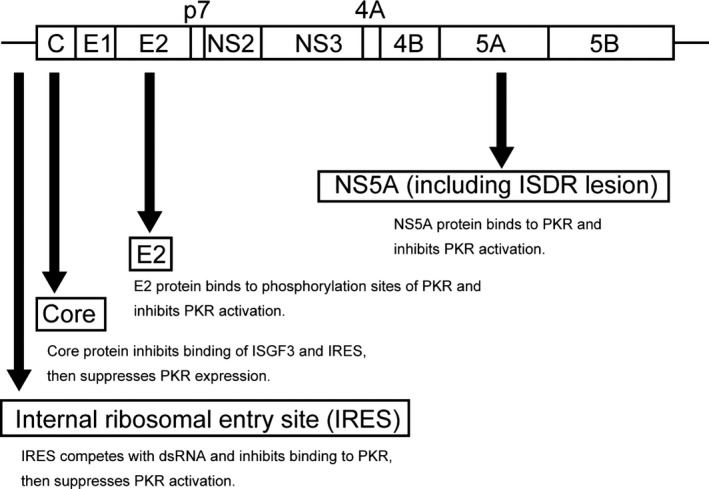
Anti‐double‐stranded RNA‐dependent protein kinase (PKR) effects as a result of hepatitis C virus (HCV). HCV has developed PKR inhibitory strategies for their persistence. The NS5A of HCV‐1b with a wild‐type interferon sensitivity determining region (ISDR) sequence within the PKR‐binding domain has the potential to block the interferon (IFN)‐induced PKR that mediates various aspects of the antiviral effects. The E2 gene of HCV interacts with the PKR‐eukaryotic initiation factor‐2 alpha phosphorylation homology domain and inhibits PKR activation by IFN
Figure 3.
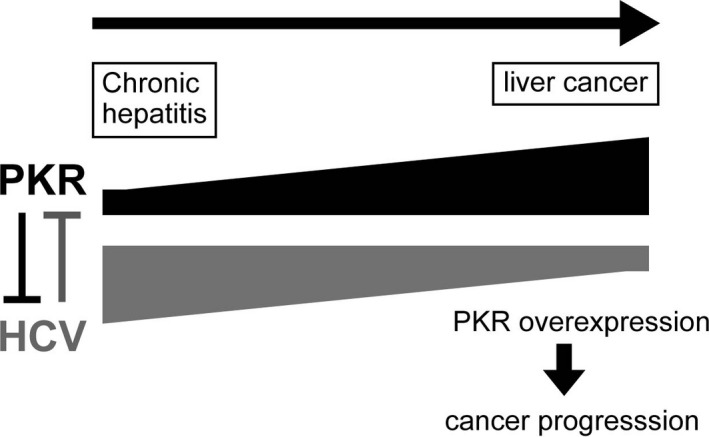
Relationship between double‐stranded RNA‐dependent protein kinase (PKR) and hepatitis C virus (HCV). In patients with chronic HCV infection, HCV function against PKR is greater than the anti‐HCV function of PKR, so active inflammation is maintained. However, in liver cancer, PKR is overexpressed, and its functions dominate, resulting in cancer progression
3. PKR AND MALIGNANT DISEASES
Initially, PKR was thought to be a tumor suppressor as its overexpression in mammalian, insect, and yeast cells led to the suppression of cell growth,44, 45 indicating its role in inhibiting cell proliferation. In addition, the expression of several PKR dominant‐negative mutants led to the malignant transformation of NIH 3T3 cells, causing tumorigenesis in nude mice.46, 47 These results suggested that PKR was able to activate some apoptotic signals, thereby supporting the notion that it may be a tumor suppressor.27, 48 Moreover, PKR interacts with major tumor suppressor genes such as p53 and phosphatase and tensin homolog, and plays essential roles in their tumor suppressor functions.49, 50 However, the role of PKR in cancer biology remains a subject of debate. In Jurkat T cells, the reduced expression of PKR also corresponded with reduced PKR activity,51 and the resultant effects were characterized in the promonocytic leukemia cell line U937.52 Furthermore in 21 of 28 chronic lymphoid leukemia cells, PKR was expressed but not active.53 However, several reports have indicated that PKR is overexpressed and activated in several hematopoietic malignancies. For example, PKR is generally overexpressed in AML and ALL.54 In addition, the status of PKR activity was recently examined in AML and ALL cell lines, and patient samples and both cell types had significantly higher levels of phosphorylated PKR/PKR activation compared with normal controls.55 In thyroid carcinoma, there was a reverse correlation between PKR expression and Ki‐67 labeling, suggesting that tumor cells with low PKR expression had much higher proliferative activity than those with high PKR expression in thyroid carcinoma.56 The reverse correlation between PKR expression and tumor cell proliferation has been reported in head and neck squamous cell carcinoma.57 Furthermore, He et al58 reported that in patients with non‐small‐cell lung carcinoma, those with high levels of phosphorylated PKR or phosphorylated eIF2α had significantly longer median survival than those with little or no expression. By contrast, Kim et al59 showed by immunohistochemical analysis that primary melanomas had minimal PKR immunoreactivity, but lymph node metastases expressed high levels of PKR protein. In the same report, analyses of colon cancer specimens showed that malignant transformation from normal mucosa to adenoma and adenocarcinoma was coincident with an increase in PKR expression. Kim et al60 also reported that PKR autophosphorylation and phosphorylation were much higher in human breast cancer cell lines than in non‐transformed mammary epithelial cell lines. Moreover, Roh et al61 demonstrated that patients with small‐sized peripheral lung cancer and high‐grade PKR expression had significantly shorter survival than those with low‐grade expression. These conflicting data suggest that the precise role of PKR in cancer may differ depending upon the pathological type of tumor, stage of tumor development, and tumor microenvironment.
4. PKR AND LIVER CANCER
Chronic HCV infection is specific to liver cancer (not to other types of cancer); hence, among the many PKR upstream triggers previously described, HCV infection and IFN signaling might be liver cancer‐specific triggers. Moreover, in HCC related to NASH, nutrition or energy excess might contribute to liver cancer. The first report on the relationship between PKR and HCC was by Shimada et al62 in 1998. In that report, the authors showed that PKR expression in HCV‐related HCC was higher in moderate‐ to well‐differentiated carcinomas compared with poorly differentiated HCC or LC. Because this study was not carried out using HCC/non‐HCC tissues pairs, it was not determined whether PKR levels in the surrounding tissue were also variable. To clarify the role of PKR in HCV‐related hepatocarcinogenesis, expression of PKR protein in paired malignant and surrounding non‐malignant tissues was examined from patients with HCV‐related HCC.63 The results showed that PKR protein levels were consistently increased in HCC‐related HCC tissues compared with surrounding non‐HCC tissue, and a similar increase was seen in eIF2α expression. In addition, HCV copy number was reduced in HCC compared with LC tissue, indicating that overexpressed PKR in HCC tissues retained its antiviral function against HCV. Importantly, sequence data indicated that increased PKR expression was not only functional but was also wild type, showing that mutant PKR had not accumulated. We studied the molecular mechanisms of PKR in HCV‐related HCC using two permissive cell lines for HCV replication (JFH1 and H77s), as well as human HCC specimens with HCV infection.64 In HCV‐related HCC cell lines, PKR upregulated c‐Fos and c‐Jun activities through activation of ERK1/2 and JNK, respectively, subsequently increasing HCC cell proliferation. Moreover, coordinated upregulation of c‐Fos and c‐Jun signaling was confirmed in human HCC specimens. c‐Fos and c‐Jun transcription factors have been implicated in carcinogenesis, and they regulate genes including important regulators of invasion and metastasis, proliferation, differentiation, and survival in several types of cancer.65, 66 However c‐Fos and c‐Jun activation through PKR signaling has been reported in HCV‐related liver cancer only.63, 64 c‐Fos and c‐Jun activation by PKR signal may be as a result of HCV chronic infection, so this signaling in liver cancer associated with other etiologies is not yet clear.
Recently, Wang et al67 reported that PKR plays a key role in increasing the proliferation and migration of HepG2 human HCC cells, and mouse xenograft models also confirmed the tumorigenic role of PKR in HepG2 cells. In that report, the tumor‐promoting function of PKR was mediated by the STAT3 transcription factor. The authors used human primary tumor samples and the surrounding tissue to show that the expression of total and phosphorylated PKR was upregulated in tumor tissues compared with the surrounding tissues. Importantly, patients did not have HBV or HCV infection, indicating that PKR is also activated in non‐HCV‐related liver cancer. These data do not support the role of PKR as a classic tumor suppressor, but suggest that PKR may have a positive regulatory role in controlling tumor growth and progression in liver cancer (Figure 4).
Figure 4.
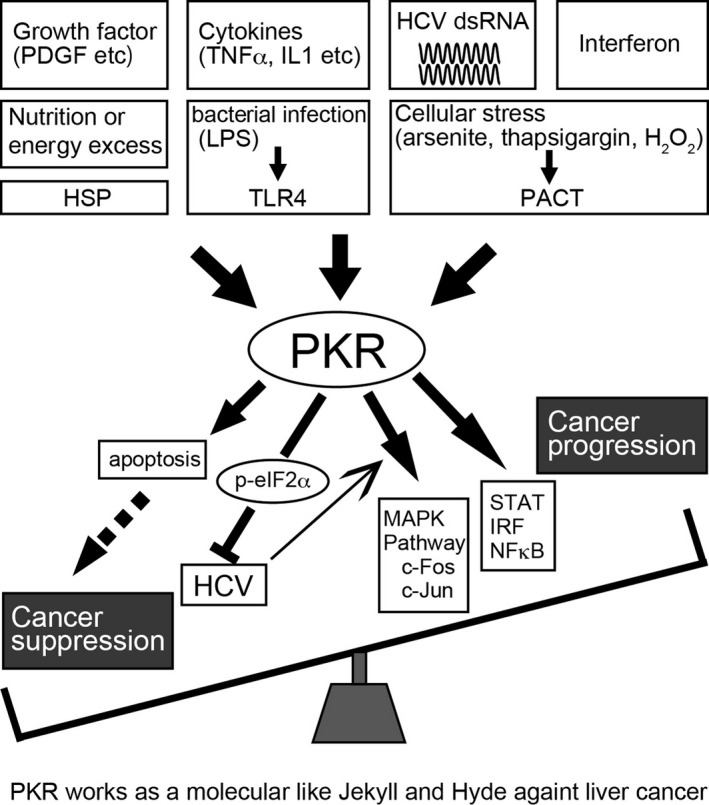
Cancer suppression and progression effects of double‐stranded (ds) RNA‐dependent protein kinase (PKR). PKR influences liver cancer through mechanisms that function either against viral infection or through the growth of cancer cells. PKR during viral infection in hepatocytes or apoptotic effect shows its function of tumor suppression. In contrast, PKR acts as a tumor promoter through enhancement of cancer cell growth. Thus, PKR works as a molecular Jekyll and Hyde in liver cancer. eIF2α, eukaryotic initiation factor 2 alpha; HCV, hepatitis C virus; HSP, heat shock protein; IL, interleukin; IRF, interferon regulatory factor; MAPK, mitogen‐activated protein kinase; NF‐κB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells; PACT, protein activator of interferon‐induced protein kinase; PDGF, platelet‐derived growth factor; STAT, signal transducer and activator of transcription; TLR4, Toll‐like receptor 4; TNFα, tumor necrosis factor alpha
5. POSSIBILITY OF PKR AS A THERAPEUTIC TARGET AGAINST LIVER CANCER
Lu et al68 reported that PKR is required for the activation of inflammasomes and the subsequent release of HMGB1 protein, a pro‐inflammatory cytokine. The functions of inflammasomes were recently reported in various inflammation‐induced cancers.69 With the prevalence of HCV expected to decline, the proportion of HCC related to NASH is anticipated to significantly increase as a result of the growing epidemic of obesity and diabetes.70 Nakamura et al71 reported that PKR can respond to nutrient signals, as well as to ER stress, and coordinates the activity of other critical inflammatory kinases, such as JNK, to regulate insulin signaling and metabolism. Dietary and genetic obesity features indicated the activation of PKR in liver tissue, and deletion of PKR led to decrease metabolic disorder as a result of nutrition or energy excess in mice. This report suggests that PKR may be the key molecule in HCC associated with NASH or metabolic disorders. Taken together with the aforementioned reports on the relationship between PKR and liver cancer, it appears that PKR has multifaceted roles in liver cancer, such as antiviral function, promotion of tumor cell growth, regulation of the inflammatory immune response, and maintenance of metabolic homeostasis. Thus, PKR might be an effective therapeutic target in human liver cancer (Figure 5). We consider that inhibition of HCV replication by PKR could indirectly affect tumor suppression. However, once liver cancer has developed, the effects of PKR on decreasing HCV are not sufficient for tumor suppression, whereas overexpressed PKR contributes to tumor progression. It has been proposed that PKR might activate some apoptotic signals or interact with several tumor suppressor genes.26, 72, 73 However, in liver cancer, these tumor suppressor effects have not yet been confirmed. Considering other functions underlying cancer progression in liver cancer, we believe that a strategy for inhibiting PKR might be effective against liver cancer caused by several etiologies. The negative effects against liver cancer by inhibiting PKR, for example, by increasing HCV replication, need to be verified in future studies.
Figure 5.
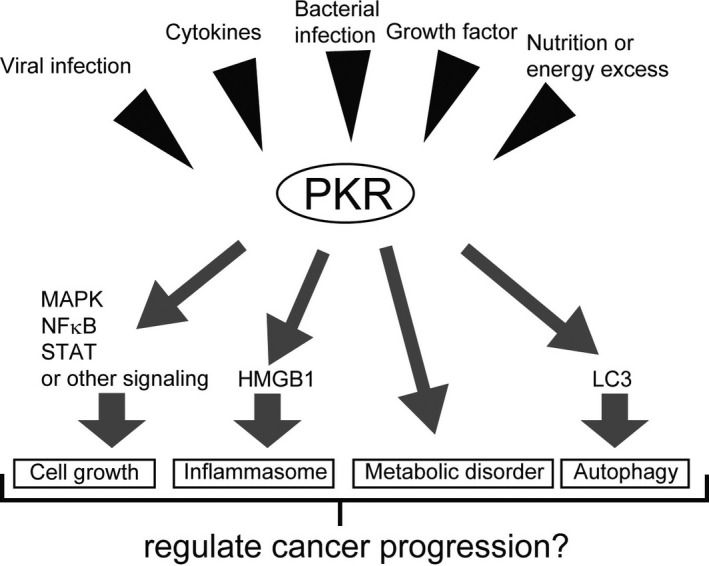
Multi‐functional roles of double‐stranded RNA‐dependent protein kinase (PKR) against liver cancer. PKR has multifaceted roles in liver cancer including antiviral functions, promotion of tumor cell growth, regulation of the inflammatory immune response, induction of autophagy, and maintenance of metabolic homeostasis. PKR may be an attractive therapeutic target for human liver cancer. HMGB1, high mobility group box 1; LC3, light chain 3; MAPK, mitogen‐activated protein kinase; NF‐κB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells; STAT, signal transducer and activator of transcription
6. CONCLUSIONS AND PERSPECTIVES
Recent progress on the roles of PKR in liver cancer has shown that PKR influences liver cancer through mechanisms via its antiviral properties or by negatively regulating the growth of cancer cells. Studies on PKR during the virus infection of hepatocytes showed its tumor suppression function. However, it can also act as a tumor promoter through enhancement of cancer cell growth. Thus, PKR functions as a molecular Jekyll and Hyde against liver cancer. The complex nature of the relationship between PKR and virus infection in liver cells and liver cell growth presents a unique challenge and opportunity to develop therapeutic intervention strategies for targeting liver cancer. Further studies examining the role of additional molecules in regulating PKR signaling in liver cancer will provide additional insights into the development of small‐molecule or peptide‐based inhibitors for future therapeutic treatments.
CONFLICT OF INTEREST
Research finding: Takeshi Imamura from SONY Corporation and Chugai Corporation.
ACKNOWLEDGMENTS
There are many important papers in this field, but as a result of the limited space, we were unable to mention all of them. This work was supported by JSPS KAKENHI (No. JP16K19349) to TW, JSPS KAKENHI (Nos. JP15H04962, JP16H06280, JP15H05952) to TI, JSPS KAKENHI (No. JP15K09006) and the Translational Research program; Strategic PRomotion for practical application of INnovative medical Technology (TR‐SPRINT) from Japan Agency for Medical Research and Development, AMED to YH.
Watanabe T, Imamura T, Hiasa Y. Roles of protein kinase R in cancer: Potential as a therapeutic target. Cancer Sci. 2018;109:919–925. https://doi.org/10.1111/cas.13551
Funding information
This work was supported by JSPS KAKENHI (Nos. JP16K19349, JP15H04962, JP16H06280, JP15H05952, JP15K09006), The Translational Research program. Strategic Promotion for practical application of Innovative medical Technology (TR‐SPRINT) from Japan Agency for Medical Research and Development, Research Institute of Science and Technology for Society, AMED.
REFERENCES
- 1. Meurs E, Chong K, Galabru J, et al. Molecular cloning and characterization of the human double‐stranded RNA‐activated protein kinase induced by interferon. Cell. 1990;62:379‐390. [DOI] [PubMed] [Google Scholar]
- 2. Sen GC, Ransohoff RM. Interferon‐induced antiviral actions and their regulation. Adv Virus Res. 1993;42:57‐102. [DOI] [PubMed] [Google Scholar]
- 3. Friedman RM, Metz DH, Esteban RM, Tovell DR, Ball LA, Kerr IM. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L‐cell extracts. J Virol. 1972;10:1184‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerr IM, Brown RE, Ball LA. Increased sensitivity of cell‐free protein synthesis to double‐stranded RNA after interferon treatment. Nature. 1974;250:57‐59. [DOI] [PubMed] [Google Scholar]
- 5. Roberts WK, Hovanessian A, Brown RE, Clemens MJ, Kerr IM. Interferon‐mediated protein kinase and low‐molecular‐weight inhibitor of protein synthesis. Nature. 1976;264:477‐480. [DOI] [PubMed] [Google Scholar]
- 6. Sen GC, Taira H, Lengyel P. Interferon, double‐stranded RNA, and protein phosphorylation. Characteristics of a double‐stranded RNA‐activated protein kinase system partially purified from interferon treated Ehrlich ascites tumor cells. J Biol Chem. 1978;253:5915‐5921. [PubMed] [Google Scholar]
- 7. Clemens MJ, Hershey JW, Hovanessian AC, et al. PKR: proposed nomenclature for the RNA‐dependent protein kinase induced by interferon. J Interferon Res. 1993;13:241. [DOI] [PubMed] [Google Scholar]
- 8. Dey M, Cao C, Dar AC, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell. 2005;23:901‐913. [DOI] [PubMed] [Google Scholar]
- 9. Taylor DR, Tian B, Romano PR, Hinnebusch AG, Lai MM, Mathews MB. Hepatitis C virus envelope protein E2 does not inhibit PKR by simple competition with autophosphorylation sites in the RNA‐binding domain. J Virol. 2001;75:1265‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor DR, Lee SB, Romano PR, et al. Autophosphorylation sites participate in the activation of the double‐stranded‐RNA‐activated protein kinase PKR. Mol Cell Biol. 1996;16:6295‐6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang F, Romano PR, Nagamura‐Inoue T, et al. Binding of double‐stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J Biol Chem. 2001;276:24946‐24958. [DOI] [PubMed] [Google Scholar]
- 12. Samuel CE. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF‐2 in interferon‐treated human cells by a ribosome‐associated kinase processing site specificity similar to hemin‐regulated rabbit reticulocyte kinase. Proc Natl Acad Sci USA. 1979;76:600‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeung MC, Liu J, Lau AS. An essential role for the interferon‐inducible, double‐stranded RNA‐activated protein kinase PKR in the tumor necrosis factor‐induced apoptosis in U937 cells. Proc Natl Acad Sci USA. 1996;93:12451‐12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goh KC, deVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292‐4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835‐841. [DOI] [PubMed] [Google Scholar]
- 16. Deb A, Zamanian‐Daryoush M, Xu Z, Kadereit S, Williams BR. Protein kinase PKR is required for platelet‐derived growth factor signaling of c‐fos gene expression via Erks and Stat3. EMBO J. 2001;20:2487‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donzé O, Abbas‐Terki T, Picard D, et al. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA‐dependent kinase PKR. EMBO J. 2001;16:3771‐3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ito T, Yang M, May WS. RAX, a cellular activator for double‐stranded RNA‐dependent protein kinase during stress signaling. J Biol Chem. 1999;274:15427‐15432. [DOI] [PubMed] [Google Scholar]
- 19. Ruvolo PP, Gao F, Blalock WL, Deng X, May WS. Ceramide regulates protein synthesis by a novel mechanism involving the cellular PKR activator RAX. J Biol Chem. 2001;276:11754‐11758. [DOI] [PubMed] [Google Scholar]
- 20. Takada Y, Ichikawa H, Pataer A, Swisher S, Aggarwal BB. Genetic deletion of PKR abrogates TNF‐induced activation of IkappaBalpha kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene. 2007;22:1201‐1212. [DOI] [PubMed] [Google Scholar]
- 21. Wong AH, Tam NW, Yang YL, et al. Physical association between STAT1 and the interferon‐inducible protein kinase PKR and implications for interferon and double‐stranded RNA signaling pathways. EMBO J. 1997;17:1291‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonnet MC, Weil R, Dam E, Hovanessian AG, Meurs EF. PKR stimulates NF‐kappaB irrespective of its kinase function by interacting with the IkappaB kinase complex. Mol Cell Biol. 2000;20:4532‐4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirchhoff S, Koromilas AE, Schaper F, Grashoff M, Sonenberg N, Hauser H. IRF‐1 induced cell growth inhibition and interferon induction requires the activity of the protein kinase, PKR. Oncogene. 1995;11:439‐445. [PubMed] [Google Scholar]
- 24. Zhang P, Samuel CE. Induction of protein kinase PKR‐dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS‐1 signaling. J Biol Chem. 2008;283:34580‐34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guerra S, López‐Fernández LA, García MA, Zaballos A, Esteban M. Human gene profiling in response to the active protein kinase, interferon‐induced serine/threonine protein kinase (PKR), in infected cells. Involvement of the transcription factor ATF‐3 IN PKR‐induced apoptosis. J Biol Chem. 2006;281:18734‐18745. [DOI] [PubMed] [Google Scholar]
- 26. Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA‐dependent protein kinase, PKR, induces apoptosis through FADD‐mediated death signaling. EMBO J. 1998;17:6888‐6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123‐138. [DOI] [PubMed] [Google Scholar]
- 28. Tallóczy Z, Jiang W, Virgin HW IV, et al. Regulation of starvation‐ and virus‐induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci USA. 2002;99:190‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic‐reticulum‐resident kinase. Nature. 1999;397:271‐274. [DOI] [PubMed] [Google Scholar]
- 30. Peel AL, Bredesen DE. Activation of the cell stress kinase PKR in Alzheimer's disease and human amyloid precursor protein transgenic mice. Neurobiol Dis. 2003;14:52‐62. [DOI] [PubMed] [Google Scholar]
- 31. Bando Y, Onuki R, Katayama T, et al. Double‐strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson's disease and Huntington's disease. Neurochem Int. 2005;46:11‐18. [DOI] [PubMed] [Google Scholar]
- 32. Lee ES, Yoon CH, Kim YS, Bae YS. The double‐strand RNA‐dependent protein kinase PKR plays a significant role in a sustained ER stress‐induced apoptosis. FEBS Lett. 2007;581:4325‐4332. [DOI] [PubMed] [Google Scholar]
- 33. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245‐264. [DOI] [PubMed] [Google Scholar]
- 34. Manns MP, von Hahn T. Novel therapies for hepatitis C ‐ one pill fits all? Nat Rev Drug Discov. 2013;12:595‐610. [DOI] [PubMed] [Google Scholar]
- 35. Delhem N, Sabile A, Gajardo R, et al. Activation of the interferon‐inducible protein kinase PKR by hepatocellular carcinoma derived‐hepatitis C virus core protein. Oncogene. 2001;20:5836‐5845. [DOI] [PubMed] [Google Scholar]
- 36. Tanaka Y, Shimoike T, Ishii K, et al. Selective binding of hepatitis C virus core protein to synthetic oligonucleotides corresponding to the 5' untranslated region of the viral genome. Virology. 2000;270:229‐236. [DOI] [PubMed] [Google Scholar]
- 37. Yu SH, Nagayama K, Enomoto N, Izumi N, Marumo F, Sato C. Intrahepatic mRNA expression of interferon‐inducible antiviral genes in liver diseases: dsRNA‐dependent protein kinase overexpression and RNase L inhibitor suppression in chronic hepatitis C. Hepatology. 2000;32:1089‐1095. [DOI] [PubMed] [Google Scholar]
- 38. MacQuillan GC, Mamotte C, Reed WD, Jeffrey GP, Allan JE. Upregulation of endogenous intrahepatic interferon stimulated genes during chronic hepatitis C virus infection. J Med Virol. 2003;70:219‐227. [DOI] [PubMed] [Google Scholar]
- 39. François C, Duverlie G, Rebouillat D, et al. Expression of hepatitis C virus proteins interferes with the antiviral action of interferon independently of PKR‐mediated control of protein synthesis. J Virol. 2000;74:5587‐5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Enomoto N, Sakuma I, Asahina Y, et al. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;334:77‐81. [DOI] [PubMed] [Google Scholar]
- 41. Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon‐inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107‐110. [DOI] [PubMed] [Google Scholar]
- 42. Gerotto M, Dal Pero F, Pontisso P, Noventa F, Gatta A, Alberti A. Two PKR inhibitor HCV proteins correlate with early but not sustained response to interferon. Gastroenterology. 2000;119:1649‐1655. [DOI] [PubMed] [Google Scholar]
- 43. Tokumoto Y, Hiasa Y, Horiike N, et al. Hepatitis C virus expression and interferon antiviral action is dependent on PKR expression. J Med Virol. 2007;79:1120‐1127. [DOI] [PubMed] [Google Scholar]
- 44. Chong KL, Feng L, Schappert K, et al. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992;11:1553‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meurs EF, Galabru J, Barber GN, Katze MG, Hovanessian AG. Tumor suppressor function of the interferon‐induced double‐stranded RNA‐activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koromilas AE, Roy S, Barber GN, Katze MG, Sonenberg N. Malignant transformation by a mutant of the IFN‐inducible dsRNA‐dependent protein kinase. Science. 1992;257:1685‐1689. [DOI] [PubMed] [Google Scholar]
- 47. Barber GN, Jagus R, Meurs EF, Hovanessian AG, Katze MG. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon‐induced enzyme RNA‐dependent protein kinase. J Biol Chem. 1995;270:17423‐17428. [DOI] [PubMed] [Google Scholar]
- 48. Gil J, García MA, Esteban M. Caspase 9 activation by the dsRNA‐dependent protein kinase, PKR: molecular mechanism and relevance. FEBS Lett. 2002;529:249‐255. [DOI] [PubMed] [Google Scholar]
- 49. Yoon CH, Lee ES, Lim DS, Bae YS. PKR, a p53 target gene, plays a crucial role in the tumor‐suppressor function of p53. Proc Natl Acad Sci USA. 2009;106:7852‐7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mounir Z, Krishnamoorthy JL, Robertson GP, et al. Tumor suppression by PTEN requires the activation of the PKR‐eIF2alpha phosphorylation pathway. Sci Signal 2009;2:ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li S, Koromilas AE. Dominant negative function by an alternatively spliced form of the interferon‐inducible protein kinase PKR. J Biol Chem. 2001;276:13881‐13890. [DOI] [PubMed] [Google Scholar]
- 52. Beretta L, Gabbay M, Berger R, Hanash SM, Sonenberg N. Expression of the protein kinase PKR in modulated by IRF‐1 and is reduced in 5q‐associated leukemias. Oncogene. 1996;12:1593‐1596. [PubMed] [Google Scholar]
- 53. Hii SI, Hardy L, Crough T, et al. Loss of PKR activity in chronic lymphocytic leukemia. Int J Cancer. 2004;109:329‐335. [DOI] [PubMed] [Google Scholar]
- 54. Basu S, Panayiotidis P, Hart SM, et al. Role of double‐stranded RNA‐activated protein kinase in human hematological malignancies. Cancer Res. 1997;57:943‐947. [PubMed] [Google Scholar]
- 55. Blalock WL, Grimaldi C, Fala F, et al. PKR activity is required for acute leukemic cell maintenance and growth: a role for PKR‐mediated phosphatase activity to regulate GSK‐3 phosphorylation. J Cell Physiol. 2009;221:232‐241. [DOI] [PubMed] [Google Scholar]
- 56. Terada T, Maeta H, Endo K, Ohta T. Protein expression of double‐stranded RNA‐activated protein kinase in thyroid carcinomas: correlations with histologic types, pathologic parameters, and Ki‐67 labeling. Hum Pathol. 2000;31:817‐821. [DOI] [PubMed] [Google Scholar]
- 57. Haines GK III, Becker S, Ghadge G, Kies M, Pelzer H, Radosevich JA. Expression of the double‐stranded RNA‐dependent protein kinase (p68) in squamous cell carcinoma of the head and neck region. Arch Otolaryngol Head Neck Surg. 1993;119:1142‐1147. [DOI] [PubMed] [Google Scholar]
- 58. He Y, Correa AM, Raso MG, et al. The role of PKR/eIF2α signaling pathway in prognosis of non‐small cell lung cancer. PLoS ONE. 2011;6:e24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim SH, Gunnery S, Choe JK, Mathews MB. Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon‐inducible protein kinase, PKR. Oncogene. 2002;21:8741‐8748. [DOI] [PubMed] [Google Scholar]
- 60. Kim SH, Forman AP, Mathews MB, Gunnery S. Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene. 2000;19:3086‐3094. [DOI] [PubMed] [Google Scholar]
- 61. Roh MS, Kwak JY, Kim SJ, et al. Expression of double‐stranded RNA‐activated protein kinase in small‐size peripheral adenocarcinoma of the lung. Pathol Int. 2005;55:688‐693. [DOI] [PubMed] [Google Scholar]
- 62. Shimada A, Shiota G, Miyata H, et al. Aberrant expression of double‐stranded RNA‐dependent protein kinase in hepatocytes of chronic hepatitis and differentiated hepatocellular carcinoma. Cancer Res. 1998;58:4434‐4438. [PubMed] [Google Scholar]
- 63. Hiasa Y, Kamegaya Y, Nuriya H, et al. Protein kinase R is increased and is functional in hepatitis C virus‐related hepatocellular carcinoma. Am J Gastroenterol. 2003;98:2528‐2534. [DOI] [PubMed] [Google Scholar]
- 64. Watanabe T, Hiasa Y, Tokumoto Y, et al. Protein kinase R modulates c‐Fos and c‐Jun signaling to promote proliferation of hepatocellular carcinoma with hepatitis C virus infection. PLoS ONE. 2013;8:e67750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Milde‐Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449‐2461. [DOI] [PubMed] [Google Scholar]
- 66. Shaulian E. AP‐1–The Jun proteins: oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22:894‐899. [DOI] [PubMed] [Google Scholar]
- 67. Wang X, Dong JH, Zhang WZ, et al. Double stranded RNA‐dependent protein kinase promotes the tumorigenic phenotype in HepG2 hepatocellular carcinoma cells by activating STAT3. Oncol Lett. 2014;8:2762‐2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lin C, Zhang J. Inflammasomes in inflammation‐induced cancer. Front Immunol 2017;8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cholankeril G, Patel R, Khurana S, Satapathy SK. Hepatocellular carcinoma in non‐alcoholic steatohepatitis: current knowledge and implications for management. World J Hepatol. 2017;9:533‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nakamura T, Furuhashi M, Li P, et al. Double‐stranded RNA‐dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yoon CH, Lee ES, Lim DS, Bae YS. PKR, a p53 target gene, plays a crucial role in the tumor‐suppressor function of p53. Proc Natl Acad Sci USA. 2009;12:7852‐7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mounir Z, Krishnamoorthy JL, Robertson GP, et al. Tumor suppression by PTEN requires the activation of the PKR‐eIF2alpha phosphorylation pathway. Sci Signal 2009;22:ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]


